Abstract
Following injury to different tissues, macrophages can contribute to both regenerative and fibrotic healing. These seemingly contradictory roles of macrophages may be related to the markedly different phenotypes that macrophages can assume upon exposure to different stimuli. We hypothesized that fibrotic healing after traumatic muscle injury would be dominated by a pro-fibrotic M2a macrophage phenotype, with M1 activation limited to the very early stages of repair. We found that macrophages accumulated in lacerated mouse muscle for at least 21 days, accompanied by limited myofiber regeneration and persistent collagen deposition. However, muscle macrophages did not exhibit either of the canonical M1 or M2a phenotypes, but instead upregulated both M1- and M2a-associated genes early after injury, followed by downregulation of most markers examined. Particularly, IL-10 mRNA and protein were markedly elevated in macrophages from 3 day injured muscle. Additionally, though flow cytometry identified distinct subpopulations of macrophages based on high or low expression of TNFα, these subpopulations did not clearly correspond to M1 or M2a phenotypes. Importantly, cell therapy with exogenous M1 macrophages but not non-activated macrophages reduced fibrosis and enhanced muscle fiber regeneration in lacerated muscles. These data indicate that manipulation of macrophage function has potential to improve healing following traumatic injury.
Keywords: Macrophage, tissue repair, inflammation, skeletal muscle, injury
Introduction
Skeletal muscle injuries are among the most common injuries suffered during sporting activities, military combat, and accidents of daily life. Repair of the muscle after traumatic or exercise-induced injury is prolonged and incomplete. Return to pre-injury activity often takes months, while injured muscle tissue is replaced by fibrotic scar tissue, which persists indefinitely [1]. Muscle force production is decreased due to the non-contractile nature of the scar, and the scar site is highly susceptible to re-injury [1]. In order to develop improved treatments for traumatic muscle injuries, a better understanding of the mechanisms underlying muscle regeneration and fibrosis is necessary.
Numerous studies have demonstrated that macrophages are essential for skeletal muscle regeneration [2–4]; however macrophages have also been implicated in fibrosis of numerous tissues [5–8]. The diverse functions of macrophages in tissue repair may be explained in part by the remarkable plasticity of these cells, which exhibit diverse and often opposing phenotypes when exposed to different environmental stimuli. Inflammatory stimuli such as bacterial components or IFNγ produce a “classically activated” macrophage phenotype, also called “M1”, characterized by production of reactive oxygen and nitrogen species and pro-inflammatory cytokines [9]. Additionally, M1 macrophages promote myoblast proliferation and reduce fibroblast collagen production in culture [2,10–12]. Macrophages also exhibit a spectrum of non-inflammatory, “alternatively activated” phenotypes, also referred to as “M2” activation [9,13]. One of the most well described M2 phenotypes, “M2a”, results from stimulation with IL-4 or IL-13, which increases macrophage expression of the mannose receptor CD206 and the scavenger receptor CD36 [14–17]. Additionally, because M2a macrophages express high levels of TGFβ and arginase in vitro [12,18], these cells are frequently termed “wound healing” macrophages [13,19–21] and have been implicated in fibrosis of numerous tissues [5,22–25]. In culture, M2a macrophages promote myoblast differentiation and fusion, but also increase collagen production by fibroblasts [2,12]. However, current knowledge of macrophage phenotype is largely derived from in vitro studies, and an M1 or M2a phenotype of macrophages in vivo is frequently extrapolated based solely on expression of a small set of phenotypic “markers” [26–30]. Due to the remarkable responsiveness of macrophages to their environment, in vitro-defined phenotypes rarely have precise in vivo counterparts [13,31], and convincing evidence that macrophages of a strictly M1 or M2a phenotype are present during tissue repair in vivo is lacking.
After toxin-induced muscle injuries that result in good regenerative outcomes, invading macrophages transition from a pro-inflammatory to an anti-inflammatory phenotype, then disappear from the muscle as healing progresses [2,3]. However, whether macrophages during the different stages of muscle healing correspond to in vitro-defined M1 and M2 phenotypes remains to be determined. Additionally, little is known about the contribution of macrophages to repair of exercise- or trauma-induced muscle injuries, which are more representative of common human injuries and result in persistent fibrous scars.
We hypothesized that fibrosis after traumatic skeletal muscle injury would be accompanied by prolonged macrophage accumulation, and that macrophages would be biased toward the pro-fibrotic M2a phenotype, especially during later stages of repair. We also hypothesized that cell therapy with exogenously activated M1 macrophages would enhance regeneration and reduce fibrosis. However, while persistent macrophage accumulation was observed after laceration, macrophages did not exhibit either of the canonical in vitro-defined phenotypes. Instead, macrophages upregulated both M1 and M2a markers early after injury, followed by an apparent deactivation. Although flow cytometry identified distinct subpopulations of macrophages at day 3 based on high or low expression of TNFα, these did not clearly correspond to M1 or M2a phenotypes. In contrast to the common view that M1 macrophages exacerbate tissue damage [13,20,32], treatment of injured muscles with exogenous M1 macrophages reduced fibrosis and enhanced muscle regeneration.
Materials and Methods
An expanded Materials and Methods is available as supplemental material.
Muscle Injury
Bilateral laceration of the gastrocnemius muscles of C57BL/6 mice was adapted from a previously described protocol [33]. Briefly, mice were anesthetized, a longitudinal incision was made on the posterior hindlimb, and a scalpel was used to lacerate the muscle transversely at its widest point through the entire thickness of the lateral half of the muscle, taking care to preserve the central neurovascular complex. The skin was closed, and the procedure was repeated on the contralateral leg.
Ethical Aspects
The Animal Care Committee at the University of Illinois at Chicago approved all experimental procedures.
Histology
Cryosections from the center of the injury site were stained by hematoxylin and eosin, Masson's trichrome, or immunohistochemistry. Regeneration was quantified in hematoxylin and eosin-stained sections, with normal or regenerating fibers identified by peripherally or centrally located nuclei, respectively, without evidence of damage; damaged area was defined as area not occupied by either normal or regenerating muscle fibers. Collagen accumulation was quantified as percent blue area in cryosections stained by Masson's trichrome. Macrophage or neutrophil accumulation was quantified as percent F4/80 or Ly6G-stained area, respectively, by standard immunohistochemical methods.
Muscle macrophage isolation by MACS
Lateral heads of the gastrocnemius muscles were enzymatically digested; cell suspensions were depleted of neutrophils, T-cells, and B-cells by MACS negative selection with anti-Ly6G, anti-CD3, and anti-CD19 (Biolegend and Miltenyi Biotec); and macrophages were isolated by MACS positive selection with anti-CD11b (Miltenyi Biotec). Thus, macrophages were obtained as the CD11b-positive, Ly6G/CD3/CD19-negative fraction.
Culture and activation of bone marrow-derived macrophages
Bone marrow derived macrophages were cultured as previously described [3], and day 6 cultures were treated with 10ng/ml each IFNγ and TNFα, or 10ng/ml IL-4, or 10ng/ml IL-10 to obtain M1, M2a, and M2c macrophages, respectively. After 24h of cytokine exposure, macrophages were harvested for RNA isolation or macrophage cell therapy.
RNA isolation and real-time PCR
Total RNA was isolated from muscle-derived macrophages and bone marrow derived macrophages using the RNeasy kit (Qiagen) and reverse-transcribed using the Thermoscript RT-PCR system (Invitrogen). Real-time PCR was performed using TaqMan Gene Expression Assays (Applied Biosystems) for mouse IL-1β, TNFα, inducible nitric oxide synthase (iNOS), IDO1, CXCL10, CD36, CD206, TGFβ, Retnla (Ym1), IL-10, and GAPDH. Relative expression was determined using the 2−ΔΔCT method [34]. Supplemental Figure 1 presents data from the same set of experiments, normalized as 2−ΔCT.
IL-10 secretion
Muscle-derived macrophages were cultured for 20 hours, conditioned media were collected and centrifuged, and the supernatant was analyzed for IL-10 using the Mouse IL-10 ELISA Ready-SET-Go kit (eBioscience).
Flow cytometry
Total cells present in muscle following laceration injury were isolated via enzymatic digest. Fcγ receptors were blocked with anti-mouse CD16/CD32 (BD Biosciences), and extracellular antigens were labeled with FITC-, Alexa488-, or PE-conjugated antibodies against F4/80 (Biolegend), CD206, or CD36 (Serotec), or with non-specific isotype controls. Cells were then fixed and permeabilized, and intracellular antigens were labeled with PE-, PerCP-Cy5.5-, or APC-conjugated antibodies against TGFβ, TNFα (Biolegend), or IL-10 (eBioscience), or with non-specific isotype controls.
Macrophage cell therapy
Gastrocnemius muscles were lacerated and bone marrow macrophages were grown as described above. Injection of M1 macrophages, non-activated macrophages, or PBS vehicle was performed at 7 days post-laceration. After an additional 7 or 14 days (i.e. 14 or 21 days post-injury), muscles were collected for histological analysis of regeneration and fibrosis.
Results
Muscle laceration heals by both regeneration and fibrosis
Consistent with previous studies [35,36], lacerated gastrocnemius muscle healed by a combination of regeneration and fibrosis. At 3 days post-laceration, the injured site was largely comprised of loose granulation tissue and degenerating myofibers (Figure 1A & E). By 7 days post-injury, centrally nucleated regenerating myofibers began to appear. The percent of total muscle area occupied by regenerating myofibers increased from 7 to 14 days post-injury, with a corresponding decrease in damaged, non-regenerated area. Subsequently, at 21 and 28 days post-injury, there was a gradual increase in percent area composed of mature, peripherally nucleated myofibers and a corresponding decrease in regenerating area. However, the percent damaged, non-regenerated area did not significantly decrease below ~40% after 14 days post-injury, indicating incomplete regeneration (Figure 1A). This contrasts with healing after toxin-induced muscle injury, which results in complete regeneration [3,37,38]. Furthermore, after 14 days, injured muscles contained a larger total number of myofibers versus uninjured (Figure 1B). However, the average cross sectional area of individual myofibers failed to increase with time (Figure 1C), remaining at ~30% of the average uninjured myofiber size. Additionally, collagen accumulation was evident by 14 days post-injury, and this fibrosis persisted for at least 28 days (Figure 1D & E).
Figure 1. Lacerated muscle heals by both regeneration and fibrosis.
Gastrocnemius muscles were lacerated and collected for histological analysis at the indicated time-points. For each muscle, sections with the largest percent damaged area were selected for analysis. Uninjured muscles served as controls. (A) Percent of total cross-sectional area occupied by centrally nucleated (“Regenerating”) or peripherally nucleated (“Normal”) fibers was quantified in five 40× fields per muscle in hematoxylin and eosin stained sections. “Damaged” area was defined as area not occupied by either type of myofiber. (B) Number of myofibers per mm2 in uninjured and injured muscles. (C) Average cross-sectional area of individual myofibers. (D) Collagen accumulation was quantified as percent blue pixels in three to six 20× fields per muscle in Masson's trichrome stained sections. (E) Representative images of trichrome stained sections in uninjured muscle and at indicated time points post-injury. Data are presented as mean +/− SD. * p<0.05 versus uninjured. n=2–5 per time point. In (A), * indicates significance for percent damage area.
Muscle laceration results in persistent macrophage accumulation, but these macrophages are not of M1 or M2a phenotype
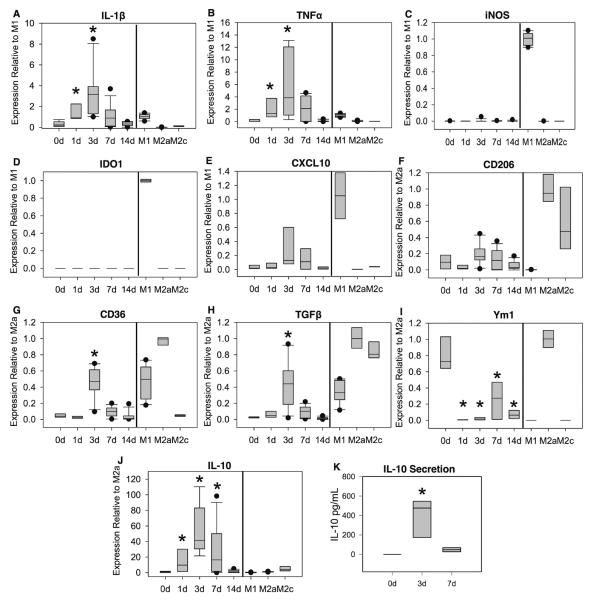
Macrophage accumulation was elevated by 3 days post-injury, peaked around 7 days, and subsequently declined (Figure 2A). However, macrophage accumulation remained elevated over uninjured levels for at least 21 days post-injury. In contrast, neutrophil accumulation peaked around 1–3 days post-injury, then rapidly returned to uninjured levels (Figure 2B). To determine whether macrophages transition from an early M1-like to a prolonged M2a-like phenotype, we isolated macrophages from injured and uninjured muscles by magnetic separation and examined expression of M1 and M2a-associated genes. Compared to resident macrophages isolated from non-injured muscle, expression of the M1-associated cytokines IL-1β and TNFα were elevated in muscle macrophages at 1 and 3 days post-injury and subsequently declined towards uninjured levels (Figure 3A&B). Macrophage expression of both of these cytokines at 1 and 3 days post-injury was comparable to that of in vitro activated M1 macrophages. In contrast, message levels of iNOS and IDO1 were low or undetectable in muscle macrophages at all time points, despite robust expression in cultured M1 macrophages (Figure 3C&D). Additionally, the M1-associated chemokine CXCL10 exhibited a trend for increased expression in muscle macrophages after injury (Figure 3E; ANOVA on ranks p=0.054 for time effect), but remained low in comparison to in vitro activated M1 macrophages. Contrary to expectations of an M1-to-M2a transition, expression of the M2a marker CD206 was not elevated over resident macrophage expression at any time point after injury, and was expressed at low levels by muscle macrophages compared to in vitro M2a positive controls (Figure 3F). Furthermore, macrophage expression of the M2-associated genes CD36, TGFβ, and IL-10 peaked relatively early at 3 days post-injury (Figure 3G, H, & J). Ym1 expression in resident macrophages was comparable to that of in vitro activated M2a macrophages (Figure 3I); after injury, muscle macrophage expression of Ym1 was significantly decreased for at least 14 days relative to resident macrophage expression, with the most pronounced reduction occurring at 1–3 days after injury (Figure 3I).
Figure 2. Muscle laceration results in prolonged accumulation of macrophages but not neutrophils.
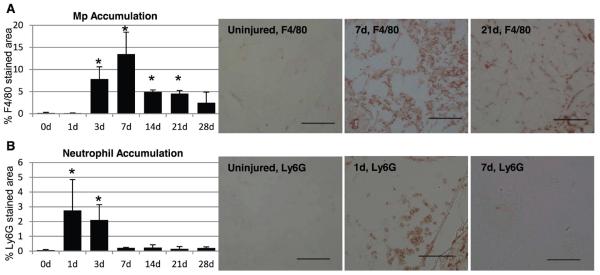
Gastrocnemius muscles were lacerated and collected for histological analysis at the indicated time-points. For each muscle, sections with the largest percent damaged area were selected for analysis. Uninjured muscles (0d) served as controls. (A) Macrophage accumulation was quantified as percent F4/80-stained area in three to six 20x fields per muscle. (B) Neutrophil accumulation was quantified as percent Ly6G-stained area in three to six 20x fields per muscle. Data are presented as mean +/− SD. * p<0.05 versus uninjured. n=2–6 per time point. Representative 20x images are shown for F4/80 (A) and Ly6G (B) labeling in uninjured muscle, at peak macrophage or neutrophil accumulation, and after peak accumulation. Scale bar =100μm.
Figure 3. Muscle macrophage phenotype.
Macrophages were isolated by magnetic separation from uninjured (0d) or lacerated gastrocnemius muscles at the indicated time points. Muscle macrophages were obtained as the CD11b-positive, Ly6G/CD3/CD19-negative cell fraction. As positive and negative controls, bone marrow derived macrophages (right side of vertical bar in panels A–J) were activated with IFNγ and TNFα (M1), IL-4 (M2a), or IL-10 (M2c). Total RNA was isolated and reverse transcribed, and expression of IL-1β (A), TNFα (B), iNOS (C), IDO1 (D), CXCL10 (E), CD206 (F), CD36 (G), TGFβ (H), Ym1 (I), and IL-10 (J) was analyzed by real-time PCR. Expression of each gene was determined by the 2−ΔΔCT method using GAPDH as endogenous control. M1-associated genes (A–E) were normalized to in vitro activated M1 macrophages, and M2-associated genes (F–J) were normalized to in vitro activated M2a macrophages. (K) Macrophages were isolated by magnetic separation from uninjured or injured muscles, equal numbers of macrophages were incubated for 20 hours, and IL-10 secretion was measured by ELISA on the conditioned medium. Data did not pass tests of normality and equal variance, and are presented with center line as median, boxes representing the 25th and 75th percentiles, whiskers representing the 10th and 90th percentiles, and outliers as dots. * p<0.05 versus macrophages from uninjured (0d) muscle. n=5–10 per time-point. In vitro activated macrophages were not included in statistical comparisons.
In contrast to the relatively modest upregulation of CD36 and TGFβ in muscle macrophages after injury, median macrophage expression of IL-10 at 3 days post-injury was elevated approximately 11- and 40-fold over in vitro M2c and M2a positive controls, respectively, and 40-fold versus resident muscle macrophage expression (Figure 3J). To determine whether this powerfully anti-inflammatory cytokine was also upregulated at the protein level, we isolated macrophages from injured and uninjured muscles and measured cytokine secretion. Resident macrophages from uninjured muscle did not secrete detectable amounts of IL-10. However, IL-10 secretion was markedly elevated in macrophages from 3 day injured muscle, and declined by 7 days post-injury (Figure 3K). Interestingly, in vitro-activated M2a and M2c bone marrow-derived macrophages did not secrete detectable amounts of IL-10 (not shown).
Though the absence of iNOS and IDO1 and low levels of CD206 and Ym1 mRNA expression indicate that canonical M1 and M2a macrophages are not present in lacerated muscle, these mRNA data do not preclude the presence of separate M1-like and M2-like populations, which could result in the observed simultaneous expression of M1- and M2a-associated genes at 3 days post-injury. Alternatively, mixed gene expression at day 3 may reflect a homogeneous macrophage population that exhibits both M1-like and M2-like characteristics. To distinguish between these possibilities, we analyzed intracellular cytokine content and cell surface activation markers by flow cytometry. At 3 days post-injury, muscle macrophages (gated as F4/80+ cells (Figure 4A&C)) were separable into distinct TNFα-high and –low subpopulations (Figure 4E). Both TNFα-high and -low macrophages exhibited similar levels of TGFβ and IL-10 expression (Figure 4E&F). CD206 was barely detectable over isotype control labeling (not shown), consistent with low levels of mRNA expression. CD36 exhibited a wide range of expression; however, CD36-high and -low macrophages exhibited similar levels of IL-10 and IL-1β (Figure 4G&H). There was also no clear correlation, negative or otherwise, between expression of CD36 and expression of TNFα. While a distinct population of TNFα-high, CD36-mid macrophages was observed, macrophages at all levels of CD36 expression exhibited a wide range of TNFα expression (Figure 4I). Taken together, these data indicated that muscle macrophages at 3 days post-injury do not clearly correspond to M1 and M2a phenotypes, which would be expected to be TNFα-high//IL-1β-high/CD36-low/IL-10-low and TNFα-low/IL-1β-low/CD36-high/IL-10-high, respectively. Nonetheless, day 3 macrophages can be partitioned based on high or low TNFα expression, which may correspond to more general pro- and non-/anti-inflammatory phenotypes.
Figure 4. Macrophages at 3 days after muscle laceration are not separable into M1 and M2a subsets.
Cells were isolated from gastrocnemius muscles at 3 days post-injury and labeled for flow cytometry. Macrophages were defined as FITC-F4/80+ cells (A) or PEF4/80+ cells (C), with lower threshold set based on background FL1 (B) or FL2 (D) fluorescence in unlabeled cells. Density plots display macrophage expression of TGFβ versus TNFα (E), IL-10 versus TNFα (F), CD36 versus IL-10 (G), CD36 versus IL-1b (H) and CD36 versus TNFα (I). In panel G, cells in the upper left quadrant (arrow) likely represent non-specific labeling, as this population was also seen in IgG control plots (not shown). Data are representative of 5 independent experiments of n=1–2 per experiment.
Cell therapy with M1 macrophages reduces muscle fibrosis and enhances myofiber regeneration after traumatic injury
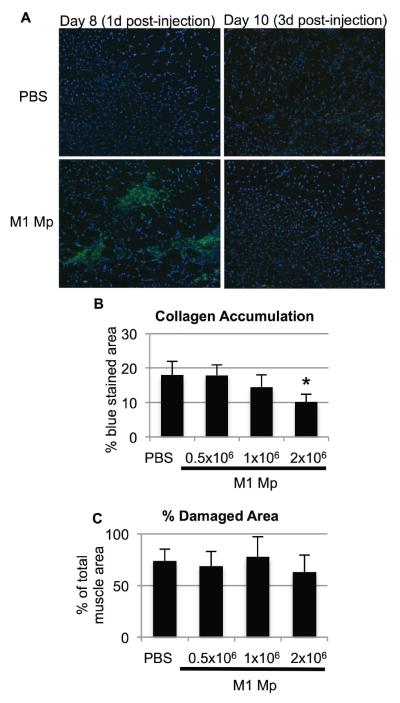
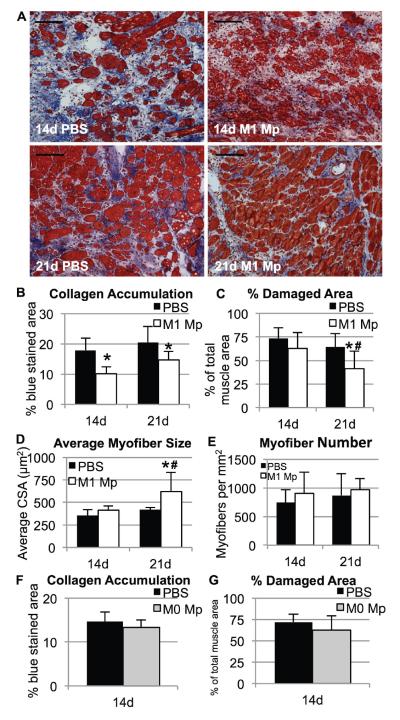
While canonical M1 and M2a macrophages do not appear to be present in lacerated muscle, in vitro studies have demonstrated that M1 macrophages can enhance myoblast proliferation and decrease fibroblast collagen production [2,12]. Therefore, we hypothesized that cell therapy with exogenous M1 macrophages would improve muscle healing after laceration. Bone marrow-derived macrophages were cultured and activated to an M1 phenotype, then injected into lacerated gastrocnemius muscles at 7 days post-injury. Preliminary experiments showed that injected M1 macrophages remained within the muscle for only a few days, as CFSE-labeled donor macrophages were detected within the muscle at 1 day post-injection but not at 3 days (Figure 5A); similar results were obtained using macrophages from CD45.1 donor mice (not shown). Cell therapy with M1 macrophages caused a dose-dependent reduction in fibrosis at 14 days post injury; 2×106 M1 macrophages produced a significant reduction in collagen accumulation, while lower doses produced a non-significant trend or had no effect (Figure 5B&C). Therefore, a dose of 2×106 M1 macrophages was used for all subsequent experiments. M1 macrophage cell therapy significantly reduced collagen accumulation in lacerated muscles at 14 and 21 days post-injury (Figure 6A&B) and also reduced percent damaged area at 21 days post-injury (Figure 6C). This reduction in damaged area was due to an increase in myofiber cross-sectional area (Figure 6D); myofiber number was unaffected by M1 macrophage therapy (Figure 6E). Importantly, M1 activation was required for macrophage cell therapy to be effective, since injection of 2×106 non-activated bone marrow-derived macrophages did not alter collagen accumulation or regeneration at 14 days post-injury (Figure 6F&G). In summary, cell therapy with exogenous M1 macrophages reduces fibrosis and enhances regeneration in lacerated skeletal muscle.
Figure 5. Dose-dependent reduction in collagen accumulation with M1 macrophage cell therapy.
PBS vehicle or 0.5×106, 1.0×106, or 2.0×106 M1-activated bone marrow-derived macrophages were injected into lacerated gastrocnemius muscles at 7 days post-injury. (A) Donor macrophages were labeled with CFSE (green) prior to injection, muscles were collected at the indicated time points, and cryosections were mounted with DAPI to label nuclei (blue). (B) Collagen accumulation at 14 days post-injury (i.e. 7 days post-injection) was quantified as percent blue pixels in three to six 20x fields per muscle in Masson's trichrome stained sections. (C) Damaged area (i.e. area not occupied by myofibers) at 14 days post-injury was quantified in five 40x fields per muscle in hematoxylin and eosin stained sections. Data are presented as mean +/− SD. * p<0.05 versus PBS control. n=4–8 muscles per time point and treatment condition.
Figure 6. Cell therapy with M1 macrophages reduces collagen accumulation and enhances muscle regeneration.
(A–E) M1-activated bone marrow-derived macrophages or PBS vehicle were injected into lacerated gastrocnemius muscles at 7 days post-injury and analyzed at 14 or 21 days post-injury. (A) Representative trichrome stained images of lacerated muscles treated with PBS (left) or 2 ×106 M1 macrophages (right) at 14 (top) or 21 (bottom) days post-injury. Scale bar = 100μm. (B) Collagen accumulation was quantified as percent blue pixels in three to six 20x fields per muscle in Masson's trichrome stained sections from muscles injected with PBS vehicle or 2 ×106 M1 macrophages. (C) Damaged area (i.e. area not occupied by myofibers) was quantified in five 40x fields per muscle in hematoxylin and eosin stained sections. (D) Average cross-sectional area of individual myofibers in hematoxylin and eosin stained sections. (E) Number of myofibers per mm2 in hematoxylin and eosin stained sections. (F&G) 2 ×106 non-activated bone marrow-derived macrophages or PBS vehicle were injected into lacerated gastrocnemius muscles at 7 days post-injury and analyzed at 14 days post-injury. (F) Collagen accumulation was quantified as percent blue pixels in trichrome stained sections. (G) Damaged area (i.e. area not occupied by myofibers) was quantified in hematoxylin and eosin stained sections. Data are presented as mean +/− SD. * p<0.05 versus PBS control at same time point; # p<0.05 versus 14d time point within same treatment group. n=4–7 per time point and treatment condition.
Discussion
Macrophages are essential for efficient muscle regeneration but can also contribute to fibrosis across numerous tissues. These contrasting roles of macrophages during tissue repair may be due to the ability of macrophages to assume a variety of functional phenotypes upon exposure to different stimuli. Data from the present study demonstrate prolonged macrophage accumulation during fibrotic healing after traumatic muscle injury. However, macrophages in injured muscle exhibited neither a strictly M1 nor M2a phenotype. Instead, macrophages at early time points after injury expressed a mix of both M1- and M2a associated markers, followed by a decrease in expression of most markers examined. In particular, IL-10 mRNA and protein secretion were highly elevated in macrophages from 3 day injured muscle. Additionally, though pro- and non-inflammatory macrophage subpopulations were identified based on levels of TNFα, these did not clearly correspond to M1 or M2a phenotypes. Importantly, cell therapy with M1 macrophages reduced collagen accumulation and enhanced regeneration in injured muscles. These findings improve our understanding of macrophage phenotypes during in vivo tissue repair following traumatic injury, and provide insight into potential macrophage-based therapies to improve muscle healing.
While macrophages are widely acknowledged to exhibit a broad spectrum of phenotypes that can only be partially mimicked in culture, the supposition that M2a or M2a-like macrophages perform tissue repair or wound healing functions in vivo remains remarkably persistent [13,19–21]. In cell culture, M2a macrophages produce a variety of factors that are important for tissue repair, including TGFβ, arginase, growth factors, and ECM components [12,13,18]. In vivo, M2a “markers” such as CD206 and arginase are expressed by macrophages in skin wounds [39,40], dystrophic muscle lesions [24,26], and ischemic kidneys [28]. However, while M2a macrophages are defined in vitro by IL-4/-13 activation, neither IL-4 nor its receptor is required for expression of CD206 by wound macrophages [40]. Additionally, CD206 is expressed at similar levels by wound macrophages with both high and low expression of the M1-associated cytokine TNFα [40]. These data indicate that bona fide M2a macrophages are not present in murine skin wounds [41], and caution against extrapolating a complete macrophage phenotype from a small number of phenotypic “markers” such as CD206.
The present study assessed a battery of mRNA and protein markers associated with M1 and M2a activation, and found that macrophages after muscle laceration injury do not exhibit either strictly M1 or M2a phenotypes. The muscle macrophage population at 1 day post-injury bears only a limited resemblance to in vitro M1 macrophages: both types of macrophages express similarly high levels of IL-1β and TNFα and low levels of CD206; however, IL-10 is expressed by day 1 muscle macrophages but not M1 macrophages, and iNOS and IDO1 are expressed in M1 macrophages but low or absent in muscle macrophages (Figure 3). Subsequently, at 3 days post-injury both M1 and M2a markers are expressed simultaneously by muscle macrophages, followed by a decrease in expression of most markers examined. At 3 days post-injury, muscle-derived macrophages were comparable to cultured M1 macrophages in expression levels of numerous genes, including IL-1β, TNFα, CD206, CD36, and TGFβ (Figure 3). However, very high expression of IL-10, low expression of CXCL10, and complete absence of iNOS and IDO1 in day 3 muscle macrophages indicate that these macrophages are not entirely of the M1 phenotype. Additionally, contrary to our hypothesis of an M1-to-M2a switch, macrophage expression of the M2a-associated genes CD36 and TGFβ decreased after day 3, Ym1 expression was low at all time points after injury, and CD206 expression was not significantly elevated over resident macrophage expression at any time point and was very low compared to cultured M2a macrophages (Figure 3). A limitation of this study is that direct comparisons of gene expression between cultured macrophages and muscle-derived macrophages are necessarily complicated by the additional isolation steps needed to obtain macrophages from muscles, and potential phenotypic changes during digestion and magnetic separation cannot be ruled out. However, muscle-derived macrophages at all time points underwent identical processing, and therefore relative gene expression within the in vivo time course should not be affected by the isolation procedure. In summary, data from the present study indicate that, during repair of skeletal muscle laceration, the macrophage population transitions from an early heterogeneous and hybrid phenotype expressing both M1- and M2-associated markers to an apparently deactivated phenotype during the later stages of repair.
At 3 days post-injury, muscle macrophages exhibited high levels of IL-10 mRNA expression and protein secretion, which may contribute to the apparent macrophage deactivation observed at subsequent time points. IL-10 is a powerful anti-inflammatory cytokine that can suppress M1 activation of macrophages [42–44] and may also inhibit M2a activation [45]. In addition to modulating activation by other cytokines, IL-10 exposure in vitro produces an “M2c” phenotype [9]; however, the mRNA expression profile of muscle macrophages at 14 days post-injury bore only a partial resemblance to in vitro activated M2c macrophages (Figure 3). Specifically, while M2c macrophages and 14 day muscle macrophages both exhibited low expression of pro-inflammatory cytokines and CD36, expression of TGFβ and CD206 was considerably lower in 14 day muscle macrophages versus M2c macrophages. These data, while limited, suggest that muscle macrophages during the later stages of repair are not analogous to in vitro activated M2c macrophages, despite the ample production of IL-10 by muscle macrophages during earlier stages of repair. Future studies will determine whether IL-10 production by muscle macrophages contributes to their subsequent deactivation.
While the macrophage populations present after muscle injury do not conform to M1/M2 classification, knowledge of the actual phenotypes present may provide insight into strategies for improving healing. For example, the apparent deactivated phenotype present during the later stages of repair suggests that increasing macrophage activation may improve healing. Exogenous M1 macrophages have previously been shown to improve engraftment of human myoblasts into injured or dystrophic muscles of immunodeficient mice [46]; however this previous study did not examine regeneration or fibrosis after injury. In the present study, donor M1 macrophages reduced collagen accumulation and enhanced myofiber regeneration in injured muscle, while non-activated macrophages had no effect. A limitation of this study is that we have not yet characterized potential phenotypic changes of exogenous M1 macrophages, and have only begun to examine their mechanism of action. Introduction of M1 macrophages into the muscle may accelerate clearance of necrotic myofibers, though phagocytosis was not directly measured in this study. In vitro studies have shown that M1 macrophages promote myoblast proliferation and suppress fibroblast collagen production [2,12]; these mechanisms may also contribute to improved healing and reduced fibrosis with M1 macrophage therapy. However, M1 macrophage therapy did not alter the number of regenerating myofibers but instead increased myofiber cross sectional area, arguing against increased myoblast proliferation as the mechanism of improved healing. Instead, accelerated debris clearance and/or reduction of collagen accumulation with M1 macrophage therapy may allow for myofiber hypertrophy and enhanced regeneration. Alternately, exogenous M1 macrophages may alter their phenotype, or the phenotypes of endogenous macrophages, upon delivery to the injured muscle, resulting in production of factors promoting myoblast differentiation and fusion [2,46]. Future studies will address potential phenotypic changes of exogenous M1 macrophages, as well as interactions between exogenous and endogenous macrophages.
Macrophage cell therapy has been useful in improving healing of numerous tissues, and activation to an M1, M2a, or other state may alter their ability to promote repair [6,47–51]. Our present demonstration of the beneficial effects of M1 macrophage therapy after muscle injury contrast with a previous study of chronic renal inflammatory disease, in which transfusion of M1 macrophages increased fibrosis and renal injury, treatment with M2a macrophages reduced fibrosis, and non-activated macrophages had no effect [6]. Prior macrophage activation may not be necessary to promote repair in all tissues, however, as naive bone-marrow derived macrophages reduced fibrosis in a mouse model of liver injury [47]. A variant of macrophage cell therapy has also been used to enhance healing of human wounds. Hypoosmotically activated blood cell suspensions, which contain monocytes/macrophages and other leukocytes [52], enhance healing of ulcers in elderly and diabetic patients [48–50] and also reduce mortality and duration of hospitalization in patients with infected sternal wounds [51]. Together, these studies in rodents and humans highlight the promise of macrophage cell therapy for enhancement of tissue repair, but also indicate that the ideal macrophage phenotype may differ based on the specific needs of the damaged tissue. Additionally, the well-known plasticity of macrophages may allow for phenotypic changes of donor cells upon introduction to a tissue repair environment; this may complicate maintenance of an “ideal” phenotype by the donor cells, but also presents opportunities for investigation of factors that regulate macrophage phenotype in vivo, or potentially guiding phenotypic changes of macrophages over the course of healing.
In conclusion, this study demonstrates that macrophages during repair of traumatic skeletal muscle injuries do not correspond to in vitro-defined M1 or M2a phenotypes. Instead, the muscle macrophage population exhibits a transition from an early heterogeneous and hybrid phenotype to a subsequent deactivated state, possibly due to macrophage production of IL-10. Because the functions of such hybrid macrophages are difficult to predict, we suggest that, for future studies, investigation of macrophage functions important for tissue repair, such as phagocytosis or matrix remodeling, may prove more useful than attempts to match endogenous tissue repair macrophages to in vitro-defined phenotypic categories that may not actually exist in vivo. The present study also provides proof-of-concept for in vitro-activated macrophages as cell therapy, as exogenous M1 macrophages decreased fibrosis and enhanced regeneration in lacerated muscle. An improved understanding of the reciprocal regulation of macrophage phenotype and the tissue repair environment may help to develop and refine macrophage-based therapies to promote healing.
Supplementary Material
Supplemental Figure 1. Muscle macrophage phenotype. Macrophages were isolated by magnetic separation from uninjured (0d) or lacerated gastrocnemius muscles at the indicated time points. RNA was obtained from muscle macrophages and activated bone marrow derived macrophages as described in Materials and Methods. Total RNA was reverse transcribed, and expression of IL-1β (A), TNFα (B), iNOS (C), IDO1 (D), CXCL10 (E), CD206 (F), CD36 (G), TGFβ (H), Ym1 (I), and IL-10 (J) was analyzed by real-time PCR. Expression of each gene is presented relative to GAPDH using the 2−ΔCT method. Data did not pass tests of normality and equal variance, and are presented with center line as median, boxes representing the 25th and 75th percentiles, whiskers representing the 10th and 90th percentiles, and outliers as dots. * p<0.05 versus macrophages from uninjured (0d) muscle. n=5–10 per time-point. In vitro activated macrophages were not included in statistical comparisons.
Acknowledgements
The authors would like to thank MN's dissertation committee, Drs. Alan Diamond, Luisa DiPietro, Giamila Fantuzzi, and John M. Kennedy, for their helpful suggestions throughout the development and execution of this research. This work was funded by Department of Defense grant #W81XWH-05-1-0159 (to TK), NIH grant #R01GM092850 (to TK), American College of Sports Medicine Doctoral Student Research Grant #2011-03604-00-00 (to MN), and University of Illinois at Chicago Graduate College Dean's Scholar Award (to MN).
Footnotes
The authors declare that no conflicts of interest exist and that the manuscript is original work and not currently being considered by another publication.
Author Contributions TK and MN designed the experiments, interpreted data, and wrote the paper. MN carried out the experiments, analyzed data, and generated the figures. EWH carried out the experiments and analyzed data.
Online Supporting Information 1. Supplemental Materials and Methods contains additional experimental detail.
2. Supplemental Figure 1 presents gene expression data normalized by the 2−ΔCT method.
References
- 1.Copland ST, Tipton JS, Fields KB. Evidence-based treatment of hamstring tears. Curr Sports Med Rep. 2009;8:308–314. doi: 10.1249/JSR.0b013e3181c1d6e1. [DOI] [PubMed] [Google Scholar]
- 2.Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryer SC, Fantuzzi G, Van Rooijen N, et al. Urokinase-type plasminogen activator plays essential roles in macrophage chemotaxis and skeletal muscle regeneration. J Immunol. 2008;180:1179–1188. doi: 10.4049/jimmunol.180.2.1179. [DOI] [PubMed] [Google Scholar]
- 4.Summan M, Warren GL, Mercer RR, et al. Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1488–R1495. doi: 10.1152/ajpregu.00465.2005. [DOI] [PubMed] [Google Scholar]
- 5.Spencer M, Yao-Borengasser A, Unal R, et al. Adipose tissue macrophages in insulin-resistant subjects are associated with collagen VI and fibrosis and demonstrate alternative activation. Am J Physiol Endocrinol Metab. 2010;299:E1016–E1027. doi: 10.1152/ajpendo.00329.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Wang YP, Zheng G, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72:290–299. doi: 10.1038/sj.ki.5002275. [DOI] [PubMed] [Google Scholar]
- 7.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villalta SA, Rinaldi C, Deng B, et al. Interleukin-10 reduces the pathology of mdx muscular dystrophy by deactivating M1 macrophages and modulating macrophage phenotype. Hum Mol Genet. 2011;20:790–805. doi: 10.1093/hmg/ddq523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mantovani A, Sica A, Sozzani S, et al. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Chazaud B, Sonnet C, Lafuste P, et al. Satellite cells attract monocytes and use macrophages as a support to escape apoptosis and enhance muscle growth. J Cell Biol. 2003;163:1133–1143. doi: 10.1083/jcb.200212046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cantini M, Giurisato E, Radu C, et al. Macrophage-secreted myogenic factors: a promising tool for greatly enhancing the proliferative capacity of myoblasts in vitro and in vivo. Neurol Sci. 2002;23:189–194. doi: 10.1007/s100720200060. [DOI] [PubMed] [Google Scholar]
- 12.Song E, Ouyang N, Hörbelt M, et al. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 13.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stein M, Keshav S, Harris N, et al. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yesner LM, Huh HY, Pearce SF, et al. Regulation of monocyte CD36 and thrombospondin-1 expression by soluble mediators. Arterioscler Thromb Vasc Biol. 1996;16:1019–1025. doi: 10.1161/01.atv.16.8.1019. [DOI] [PubMed] [Google Scholar]
- 16.Bhattacharjee A, Shukla M, Yakubenko VP, et al. IL-4 and IL-13 employ discrete signaling pathways for target gene expression in alternatively activated monocytes/macrophages. Free Radic Biol Med. 2013;54:1–16. doi: 10.1016/j.freeradbiomed.2012.10.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng J, Han J, Pearce SF, et al. Induction of CD36 expression by oxidized LDL and IL-4 by a common signaling pathway dependent on protein kinase C and PPAR-gamma. J Lipid Res. 2000;41:688–696. [PubMed] [Google Scholar]
- 18.Louis CA, Mody V, Henry WL, et al. Regulation of arginase isoforms I and II by IL-4 in cultured murine peritoneal macrophages. Am J Physiol. 1999;276:R237–R242. doi: 10.1152/ajpregu.1999.276.1.R237. [DOI] [PubMed] [Google Scholar]
- 19.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moyer AL, Wagner KR. Regeneration versus fibrosis in skeletal muscle. Curr Opin Rheumatol. 2011;23:568–573. doi: 10.1097/BOR.0b013e32834bac92. [DOI] [PubMed] [Google Scholar]
- 21.Mahdavian Delavary B, van der Veer WM, van Egmond M, et al. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Prasse A, Pechkovsky DV, Toews GB, et al. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–792. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 23.Reiman RM, Thompson RW, Feng CG, et al. Interleukin-5 (IL-5) augments the progression of liver fibrosis by regulating IL-13 activity. Infect Immun. 2006;74:1471–1479. doi: 10.1128/IAI.74.3.1471-1479.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidal B, Serrano AL, Tjwa M, et al. Fibrinogen drives dystrophic muscle fibrosis via a TGFbeta/alternative macrophage activation pathway. Genes Dev. 2008;22:1747–1752. doi: 10.1101/gad.465908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wehling-Henricks M, Jordan MC, Gotoh T, et al. Arginine metabolism by macrophages promotes cardiac and muscle fibrosis in mdx muscular dystrophy. PLoS One. 2010;5:e10763. doi: 10.1371/journal.pone.0010763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villalta SA, Nguyen HX, Deng B, et al. Shifts in macrophage phenotypes and macrophage competition for arginine metabolism affect the severity of muscle pathology in muscular dystrophy. Hum Mol Genet. 2009;18:482–496. doi: 10.1093/hmg/ddn376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawao N, Nagai N, Tamura Y, et al. Urokinase-type plasminogen activator contributes to heterogeneity of macrophages at the border of damaged site during liver repair in mice. Thromb Haemost. 2011;105:892–900. doi: 10.1160/TH10-08-0516. [DOI] [PubMed] [Google Scholar]
- 28.Lee S, Huen S, Nishio H, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. doi: 10.1681/ASN.2009060615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anzai A, Anzai T, Nagai S, et al. Regulatory role of dendritic cells in postinfarction healing and left ventricular remodeling. Circulation. 2012;125:1234–1245. doi: 10.1161/CIRCULATIONAHA.111.052126. [DOI] [PubMed] [Google Scholar]
- 30.Miao M, Niu Y, Xie T, et al. Diabetes-impaired wound healing and altered macrophage activation: a possible pathophysiologic correlation. Wound Repair Regen. 2012;20:203–213. doi: 10.1111/j.1524-475X.2012.00772.x. [DOI] [PubMed] [Google Scholar]
- 31.Weidenbusch M, Anders HJ. Tissue Microenvironments Define and Get Reinforced by Macrophage Phenotypes in Homeostasis or during Inflammation, Repair and Fibrosis. J Innate Immun. 2012;4:463–477. doi: 10.1159/000336717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Duffield JS, Lupher M, Thannickal VJ, et al. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–276. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fukushima K, Badlani N, Usas A, et al. The use of an antifibrosis agent to improve muscle recovery after laceration. Am J Sports Med. 2001;29:394–402. doi: 10.1177/03635465010290040201. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Menetrey J, Kasemkijwattana C, Fu FH, et al. Suturing versus immobilization of a muscle laceration. A morphological and functional study in a mouse model. Am J Sports Med. 1999;27:222–229. doi: 10.1177/03635465990270021801. [DOI] [PubMed] [Google Scholar]
- 36.Shen W, Li Y, Tang Y, et al. NS-398, a cyclooxygenase-2-specific inhibitor, delays skeletal muscle healing by decreasing regeneration and promoting fibrosis. Am J Pathol. 2005;167:1105–1117. doi: 10.1016/S0002-9440(10)61199-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryer SC, Koh TJ. The urokinase-type plasminogen activator receptor is not required for skeletal muscle inflammation or regeneration. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1152–R1158. doi: 10.1152/ajpregu.00132.2007. [DOI] [PubMed] [Google Scholar]
- 38.Koh TJ, Bryer SC, Pucci AM, et al. Mice deficient in plasminogen activator inhibitor-1 have improved skeletal muscle regeneration. Am J Physiol Cell Physiol. 2005;289:C217–C223. doi: 10.1152/ajpcell.00555.2004. [DOI] [PubMed] [Google Scholar]
- 39.Mirza R, Koh TJ. Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine. 2011;56:256–264. doi: 10.1016/j.cyto.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 40.Daley JM, Brancato SK, Thomay AA, et al. The phenotype of murine wound macrophages. J Leukoc Biol. 2010;87:59–67. doi: 10.1189/jlb.0409236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol. 2011;178:19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamilton TA, Ohmori Y, Tebo J. Regulation of chemokine expression by antiinflammatory cytokines. Immunol Res. 2002;25:229–245. doi: 10.1385/IR:25:3:229. [DOI] [PubMed] [Google Scholar]
- 43.Stout RD, Jiang C, Matta B, et al. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 44.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Waal Malefyt R, Figdor CG, Huijbens R, et al. Effects of IL-13 on phenotype, cytokine production, and cytotoxic function of human monocytes. Comparison with IL-4 and modulation by IFN-gamma or IL-10. J Immunol. 1993;151:6370–6381. [PubMed] [Google Scholar]
- 46.Bencze M, Negroni E, Vallese D, et al. Proinflammatory macrophages enhance the regenerative capacity of human myoblasts by modifying their kinetics of proliferation and differentiation. Mol Ther. 2012;20:2168–2179. doi: 10.1038/mt.2012.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thomas JA, Pope C, Wojtacha D, et al. Macrophage therapy for murine liver fibrosis recruits host effector cells improving fibrosis, regeneration, and function. Hepatology. 2011;53:2003–2015. doi: 10.1002/hep.24315. [DOI] [PubMed] [Google Scholar]
- 48.Zuloff-Shani A, Kachel E, Frenkel O, et al. Macrophage suspensions prepared from a blood unit for treatment of refractory human ulcers. Transfus Apher Sci. 2004;30:163–167. doi: 10.1016/j.transci.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 49.Zuloff-Shani A, Adunsky A, Even-Zahav A, et al. Hard to heal pressure ulcers (stage III–IV): efficacy of injected activated macrophage suspension (AMS) as compared with standard of care (SOC) treatment controlled trial. Arch Gerontol Geriatr. 2010;51:268–272. doi: 10.1016/j.archger.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 50.Danon D, Madjar J, Edinov E, et al. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp Gerontol. 1997;32:633–641. doi: 10.1016/s0531-5565(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 51.Orenstein A, Kachel E, Zuloff-Shani A, et al. Treatment of deep sternal wound infections post-open heart surgery by application of activated macrophage suspension. Wound Repair Regen. 2005;13:237–242. doi: 10.1111/j.1067-1927.2005.130304.x. [DOI] [PubMed] [Google Scholar]
- 52.Leor J, Rozen L, Zuloff-Shani A, et al. Ex vivo activated human macrophages improve healing, remodeling, and function of the infarcted heart. Circulation. 2006;114:I94–100. doi: 10.1161/CIRCULATIONAHA.105.000331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Muscle macrophage phenotype. Macrophages were isolated by magnetic separation from uninjured (0d) or lacerated gastrocnemius muscles at the indicated time points. RNA was obtained from muscle macrophages and activated bone marrow derived macrophages as described in Materials and Methods. Total RNA was reverse transcribed, and expression of IL-1β (A), TNFα (B), iNOS (C), IDO1 (D), CXCL10 (E), CD206 (F), CD36 (G), TGFβ (H), Ym1 (I), and IL-10 (J) was analyzed by real-time PCR. Expression of each gene is presented relative to GAPDH using the 2−ΔCT method. Data did not pass tests of normality and equal variance, and are presented with center line as median, boxes representing the 25th and 75th percentiles, whiskers representing the 10th and 90th percentiles, and outliers as dots. * p<0.05 versus macrophages from uninjured (0d) muscle. n=5–10 per time-point. In vitro activated macrophages were not included in statistical comparisons.