Abstract
Background:
Repeated exposure to the traumatic memory (RETM) is a common component of treatments for posttraumatic stress disorder (PTSD). This treatment is based on a fear extinction model; however, the degree to which this treatment actually engages and modifies neural networks mediating fear extinction is unknown. Therefore, the purpose of the current exploratory study was to define the dynamic changes in neural processing networks while participants completed a novel adaptation of RETM.
Method:
Participants were adult women (N=16) with PTSD related to physical or sexual assault. Prior to scanning, participants provided written narratives of a traumatic event related to their PTSD as well as a neutral control event. RETM during fMRI consisted of 5 sequential presentations of the blocked narrative types, lasting three minutes each. Self-reported anxiety was assessed after each presentation.
Results:
Relative to changes in functional connectivity during the neutral control script, RETM was associated with strengthened functional connectivity of the right amygdala with the right hippocampus and right anterior insular cortex, left amygdala with the right insular cortex, medial PFC with right anterior insula, left hippocampus with striatum and dorsal cingulate cortex, and right hippocampus with striatum and orbitofrontal cortex. Greater PTSD severity generally led to less changes in functional connectivity with the right insular cortex.
Conclusions:
These results provide evidence that RETM engages and modifies functional connectivity pathways with neural regions implicated in fear extinction. The results also implicate the engagement of the right insular cortex and striatum during RETM and suggest their importance in human fear extinction to trauma memories. However, comorbidity in the sample and the lack of a control group limit inferences regarding RETM with PTSD populations specifically.
Keywords: PTSD, fear extinction, neuroimaging, exposure therapy
Posttraumatic stress disorder is characterized by re-experiencing, avoidance, and hyperarousal symptoms (APA, 2000) and is associated with marked quality of life impairments (Olatunji, Cisler, & Tolin, 2007). To date, the most evidence-based and widely disseminated psychological treatment for PTSD is prolonged exposure (PE) (Foa et al., 1999; Foa, Rothbaum, Riggs, & Murdock, 1991). While PE is a well-supported intervention, it is of limited efficacy with only ~60% of treatment completers entering remission (Foa et al., 1999; Resick, Nishith, Weaver, Astin, & Feuer, 2002; Schnurr et al., 2007). Thus, research efforts directed at improving the efficacy of PE is necessary.
One important treatment component of PE is repeated exposure to the trauma memory (RETM). In PE, this is termed imaginal exposure and takes the form of the individual recounting the trauma narrative repeatedly while providing indices of distress. Therapeutic response to traumatic memory exposure is based on a fear extinction model (Foa & Kozak, 1986; Foa et al., 1991; Rothbaum & Davis, 2003): thoughts and memories of the traumatic event are conceptualized as conditioned stimuli (CS+) that trigger anxiety responses (i.e., the conditioned response) due to their association with the traumatic event (i.e., the unconditioned stimulus, US). Repeated exposure to the traumatic memory (CS+) in a safe context is theorized to weaken the predictive value of the CS+ to predict the US and thereby weaken the ability of the traumatic memory or reminders to elicit anxiety/distress responses.
The purpose of the present exploratory study was to identify the in vivo neural mechanisms engaged and modified during repeated exposure to the traumatic memory. This intent was motivated by the assumptions that this would 1) provide important inferences regarding the mechanisms of treatment action and 2) facilitate development and testing of adjunctive methods methods to enhance these mechanisms (e.g., pharmacological agonists such as d-cycloserine). We focused on mechanisms of neural functional connectivity changes with neural regions implicated in fear extinction during analogue exposure therapy conducted during fMRI, given that exposure to the trauma narrative is based on a fear extinction model (Foa et al., 1986; Foa et al., 1991; Rothbaum et al., 2003). Extensive basic science research has demonstrated that fear extinction involves the interaction between three separate neural structures (Milad et al., 2007; Myers & Davis, 2007; Phelps, Delgado, Nearing, & LeDoux, 2004; Sotres-Bayon, Cain, & LeDoux, 2006). First, the amygdala is critical for the detection and valuation of the CS+ and for motivating the expression of fear-relevant behavioral responding. Second, the hippocampus is involved in contextual modulation of amygdala processing of the CS+, such as learning that the CS+ does not predict a shock when a safety signal is present. Third, the medial prefrontal cortex (mPFC) has direct anatomical projections to the amygdala (Ghashghaei, Hilgetag, & Barbas, 2007) and is critical for regulation of amygdala processing. Indeed, some rodent studies have found that lesion of the mPFC impairs fear-extinction (Morgan & LeDoux, 1995; Morgan, Romanski, & LeDoux, 1993; Morgan, Schulkin, & LeDoux, 2003), albeit inconsistently (Myers et al., 2007). Based on this research, we focused our characterization of the neural mechanisms engaged and modified in vivo during imaginal trauma exposure on regions of the brain functionally connected with the mPFC, bilateral amygdala, and bilateral hippocampus. One important caveat, though, is that this network of three regions is likely not specific to fear extinction; indeed, these three regions are also generally implicated in salience detection (Davis & Whalen, 2001), memory (Squire, 1992), and emotion regulation (Etkin, Egner, Peraza, Kandel, & Hirsch, 2006).
An extensive amount of neuroimaging research has focused on identifying neural mechanisms mediating PTSD (Hayes, Hayes, & Mikedis, 2012; Patel, Spreng, Shin, & Girard, 2012; Sartory et al., 2013). In regards to brain function during emotion processing and cognitive tasks, individuals with PTSD demonstrate greater amygdala and dorsomedial PFC activation relative to controls, and less ventral medial PFC activation relative to controls (Hayes et al., 2012). In regards to brain function during symptom provocation, which in the case of PTSD involves a single exposure to a trauma narrative (i.e.,. script-driven imagery), individuals with PTSD demonstrate greater activation of the posterior cingulate, retrosplenial cortex, dorsal anterior cingulate cortex, and striatum compared to controls (Sartory et al., 2013). When collapsed across the type of study (cognitive or emotional task studies and symptom provocation studies), individuals with PTSD demonstrate greater activation in the anterior insula, hippocampus, amygdala, and lateral frontal gyri (Patel et al., 2012). Overall, these results suggest dysfunction in regions implicated in salience detection (amygdala, anterior insula), reward valuation (striatum), emotion regulation (ventral medial PFC), cognitive control (lateral PFC), and autobiographical recall (posterior cingulate cortex). Further, these meta-analyses also implicate dysfunction within the regions implicated in fear extinction noted above (amygdala, hippocampus, and medial PFC). Accordingly, based on 1) the network of regions hypothesized to mediate fear extinction, 2) the conceptualization of RETM as a process of fear extinction, and 3) the meta-analytic findings of dysfunctional activation in amygdala, hippocampus, and medial PFC, we broadly hypothesize that RETM works through engagement and modification of functional connectivity with these three regions implicated in fear extinction.
Note that, because we use a single analogue session of repeated exposure to the traumatic memory (RETM), a therapeutic response is not expected (e.g., psychological treatment for PTSD typically lasts ~12 weeks). Thus, the current investigation is not a probe of the changes in neural mechanisms that underlie therapeutic response to RETM; instead, the current investigation probes the neural mechanisms that are engaged by the therapeutic procedure of RETM. We only examine the neural mechanism change during RETM among individuals with a current diagnosis if PTSD and we did not recruit a trauma-exposed group without PTSD as a comparison sample. First, RETM as a treatment would not be provided to a trauma-exposed individual without PTSD, thus there would be little clinical utility of identifying neural mechanism changes during RETM among these individuals. Second, trauma-exposed individuals without PTSD exhibited resilience, thus it might expected that they would exhibit significantly different neural responses to RETM that would not necessarily be informative about the neural mechanisms of change among individuals with PTSD, which again limits the clinical utility of this comparison. Accordingly, given that this is the first investigation of the in vivo changes in neural mechanisms during RETM among individual with PTSD, this study is exploratory in nature, conducted specifically among individual with a current diagnosis of PTSD, and we broadly hypothesized that RETM engages and modifies functional connectivity with the key nodes implicated in fear extinction.
Method
Participants
Seventeen adult women with PTSD related to either physical or sexual assault were enrolled into the study. One woman moved excessively during the scan causing intractable signal artifact, and her data were subsequently removed from analyses. This resulted in a final sample of 16 participants. Table 1 lists demographic and clinical characteristics of this sample. Inclusion criteria were 1) a history of either physical or sexual assault, 2) a current diagnosis of PTSD, and 3) that participants were stable on any psychiatric medications for at least 4 weeks. Exclusion criteria included psychotic disorders, a primary substance use disorder, or internal metal objects. Participants were recruited from outpatient mental health clinics and from community wide advertisements. All study procedures were approved by the local institutional review board.
Table 1.
Demographic and clinical characteristics of the 16 adult women in this sample.
| Variable | Mean (or frequency) | SD |
|---|---|---|
| Age | 33.8 | 10.8 |
| Ethnicity | 44% Caucasian 50% African-American 6% Other |
- |
| Marital Status | 19% married 25% divorced 19% separated 38% never married |
- |
| Education | 6% not graduate high school 38% graduate high school or GED 38% some college 18% graduate 2 year college or more education |
- |
| Current Job | 44% unemployed | - |
| Ever sought mental health services | 75% yes | - |
| Ever been hospitalized for psychiatric reason |
38% yes | - |
| PCL score | 62.3 | 13.5 |
| BDI-II score | 24 | 11.7 |
| Number of total direct assaults | 7.6 | 2.4 |
| Number of physical assaults from non-caregiver | 3.0 | 1.2 |
| Number of physical assaults from caregiver | 1.6 | 1.2 |
| Number of sexual assaults | 2.9 | 1.4 |
| Current Major Depressive Disorder | 44% | - |
| Current Panic Disorder | 44% | - |
| Current Generalized Anxiety Disorder | 56% | - |
| Current PTSD | 100% | - |
| Current Substance Use Disorder | 25% | - |
Note. PCL = Posttraumatic Checklist – civilian version; BDI-II = Beck Depression Inventory-II.
Assaultive trauma histories were characterized using the trauma assessment section of the National Women’s Survey and National Survey of Adolescents (Kilpatrick et al., 2000; Kilpatrick et al., 2003; Resnick, Kilpatrick, Dansky, Saunders, & Best, 1993), a structured interview used in prior epidemiological studies of assault and mental health functioning among adult women and adolescents. Specific assaultive events were assessed with behaviorally specific dichotomous questions and included: 1) sexual assault (i.e., anal penetration, vaginal penetration, oral sex on the perpetrator, oral sex from the perpetrator, digital penetration, fondling, forced fondling of the perpetrator), 2) physical assault (i.e., attacked with a weapon, attacked with a stick, club, or bottle, attacked without a weapon, threatened with a weapon, attacked with fists), and 3) severe abuse from a caregiver (i.e., beaten with fists or an object to the point where bruising or bleeding occurred).
Psychological disorders were assessed with the Structured Clinical Interview for DSM-IV Disorders (SCID) (First, 2002) administered by a trained clinical interviewer and supervised by a licensed clinical psychologist. Participants additionally completed the Posttraumatic Stress Checklist-Civilian Version (Blanchard, Jones-Alexander, Buckley, & Forneris, 1996) and Beck Depression Inventory-II (Beck, Steer, & Brown, 1996).
Repeated Exposure to the Trauma Memory Methodology
Participants were given a description and therapeutic rationale for RETM (e.g., “coming in repeated contact with the memory allows your brain to process the memory and help you learn to feel more comfortable with it”). Participants were asked to provide a written narrative of their primary traumatic event and also of a neutral control event. The written narratives were collected using standardized methodology commonly used in the literature (Orr et al., 1998; Orr, Pitman, Lasko, & Herz, 1993; Pitman, Orr, Forgue, de Jong, & Claiborn, 1987; Rauch et al., 1996; Shalev, Orr, & Pitman, 1992; 1993) that facilitates collection of relevant sensory detail (e.g., physical reactions, contextual stimuli, etc) during the event. The trauma and neutral scripts were matched in length and audiorecorded by a female research assistant.
Prior to fMRI, participants practiced the rating scale they would use during the scan. Participants were asked to provide ratings of anxiety, vividness of the memory, and dissociation during the memory recall on Likert scales of 1-10. During fMRI, participants were presented with five successive presentations of each memory type, with each presentation lasting three minutes. The narratives were presented both aurally through headphones and visually, such that the written narrative was presented to them on a projected screen that participants viewed through a mirror attached to the MRI head coil. Each three minute presentation was preceded by 30 seconds of a resting-state. The subjective ratings were collected after each individual presentation of each narrative. The trauma and neutral narratives were presented sequentially (e.g., the trauma narrative was repeated five times, then the neutral narrative was repeated five times), with the order (trauma or neutral first) counterbalanced across participants. To ensure the participants’ comfort during the scan, participants were told they could stop the scan any time they wanted and still receive full compensation for the study.
fMRI acquisition and image preprocessing
See the supplementary material for description of 3T fMRI acquisition and preprocessing.
Data Analysis
We probed in vivo changes in functional organization of a fear extinction network through whole-brain seedmaps of functional connectivity with the mPFC, bilateral amygdala, and bilateral hippocampi (see Supplementary Figure 1). The anatomical coordinates were taken from prior fear extinction, fear reversal, and emotion regulation fMRI studies (Delgado, Nearing, LeDoux, & Phelps, 2008; Phelps et al., 2004; Schiller & Delgado, 2010; Schiller, Levy, Niv, LeDoux, & Phelps, 2008). While the amygdala and hippocampus seed regions were 6 mm radius spheres (48 3×3×3mm voxels), the mPFC seed region was an 8 mm radius sphere (100 3×3×3mm voxels) in order to extend into both the left and right subgenual cingulate cortex. The specific anatomical coordinates of the centroid of each ROI (XYZ), in MNI space, were: mPFC = 0, 36, 0; right amygdala = 19,−5,−15; left amygdala = −19, −5, −15; right hippocampus = 27,−17,−13; left hippocampus = −28,−17,−13.
For each exposure presentation singular value decomposition was used to extract the first principal component of the voxels’ timecourses within each ROI separately. These ROI principal component timecourses were then separately regressed onto the timecourses of every other voxel in the brain, for each exposure epoch and for each narrative type separately, resulting in whole-brain seedmaps of functional connectivity with the seed region. We then used mixed models (Bryk & Raudenbush, 1987; Raudenbush & Byrk, 2002) to characterize growth curves of brain-wide functional connectivity with the seed regions over the five exposure epochs. Briefly, this approach models repeated measurements as ‘nested’ within the individual, such that a growth curve (for each voxel) is characterized separately for each individual (i.e., first-level analyses), and group level statistics are then computed on these individual-level parameters (i.e., second-level analyses). We modeled linear growth curves, using ordinary least squares regression, consisting of two parameters: an intercept (representing the initial degree of functional connectivity) and a slope (representing the linear change in functional connectivity across the five exposure epochs). The sign of the slope (positive or negative) indicates whether the functional connectivity increased or decreased across the exposure epochs. This was done in a mass-univariate whole brain approach separately for each ROI. That is, we characterized the growth curves of functional connectivity between the right amygdala and every other voxel in the brain, between the left amygdala and every other voxel in the brain, the mPFC and every other voxel in the brain, etc. We then created whole-brain contrast maps, in which we compared the growth curve parameter of interest (i.e., the slope) between the trauma narrative exposure and the neutral narrative exposure. This comparison controls for the effect of repeated recounting of a memory per se and other confounds such as change over time and scanner drift, allowing more precise inferences regarding dynamic changes in neural processing mechanisms that are specific to trauma narrative exposure. We controlled for whole-brain multiple corrections with cluster-level thresholding, with a corrected p < .05 defined as 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations (Forman et al., 1995).
The relationship between individual differences in PTSD symptom severity and in vivo brain connectivity changes were assessed through whole-brain robust regression analyses, in which PCL scores were regressed onto each individual’s voxel-wise slope parameters while also controlling for the intercept and slope parameters of the anxiety ratings during the trauma exposure (to control for the relationships between individual differences in PTSD symptoms and anxiety ratings during the trauma exposure). We again controlled for multiple comparisons with cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005.
Results
Behavioral
As can be seen in Table 2, mean anxiety ratings (collapsed across exposure epochs) were significantly higher during the trauma exposure relative to the neutral exposure (t = 6.4, p < .001). By contrast, mean dissociation and vividness ratings did not significantly differ between the trauma and neutral exposures (ps > .2). The slope for the change in anxiety ratings during the trauma exposure across the 5 exposures was significantly positive (t = 2.5, p = .024), indicating increased emotional engagement with the trauma memory.
Table 2.
Mean (SD) subjective ratings during the trauma and neutral memory exposures.
| Anxiety Ratings | Vividness Ratings | Dissociation Ratings | ||||
|---|---|---|---|---|---|---|
| Exposure Epoch | Trauma | Neutral | Trauma | Neutral | Trauma | Neutral |
| 1 | 6.6 (2.6) | 3.3 (2.6) | 7.4 (3.1) | 6.9 (2.7) | 2.8 (2.8) | 2.5 (2.6) |
| 2 | 7.6 (2.5) | 2.7 (2.5) | 8 (2) | 7.6 (2.4) | 2.2 (2) | 2.4 (2.6) |
| 3 | 7.3 (2.3) | 2.5 (2.4) | 8.1 (1.7) | 7.5 (2.5) | 2.4 (1.9) | 2.9 (2.4) |
| 4 | 7.4 (2.4) | 2.2 (2.8) | 8.6 (1.5) | 7.3 (2.4) | 2.3 (1.8) | 2.5 (2.4) |
| 5 | 7.4 (2.4) | 2.4 (3) | 8.4 (1.8) | 7.3 (2.5) | 2.5 (1.9) | 2.9 (2.6) |
Imaging
Functional connectivity with the right amygdala significantly increased across the imaginal trauma exposures versus the neutral exposures for the bilateral anterior insula and right hippocampus (Figure 1), and also with right inferior frontal gyrus, and ventral visual cortex (whole-brain statistical map provided in Supplemental Figure 2). Functional connectivity with the left amygdala significantly increased across the imaginal trauma exposures versus the neutral exposures for the right anterior insula and decreased with the right superior frontal gyrus (Figure 2; whole-brain statistical map provided in Supplemental Figure 3). Trauma exposure-related functional connectivity with the right hippocampus significantly increased for the right putamen and dorsomedial PFC / dorsal anterior cingulate cortex (ACC) (Figure 3; whole-brain statistical map provided in Supplemental Figure 4). Trauma exposure-related functional connectivity with the left hippocampus significantly increased for the right putamen and right orbitofrontal gyrus (Figure 4; whole-brain statistical map provided in Supplemental Figure 5). Finally, functional connectivity with the medial PFC significantly increased for the bilateral anterior insula (Figure 5) as well as right inferior frontal gyrus, right parietal cortex, and portions of the ventral visual stream across imaginal trauma exposures (whole-brain statistical map provided in Supplemental Figure 6).
Figure 1.
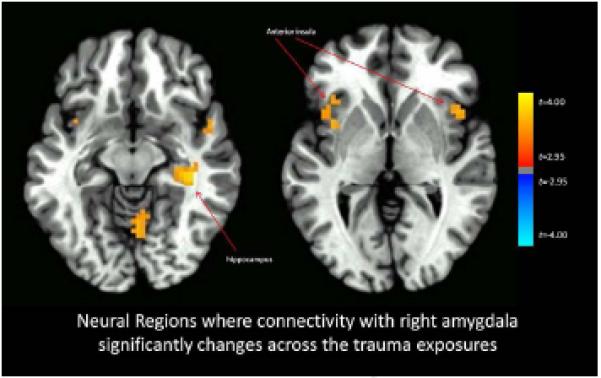
Neural Regions where connectivity with the right amygdala significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Figure 2.
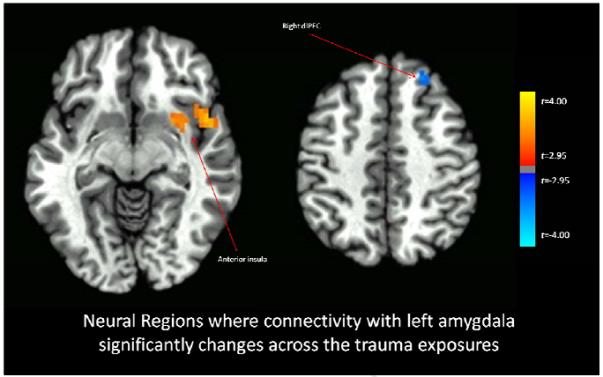
Neural Regions where connectivity with the left amygdala significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Figure 3.
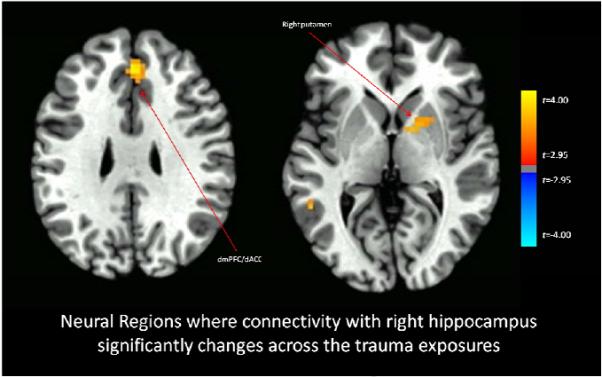
Neural Regions where connectivity with the right hippocampus significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Figure 4.
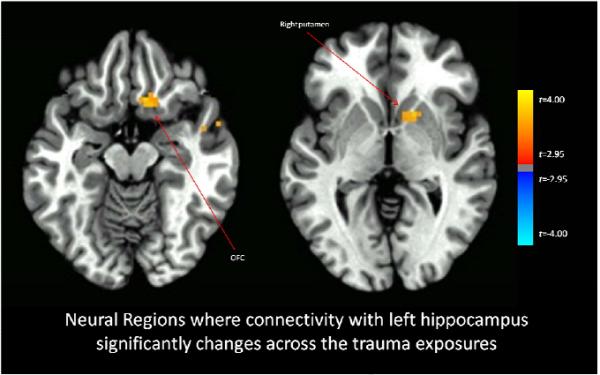
Neural Regions where connectivity with the left hippocampus significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Figure 5.
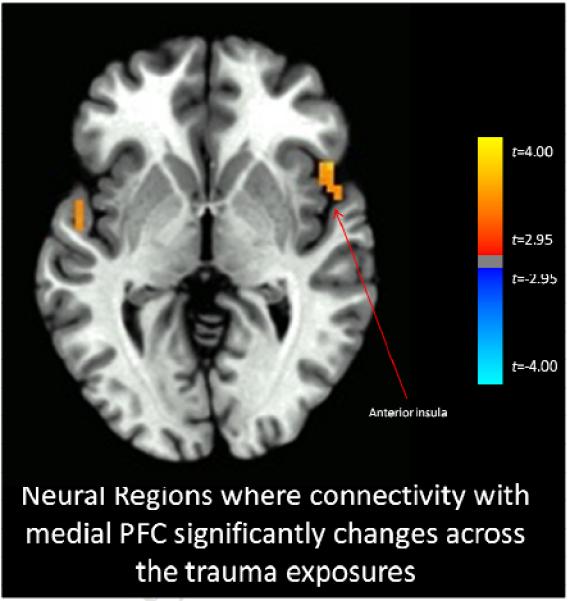
Neural Regions where connectivity with the medial PFC significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Given that the analyses are based on contrasts of changes in functional connectivity during trauma versus neutral exposures, the direction of the observed changes in connectivity are equivocal (e.g., a positive contrast value could indicate that connectivity increased during the trauma exposures and did not change during the neutral exposures or that connectivity did not change during the trauma exposures but decreased during the neutral exposures). Therefore, we also plotted the mean functional connectivity values across the trauma and neutral exposures for the main regions identified in the above contrasts (Supplementary Figures 7-11). As can be seen, in each case, the observed fcMRI changes are represented by the direction of the contrast effect: that is, a positive contrast value indicates increasing connectivity during the trauma exposures and decreasing or static connectivity during the neutral exposures.
Correlations between PTSD symptom severity and functional connectivity changes related to trauma exposures
As indicated in Figure 6, we found an overlapping region in the right anterior insula / inferior frontal gyrus where greater PTSD symptoms were associated with less strengthening of functional connectivity across trauma exposures with both the right hippocampus (Figures 6a) and right amygdala (Figure 6b). Similarly, there was a region in the right posterior insula / inferior frontal gyrus (Figure 6c) where greater PTSD symptoms were associated with less trauma exposure-related strengthening of functional connectivity with the mPFC.
Figure 6.
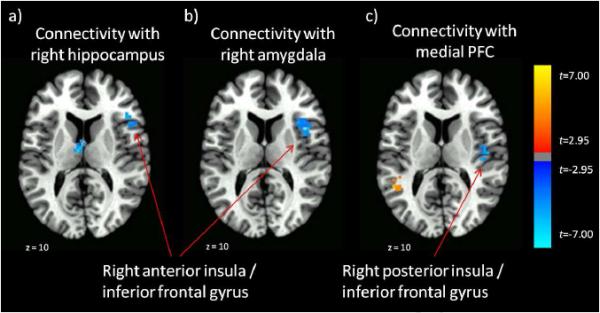
Neural Regions where PTSD symptoms significantly negatively correlate with a) degree of connectivity changes between the right anterior insula / inferior frontal gyrus and right hippocampus, b) degree of connectivity changes between the right anterior insula / inferior frontal gyrus and right amygdala, and c) degree of connectivity chnages between the right posterior insula / inferior frontal gyrus and medial PFC
Comorbidity
Finally, given the comorbidity in the present sample, we also conducted exploratory tests of whether the observed functional connectivity results differed as a function of comorbidity with major depressive disorder, panic disorder, and generalized anxiety disorder. We observed that the presence of these comorbidities (versus their absence) did not significantly affect the degree of observed functional connectivity changes during RETM nor did it alter the direction of the observed effects (results available upon request from first author).
Discussion
The results of this initial exploratory study suggest that functional connectivity of the key nodes implicated in fear extinction changes with repeated exposure to a traumatic memory. There was consistency in the brain regions whose functional connectivity with these nodes changed, such that strengthening of functional connectivity with the striatum was observed for both the right and left hippocampus, and strengthening of functional connectivity with the anterior insular cortices was observed for both left and right amygdala as well as mPFC. These findings implicate the integration of the striatum and anterior insula with key nodes implicated in fear extinction as a core process occurring during repeated exposure to the traumatic memory. Given the role of both the striatum and anterior insular cortices in reward learning and, more specifically, in tracking prediction errors during learning (Bossaerts, 2010; Oyama, Hernadi, Iijima, & Tsutsui, 2010; Preuschoff, Quartz, & Bossaerts, 2008; Schultz & Dickinson, 2000; Stalnaker, Calhoon, Ogawa, Roesch, & Schoenbaum, 2012), one hypothesis explaining the observed findings is that fear extinction induced through repeated exposure to a traumatic memory draws heavily on tracking prediction errors to facilitate therapeutic learning processes. That is, from a fear conditioning perspective, trauma reminders (CS+) signal the occurrence of the traumatic event (US). Repeated presentation of the CS+ in the absence of the US would therefore be expected to generate prediction errors, and recent evidence among humans indeed suggests that striatal activity tracks prediction errors during fear extinction learning (Li, Schiller, Schoenbaum, Phelps, & Daw, 2011). Accordingly, the observed increased functional connectivity with the striatum and anterior insular cortices suggests that the tracking of prediction errors becomes increasingly integrated into the fear extinction neural network during repeated exposure to the traumatic memory and that this influence underlies the learning that the CS+ no longer predicts the US. While this is one plausible hypothesis explaining the observed changes in functional connectivity, it should be emphasized that this hypothesis is based on patterns of observed brain data (i.e., reverse inference) and more data is needed to corroborate this tentative hypothesis.
We also found evidence that greater severity of baseline PTSD symptoms was associated with less functional integration of the right insular cortex and neighboring inferior frontal gyrus into the fear extinction network across trauma exposures. As individual differences in anxiety responses to the trauma exposure were controlled in the analysis, the observed relationship with baseline PTSD symptoms cannot be attributed to differences in emotional responses to the trauma exposures. One hypothesis explaining this finding is that those individuals with more severe PTSD symptoms may generate less prediction errors during the fear extinction process, which might be expected to confer risk for poorer treatment outcomes. Prior research has found that more severe pre-treatment PTSD symptoms are associated with worse treatment outcomes (Karatzias et al., 2007), and the present finding suggests a possible neural mechanism mediating the worse outcomes associated with more severe PTSD symptoms. If this explanation is valid, then it might be expected that supplemental therapeutic procedures that can potentiate connectivity between the fear extinction network and right insular cortex/inferior frontal gyrus may lead to better treatment outcomes among individuals with greater PTSD symptoms severity.
In addition to finding increased integration of neural regions associated with tracking prediction errors into the fear extinction network, we also observed several other changes in functional connectivity with regions of theoretical interest. Functional connectivity between the right amygdala and right hippocampus was found to increase across the trauma exposure. Given the role of the hippocampus in memory consolidation and contextual retrieval during extinction (Myers et al., 2007), it seems possible that the increasing functional connectivity between these regions is indicative of encoding of the new association that, in a certain context, trauma reminders (CS+) do not signal the occurrence of the US. We also observed strengthening of functional connectivity between the right hippocampus and a region extending from the dorsomedial PFC into the dorsal anterior cingulate cortex (ACC). Given the role of this region in conflict monitoring (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Kerns et al., 2004), this observation may reflect the integration of conflict monitoring processes during fear extinction. Indeed, the dACC is also implicated in tracking prediction errors and volatility of expected outcomes (Behrens, Woolrich, Walton, & Rushworth, 2007; Rushworth & Behrens, 2008); thus, the ‘conflict’ during fear extinction to traumatic memories may involve changes in the discrepancy between actual and expected threat outcomes. Again, these hypotheses about cognitive functions are based on observed patterns of brain activity (i.e., reverse inferences), and it must be emphasized that these are hypotheses in need of further corroboration.
Finally, we did not observe changes in connectivity between the amygdala and mPFC. Given the hypothesized role of the mPFC in fear extinction learning (Maren, Phan, & Liberzon, 2013; Milad et al., 2007; Myers et al., 2007; Orsini, Kim, Knapska, & Maren, 2011; Quirk & Beer, 2006), it may have been expected that we would observe increasing modular relationships (i.e., negative functional connectivity) between the regions, such that more mPFC activity is associated with less amygdala activity. One possible explanation for the failure to observe this relationship in the present data is that it may require more than 5 repeated exposures for sufficient learning to occur that would facilitate mPFC inhibition of the amygdala. The failure to observe this finding also highlights the need to continue studying the in vivo neural mechanisms of change that occur during actual therapies in order to better inform how to implement and improve them.
Several study limitations of this study should temper conclusions. First, a single truncated session of RETM is unlikely to be therapeutic, as is seen clinically when PTSD patients actually sometimes show a transient increase in PTSD symptoms during the early stages of RETM (Foa, Zoellner, Feeny, Hembree, & Alvarez-Conrad, 2002). Future research is also necessary to determine the functional connectivity changes during RETM that scale with symptom reductions. Second, our sample was limited to adult women with PTSD related to assaultive violence exposure. While this increased the homogeneity of the sample, the degree to which these results generalize to more heterogeneous populations is unknown. Third, the inferences regarding increased integration of prediction error tracking during fear extinction learning need to be further corroborated with future research. Fourth, the lack of a control group limits inferences regarding whether the observed results are specific to RETM for PTSD populations specifically, to trauma exposed populations generally, or simply to exposure to stressful memories. Fifth, there was a high degree of comorbidity in the present sample, which limits inferences regarding generalizability to PTSD populations specifically.
Supplementary Material
Graphical depictions of regions of interest in bilateral amygdala, hippocampus, and mPFC.
Changes in functional connectivity between right hippocampus and right putamen and dmPFC/dACC across the trauma and neutral exposures.
Changes in functional connectivity between left hippocampus and right orbitofrontal gyrus across the trauma and neutral exposures.
Whole-brain map of neural regions where connectivity with the right amygdala significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the left amygdala significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the right hippocampus significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the left hippocampus significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the medial PFC significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Changes in functional connectivity between mPFC and right insula and left insula across the trauma and neutral exposures.
Changes in functional connectivity between left amygdala and right insula and right dlPFC across the trauma and neutral exposures.
Changes in functional connectivity between right amygdala and right insula, left insula, and right hippocampus across the trauma and neutral exposures.
Acknowledgements
We thank Cindy Mosley, Andi Ham, Shanti Tripathi, and George Andrew James for help with recruitment, analysis, and administration.
Funding sources: Portions of this work were supported through grants 1R21MH097784-01 and T32 DA022981-02. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute for Mental Health or the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- APA . Diagnostic and Statistical Manual of Mental Health Disorders. 4th. Author; Washington,DC: 2000. text rev. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the BDI-II. The Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nature Neuroscience. 2007;10:1214–1221. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Jones-Alexander J, Buckley TC, Forneris CA. Psychometric properties of the PTSD Checklist (PCL) Behaviour Research and Therapy. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- Bossaerts P. Risk and risk prediction error signals in anterior insula. Brain Struct Funct. 2010;214:645–653. doi: 10.1007/s00429-010-0253-1. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108:624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Application of Hierarchical Linear-Models to Assessing Change. Psychological Bulletin. 1987;101:147–158. [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–882. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer, Robert L, Gibbon Miriam, Williams, Janet BW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-Patient Edition. Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. Journal of Consulting and Clinical Psychology. 1999;67:194–200. doi: 10.1037//0022-006x.67.2.194. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Foa EB, Rothbaum BO, Riggs DS, Murdock TB. Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive-behavioral procedures and counseling. Journal of Consulting and Clinical Psychology. 1991;59:715–723. doi: 10.1037//0022-006x.59.5.715. [DOI] [PubMed] [Google Scholar]
- Foa EB, Zoellner LA, Feeny NC, Hembree EA, Alvarez-Conrad J. Does imaginal exposure exacerbate PTSD symptoms? Journal of Consulting and Clinical Psychology. 2002;70:1022–1028. doi: 10.1037//0022-006x.70.4.1022. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, Mikedis AM. Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood and Anxiety Disorders. 2012;2:9. doi: 10.1186/2045-5380-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatzias A, Power K, McGoldrick T, Brown K, Buchanan R, Sharp D, Swanson V. Predicting treatment outcome on three measures for post-traumatic stress disorder. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:40–46. doi: 10.1007/s00406-006-0682-2. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Acierno R, Saunders B, Resnick HS, Best CL, Schnurr PP. Risk factors for adolescent substance abuse and dependence: data from a national sample. Journal of Consulting and Clinical Psychology. 2000;68:19–30. doi: 10.1037//0022-006x.68.1.19. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. Journal of Consulting and Clinical Psychology. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Li J, Schiller D, Schoenbaum G, Phelps EA, Daw ND. Differential roles of human striatum and amygdala in associative learning. Nature Neuroscience. 2011;14:1250–1252. doi: 10.1038/nn.2904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behavioral Neuroscience. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neuroscience Letters. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morgan MA, Schulkin J, LeDoux JE. Ventral medial prefrontal cortex and emotional perseveration: the memory for prior extinction training. Behavioural Brain Research. 2003;146:121–130. doi: 10.1016/j.bbr.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, Tolin DF. Quality of life in the anxiety disorders: a meta-analytic review. Clinical Psychology Review. 2007;27:572–581. doi: 10.1016/j.cpr.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Berry NJ, Ahern CE, Pitman RK. Psychophysiologic assessment of women with posttraumatic stress disorder resulting from childhood sexual abuse. Journal of Consulting and Clinical Psychology. 1998;66:906–913. doi: 10.1037//0022-006x.66.6.906. [DOI] [PubMed] [Google Scholar]
- Orr SP, Pitman RK, Lasko NB, Herz LR. Psychophysiological assessment of posttraumatic stress disorder imagery in World War II and Korean combat veterans. Journal of Abnormal Psychology. 1993;102:152–159. doi: 10.1037//0021-843x.102.1.152. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Kim JH, Knapska E, Maren S. Hippocampal and prefrontal projections to the basal amygdala mediate contextual regulation of fear after extinction. Journal of Neuroscience. 2011;31:17269–17277. doi: 10.1523/JNEUROSCI.4095-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama K, Hernadi I, Iijima T, Tsutsui K. Reward prediction error coding in dorsal striatal neurons. Journal of Neuroscience. 2010;30:11447–11457. doi: 10.1523/JNEUROSCI.1719-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R, Spreng RN, Shin LM, Girard TA. Neurocircuitry models of posttraumatic stress disorder and beyond: a meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews. 2012;36:2130–2142. doi: 10.1016/j.neubiorev.2012.06.003. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Archives of General Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. Journal of Neuroscience. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Archives of General Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Byrk A. Hierarchical Linear Models. 2nd Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. Journal of Consulting and Clinical Psychology. 2002;70:867–879. doi: 10.1037//0022-006x.70.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. Journal of Consulting and Clinical Psychology. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE. Choice, uncertainty and value in prefrontal and cingulate cortex. Nature Neuroscience. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Sartory G, Cwik J, Knuppertz H, Schurholt B, Lebens M, Seitz RJ, Schulze R. In search of the trauma memory: a meta-analysis of functional neuroimaging studies of symptom provocation in posttraumatic stress disorder (PTSD) PLoS ONE. 2013;8:e58150. doi: 10.1371/journal.pone.0058150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14:268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. Journal of Neuroscience. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurr PP, Friedman MJ, Engel CC, Foa EB, Shea MT, Chow BK, Resick PA, Thurston V, Orsillo SM, Haug R, Turner C, Bernardy N. Cognitive behavioral therapy for posttraumatic stress disorder in women: a randomized controlled trial. JAMA. 2007;297:820–830. doi: 10.1001/jama.297.8.820. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dickinson A. Neuronal coding of prediction errors. Annual Review of Neuroscience. 2000;23:473–500. doi: 10.1146/annurev.neuro.23.1.473. [DOI] [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Pitman RK. Psychophysiologic response during script-driven imagery as an outcome measure in posttraumatic stress disorder. Journal of Clinical Psychiatry. 1992;53:324–326. [PubMed] [Google Scholar]
- Shalev AY, Orr SP, Pitman RK. Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. American Journal of Psychiatry. 1993;150:620–624. doi: 10.1176/ajp.150.4.620. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Cain CK, LeDoux JE. Brain mechanisms of fear extinction: historical perspectives on the contribution of prefrontal cortex. Biological Psychiatry. 2006;60:329–336. doi: 10.1016/j.biopsych.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stalnaker TA, Calhoon GG, Ogawa M, Roesch MR, Schoenbaum G. Reward prediction error signaling in posterior dorsomedial striatum is action specific. Journal of Neuroscience. 2012;32:10296–10305. doi: 10.1523/JNEUROSCI.0832-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Graphical depictions of regions of interest in bilateral amygdala, hippocampus, and mPFC.
Changes in functional connectivity between right hippocampus and right putamen and dmPFC/dACC across the trauma and neutral exposures.
Changes in functional connectivity between left hippocampus and right orbitofrontal gyrus across the trauma and neutral exposures.
Whole-brain map of neural regions where connectivity with the right amygdala significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the left amygdala significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the right hippocampus significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the left hippocampus significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Whole-brain map of neural regions where connectivity with the medial PFC significantly changes across the trauma exposures relative to the neutral exposures. Thresholded at p < .05 using cluster-level thresholds of 16 contiguous voxels surviving an uncorrected p < .005 based on Monte Carlo simulations.
Changes in functional connectivity between mPFC and right insula and left insula across the trauma and neutral exposures.
Changes in functional connectivity between left amygdala and right insula and right dlPFC across the trauma and neutral exposures.
Changes in functional connectivity between right amygdala and right insula, left insula, and right hippocampus across the trauma and neutral exposures.


