Abstract
NK cells are non-T, non-B lymphocytes that kill target cells without previous activation. The immunophenotype and function of these cells in humans and mice are well defined, but canine NK cells remain incompletely characterized. Our objectives were to isolate and culture canine peripheral blood NK cells, and to define their immunophenotype and killing capability. PBMC were obtained from healthy dogs and T cells were depleted by immunomagnetic separation. The residual cells were cultured in media supplemented with IL-2, IL-15 or both, or with mouse embryonic liver (EL) feeder cells. Non-T, non-B lymphocytes survived and expanded in these cultures. IL-2 was necessary and sufficient for survival; the addition of IL-15 was necessary for expansion, but IL-15 alone did not support survival. Culture with EL cells and IL-2 also fostered survival and expansion. The non-T, non-B lymphocytes uniformly expressed CD45, MHC I, and showed significant cytotoxic activity against CTAC targets. Expression of MHC II, and of CD11/18 was restricted to subsets of these cells. The data show that cells meeting the criteria for NK cells in other species, i.e., non-T, non-B lymphocytes with cytotoxic activity, can be expanded from canine PBMC by T-cell depletion and culture with cytokines or feeder cells.
Keywords: canine, NK cells, interleukins
Introduction
Natural killer (NK) cells are non-T, non-B lymphocytes, which are part of the innate immune system and are constitutively able to kill target cells without prior activation. NK cells play important roles in tumor immunity, viral disease and pregnancy (Croy et al., 2006; Vivier et al., 2008). NK cells have been characterized in a variety of mammals, including humans (Vivier et al., 2008), rhesus macaques (LaBonte et al. 2001), mice (Welsh, 1978; Dissen et al., 2008), rats (Rolstad et al., 2001; Dissen et al., 2008), cows (Dissen et al., 2008; Boysen and Storset, 2009), and pigs (Gerner et al., 2009). Expression of prototypical surface markers, including CD56 on human cells and NK1.1 on mice, allow for prospective identification of NK cells. Human and murine NK cells have been particularly well characterized and share some functional properties, such as MHC I-dependent killing. However, there are significant functional and phenotypic differences between species, including the specific molecules involved in MHC I binding, which include immunoglobulin-like killer inhibitory receptors (KIR) in humans and lectin-like Ly49 receptors in mice (Vivier et al., 2008; Boysen and Storset, 2009). These differences have made it challenging to extrapolate human NK function from murine data and indicate the need for additional models of NK function.
NK cell transfer from haploidentical donors is a promising new avenue for cancer therapy and has reached phase 1 clinical trials in patients with acute myelogenous leukemia. Preliminary data suggest that adoptive transfer of NK cells may reduce the risk of graft-versus-host disease compared to other hematologic transplantation protocols (Ruggeri et al., 2002; Giebel et al., 2003; Miller et al., 2005; Moretta et al., 2008a; Moretta et al., 2008b). Canine models were essential for the development and validation of clinical protocols for bone marrow transplantation (Ladiges et al., 1990; Panse et al., 2003; Graves et al., 2007; Jochum et al., 2007) and may be similarly useful for optimization of adoptive NK cell transfer. Nonetheless, canine NK cells remain incompletely characterized.
Putative canine NK cells have been identified primarily by NK-like cytotoxicity and/or phenotypically as large granular lymphocytes (Loughran et al., 1993; Nakada et al., 1995; Nakada et al., 1997; Nariai Nakada et al., 1999; Strasser et al., 2000; Kuwabara et al., 2006). Prototypical human or murine NK markers including CD56 (Otani et al., 2002), KIR (Hammond et al., 2009; Yoder and Litman, 2011) or Ly49 (Hammond et al., 2009) have not been documented in definable population of canine lymphocytes that can be called NK cells. Thus, many investigators have limited their focus to lymphocytes that have NK-like cytotoxic properties against canine thyroid adenocarcinoma (CTAC) cells, even though these inevitably include cells that express prototypical T cell markers (CD3 and CD5) or represent T-cell derived lymphokine activated killer (LAK) cells (Helfand et al., 1994; Funk et al., 2003; Funk et al., 2005; Huang et al., 2008; Lin et al., 2010). Huang et al (2008) recently described a novel CD3+/CD5lo/CD8+ population with NK activity, which are consistent with NK T cells from other species. Non-T, non-B lymphocytes have been identified in canine peripheral blood, but they comprise <5% of circulating lymphocytes (Otani et al., 2002; Otani et al., 2008) and their cytotoxicity has not been explored. The purpose of this project was to isolate and expand canine NK (non-T, non-B) lymphocytes in vitro and to characterize their immunophenotype and cytotoxic capability.
Materials and Methods
Animals
Thirteen peripheral blood samples were obtained from 12 healthy pet dogs with owner consent under a protocol approved by the University of Minnesota IACUC (protocol 0802A27363). All animals had received routine vaccinations and prophylactic anthelminthics. The dogs included four Labrador retrievers, one German wirehaired pointer, and seven mixed breeds, all between 1 and 7 years old.
NK cell isolation
PMBC were isolated by Ficoll-Hypaque density gradient centrifugation (Ficoll-Paque Plus, GE Healthcare, Piscataway, NJ). Cells were treated with RBC lysing agent (eBiosciences, San Diego, CA) and platelets were removed by washing and resuspending cell pellets in glass pipettes. For T-cell depletion, PMBC were resuspended in PBS with 0.5% fetal bovine serum (Atlas Biologicals, Ft. Collins, CO) and 2 mM EDTA. After blocking Fc receptors with canine gamma globulin (Jackson ImmunoResearch Laboratories Inc., West Grove, PA, USA), cells were incubated on ice for 15 min with anti-CD5 antibody conjugated to phycoerythrin (PE) (clone YKIX322.3, Serotec, Raleigh, NC). Immunomagnetic separation was used to remove CD5-positive cells with the EasySep PE positive selection kit according to the manufacturer's protocol (STEMCELL Technologies, Vancouver, Canada). The procedure was repeated three times to maximize depletion.
Immunophenotyping
Staining was performed using anti-canine CD3 conjugated to fluorescein isothiocyanate (FITC) (clone CA17.2A12, Serotec), anti-canine CD4-FITC (YKIX302.9, Serotec), anti-canine CD5-FITC, anti-canine CD5-PE, anti-canine CD8-PE (YCATE55.9, Serotec), anti-canine CD21-PE (CA2. 1D6, Serotec), anti-canine CD45-FITC or -PE (YKIX716.13, Serotec), anti-canine CD11/18-FITC (YKIX490.6.4, Serotec), anti-human CD14-PE (TÜK4, Serotec), and unconjugated anti-canine CD11b (CA16.3E10, Serotec), anti-human CD22 (RFB4, Abcam, Cambridge, MA), anti-bovine MHC I (H58A, VMRD, Pullman, WA), anti-human HLA-DR (L243, BD Biosciences, San Jose, CA), anti-human CD94 (HP-3DP, BD Biosciences), anti-human CD56 (B-159, BD Biosciences), anti-human Nkp46 (BAB281, Beckman Coulter, Miami, FL), anti-human TIM-3 (FAB2365P, R&D Systems, Minneapolis, MN). The antibodies against human CD14 (Vernau et al., 1999), CD22 (Faldyna et al., 2007), and CD94 (Schuberth et al., 2007) have been previously verified to recognize the corresponding epitopes of the homologous canine proteins. At least 2000 cells were analyzed. If insufficient cells were present for staining with all antibody combinations, staining for T and B lymphoid markers and for panleukocyte markers was prioritized. Data were collected using a FACSCalibur or LSR II (BD Biosciences) and analyzed using FlowJo software (TreeStar, Ashland, OR).
Cytology
Cytospin preparations were prepared using 100 μL of cell suspension and were stained with Diff Quick and/or modified Wright's stain for microscopic examination.
Cell culture
CD5-depleted leukocytes were cultured for 14 days (9 samples) or for 21-25 days (4 samples) days in media containing 60% DMEM, 30% HAMS F-12 + 2 mM L-glutamine, 10% heat-inactivated human serum, ß-mercaptoethanol ethanolamine, sodium selenite, and ascorbic acid, as described (Pierson et al., 1995). Samples for the 14 day culture were divided into 3 treatment groups, each containing 1,000 IU/mL of IL-2, 10 ng/mL of IL-15, or 1,000 IU/mL IL-2 + 10 ng/mL IL-15. Samples for the 21 day cultures were grown with EL08-1D2 (EL) feeder cells (McCullar et al., 2008) in media with IL-2 or in cytokine conditions (either IL-2 throughout, IL-2 for 14 days and then IL-2 + IL-15 or IL-2 + IL-15 throughout). Cultures were plated at an initial cell density of 1×106 cells/mL and were fed every 3-4 days.
Cytotoxicity
A 4-hour 51Cr release assay was performed as described (Miller et al., 1992) using serial effector to target dilutions from 20:1 to 0.08:1.
Results and Discussion
Non-T, non-B canine peripheral blood lymphocytes can be expanded in vitro
Previous work in human NK cells has shown that successful expansion of these cells in vitro with cytokine-supplementation requires T-cell depletion, since T cells are much more numerous and expand under the influence of the same cytokines. Here, we used CD5 as the target for depletion (with anti-CD5 antibodies), since this antigen is invariably and robustly expressed by normal canine T cells, and because of the empirical performance characteristics of the available anti-canine CD5 antibody as compared to anti-canine CD3 antibody. Macrophages and B cells were not depleted since these populations are reported to enhance in vitro expansion of human NK cells (Miller et al., 1992; Pierson et al., 1994). Residual PBMC after CD5-depletion ranged from 5 × 105 - 6 × 106, with 4.6-52% of these cells localizing the lymphocyte gate based on their light scatter properties. CD5-negative cells persisted after 2-3 weeks in culture with IL-2 or IL-2 + IL-15 in 8/13 samples (62%); insufficient numbers of cells (<12,500 total cells) were recovered from four samples; and there was incomplete T-cell depletion in one sample.
We report on the results from the eight samples where CD5-negative cells were recovered from culture. Cells from four of the eight dogs were in culture for 14 days and cells from the other four were in culture for 21 days.
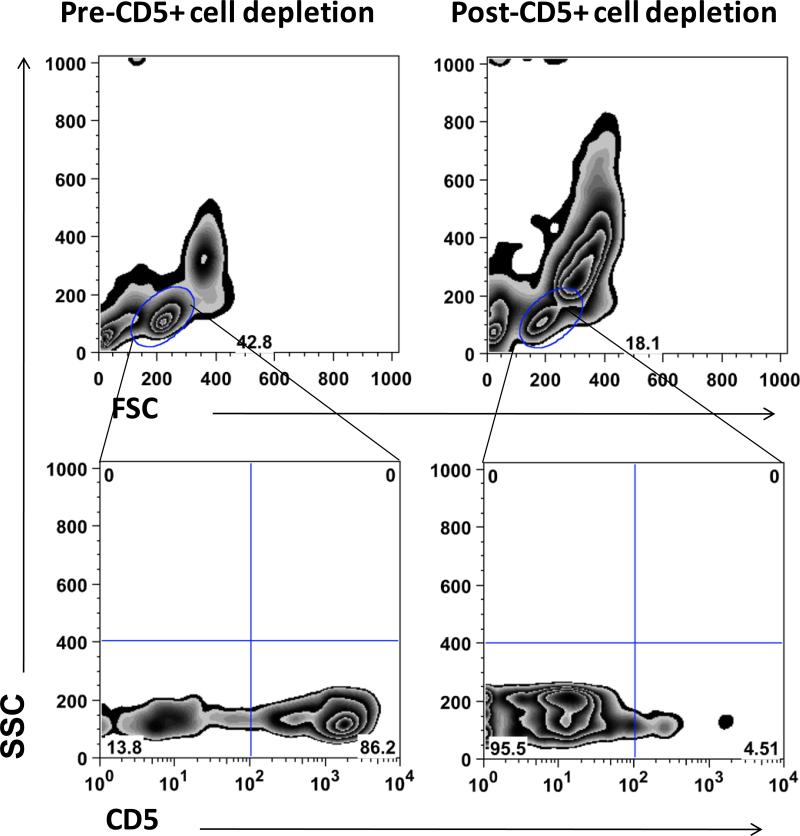
Figure 1 shows an example of the cell populations before and after T-cell depletion. On average, 82.7% (range = 76 - 90%) of the lymphoid cells were CD5+ before depletion, and 10.1% (range 0.9-22.8%) were CD5+ after depletion. The cytokine requirements for in vitro expansion of canine NK cells were unknown. Previous reports of putative canine NK cells suggested these cells express IL-2 receptors and respond to IL-2, but no information was available regarding expression of IL-15 receptors or response to IL-15, which is the prototypical NK cell growth factor in humans. Thus, we examined survival and expansion under conditions where cells were cultured with IL-2, IL-15, or both. Culture with IL-15 alone was stopped after the initial three samples, since in each of these instances fewer than 10,000 cells survived after 14 days. The expansion of CD5-negative cells observed in eight independent samples is shown in Table 1. IL-2 alone supported survival of the T-cell depleted lymphoid cells, or at least maintained a balance between proliferation and death, as the numbers at the onset (day 0) and at the end of culture (day 14) were either significantly different or only minimally increased. The addition of IL-15 resulted in reproducible expansion of the T-cell depleted lymphoid cells. In-vitro human and murine NK cell expansion is enhanced when cultured with feeder cells, so we examined NK cell growth in the EL feeder cell co-culture system. EL cells in the presence of IL-2 promoted similar levels of proliferation as cytokines alone, suggesting these cells could at least partly replace the added effects of IL-15.Variability in the magnitude of the expansion noted among the different donor dogs was likely attributable to the aforementioned heterogeneity in the number of CD5- cells present at the beginning of the culture for each dog, and possibly to the inherent variability in the response to cytokines that is expected in outbred animals.
Figure 1.
Depletion of CD5+ cells from PBMC. Immunomagnetic separation was used to obtain CD5+ cell-depleted populations for expansion of putative NK cells. The top panels show PBMC prior to (left) and after (right) CD5+ cell depletion with gating on lymphocytes based on forward and right angle (side) light scatter properties. The bottom panels show CD5 staining vs. right angle light scatter in lymphocytes prior to (left) and after (right) CD5+ cell depletion. The figure shows depletion in PBMC from one representative sample of eight used for analysis.
Table 1.
Fold-expansion of CD5-negative cells in culture
| 14-day expansion | |||
|---|---|---|---|
| IL-2 | IL-2 + IL-15 | EL08-1D2 cells + IL-2 | |
| Donor A | 0.21 | 354 | ND |
| Donor B | 1.09 | 7.75 | ND |
| Donor C | 2.26 | 4.67 | ND |
| Donor Da | 4.6 | 11 | ND |
| 21-day expansion | |||
| Donor Da | ND | 4.72 | 77.8 |
| Donor E | ND | 287 | 62.1 |
| Donor F | ND | 85.3 | 559 |
| Donor G | ND | 181 | ND |
ND = Not done.
Experiments performed on separate samples from the same donor.
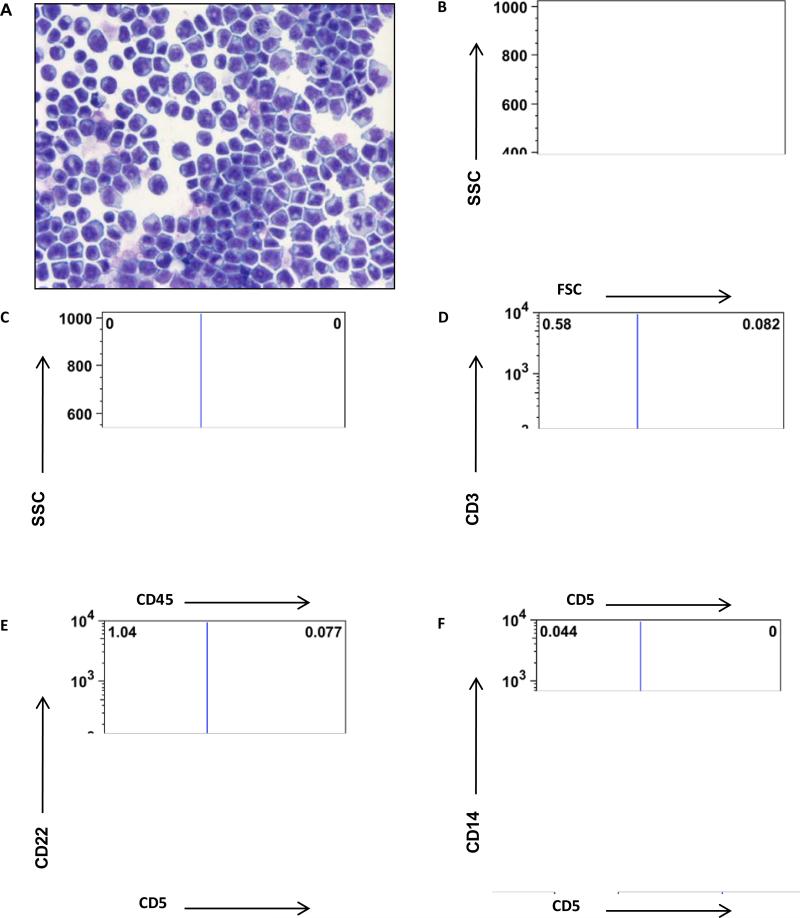
The cytology and phenotypic staining of the cells grown in culture with IL-2 or with IL-2 and IL-15 were similar, and thus are presented together. The cultured cells consisted almost exclusively of medium to large lymphocytes (Figure 2A; mean = 90.6% ± 8.0, as determined by gating on the lymphocyte gate in the flow cytometric analyses). Somewhat surprisingly, the cells had no cytoplasmic granules when visualized using Wright's stains at the end of the culture period (Figure 2B). The cells were predominately non-T, non-B cells (0.94% ± 1 expressed CD3 or CD5, 0.50% ±0.9 expressed CD4, and 1.3% ± 1 expressed CD22), and they also were not of monocyte origin (0.16% ± 0.28 expressed CD14). The cells universally (>95%) expressed CD45 and MHC I, and expression of other markers was restricted to subsets of these cells. An average of 79.6% (± 3.6) expressed MHC II, 52% (± 34) expressed CD11/18, and 42% (± 30) expressed CD11b. An average of 8.9% (± 8.0) of the cells expressed CD8. The cells did not have receptors that bound anti-human CD56 or anti-human NKp46. Few cells expressed CD94 (3.16% ± 7) and Tim-3 (2.48% ± 5). The failure to bind the antibodies for prototypical human NK markers may be due to poor cross-reactivity between the human and canine epitopes or expression of different surface markers.
Figure 2.
Morphologic and immunophenotypic properties of the non-T, non-B lymphocytes after culture. (A) Photomicrograph of a cytospin preparation of cultured non-T, non-B lymphocytes (Aqueous Romanowsky stain, 500X magnification). (B-F) Flow cytometric light scatter and expression of CD45, CD3, CD5, CD22, and CD14 in non-T, non-B lymphocytes after 14 days in culture with IL-2 + IL-15.
These results show that depletion of T cells allowed for successful expansion of a previously undescribed population of canine peripheral non-T, non-B lymphocytes and suggest that IL-2 was necessary and sufficient for survival of these cells. However, addition of IL-15 or stroma appeared to be required for their expansion. This is consistent with what is known for NK cells in other mammalian species, and underscore potential pitfalls with other studies where IL-2 has been used to expand putative NK cells without prior T cell depletion, since IL-2 (and IL-15) stimulates both T cells and NK cells. Thus, it is likely that at least some earlier studies might have selected primarily for the more numerous T cells in their cultures. One group had previously reported enhancement of canine NK-like activity against transmissible venereal tumor cells with IL-6 and IL-15 (Lin et al., 2008). IL-15 is a potent activator of quiescent human NK cells. Mechanistically, NK cells respond transiently to free IL-15 (that is, IL-15 in solution and not bound to other cells), followed by persistent responsiveness to IL-2 and trans-presented IL-15 (Pillet et al., 2009). Similar mechanisms operating in the canine cells could explain the enhanced proliferation with IL-2 + IL-15, and why IL-15 alone was insufficient for in vitro survival and expansion.
Canine NK cells have been presumed to be large granular lymphocytes, but granularity has been variable in cases of purported NK cell neoplasia (Helfand et al., 1995; Vernau and Moore, 1999; McDonough and Moore, 2000; Bonkobara et al., 2007). Staining of azurophilic granules can be variable with Romanowsky-type stains, however, granules were clearly evident in T cells cultured from several of the donor dogs (data not shown), so staining problems were unlikely to account for our observation. We favor the explanation that the absence of granules in the cells was due to degranulation in culture, although we cannot exclude the possibility that canine NK cells are medium to large nongranular lymphocytes,. Resolution of this issue will require tools for positive selection of fresh canine NK cells that will allow microscopic examination without the need for enrichment or expansion in medium to long term cultures.
In vitro expanded non-T, non-B lymphocytes show specific cytotoxicity against CTAC target cells
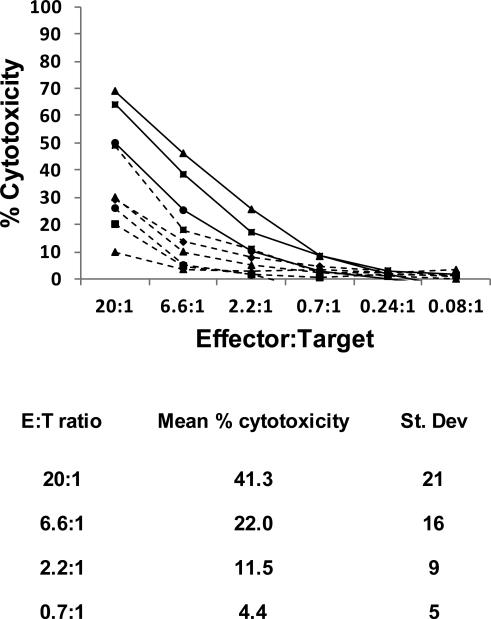
CTAC cells are the most common targets to assess NK, NK-like and LAK activity of canine lymphoid cells. Figure 3 shows specific cytotoxicity of eight independent preparations of cultured non-T, non-B lymphocytes against CTAC cells and tabulates the normalized mean (± S.D.) from all the experiments done at each effector:target (E:T) ratio used. Results include cells stimulated with IL-2 or with IL-2 + IL-15, since there was no difference in killing between these conditions. On average, the non-T, non-B lymphocytes for the cytokine-only groups showed 41% cytotoxicity at a 20:1 E:T ratio. Mean cytotoxicity of the EL group (mean of 28.9% ± 7.6 at 20:1 E:T ratio) was within the range observed in the cytokine only groups, but specific cytotoxicity from EL-cultured cells was always lower when compared in the same donor dogs (mean 28.9% ± 7.6 for EL and mean 61% ± 9.9 for cytokines). We repeatedly observed EL cell erosion in the co-culture system within ~14 days, and by 21 days, these cells were completely depleted. Similar depletion of feeder cells has been reported in co-culture systems with human NK cells (Spaggiari et al., 2006; Hoogduiin et al., 2011). This suggests possible xenorecognition of EL cells leading to canine NK-mediated cytotoxicity, which would be consistent with reduced capability of EL-cultured NK cells to kill CTAC targets if there had been prior degranulation and incomplete recovery.
Figure 3.
Specific cytotoxicity against CTAC target cells mediated by cultured non-T, non-B lymphocytes. Specific cytotoxicity was measured using a 51Cr release assay. Labeled CTAC cells were co-cultured for 4 hr with decreasing numbers of non-T, non-B lymphocytes expanded in IL-2 or IL-2 + IL-15 for 14 days. Percent specific cytotoxicity was calculated according to the formula (experimental release – spontaneous release)/(maximum release – spontaneous release) × 100. Intra-experimental variance for replicates was <15% for each condition. The top panel shows results of each of the cytoxocity experiments. Cytotoxicity was not significantly different based on the expansion conditions; the table summarizes the means and standard deviations of the % cytotoxicity at each effector:target ratio used for all the experiments.
In some experiments, cytotoxicity of cytokine-cultured cells against CTAC targets was as high as 70%. This approximates the specific cytotoxicity against CTAC cells reported for T cells and NKT cells (Huang et al., 2008; Lin et al., 2010), which would utilize recognition of allogenic MHC I for killing, unlike NK cells, which are activated by low or absent MHC I expression. We verified that MHC I levels in CTAC cells are comparable to those seen in various other tumors, and about 50% lower than the levels of MHC I observed in peripheral blood leukocytes. Only one commercially available antibody recognizes canine MHC I, and it recognizes a conserved epitope in the non-polymorphic region of the molecule. The lack of anti-canine MHC I antibodies that can block the interaction between MHC I and the surface receptors on the NK cells that recognize this complex, precluded absolute confirmation of MHC I-dependent cytotoxicity in our experiments. This confirmation will require future efforts to identify or generate MHC I-deficient canine target cells or to develop anti-canine MHC I blocking antibodies.
In summary, this is the first report showing enrichment and expansion of canine non-T, non-B lymphocytes that have cytotoxic activity and meet the standard criteria to be defined as NK cells. This establishes a foundation to develop antibodies to identify specific canine NK cell markers that can be used for positive selection. It also shows that canine NK cells retain evolutionarily conserved mechanisms of growth regulation and activation, and may provide useful models to develop and refine therapeutic strategies based on adoptive transfer of allogeneic NK cells.
Acknowledgments
We thank Megan Duckett for expert technical help. We would like to acknowledge the assistance of the Flow Cytometry Core Facility of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by grant P30 CA77598 from the National Institutes of Health of the United States Public Health Service. This project was supported in part by a University of Minnesota Companion Animal Grant and by the University of Minnesota Animal Cancer Care and Research Program/Comparative Oncology Research Fund.
References
- Bonkobara M, Saito T, Yamashita M, Tamura K, Yagihara H, Isotani M, Sato T, Washizu T. Blastic natural killer cell leukaemia in a dog--a case report. Vet. J. 2007;174:659–662. doi: 10.1016/j.tvjl.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Boysen P, Storset AK. Bovine natural killer cells. Vet. Immunol. Immunopathol. 2009;130:163–177. doi: 10.1016/j.vetimm.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Croy BA, van den Heuvel MJ, Borzychowski AM, Tayade C. Uterine natural killer cells: a specialized differentiation regulated by ovarian hormones. Immunol. Rev. 2006;214:161–185. doi: 10.1111/j.1600-065X.2006.00447.x. [DOI] [PubMed] [Google Scholar]
- Dissen E, Fossum S, Hoelsbrekken S, Saether P. NK cell receptors in rodents and cattle. Sem. Immunol. 2008;20:369–375. doi: 10.1016/j.smim.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Faldyna M, Samankova P, Leva L, Cerny J, Oujezdska J, Rehakova Z, Sinkora J. Cross-reactive anti-human monoclonal antibodies as a tool for B-cell identification in dogs and pigs. Vet. Immunol. Immunopathol. 2007;119:56–62. doi: 10.1016/j.vetimm.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Funk J, Schmitz G, Bach U, Failing K, Burkhardt E. Influence of different tumour types on natural cytotoxicity (NK cell activity) and mitogen-induced lymphocyte proliferation in isolated blood lymphocytes from 110 dogs with tumours. Res. Vet. Sci. 2003;2003:129–135. doi: 10.1016/s0034-5288(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Funk J, Schmitz G, Failing K, Burkhardt E. Natural killer (NK) and lymphokine-activated killer (LAK) cell functions from healthy dogs and 29 dogs with a variety of spontaneous neoplasms. Cancer Immunol. Immunother. 2005;54:87–92. doi: 10.1007/s00262-004-0555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerner W, Käser T, Saalmüller A. Porcine T lymphocytes and NK cells--an update. Dev. Comp. Immunol. 2009;33:310–320. doi: 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Giebel S, Locatelli F, Lamparelli T, Velardi A, Davies S, Frumento G, Maccario R, Bonetti F, Wojnar J, Martinetti M, Frassoni F, Giorgiana G, Bacigalupo A, Holowiecki J. Survival advantage with KIR ligand incompatibility in hematopoietic stem cell transplantation from unrelated donors. Blood. 2003;102:814–819. doi: 10.1182/blood-2003-01-0091. [DOI] [PubMed] [Google Scholar]
- Graves SS, Hogan W, Kuhr CS, Diaconescu R, Harkey MA, Georges GE, Sale GE, Zellmer E, Baran S, Jochum C, Stone B, Storb R. Stable trichimerism after marrow grafting from 2 DLA-identical canine donors and nonmyeloablative conditioning. Blood. 2007;110:418–423. doi: 10.1182/blood-2007-02-071282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond JA, Guethlein LA, Abi-Rached L, Moesta AK, Parham P. Evolution and survival of marine carnivores did not require a diversity of killer cell Ig-like receptors or Ly49 NK cell receptors. J. Immunol. 2009;182:3618–3627. doi: 10.4049/jimmunol.0803026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfand SC, Modiano JF, Moore PF, Soergel SA, MacWilliams PS, Dubielzig RD, Hank JA, Gelfand EW, Sondel PM. Functional interleukin-2 receptors are expressed on natural killer-like leukemic cells from a dog with cutaneous lymphoma. Blood. 1995;86:636–645. [PubMed] [Google Scholar]
- Helfand SC, Soergel SA, Modiano JF, Hank JA, Sondel PM. Induction of lymphokine-activated killer (LAK) activity in canine lymphocytes with low dose human recombinant interleukin-2 in vitro. Cancer Biother. 1994;9:237–244. doi: 10.1089/cbr.1994.9.237. [DOI] [PubMed] [Google Scholar]
- Huang YC, Hung SW, Jan TR, Liao KW, Cheng CH, Wang YS, Chu RM. CD5-low expression lymphocytes in canine peripheral blood show characteristics of natural killer cells. J. Leukoc. Biol. 2008;84:1501–1510. doi: 10.1189/jlb.0408255. [DOI] [PubMed] [Google Scholar]
- Jochum C, Beste M, Zellmer E, Graves SS, Storb R. CD154 blockade and donor-specific transfusions in DLA-identical marrow transplantation in dogs conditioned with 1-Gy total body irradiation. Biol. Blood Marrow Transplant. 2007;13:164–171. doi: 10.1016/j.bbmt.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwabara M, Nariai Y, Huriuchi Y, Nakajima Y, Yamaguchi Y, Horioka E, Kawanabe M, Kubo T, Yukawa M, Sakai T. Immunological effects of recombinant feline interferon-omega (KT-80) administration in the dog. Microbiol. Immunol. 2006;50:637–641. doi: 10.1111/j.1348-0421.2006.tb03828.x. [DOI] [PubMed] [Google Scholar]
- LaBonte ML, Hershberger KL, Korber B, Letvin NL. The KIR and CD94/NKG2 families of molecules in the rhesus monkey. Immunol. Rev. 2001;183:25–40. doi: 10.1034/j.1600-065x.2001.1830103.x. [DOI] [PubMed] [Google Scholar]
- Ladiges WC, Storb R, Thomas ED. Canine models of bone marrow transplantation. Lab Anim Sci. 1990;40:11–15. [PubMed] [Google Scholar]
- Lin CY, Chuang TF, Liao KW, Huang KY, Pai CC, Chu RM. Combined immunogene therapy of IL-6 and IL-15 enhances anti-tumor activity through augmented NK cytotoxicity. Cancer Lett. 2008;272:285–295. doi: 10.1016/j.canlet.2008.07.016. [DOI] [PubMed] [Google Scholar]
- Lin YC, Huang YC, Wang YS, Juang RH, Liao KW, Chu RM. Canine CD8 T cells showing NK cytotoxic activity express mRNAs for NK cell-associated surface molecules. Vet. Immunol. Immunopathol. 2010;133:144–153. doi: 10.1016/j.vetimm.2009.07.013. [DOI] [PubMed] [Google Scholar]
- Loughran TP, Jr., Deeg HJ, Storb R. Inhibition of canine NK activity by anti-CD18 monoclonal antibody, UV irradiation and cyclosporine. Exp. Hematol. 1993;21:411–413. [PubMed] [Google Scholar]
- McCullar V, Oostendorp R, Panoskaltsis-Mortari A, Yun G, Lutz CT, Wagner JE, Miller JS. Mouse fetal and embryonic liver cells differentiate human umbilical cord blood progenitors into CD56-negative natural killer cell precursors in the absence of interleukin-15. Exp. Hematol. 2008;36:598–608. doi: 10.1016/j.exphem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonough SP, Moore PF. Clinical, hematologic, and immunophenotypic characterization of canine large granular lymphocytosis. Vet. Pathol. 2000;37:637–646. doi: 10.1354/vp.37-6-637. [DOI] [PubMed] [Google Scholar]
- Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–2229. [PubMed] [Google Scholar]
- Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- Moore ML, Chi MH, Goleniewska K, Durbin JE, Peebles RS., Jr. Differential regulation of GM1 and asialo-GM1 expression by T cells and natural killer (NK) cells in respiratory syncytial virus infection. Viral Immunol. 2008;21:327–339. doi: 10.1089/vim.2008.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta A, Locatelli F, Moretta L. Human NK cells: from HLA class I-specific killer Ig-like receptors to the therapy of acute leukemias. Immunol. Rev. 2008a;224:58–69. doi: 10.1111/j.1600-065X.2008.00651.x. [DOI] [PubMed] [Google Scholar]
- Moretta L, Locatelli F, Moretta A. Alloreactive natural killer cells in targeting high-risk leukemias. Ann. Rheum. Dis. 2008b;67:ii39–ii43. doi: 10.1136/ard.2008.097980. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Tsukatani Y, Kosaka T, Kuwabara M, Tanaka S, Fujiwara K. Relationship between radical production and natural killer cytotoxic factor (NKCF) in canine natural killer (NK) cell-mediated cytotoxicity. Vet. Immunol. Immunopathol. 1997;55:273–282. doi: 10.1016/s0165-2427(96)05720-0. [DOI] [PubMed] [Google Scholar]
- Nakada Y, Tsukatani Y, Kosaka T, Miyamori M, Kuwabara M, Tanaka S, Koide F. Release of natural killer cytotoxic factor (NKCF) from canine natural killer (NK) cells stimulated with cytoplasmic membrane of target cells. J. Vet. Med. Sci. 1995;57:165–167. doi: 10.1292/jvms.57.165. [DOI] [PubMed] [Google Scholar]
- Nariai Nakada Y, Nariai K, Kosaka T, Kuwabara M, Kiuchi Y. Morphological observation of canine natural killer cells mediated cytotoxicity. J. Vet. Med. Sci. 1999;61:835–838. doi: 10.1292/jvms.61.835. [DOI] [PubMed] [Google Scholar]
- Otani I, Niwa T, Tajima M, Ishikawa A, Watanabe T, Tsumagari S, Takeishi M, Kanayama K. CD56 is expressed exclusively on CD3+ T lymphocytes in canine peripheral blood. J. Vet. Med. Sci. 2002;64:441–444. doi: 10.1292/jvms.64.441. [DOI] [PubMed] [Google Scholar]
- Otani I, Ohta K, Ishikawa A, Yamada T, Ishinazaka T, Ohtaki T, Tsumagari S, Kanayama K. Flow cytometric analysis of canine umbilical cord blood lymphocytes. J. Vet. Med. Sci. 2008;70:285–287. doi: 10.1292/jvms.70.285. [DOI] [PubMed] [Google Scholar]
- Panse JP, Bastianelli C, Santos EB, Schwarzinger I, Raff RF, Storb R, Sandmaier BM. Dog leukocyte antigen nonidentical unrelated canine marrow grafts: enhancement of engraftment by CD4 and CD8 T cells. Transplant. 2003;76:474–480. doi: 10.1097/01.TP.0000076625.18877.02. [DOI] [PubMed] [Google Scholar]
- Pierson BA, McGlave PB, Hu WS, Miller JS. Natural killer cell proliferation is dependent on human serum and markedly increased utilizing an enriched supplemented basal medium. J. Hematother. 1995;4:149–158. doi: 10.1089/scd.1.1995.4.149. [DOI] [PubMed] [Google Scholar]
- Pierson BA, Miller JS, Verfaillie C, McGlave PB, Hu WS. Population dynamics of human activated natural killer cells in culture. Biotechnol. Bioeng. 1994;43:685–692. doi: 10.1002/bit.260430803. [DOI] [PubMed] [Google Scholar]
- Pillet AH, Bugault F, Theze J, Chakrabarti LA, Rose T. A programmed switch from IL-15- to IL-2-dependent activation in human NK cells. J. Immunol. 2009;182:6267–6277. doi: 10.4049/jimmunol.0801933. [DOI] [PubMed] [Google Scholar]
- Rolstad B, Naper C, Lovik G, Vaage JT, Ryan JC, Backman-Petersson E, Kirsch RD, Butcher GW. Rat natural killer cell receptor systems and recognition of MHC class I molecules. Immunol. Rev. 2001;181:149–157. doi: 10.1034/j.1600-065x.2001.1810112.x. [DOI] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Schuberth J-H, Kucinskiene G, Chu R-M, Faldyna M. Reactivity of cross-reacting monoclonal antibodies with canine leukocytes, platelets and erythrocytes. Vet. Immunol. Immunopathol. 2007;119:47–55. doi: 10.1016/j.vetimm.2007.06.013. [DOI] [PubMed] [Google Scholar]
- Strasser A, Teltscher A, May B, Sanders C, Niedermuller H. Age-associated changes in the immune system of German shepherd dogs. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2000;47:181–192. doi: 10.1046/j.1439-0442.2000.00278.x. [DOI] [PubMed] [Google Scholar]
- Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet. Immunol. Immunopathol. 1999;69:145–164. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat. Immunol. 2008;9:503–510. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- Welsh RM., Jr. Mouse natural killer cells: induction specificity, and function. J. Immunol. 1978;121:1631–1635. [PubMed] [Google Scholar]
- Yang H, Yogeeswaran G, Bukowski JF, Welsh RM. Expression of asialo GM1 and other antigens and glycolipids on natural killer cells and spleen leukocytes in virus-infected mice. Nat. Immun. Cell. Growth. Regul. 1985;4:21–39. [PubMed] [Google Scholar]
- Yoder JA, Litman GW. The phylogenetic origins of natural killer receptors and recognition: relationships, possibilities, and realities. Immunogenetics. 2011;63:123–141. doi: 10.1007/s00251-010-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]