Abstract
Background
Pneumonia poses a significant risk in patients with moderate to severe chronic obstructive pulmonary disease but data are limited on the disease phenotypes most susceptible to pneumonia.
Methods
Cluster analysis using a data-driven recursive partitioning algorithm was employed using baseline data from two pooled one-year randomized exacerbation trials (n=3,255) of fluticasone furoate/vilanterol or vilanterol alone to identify distinct patient groups at greatest risk of pneumonia or serious (hospitalization or death) pneumonia.
Results
Five clusters were identified. Patients at greater risk of first pneumonia had more severe obstruction (forced expiratory volume in one second/forced vital capacity <46%) and either a body mass index <19 kg/m2 (hazard ratio 7.8, 95% confidence interval 4.7–13.0; n=144) or a pneumonia history and greater comorbidities (hazard ratio 4.8, 95% confidence interval 3.0–7.7; n=374) relative to the cluster with the lowest pneumonia risk (reference; n=1310). Multiple comorbidities and use of psychoanaleptics also contributed to an increased risk of pneumonia in more obstructed patients. Independent of cluster, use of inhaled corticosteroids was associated with pneumonia (hazard ratio 1.89, 95% confidence interval 1.25–2.84) and serious pneumonia (hazard ratio 2.92, 95% confidence interval 1.40–6.01).
Conclusion
Cluster analysis can identify patient populations at risk for serious safety outcomes and inform risk management strategies to optimize patient management. The greatest risk for pneumonia was in subjects with multiple pneumonia risk factors.
Keywords: chronic obstructive pulmonary disease, inhaled corticosteroids, long-acting β2-agonists, pneumonia, cluster analysis
Introduction
Community-acquired pneumonia results in greater morbidity and mortality in patients with chronic obstructive pulmonary disease (COPD) than in those without the disease.1 The annual incidence of community-acquired pneumonia in the COPD population is estimated to be 22.4 episodes per 1,000 patients,2 and age, severity of COPD, and presence of comorbidity further increase the risk of hospitalization with community-acquired pneumonia. Findings further suggest an increase in pneumonia when COPD is treated with inhaled corticosteroids (ICS), although ICS also reduce the rates of moderate or severe exacerbations of COPD.3 The mechanism by which ICS increase the risk of pneumonia is unclear, but may relate to reducing the inflammatory response, including neutrophils, in the respiratory tract.4
Fluticasone furoate/vilanterol (FF/VI) is a new once-daily ICS/long-acting β2-agonist (LABA) combination (FF/VI 100/25 μg, BREO™ ELLIPTA™, GlaxoSmithKline, Research Triangle Park, NC, USA) that reduces exacerbations of COPD, improves lung function, and may increase the risk of pneumonia compared with the LABA (vilanterol) alone.5 Factors indicated to be associated with this increased risk among patients with COPD treated with FF/VI are consistent with previous observations in the ICS/LABA class, and include older age, lower body mass index (BMI), current smoking, the occurrence of a previous pneumonia, and poorer lung function.5,6
We conducted a cluster analysis using data from two one-year studies of FF/VI and vilanterol to identify combinations of patient characteristics that place patients at greater risk of pneumonia for the purposes of risk management.
Materials and methods
Clinical study design and subjects
The study designs, recruitment criteria, and procedures of the two studies analyzed herein have been previously reported.5 Briefly, patients aged ≥40 years with a documented diagnosis of COPD7 and at least one moderate or severe exacerbation in the prior 12 months were randomized to 52 weeks of treatment with vilanterol 25 μg or FF/VI at strengths of 50/25, 100/25, or 200/25 μg. The primary efficacy endpoint was the annual rate of moderate or severe exacerbations (requiring antibiotics and/or oral corticosteroids and/or resulting in hospitalization) in each treatment arm. Pneumonia was also reported and recorded. The diagnosis was based on the investigator’s judgment based on the available clinical evidence, including a chest X-ray. Pneumonia was defined as an adverse event or as a serious adverse event if it resulted in hospitalization.
Cluster analysis methodology
Cluster analysis was employed using the R-package and RPART procedure (http://www.r-project.org/).8,9 Using the data-driven recursive partitioning algorithm to model time to first or severe pneumonia, the decision tree created clusters of patients based on the intent-to-treat population such that differences in risk of pneumonia were maximized among clusters. The size of the decision tree was determined by minimizing the cross validation error; and cluster membership was then assigned to each patient based on the selected tree. The three doses of FF/VI were collapsed into one group to examine treatment versus nontreatment with FF/VI. Risk of pneumonia and serious pneumonia was calculated using Cox proportional hazards, with an initial model adjusted for study, smoking status, and region as a random effect. Because these variables were part of the initial model used to calculate risk, they could not be included as variables for defining the clusters. The results of the initial analysis did not identify treatment as a variable associated with clusters (see Results section), thus treatment was included as an explanatory variable within the final model. To avoid identification of spurious clusters, the number of patients within any one cluster was set at ≥100. To avoid confounding by similar variables (eg, membership of a specific age group such as 65–75 years versus age as a continuous variable) each variable was assessed for collinearity with every other variable using Pearson’s correlation coefficients. Where two similar variables exhibited a Pearson’s correlation coefficient value ≥0.7 (eg, eosinophil count versus percentage) only one variable was retained based on the clinician’s selection. Variables included in the analysis are noted in Table 1.
Table 1.
Characteristics and variables considered in initial cluster analysis
| Characteristic/variable: class | Characteristic/variable: specific |
|---|---|
| Spirometry | • Post-bronchodilator FEV1 L • Post-bronchodilator FEV1 % predicted • FEV1% reversibility • Post-bronchodilator FEV1/FVC ratio • Post-bronchodilator FVC% predicted |
| Demographics | • Age (years) • Sex • BMI (kg/m2) • Smoking (current/former) • Smoking pack-years |
| Race | • African American/African heritage • American Indian/Alaska Native • Asian • Mixed • White |
| COPD history | • Duration of COPD (years) • COPD type (chronic bronchitis and/or emphysema) |
| History of exacerbation (past year) | • ≤1 versus ≥2 requiring oral corticosteroids and/or antibiotics • ≤1 versus ≥2 requiring hospitalization 0, 1, 2, >2 or more |
| Medical history | • Targeted medical conditions* • Vaccination for influenza (yes versus no) • Vaccination for pneumonia (yes versus no) |
| Medications at baseline/initiated prior to randomization | • ATC classification** |
| Laboratory investigations | • Blood urea nitrogen • Eosinophils • Hemoglobin • Lymphocytes • Neutrophils • Red blood cells • White blood cells |
| Randomized treatment | • VI 25 μg • FF/VI treatment (50/25 μg, 100/25 μg, 200/25 μg) |
Notes:
Targeted medical conditions: cardiovascular history/risk, vascular disorders, metabolism disorders, history of pneumonia, cardiac disorders, eye disorders, arrhythmia;
medications were coded based on the ATC of interest. Some medications have more than one indication and were categorized in more than one therapeutic class (eg, aspirin for pain relief versus cardiovascular disease prevention). Because the reasons for taking the medications were not collected systematically, the classification was based on the ATC classes and not on the underlying indication for an individual patient. It is possible that some medications were counted in more than one ATC category. Medication classes included in the analysis included: antihistamines, antihypertensives, antithrombotics, diuretics, diabetes medications, psychoanaleptics, psycholeptics, agents acting on the renin–angiotensin system (eg, angiotensin-converting enzyme inhibitors, angiotensin II agonists), antihemorrhagics, antianemic preparations, beta-blockers, cardiac therapies, calcium channel blockers, lipid-modifying agents, anti-inflammatory and antirheumatic products, medications for bone diseases (including muscle pain), antihemorrhagics, and vasodilators.
Abbreviations: ATC, Anatomic Therapeutic Category; COPD, chronic obstructive pulmonary disease; BMI, body mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FF/VI, fluticasone furoate/vilanterol; VI, vilanterol.
Hazard ratios (HR) and 95% confidence intervals (CI) were calculated for each cluster, using the cluster with the lowest incidence rate as the reference value. Baseline variables within each cluster are presented as proportions for categorical variables and medians (interquartile range) for continuous variables. Differences between variables in clusters were assessed using χ2 for categorical variables and the Wilcoxon rank sum test for continuous variables.
Results
Primary efficacy and safety
The primary data of these studies have already been reported.5 More pneumonia, serious pneumonia, and fatal pneumonia events occurred with FF/VI than vilanterol (Table 2).
Table 2.
Pneumonia, serious pneumonia, and fatal pneumonia events with FF/VI and VI
| n (%) | FF/VI 50 μg/25 μg (n=820) |
FF/VI 100 μg/25 μg (n=806) |
FF/VI 200 μg/25 μg (n=811) |
VI 25 μg (n=818) |
|---|---|---|---|---|
| Pneumonia | ||||
| Subjects | 48 (5.9) | 51 (6.3) | 55 (6.8) | 27 (3.3) |
| Events | 54 | 58 | 65 | 28 |
| Serious pneumonia | ||||
| Subjects and events | 24 (2.9) | 25 (3.1) | 23 (2.8) | 8 (<1) |
| Fatal pneumonia | ||||
| Subjects and events | 0 | 1 | 7* | 0 |
Note:
One subject in the 200/25 treatment group experienced a fatal exacerbation of chronic obstructive pulmonary disease, and was noted to have concurrent pneumonia at the time of death.
Abbreviations: FF/VI, fluticasone furoate/vilanterol; VI, vilanterol.
Cluster analysis
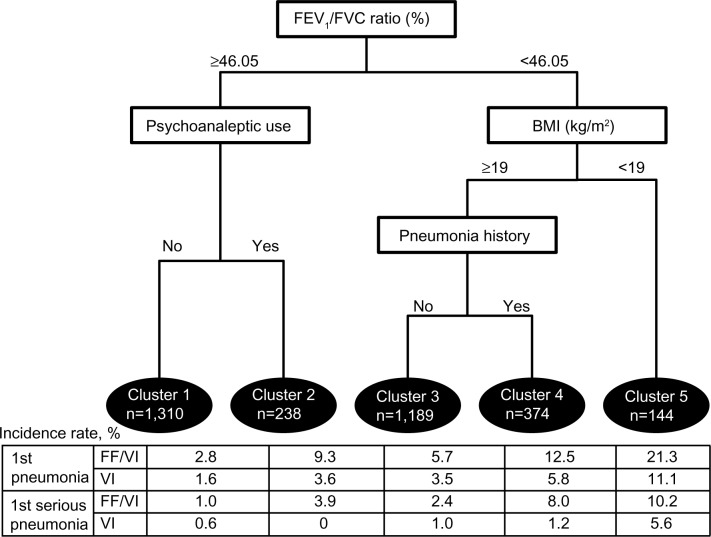
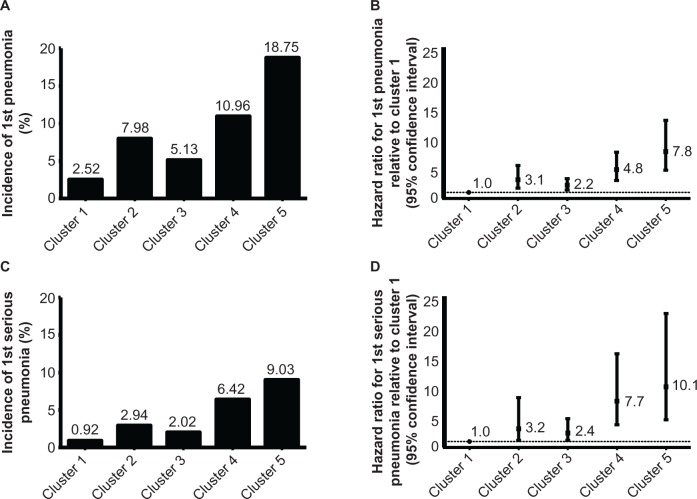
This analysis yielded five clusters combining clinical and demographic attributes, including forced expiratory volume in one second/forced vital capacity (FEV1/FVC) ratio, psychoanaleptic use, BMI, and history of pneumonia (Figure 1). The final tree was based on combining the similar trees determined for all pneumonia events (Figure S1) and serious pneumonia events (subset of pneumonia events resulting in hospitalization; Figure S2). Treatment (FF/VI or vilanterol) was not identified as a variable in the tree that maximized the difference in risk between clusters, so was included in the final Cox model. The incidence of pneumonia and serious pneumonia was lowest in cluster 1 (2.52% and 0.92%, respectively) during the one-year study period and highest in cluster 5 (18.75% and 9.03%, respectively), generally increasing from left to right (Figure 2A and C). Relative to cluster 1 (reference), HRs for the time to first pneumonia in clusters 2–5 ranged from a 2.2-fold increased risk of first pneumonia in cluster 3 versus cluster 1 to a 7.8-fold increased risk of first serious pneumonia in cluster 5 versus cluster 1 (Figure 2B and D).
Figure 1.
Cluster analysis tree of first and first serious pneumonia.
Abbreviations: BMI, bone mass index; FEV1, forced expiratory volume in one second; FVC, forced vital capacity; FF/VI, fluticasone furoate/vilanterol; VI, vilanterol.
Figure 2.
Incidence (A and C) and hazard ratios (B and D) for first pneumonia (A and B) and first serious pneumonia (C and D) by cluster.
Patient clusters
The clusters identified comprised the following: patients with poorer lung function (FEV1/FVC <46.05%) and lower BMI <19 kg/m2 (cluster 5); patients with poorer lung function, normal BMI and prior history (cluster 4) or no history (cluster 3) of pneumonia; and patients with better lung function in receipt (cluster 2) or not in receipt (cluster 1) of psychoanaleptics (89% antidepressants). There were significant baseline differences between clusters assessed by demographics, spirometry, COPD history, or COPD type (Table 3) and comorbidities or concomitant medications (Table 4). There were no clinically significant differences in laboratory measures across clusters (data not shown).
Table 3.
Patient characteristics by cluster
| Parameter | Cluster 1 (less obstruction) n=1,310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1,189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
P-value |
|---|---|---|---|---|---|---|
| Demographics | ||||||
| Age, median (IQR) | 64 (56–70) | 60 (55–67) | 65 (59–71) | 65 (59–71) | 64 (56–70) | <0.0001 |
| Male, n (%) | 701 (53.5) | 67 (28.2) | 788 (66.3) | 234 (62.6) | 80 (55.6) | <0.0001 |
| Body mass index at screening, median (IQR) | 27.3 (24.1–31.3) | 29.6 (25.0–34.1) | 25.6 (23.0–28.6) | 25.7 (22.6–29.0) | 17.6 (16.4–18.3) | <0.0001 |
| Ethnicity, n (%) | ||||||
| Not Hispanic or Latino | 1,000 (76.3) | 224 (94.1) | 986 (82.9) | 328 (87.7) | 133 (92.4) | <0.0001 |
| Hispanic or Latino | 310 (23.7) | 14 (5.9) | 203 (17.1) | 46 (12.3) | 11 (7.6) | – |
| Smoking status | ||||||
| Former, n (%) | 736 (56.2) | 99 (41.6) | 702 (59.0) | 224 (59.9) | 55 (38.2) | <0.0001 |
| Current, n (%) | 574 (43.8) | 139 (58.4) | 487 (41.0) | 150 (40.1) | 89 (61.8) | – |
| Number of pack years, median (IQR) | 38.0 (22.0–50.0) | 46.0 (35.0–60.0) | 42.0 (30.0–60.0) | 45.0 (32.0–64.0) | 43.0 (30.0–56.5) | <0.0001 |
| Spirometry | ||||||
| % predicted post-bronchodilator FEV1 at baseline, median (IQR) | 51.9 (43.9–60.3) | 52.1 (43.7–60.2) | 35.0 (27.2–43.7) | 33.5 (26.0–41.6) | 28.0 (21.8–34.9) | <0.0001 |
| Percent reversibility (%) at screening, median (IQR) | 9.0 (2.4–17.5) | 13.6 (6.9–22.6) | 14.3 (6.0–25.2) | 16.2 (8.6–25.4) | 18.1 (8.3–28.8) | <0.0001 |
| Reversible*, n (%) | 372 (28.5) | 101 (42.6) | 362 (31.2) | 101 (27.6) | 36 (25.2) | 0.0002 |
| Duration of COPD, n (%) | ||||||
| <5 years | 581 (44.4) | 113 (47.5) | 466 (39.2) | 117 (31.3) | 56 (38.9) | <0.0001 |
| 5 to <10 years | 432 (33.0) | 65 (27.3) | 347 (29.2) | 128 (34.2) | 56 (38.9) | – |
| 10 to <15 years | 158 (12.1) | 38 (16.0) | 204 (17.2) | 82 (21.9) | 17 (11.8) | – |
| ≥15 years | 139 (10.6) | 22 (9.2) | 172 (14.5) | 47 (12.6) | 15 (10.4) | – |
| Exacerbation requiring OCS/antibiotic in past 12 months, n (%) | ||||||
| 0 | 94 (7.2) | 16 (6.7) | 81 (6.8) | 40 (10.7) | 19 (13.2) | <0.0001 |
| 1 | 865 (66.0) | 140 (58.8) | 722 (60.7) | 196 (52.4) | 69 (47.9) | – |
| 2 | 248 (18.9) | 50 (21.0) | 279 (23.5) | 82 (21.9) | 34 (23.6) | – |
| ≥3 | 103 (7.9) | 32 (13.4) | 107 (9.0) | 56 (15.0) | 22 (15.3) | – |
| Exacerbation requiring hospitalization in past 12 months, n (%) | ||||||
| 0 | 1,031 (78.7) | 195 (81.9) | 1,001 (84.2) | 256 (68.4) | 110 (76.4) | <0.0001 |
| 1 | 219 (16.7) | 36 (15.1) | 157 (13.2) | 104 (27.8) | 31 (21.5) | – |
| ≥2 | 60 (4.6) | 7 (2.9) | 31 (2.6) | 14 (3.7) | 3 (2.1) | – |
| COPD type, n (%) | ||||||
| History of bronchitis | 947 (72.8) | 170 (71.4) | 756 (63.9) | 214 (57.2) | 69 (47.9) | <0.0001 |
| History of emphysema | 582 (44.8) | 128 (53.8) | 729 (61.6) | 260 (69.5) | 110 (76.4) | <0.0001 |
Notes:
Reversibility indicated by ≥12% and 200 ml change in lung function following bronchodilator. The most prevalent/greatest parameter across clusters is shown in bold.
Abbreviations: BMI, bone mass index; COPD, chronic obstructive pulmonary disease; OCS, oral corticosteroids; IQR, interquartile range; FEV1, forced expiratory volume in one second.
Table 4.
Patient comorbidities and concomitant medications by cluster
| Parameter, n (%) | Cluster 1 (less obstruction) n=1,310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1,189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
P-value |
|---|---|---|---|---|---|---|
| Comorbidities | ||||||
| Cardiovascular history/risk | 806 (61.5) | 179 (75.2) | 706 (59.4) | 253 (67.6) | 65 (45.1) | <0.0001 |
| Vascular disorders | 631 (48.2) | 134 (56.3) | 540 (45.4) | 196 (52.4) | 40 (27.8) | <0.0001 |
| Metabolism disorders | 505 (38.5) | 143 (60.1) | 413 (34.7) | 193 (51.6) | 40 (27.8) | <0.0001 |
| History of pneumonia | 241 (18.4) | 87 (36.6) | 0 (0.0) | 374 (100) | 38 (26.4) | <0.0001 |
| Cardiac disorders | 220 (16.8) | 48 (20.2) | 162 (13.6) | 74 (19.8) | 22 (15.3) | 0.0136 |
| Eye disorders | 140 (10.7) | 31 (13.0) | 130 (10.9) | 84 (22.5) | 22 (15.3) | <0.0001 |
| Arrhythmia | 80 (6.1) | 13 (5.5) | 38 (3.2) | 21 (5.6) | 10 (6.9) | 0.0107 |
| Concomitant medications | ||||||
| On-treatment pneumonia Vaccine | 117 (8.9) | 15 (6.3) | 51 (4.3) | 38 (10.2) | 6 (4.2) | <0.0001 |
| On-treatment influenza vaccine | 312 (23.8) | 55 (23.1) | 220 (18.5) | 119 (31.8) | 27 (18.8) | <0.0001 |
| Agents acting on the renin-angiotensin system | 419 (32.0) | 85 (35.7) | 365 (30.7) | 122 (32.6) | 18 (12.5) | <0.0001 |
| Anti-inflammatory and antirheumatic products | 406 (31.0) | 127 (53.4) | 388 (32.6) | 144 (38.5) | 29 (20.1) | <0.0001 |
| Lipid-modifying agents | 342 (26.1) | 110 (46.2) | 279 (23.5) | 128 (34.2) | 22 (15.3) | <0.0001 |
| Antithrombotics | 308 (23.5) | 78 (32.8) | 308 (25.9) | 112 (29.9) | 25 (17.4) | 0.0011 |
| Diuretics | 256 (19.5) | 69 (29.0) | 238 (20.0) | 79 (21.1) | 19 (13.2) | 0.0027 |
| Beta-blockers | 151 (11.5) | 33 (13.9) | 88 (7.4) | 35 (9.4) | 5 (3.5) | 0.0001 |
| Diabetes medications | 146 (11.1) | 38 (16.0) | 92 (7.7) | 39 (10.4) | 1 (0.7) | <0.0001 |
| Psycholeptics | 123 (9.4) | 102 (42.9) | 142 (11.9) | 65 (17.4) | 18 (12.5) | <0.0001 |
| Antihistamines | 116 (8.9) | 53 (22.3) | 111 (9.3) | 53 (14.2) | 10 (6.9) | <0.0001 |
| Antihypertensives | 53 (4.0) | 6 (2.5) | 51 (4.3) | 31 (8.3) | 8 (5.6) | 0.0038 |
| Antihemorrhagics/antianemia preparations | 48 (3.7) | 30 (12.6) | 43 (3.6) | 21 (5.6) | 4 (2.8) | <0.0001 |
| Drugs for bone diseases (including muscle pain) | 39 (3.0) | 15 (6.3) | 37 (3.1) | 29 (7.8) | 11 (7.6) | <0.0001 |
| Psychoanaleptics | 0 (0.0) | 238 (100) | 172 (14.5) | 66 (17.6) | 23 (16.0) | <0.0001 |
Note: Most prevalent parameter across clusters is shown in bold.
Abbreviation: BMI, body mass index.
Patients with the greatest risk of pneumonia and serious pneumonia (cluster 5) predominantly had a history of emphysema, the highest prevalence of current smoking, the greatest extent of reversibility, and were more likely to have experienced at least two exacerbations in the prior year compared with those in other clusters. Patients in cluster 5 also had considerably fewer comorbidities than other clusters and were in receipt of the fewest concomitant medications. Patients in clusters 4 and 2 showed higher rates of comorbidity and concomitant medication.
Patients in cluster 3 were predominantly male and had COPD for the greatest amount of time, but were the least likely to have experienced an exacerbation requiring hospitalization in the prior year; this cluster was primarily differentiated from the other clusters on the basis of poorer lung function and the absence of other defining characteristics.
Cluster 2 subjects were predominantly female, in addition to having a higher prevalence of comorbidity (and typically the highest receipt of concomitant medication). This cluster contained the fewest Hispanic and Latino patients, had the least impaired lung function, greatest proportion of current smokers, and greatest proportion of reversible patients. Forty-three percent of subjects in cluster 2 were also using psycholeptic medications, over half of whom were exposed to benzodiazepines (Table S1).
Cluster 1 (the reference population) contained the greatest number of patients overall, and were more likely to be Hispanic or Latino and more likely to have a history of COPD characterized by bronchitis than patients in the other clusters.
Treatment effects
In each cluster, pneumonia or serious pneumonia occurred more frequently with any strength of FF/VI therapy than with vilanterol alone (see Table in Figure 1). Across all clusters and relative to treatment with vilanterol alone, treatment with FF/VI at any strength (ie, all strengths combined) was associated with a significantly greater risk of first pneumonia (HR 1.89, CI 1.25–2.84) and first serious pneumonia (HR 2.92, CI 1.40–6.01). No interaction was found between treatment and cluster for first pneumonia (P=0.9741) or first serious pneumonia (P=0.8089). Doses of FF/VI were balanced across the clusters (P=0.2064, Table S2).
Effects of variables used in the model
Smoking status (current) as a main effect was not associated with a significantly increased risk of first pneumonia (HR 1.0, CI 0.7–1.4) or first serious pneumonia (HR 0.9, CI 0.6–1.5). The distribution of geographic region across clusters was significantly different (P≤0.0001) relative to the total distribution (Table 5). Overall, approximately one third of patients were from the USA, a quarter were from Europe, and the remainder were balanced between the two “other” regions (Argentina, Chile, Mexico, Peru, and the Philippines as other region 1, and Australia, Canada, and South Africa as other region 2). In cluster 2 (in receipt of psychoanaleptics, higher lung function, more comorbidity, predominantly female), 61.3% of the population were from the USA, while compared with the total population proportionately fewer patients were from Europe or “other” region 1. The USA also provided the majority of patients in cluster 4 (poorer lung function, higher proportions of comorbidity, prior pneumonia) and cluster 5 (poorer lung function, lower BMI, greatest pneumonia risk).
Table 5.
Geographic contribution to total population and each cluster
| Region, % patients | Total n=3,255 |
Cluster 1 (less obstruction) n=1,310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1,189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
|---|---|---|---|---|---|---|
| USA | 34.0 | 26.6 | 61.3 | 30.3 | 53.7 | 36.8 |
| Europe | 26.1 | 27.7 | 14.3 | 30.5 | 18.2 | 13.9 |
| Other 1 | 21.4 | 28.8 | 6.3 | 19.2 | 11.8 | 22.2 |
| Other 2 | 18.5 | 17.0 | 18.1 | 20.0 | 16.3 | 27.1 |
Notes: Other 1, Argentina, Chile, Mexico, Peru, Philippines; Other 2, Australia, Canada, South Africa. The most prevalent region in each cluster is shown in bold.
Abbreviation: BMI, body mass index.
Discussion
Cluster analysis of two replicate one-year studies of FF/VI versus vilanterol identified five clusters of patients with differing risks for occurrence of pneumonia or serious pneumonia. Using the cluster with the lowest incidence of pneumonia (cluster 1) as a reference, the risk of pneumonia or serious pneumonia was significantly increased in each of the remaining clusters. The clusters identified were defined by combinations of the established pneumonia risk factors of lung function, BMI, and prior history of pneumonia, and also by the use of psychoanaleptic medication. FEV1/FVC was the main characteristic used to describe the clusters, and clusters 3, 4, and 5 were characterized by greater obstruction, as defined by FEV1/FVC, than clusters 1 and 2.
Cluster 5 had the highest risk of pneumonia, and subjects in this cluster showed the greatest extent of lung function impairment, defined as FEV1% predicted, and the lowest BMI of all the clusters. Cluster 4 subjects had more impaired lung function (FEV1% predicted), more comorbid conditions, and a prior history of pneumonia compared with cluster 1, while those in cluster 3 had only more impaired lung function (FEV1% predicted) compared with cluster 1. Cluster 2 subjects also showed an increased risk of pneumonia relative to cluster 1; cluster 2 subjects differed from those in cluster 1 by use of psychoanaleptics and an increased prevalence of comorbidities. The algorithm did not identify treatment with FF/VI versus vilanterol as a variable maximizing the difference in risk of pneumonia between clusters; however, treatment with FF/VI versus vilanterol alone did result in a significantly increased risk of pneumonia or serious pneumonia in each cluster. There was no differential treatment effect across clusters.
Vanfletern et al10 have identified different COPD phenotypes based on the number and types of comorbidities, and the phenotypes identified loosely track with the clusters of varying pneumonia risk identified in our analysis, including low comorbidity (cluster 1), psychological (cluster 2), cardiovascular (clusters 2 and 4), metabolic (clusters 2 and 4), and cachectic (cluster 5) groups. In our analysis, the initial split of the population occurred based on lung function (FEV1/FVC ratio <46%). Poorer lung function is known to increase the risk of pneumonia in patients with COPD,1,11 so it is perhaps unsurprising that our analysis defined lung function as the initial variable associated with an increased/decreased risk of pneumonia. Increasing obstruction measured as FEV1 and increasing prevalence of emphysematous COPD generally tracked with increasing risk among the cohorts.
The poorer lung function population subsequently split on the basis of BMI and patients with both poorer lung function and a BMI <19 kg/m2 formed the cluster (cluster 5) with the greatest risk and incidence of pneumonia and serious pneumonia. This finding in part confirms that of the initial analysis, in which BMI was associated with the occurrence of pneumonia,12 and also aligns with other findings.11 It follows that in patients with both poor lung function and low BMI, the risk of pneumonia would be increased as a consequence of the presence of both risk factors.
Lower lung function, a prior pneumonia event, and greater prevalence of comorbidities were the defining characteristics of cluster 4. In COPD, it is known that the incidence of community-acquired pneumonia is approximately doubled in patients with a prior event,2 which suggests that, as has been shown for exacerbations of COPD,13 the occurrence of a prior event may increase the risk of a recurrent event.
The finding that cluster 2 comprised patients with better lung function (FEV1/FVC >46%), more psychoanaleptic use, and frequent psycholeptic use suggests that receipt of psychotropic medications presents an increased risk of pneumonia in COPD that is distinct from established factors. Other studies have reported that both psychotropic drugs14 and the underlying psychiatric/neurological disorders they treat15,16 increase the risk of pneumonia. Patients with psychiatric and neurological disorders can have impairment in both the perception of symptoms and the seeking of health care, and may be more likely to smoke.17 Patients with COPD who smoke have a higher prevalence of depression and are at greater risk of developing depression than those who do not.18 Depressive symptoms are known to pose a greater risk of exacerbation, exacerbation-related hospitalization, and death in COPD.19
Patients taking psychotropic medications are also at risk for aspiration pneumonia because of dysphagia14,17 and relaxation of lower esophageal sphincter tone that can aggravate gastroesophageal reflux.20 The most common class of psychotropic drugs used by patients in these studies was benzodiazepines, which were estimated to be dispensed to nearly one third of patients with COPD in a Canadian study.21 A recent nested case-control and survival analysis in a population-based cohort indicated that benzodiazepines increase the risk of pneumonia by nearly 50%,22 although an earlier study suggested that this risk was related to concomitant opioid use.23 Aside from the aspiration risk cited, it has been suggested that benzodiazepine-related pneumonia may be due to immunosuppression that is mediated via activation of gamma-aminobutyric acid A receptors on macrophages.24
Another important aspect related to increased risk of pneumonia identified in this analysis is the presence of multiple comorbidities. The presence of a history or risk of cardiovascular disease and the incidence of coronary artery disease and cardiac, metabolic, or vascular disorders was highest in clusters 2 and 4, in whom the risk of pneumonia was greater than in both cluster 1 (the reference population) and cluster 3. Both congestive heart failure and peripheral vascular disease are independent risk factors for the occurrence of community-acquired pneumonia.2 Similarly, both cardiovascular disease and diabetes place patients with COPD at an increased risk of hospitalization for pneumonia. This would suggest that an increased risk of pneumonia would be most likely to be present in subjects with comorbid cardiovascular disease treated with FF/VI versus vilanterol. However, in other analyses of the data utilized here (not shown), subjects with comorbid cardiovascular disease treated with FF/VI had a numerically lower risk for occurrence of pneumonia when compared with subjects with no comorbid cardiovascular disease treated with vilanterol. The definition of comorbid cardiovascular disease used in this analysis was a prior or current diagnosis of coronary artery disease, myocardial infarction, arrhythmia, congestive heart failure, hypertension, cerebrovascular accident, diabetes mellitus, or hypercholesterolemia. This broad definition may have had a confounding effect by classing together morbidities, and perhaps does not adequately reflect the complexity of COPD (eg, a single factor versus multiple factors contributing to risk of pneumonia).
It is also notable that only a minority of patients were vaccinated for pneumonia (range across clusters, 4%–9%) or influenza (range 19%–23%). Vaccination rates were highest for cluster 4, which had a higher level of obstruction, pneumonia history, and more frequent comorbidities relative to other clusters. Cluster 4 patients included disproportionate numbers from the USA, suggesting that greater vaccination rates and comorbidities in this cluster could relate to differences in health systems and lifestyle or nutrition relative to other regions. Imbalances in geographic distribution across other clusters support this possibility. The low vaccination rates in these COPD study patients suggests a modifiable risk of pneumonia exists that can be further addressed within clinical practice settings and by vaccine uptake strategies. Although the evidence is not consistent, the GOLD (Global Initiative for Chronic Obstructive Lung Disease) consensus document25 and Centers for Disease Control and Prevention26 recommend that influenza and pneumonia vaccination be offered to every COPD patient, where vaccination is more effective in older patients, severe COPD patients, and those with comorbid cardiovascular disease. Despite the increased risk of pneumonia in these groups, the patients with greatest risk of pneumonia are also those who receive the greatest benefit of ICS, including those with increased obstruction and low BMI.6
Treatment allocation was balanced across clusters, which would be expected due to randomization. There was a small imbalance in cluster 5, with a slight underrepresentation of FF/VI 100/25 μg (17.4%) and overrepresentation of FF/VI 200/25 μg (31.3%), compared with the 25% expected. This variation likely resulted due to chance because this was the smallest of the clusters (n=144), but could have contributed to the observed increased risk of pneumonia. Based on the benefit-risk profile,5 the 200/25 dose of FF/VI was not progressed for registration in COPD.
Regardless of cluster, there is an approximate two-fold increase in the relative risk of pneumonia and a nearly three-fold increase of serious pneumonia among patients treated with FF/VI relative to vilanterol; however, the absolute risk of pneumonia is greatest among those who benefit most from FF/VI (eg, clusters 4 and 5).6 Risk factors that put individuals at greatest risk of pneumonia (eg, low BMI, increased airflow obstruction, multiple morbidities, and prior history of pneumonia) can be collectively assessed when making treatment decisions. If the greatest risk of pneumonia had been found among patients with modest benefit from FF/VI, the benefit–risk profile may merit a bronchodilator alone (eg, vilanterol) rather than FF/VI, to maintain a positive benefit–risk profile.
Cluster analysis is a useful tool to examine heterogeneous diseases such as COPD27 and has been employed to aid in the identification of distinct clusters or phenotypes of COPD,28 including those at greater risk of mortality.29,30 Interestingly, there is some overlap in the phenotypes identified as being at greater risk of mortality and pneumonia, including those with multiple comorbidities and moderate to severe airflow limitation29,30 and those with severe airflow limitation with low BMI.29 Cluster analysis differs principally from subgroup analysis in that it seeks to first maximize differences between groups of patients using a data-driven algorithm and then identify multiple characteristics which define those patients. In comparison, subgroup analysis first defines patients by individual characteristics one at a time.
This analysis benefited from a large dataset representing a broad international patient population, which is an important element in allowing robust clusters to be identified. However, the generalizability of these findings is limited to patients with a history of COPD exacerbation in the past year. An additional limitation is the lack of validation via a second dataset or split sample; however, results are consistent with what is known about risk factors for pneumonia. Given the small number of pneumonia events, we were not able to evaluate the effect of ICS dose on pneumonia within clusters. Finally, definitions of pneumonia employed in the original studies and in this analysis did not include any standardized assessment of pneumonia severity, such as CURB-65 or Pneumonia Severity Index.31 The definition of serious pneumonia was based on hospitalization due to pneumonia, which may vary across health systems, relative to standardized criteria.
The present analysis identified five clusters of patients with differing risks of pneumonia occurrence over one year of therapy with FF/VI or vilanterol. The clusters were in part defined by well established risk factors, such as BMI and lung function and as such, the outcomes can be considered confirmatory of other findings. The clusters were also defined by less established risk factors, such as prior occurrence of pneumonia, receipt of psychotropic medications, and presence of multiple comorbidities, and as such can be considered hypothesis-generating. Our analysis suggests that physicians should consider many factors and combination of risk factors when treating COPD patients with an ICS/LABA such as FF/VI, balancing the risks and benefit in the individual patient.
Supplementary materials
Cluster analysis tree of first pneumonia.
Abbreviations: BMI, bone mass index; FEV1, forced expiratory volume in one second.
Cluster analysis tree of first serious pneumonia.
Abbreviations: FEV1, forced expiratory volume in one second; BMI, body mass index.
Table S1.
Psychoanaleptic and psycholeptic prescription in the five clusters
| n (% of cluster population) | Cluster 1 (less obstruction) n=1,310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1,189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
|---|---|---|---|---|---|
| Psychoanaleptics | |||||
| Any | 0 (0.0) | 238 (100) | 172 (14.5) | 66 (17.6) | 23 (16.0) |
| Antidepressants | 0 (0.0) | 212 (89.1) | 147 (12.4) | 64 (17.1) | 22 (15.3) |
| Psychostimulants for ADHD/nootropics | 0 (0.0) | 33 (13.9) | 27 (2.3) | 3 (0.8) | 1 (0.7) |
| Antidementia medication | 0 (0.0) | 7 (2.9) | 4 (0.3) | 2 (0.5) | 0 (0.0) |
| Psycholeptics | |||||
| Any | 123 (9.4) | 102 (42.9) | 142 (11.9) | 65 (17.4) | 18 (12.5) |
| Antianxiety medication | 82 (6.3) | 68 (28.6) | 100 (8.4) | 38 (10.2) | 13 (9.0) |
| Hypnotics/sedatives | 53 (4.0) | 40 (16.8) | 56 (4.7) | 31 (8.3) | 6 (4.2) |
| Antipsychotics | 10 (0.8) | 21 (8.8) | 12 (1.0) | 9 (2.4) | 1 (0.7) |
| Benzodiazepine/derivative | 70 (5.3) | 59 (24.8) | 87 (7.3) | 34 (9.1) | 9 (6.3) |
Abbreviation: ADHD, attention-deficit hyperactivity disorder; BMI, body mass index.
Table S2.
Distribution of treatment by cluster
| Treatment (% of patients) | Total N=3255 |
Cluster 1 (less obstruction) n=1310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
|---|---|---|---|---|---|---|
| VI 25 | 25.1 | 24.7 | 23.5 | 26.6 | 23.3 | 25.0 |
| FF/VI 50/25 | 25.2 | 24.7 | 27.3 | 23.5 | 30.5 | 26.4 |
| FF/VI 100/25 | 24.8 | 25.7 | 25.6 | 24.6 | 24.3 | 17.4 |
| FF/VI 200/25 | 24.9 | 25.0 | 23.5 | 25.3 | 22.0 | 31.3 |
Abbreviations: BMI, body mass index; FF/VI, fluticasone furoate/vilanterol; VI, vilanterol.
Footnotes
Author contributions
RLD and DBR conceived the analysis; RLD, HL and DH conducted the analysis; all authors interpreted the results, developed the manuscript, and approved the final draft for submission.
Disclosure
This work was supported by GlaxoSmithKline (protocol number WEUKBRE6624). Editorial support in the form of development of an outline and first draft under the guidance of the lead author, collation of author comments on subsequent drafts, referencing, copyediting, and generation of tables and figures was provided by Geoff Weller, Gardiner-Caldwell Communications (Macclesfield, UK). Funding for this support was provided by GlaxoSmithKline. All authors are employees of and hold shares in GlaxoSmithKline.
References
- 1.Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community-acquired pneumonia. Eur Respir J. 2006;28(2):346–351. doi: 10.1183/09031936.06.00131905. [DOI] [PubMed] [Google Scholar]
- 2.Müllerova H, Chigbo C, Hagan GW, et al. The natural history of community-acquired pneumonia in COPD patients: a population database analysis. Respir Med. 2012;106(8):1124–1133. doi: 10.1016/j.rmed.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 3.Nannini LJ, Poole P, Milan SJ, Kesterton A. Combined corticosteroid and long-acting beta(2)-agonist in one inhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;8:CD006826. doi: 10.1002/14651858.CD006826.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnes NC, Qiu YS, Pavord ID, et al. Antiinflammatory effects of salmeterol/fluticasone propionate in chronic obstructive lung disease. Am J Respir Crit Care Med. 2006;173(7):736–743. doi: 10.1164/rccm.200508-1321OC. [DOI] [PubMed] [Google Scholar]
- 5.Dransfield MT, Bourbeau J, Jones PW, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1(3):210–223. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 6.BREO™ ELLIPTA™ (fluticasone furoate/vilanterol inhalation powder) for treatment of chronic obstructive pulmonary disease. NDA204275. [Accessed January 6, 2014]. Available from: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/Pulmonary-AllergyDrugsAdvisoryCommittee/UCM347931.pdf.
- 7.Celli BR, MacNee W, ATS/ERS Task Force Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 8.Breiman L. Classification and Regression Trees. New York, NY, USA: Chapman and Hall; 1983. [Google Scholar]
- 9.Therneau T, Atkinson B, Ripley B. RPART: Recursive Partitioning. R package version 4.0-1. 2012. [Accessed January 6, 2014]. Available from: http://cran.r-project.org/web/packages/rpart/index.html.
- 10.Vanfleteren LE, Spruit MA, Groenen M, et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(7):728–735. doi: 10.1164/rccm.201209-1665OC. [DOI] [PubMed] [Google Scholar]
- 11.Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647. doi: 10.1183/09031936.00193908. [DOI] [PubMed] [Google Scholar]
- 12.Singh S, Loke YK. Risk of pneumonia associated with long-term use of inhaled corticosteroids in chronic obstructive pulmonary disease: a critical review and update. Curr Opin Pulm Med. 2010;16(2):118–122. doi: 10.1097/MCP.0b013e328334c085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 14.Knol W, van Marum RJ, Jansen PA, Souverein PC, Schobben AF, Egberts AC. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56(4):661–666. doi: 10.1111/j.1532-5415.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 15.Seminog OO, Goldacre MJ. Risk of pneumonia and pneumococcal disease in people with severe mental illness: English record linkage studies. Thorax. 2013;68(2):171–176. doi: 10.1136/thoraxjnl-2012-202480. [DOI] [PubMed] [Google Scholar]
- 16.Chou FH, Tsai KY, Chou YM. The incidence and all-cause mortality of pneumonia in patients with schizophrenia: a nine-year follow-up study. J Psychiatr Res. 2013;47(4):460–146. doi: 10.1016/j.jpsychires.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Quint JK, Brown JS. Weighing up risk factors for pneumonia: the role of mental illness and benzodiazepine use. Thorax. 2013;68(2):121–122. doi: 10.1136/thoraxjnl-2012-202818. [DOI] [PubMed] [Google Scholar]
- 18.Hanania NA, Müllerova H, Locantore NW, et al. Determinants of depression in the ECLIPSE chronic obstructive pulmonary disease cohort. Am J Respir Crit Care Med. 2011;183(5):604–611. doi: 10.1164/rccm.201003-0472OC. [DOI] [PubMed] [Google Scholar]
- 19.Papaioannou AI, Bartziokas K, Tsikrika S, et al. The impact of depressive symptoms on recovery and outcome of hospitalised COPD exacerbations. Eur Respir J. 2013;41(4):815–823. doi: 10.1183/09031936.00013112. [DOI] [PubMed] [Google Scholar]
- 20.Rushnak MJ, Leevy CM. Effect of diazepam on the lower esophageal sphincter. A double-blind controlled study. Am J Gastroenterol. 1980;73(2):127–130. [PubMed] [Google Scholar]
- 21.Vozoris NT, Fischer HD, Wang X, Anderson GM, et al. Benzodiazepine use among older adults with chronic obstructive pulmonary disease: a population-based cohort study. Drugs Aging. 2013;30(3):183–192. doi: 10.1007/s40266-013-0056-1. [DOI] [PubMed] [Google Scholar]
- 22.Obiora E, Hubbard R, Sanders RD, Myles PR. The impact of benzodiazepines on occurrence of pneumonia and mortality from pneumonia: a nested case-control and survival analysis in a population-based cohort. Thorax. 2013;68(2):163–170. doi: 10.1136/thoraxjnl-2012-202374. [DOI] [PubMed] [Google Scholar]
- 23.Dublin S, Walker RL, Jackson ML, et al. Use of opioids or benzodiazepines and risk of pneumonia in older adults: a population-based case-control study. J Am Geriatr Soc. 2011;59(10):1899–1907. doi: 10.1111/j.1532-5415.2011.03586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanders RD, Degos V, Young WL. Cerebral perfusion under pressure: is the autoregulatory ‘plateau’ a level playing field for all? Anaesthesia. 2011;66(11):968–972. doi: 10.1111/j.1365-2044.2011.06915.x. [DOI] [PubMed] [Google Scholar]
- 25.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention Advisory Committee on Immunization Practices recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older – United States, 2013. MMWR Surveill Summ. 2013;62(Suppl 1):1. [PubMed] [Google Scholar]
- 27.Weatherall M, Shirtcliffe P, Travers J, Beasley R. Use of cluster analysis to define COPD phenotypes. Eur Respir J. 2010;36(3):472–474. doi: 10.1183/09031936.00035210. [DOI] [PubMed] [Google Scholar]
- 28.Travers J, Weatherall M, Fingleton J, Beasley R. Towards individualised medicine for airways disease: identifying clinical phenotype groups. Eur Respir J. 2012;39(4):1033–1034. doi: 10.1183/09031936.00122811. [DOI] [PubMed] [Google Scholar]
- 29.Burgel PR, Roche N, Paillasseur JL, et al. Clinical COPD phenotypes identified by cluster analysis: validation with mortality. Eur Respir J. 2012;40(2):495–496. doi: 10.1183/09031936.00228511. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Aymerich J, Gómez FP, Benet M, et al. Identification and prospective validation of clinically relevant chronic obstructive pulmonary disease (COPD) subtypes. Thorax. 2011;66(5):430–437. doi: 10.1136/thx.2010.154484. [DOI] [PubMed] [Google Scholar]
- 31.Loke YK, Kwok CS, Niruban A, Myint PK. Value of severity scales in predicting mortality from community-acquired pneumonia: systematic review and meta-analysis. Thorax. 2010;65(10):884–890. doi: 10.1136/thx.2009.134072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cluster analysis tree of first pneumonia.
Abbreviations: BMI, bone mass index; FEV1, forced expiratory volume in one second.
Cluster analysis tree of first serious pneumonia.
Abbreviations: FEV1, forced expiratory volume in one second; BMI, body mass index.
Table S1.
Psychoanaleptic and psycholeptic prescription in the five clusters
| n (% of cluster population) | Cluster 1 (less obstruction) n=1,310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1,189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
|---|---|---|---|---|---|
| Psychoanaleptics | |||||
| Any | 0 (0.0) | 238 (100) | 172 (14.5) | 66 (17.6) | 23 (16.0) |
| Antidepressants | 0 (0.0) | 212 (89.1) | 147 (12.4) | 64 (17.1) | 22 (15.3) |
| Psychostimulants for ADHD/nootropics | 0 (0.0) | 33 (13.9) | 27 (2.3) | 3 (0.8) | 1 (0.7) |
| Antidementia medication | 0 (0.0) | 7 (2.9) | 4 (0.3) | 2 (0.5) | 0 (0.0) |
| Psycholeptics | |||||
| Any | 123 (9.4) | 102 (42.9) | 142 (11.9) | 65 (17.4) | 18 (12.5) |
| Antianxiety medication | 82 (6.3) | 68 (28.6) | 100 (8.4) | 38 (10.2) | 13 (9.0) |
| Hypnotics/sedatives | 53 (4.0) | 40 (16.8) | 56 (4.7) | 31 (8.3) | 6 (4.2) |
| Antipsychotics | 10 (0.8) | 21 (8.8) | 12 (1.0) | 9 (2.4) | 1 (0.7) |
| Benzodiazepine/derivative | 70 (5.3) | 59 (24.8) | 87 (7.3) | 34 (9.1) | 9 (6.3) |
Abbreviation: ADHD, attention-deficit hyperactivity disorder; BMI, body mass index.
Table S2.
Distribution of treatment by cluster
| Treatment (% of patients) | Total N=3255 |
Cluster 1 (less obstruction) n=1310 |
Cluster 2 (less obstruction, psychoanaleptic users, high comorbidity) n=238 |
Cluster 3 (greater obstruction, no pneumonia history) n=1189 |
Cluster 4 (greater obstruction, high comorbidity, pneumonia history) n=374 |
Cluster 5 (greater obstruction, low BMI) n=144 |
|---|---|---|---|---|---|---|
| VI 25 | 25.1 | 24.7 | 23.5 | 26.6 | 23.3 | 25.0 |
| FF/VI 50/25 | 25.2 | 24.7 | 27.3 | 23.5 | 30.5 | 26.4 |
| FF/VI 100/25 | 24.8 | 25.7 | 25.6 | 24.6 | 24.3 | 17.4 |
| FF/VI 200/25 | 24.9 | 25.0 | 23.5 | 25.3 | 22.0 | 31.3 |
Abbreviations: BMI, body mass index; FF/VI, fluticasone furoate/vilanterol; VI, vilanterol.