Abstract
Objective
To evaluate the impact of concurrent substance use disorder (SUD) and nicotine-dependence treatment for stimulant-dependent patients.
Method
A randomized, 10-week trial with follow-up at 3 and 6 months post-smoking quit date conducted at 12 SUD treatment programs between February 2010 and July 2012. Adults, meeting DSM-IV-TR criteria for cocaine and/or methamphetamine-dependence and interested in quitting smoking were randomized to treatment as usual (TAU; n=271) or TAU with smoking-cessation treatment (TAU+SCT, n=267). All participants received SUD TAU. TAU+SCT participants received weekly individual smoking cessation counseling and extended-release (XL) bupropion (300 mg/day) during weeks 1–10. During post-quit treatment (weeks 4–10), TAU+SCT participants received a nicotine inhaler and contingency management for smoking abstinence. Weekly proportion of stimulant-abstinent participants during the treatment phase, as assessed by urine drug screens and self-report, was the primary outcome. Secondary measures included other substance/nicotine use outcomes and treatment attendance.
Results
There were no significant treatment effects on stimulant-use outcomes, as measured by the primary outcome and stimulant-free days, on drug-abstinence, or on attendance. TAU+SCT, relative to TAU, participants had significantly better outcomes for drug-free days at 6-month follow-up (X2(1)=4.09, p<.05), with a decrease in drug-free days from baseline of −1.3% in TAU+SCT and of −7.6% in TAU. TAU+SCT, relative to TAU, participants had significantly better outcomes on smoking point-prevalence abstinence (25.5% vs. 2.2%; X2(1)=44.69, p<.001; OR=18.2).
Conclusions
These results suggest that providing smoking-cessation treatment to illicit stimulant-dependent patients in outpatient SUD treatment will not worsen, and may enhance, abstinence from non-nicotine substance use.
Keywords: Smoking cessation, stimulant dependence, treatment, clinical trial
INTRODUCTION
Cigarette smoking, which accounts for 443,000 deaths annually in the United States,1 has a prevalence rate of 49 – 98% in illicit drug abusers, a rate substantially higher than the 19.8% rate in the general population.2 Despite the pervasiveness and deadly consequences of smoking in addicted individuals,3 smoking-cessation treatment (SCT) is typically not provided in substance use disorder (SUD) treatment programs due, in part, to concern that it might impact negatively on non-nicotine substance use outcomes.4,5 Prochaska and colleagues6 completed a meta-analysis of 19 studies in which the impact of SCT on non-nicotine drug/alcohol use was assessed; the findings suggested that SCT may actually improve substance use outcomes. However, the 19 studies analyzed included mainly alcohol-dependent, and, to a lesser extent, methadone-maintained participants; the potential impact of concurrent treatment for illicit stimulant and nicotine dependence is unknown.
Clinical and laboratory studies have established a link between cigarette smoking and nonnicotine stimulant abuse. The rate of smoking in cocaine abusers is 75–80% 7–9 and is 87% or higher in methamphetamine abusers10,11 and is associated with more severe cocaine addiction.7,12 Human laboratory studies have found that cocaine administration increases cigarette smoking,13,14 and that mecamylamine, a nicotine antagonist, reduces cue-induced cocaine craving15 while nicotine increases it.16 The present trial addressed this research gap by evaluating the impact of concurrent SUD treatment and SCT in cocaine- and/or methamphetamine-dependent patients. Past research suggests that smoking-cessation rates in substance abusing populations are poor17,18 but that smoking-cessation rates can be improved by combining psychosocial and pharmacological smoking-cessation interventions.19,20 Therefore, the SCT utilized in the present trial combined psychosocial interventions with FDA-approved smoking cessation medications. It was predicted that the concurrent provision of SUD and SCT would improve, rather than worsen, stimulant-use outcomes.
METHODS
Study Design
This was a 10-week, intent-to-treat (ITT), 2-group randomized trial with follow-up visits at 3 and 6 months post-smoking quit date. The trial was conducted by the National Institute on Drug Abuse (NIDA) National Drug Abuse Treatment Clinical Trials Network (CTN) at twelve SUD treatment programs. A full discussion of design considerations has been published previously.21
Participants
Recruitment was primarily from clinic patients entering treatment at a participating site; secondary recruitment methods included advertising and direct community promotions, such as networking with community professionals. Eligible participants were adults enrolled in outpatient SUD treatment, and interested in quitting smoking. Participants were required to meet DSM-IV-TR criteria for current cocaine- or methamphetamine-dependence, to smoke at least 7 cigarettes per day (CPD), to have a Carbon Monoxide (CO) level ≥ 8 ppm, and to have smoked cigarettes for at least 3 months. The decision to require 7 CPD was based on a prior trial completed by our group in which the more standard ≥10 CPD criterion was a primary reason for excluding African-American, but not Caucasian, smokers. Exclusion criteria included a medical or psychiatric condition potentially making participation unsafe, current treatment for nicotine dependence; for women, pregnancy, breastfeeding, or unwillingness to use adequate birth control. Candidates were excluded if they had used tobacco products other than cigarettes in the past week, had all stimulant-positive urine drug screen (UDS) results during screening/baseline, or were seeking or receiving opioid-agonist treatment. All participants were given a thorough explanation of the study and signed an informed consent form approved by the Institutional Review Boards of the participating sites.
Procedures
Participants were randomized to treatment as usual (TAU) or TAU with smoking-cessation treatment (TAU+SCT) in a 1:1 ratio stratified by site and baseline UDS results (stimulant-negative vs. positive). During the 10-week treatment phase, participants were scheduled to attend two research visits per week for efficacy and safety assessments, with identical assessments completed for both TAU and TAU+SCT participants. There were single follow-up visits at 3-months and 6-months post-quit date. Participants received $15 for shorter, and $25 for longer, weekly visits; at the week 10 longer visit, participants received an additional $25 based on the visit's larger assessment burden. Participants were reimbursed $40 per follow-up visit. TAU+SCT participants could also earn monetary rewards through the contingency management (CM) intervention.
Treatment
All participants received SUD as typically provided by the study site, which consisted of at least one treatment session per week during the 10-week treatment phase. TAU+SCT participants also received SCT consisting of extended-release (XL) bupropion, nicotine inhaler, smoking-cessation counseling, and CM for smoking abstinence. Bupropion hydrochloride XL 150 mg (for dose escalation and taper) and 300 mg tablets, manufactured by GlaxoSmithKline, were used. The bupropion-XL dosing schedule was 150 mg/day for study days 1–3, 300 mg/day for study days 4 through week 10, and a 3-day dose taper of 150 mg following week 10. The NICOTROL® inhaler, manufactured by Pfizer, was also used for the trial. Starting with the target quit date (study day 20) through week 10, TAU+SCT participants were prescribed 6–16 nicotine cartridges per day ad libitum; participants received a 3-week taper following week 10.
TAU+SCT participants received weekly 10 minute smoking-cessation counseling sessions during study weeks 1–10, using the “Smoke Free and Living It©" 22 manual. Interventionists were trained on the manual and certified after a successful mock session. All sessions were video-recorded to monitor adherence; of the 283 sessions rated, 271 (95.8%) were rated as adherent. Prize-based CM (i.e., using a fishbowl from which chips were drawn) was used to reinforce negative CO (i.e., CO < 4 ppm) results by the TAU+SCT participants during the post-quit phase. In order to encourage continuous abstinence, the number of draws earned escalated with each consecutive week of abstinence and reset if evidence of smoking was obtained. The maximum number of draws that could be earned by a participant was 110, which equates to approximately $380 in prizes.
Measures
The primary outcome was the weekly proportion of stimulant-abstinent participants during the treatment phase, as assessed by stimulant-negative UDS and self-report of no stimulant use. A rapid UDS system that screened for cocaine, methamphetamine, amphetamine, opioids, benzodiazepines, and marijuana was used to analyze the urine samples (Branan Medical Corporation). To avoid falsification, urine samples were collected using temperature monitoring and the validity of urine samples was checked with the use of a commercially available adulterant test. Self-report of substance use was assessed using the Timeline Follow-back (TLFB) method,23,24 which is a widely employed and well-validated method.
Secondary outcomes included proportion of stimulant-abstinent participants at follow-up, proportion of drug-abstinent participants during active treatment and follow-up, stimulant-free and drug-free days during the active treatment phase and follow-up, smoking point-prevalence abstinence at the end of treatment and follow-up, and SUD treatment attendance during the active treatment phase. Drug-abstinence was assessed by negative UDS and self-report of no substance use (i.e., alcohol, and/or other non-nicotine substance use). Stimulant-free and drug-free days were assessed by the TLFB. Smoking point-prevalence abstinence was assessed by self-report of not smoking in the previous seven days, confirmed by a CO level <8 ppm.25 Treatment attendance was defined as the percent of scheduled treatment hours attended as obtained from clinic records. Safety was assessed through adverse event (AE). Bupropion-XL adherence was assessed weekly via self-report and pill count; nicotine inhaler use was assessed weekly during the post-quit period via self-report and inhaler count.
Data Analysis
All analyses were completed on the ITT sample using SAS, Version 9.1.3 (SAS Institute, Inc.). Statistical tests were conducted at a 5% Type I error rate (two-sided) for all measures. It has been recommended that effect sizes be provided rather than using the Bonferroni procedure to adjust for multiple-comparisons;26 thus, effect sizes and 95% confidence intervals (CI’s) are provided for each statistically significant treatment effect. Statistical models for longitudinal data included treatment, week, and treatment-by-week interaction effects. For each model, the baseline of the outcome measure being analyzed was a covariate that could be selected for inclusion in the model by the corrected Akaike Information Criterion (AICC) as the optimizing criterion.
All repeated-measures regressions were random intercept mixed model (or generalized mixed model) regressions using participant as random effect. Regressions with binary response variables used logistic mixed models and the remaining regressions used ordinary mixed models. AEs included all untoward events reported by the participants as well as clinically significant changes in vital signs. AEs were coded using the medical dictionary for regulatory activities (MedDRA® Version 15) and tabulated by body system and preferred term, seriousness, and relationship to study medication. AEs were compared between treatment groups using Pearson chi square, or the Fisher Exact test depending on marginal frequency counts.
The most conservative estimate for missing data was calculated, which was for missing urine samples including those missed due to participants failing to attend visits as well as those missing due to technical issues. This estimate yielded a missing data rate of 13.8%. The approach taken to handling missing data depended on the outcome measure. For the primary outcome (stimulant abstinence), data for a week in which both urine samples were missing and the participant self-reported no illicit stimulant use was treated as missing. For a week in which one stimulant-free UDS was produced, the second urine sample was missing, and the participant self-reported no illicit stimulant use or the self-report data were missing, was imputed as stimulant-abstinent. For the smoking abstinence measure, missing days were coded as smoking days, which is a standard approach in smoking-cessation trials. Missing data for other measures were treated as missing.
RESULTS
Participants and Disposition
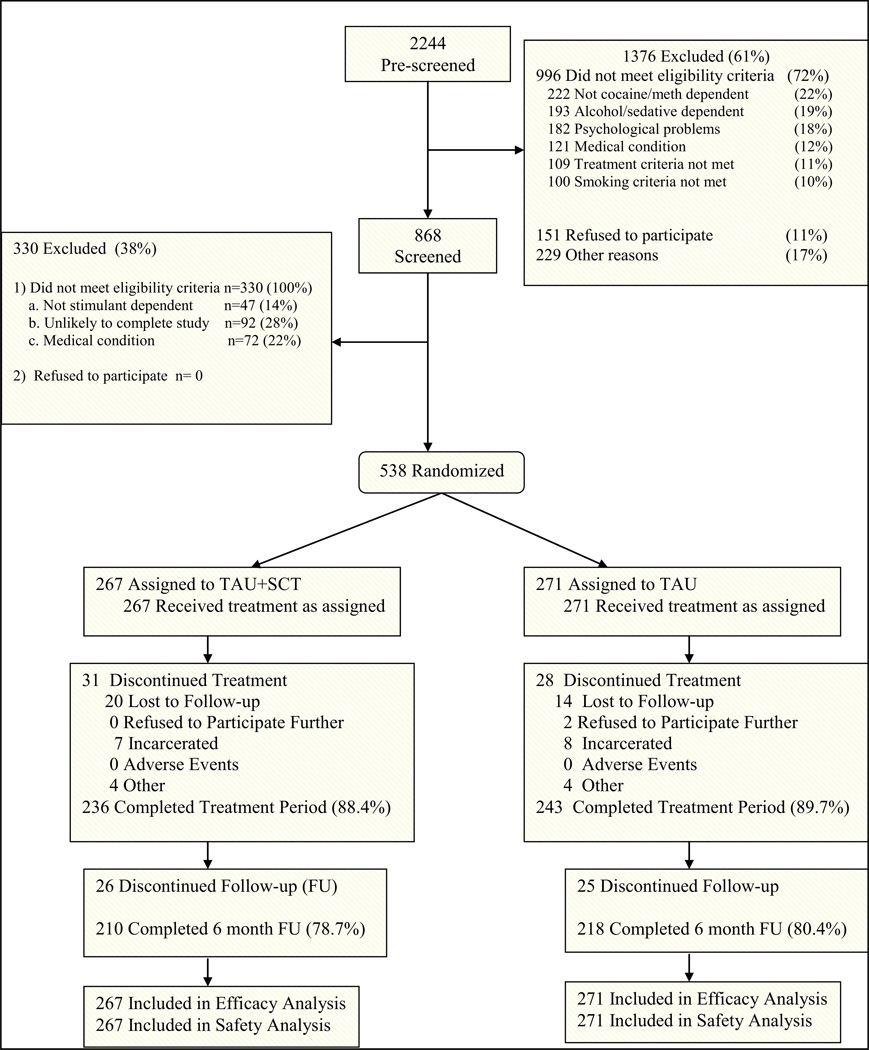
As shown in Figure 1, 2244 candidates were pre-screened, 868 were consented and screened, and 538 were randomized to TAU+SCT or TAU. Approximately 89% of participants completed the 10-week active treatment period, 85% completed the 3-month follow-up, and 78.7% completed the 6-month follow-up, with no group differences on completion rate or reasons for non-completion. No participant discontinued the study due to an AE. Demographic and baseline characteristics did not differ significantly between groups. The sample was approximately 52% male and 60% Caucasian, and participants were 36 years of age on average (Table 1). Approximately 56% of the sample was cocaine-dependent, 39% were methamphetamine-dependent, and 5% were dependent on both substances. The sample had a medium level of nicotine dependence as assessed by the Fagerström score, and, on average, smoked 16 CPD.
Figure 1.
Participant Disposition
Table 1.
Participant Demographic and Baseline Characteristics
| Characteristic | TAU+SCT (n=267) |
TAU (n=271) |
Total (n=538) |
|---|---|---|---|
| Age, mean (SD), y | 36.9 (10.0) | 36.0 (10.1) | 36.4 (10.0) |
| Sex, male, n (%) | 145 (54.3) | 135 (49.8) | 280 (52.0) |
| Race, n (%) | |||
| African-American | 83 (31.2) | 88 (32.5) | 171 (31.8) |
| Caucasian | 162 (60.9) | 158 (58.3) | 320 (59.6) |
| Other/mixed | 21 (7.9) | 25 (9.2) | 56 (8.6) |
| Ethnicity, Hispanic, n (%) | 34 (12.9) | 33 (12.3) | 67 (12.6) |
| Marital Status, n (%) | |||
| Married | 32 (12.0) | 26 (9.6) | 58 (10.8) |
| Separated/Divorced/Widowed | 89 (33.3) | 104 (38.4) | 193 (35.9) |
| Never Married | 146 (54.7) | 141 (52.0) | 287 (53.3) |
| Education, mean (SD), y | 11.7 (1.9) | 12.0 (1.9) | 11.9 (1.9) |
| Employment n(%) | |||
| Full Time | 74 (27.7) | 86 (31.7) | 160 (29.7) |
| Part Time | 61 (22.8) | 62 (22.9) | 123 (22.9) |
| Other | 132 (49.4) | 123 ( 45.4) | 255 (47.4) |
| Stimulant dependence diagnosis, n (%) | |||
| Cocaine only | 147 (55.1) | 154 (57.0) | 301 (56.1) |
| Methamphetamine only | 102 (38.2) | 107 (39.6) | 209 (38.9) |
| Both cocaine and methamphetamine | 18 (6.7) | 9 (3.3) | 27 (5.0) |
| Alcohol/Non-stimulant diagnosis*, n (%) | 120 (44.9)% | 121 (44.6) | 241 (44.8) |
| Days/stimulant use at baseline (previous 28) | 1.9 (4.9) | 1.5 (3.7) | 1.7 (4.3) |
| Stimulant-free (previous 28), n (%) | 194 (72.7) | 199 (73.4) | 393 (73.0) |
| Days/drug use at baseline (previous 28) | 4.2 (7.9) | 3.5 (6.9) | 3.8 (7.4) |
| Drug-free (previous 28), n (%) | 155 (58.1) | 164 (60.5) | 319 (59.3) |
| Smoking history | |||
| Fagerström score, mean (SD) | 5.7 (2.3) | 5.6 (2.1) | 5.6 (2.2) |
| No. of Smoking years, mean (SD) | 20.3 (9.6) | 19.0 (10.3) | 19.7 (9.9) |
| No. of cigarettes/day, mean (SD) | 16.7 (8.4) | 16.0 (7.4) | 16.3 (7.9) |
Note. Demographic and baseline characteristics did not differ significantly between the treatment groups;
A diagnosis of substance abuse or dependence (The most common diagnoses were alcohol dependence at 14.7%, marijuana abuse at 13.8%, and alcohol abuse at 11.8%).
SCT Adherence
Table 2 provides adherence and tolerability data for the study medications. Based on pill count and self-report, approximately 93% of prescribed bupropion pills were taken. In contrast, the nicotine inhaler was used as prescribed by only 7.3% of participants based on self-report and by 5.5% of participants based on cartridge count. For smoking-cessation counseling, participants attended 8.6 of 10 possible sessions on average. For CM, 187 of the TAU+SCT participants earned at least one draw.
Table 2.
Summary of Medication Adherence and Tolerability for TAU+SCT participants
| Self-report | Pill/Cartridge Count |
|
|---|---|---|
| Medication Adherence | ||
| Percentage of bupropion taken, mean (std. dev.) | 92.4%a(15.6%) | 94.5%b(14.9%) |
| Average nicotine inhaler cartridges used per day (over all active treatment weeks): | ||
| < 1 Cartridge, n (%) | 139 (56.3)c | 148 (62.2)d |
| ≥ 1 but < 4 Cartridges, n (%) | 69 (27.9)c | 63 (26.5)d |
| ≥ 4 but < 6 Cartridges, n (%) | 21 (8.5)c | 14 (5.9)d |
| ≥ 6 Cartridges (as prescribed), n (%) | 18 (7.3)c | 13 (5.5)d |
| Medication Tolerability | ||
| Tolerability of maximum bupropion dose: | ||
| Reached maximum, n (%) | 258 (97.7) | NA |
| Sustained dose at maximum, n (%) | 190 (72.0) | NA |
Self-reported adherence was calculated by dividing the number of milligrams reported taken by the number of milligrams prescribed, and multiplying by 100;
pill count adherence was calculated by taking the number of pills dispensed minus the number returned or reported lost divided by the number of pills prescribed to be taken and multiplying by 100.
Self-reported adherence was calculated by dividing the number of cartridges reported taken by the number of days assessed, and multiplying by 100;
cartridge count adherence was calculated by dividing the number of used cartridges returned by the number of days assessed and multiplying by 100. In cases where participants failed to return their medication bottles/cartridges, those bottles/ cartridges were excluded from the analysis.
Efficacy Outcomes
Stimulant-use outcomes
The treatment groups did not differ significantly on the primary outcome measure of weekly proportion of stimulant-abstinent participants during active treatment, with non-significant treatment (X2(1)=0.65, p=.42) and treatment-by-week interaction (X2(1)=0.86, p=.35) effects. Overall, TAU+SCT participants averaged 77.2% stimulant-abstinent weeks compared to 78.1% stimulant-abstinent weeks for TAU participants. There was a similar lack of significant treatment effect on stimulant-abstinence at 3-month (X2(1)=2.45, p=.12) and 6-month (X2(1)=0.10, p=.75) follow-ups. For stimulant-free days, there were no significant treatment (X2(1)=0.06, p=.80) or treatment-by-week interaction (X2(1)=0.24, p=.62) effects for weekly proportion of stimulantfree days during active treatment, and no significant treatment effect for stimulant-free days at 3-month (X2(1)=0.63, p=.43) and 6-month (X2(1)=1.26, p=.26) follow-ups.
Smoking outcomes
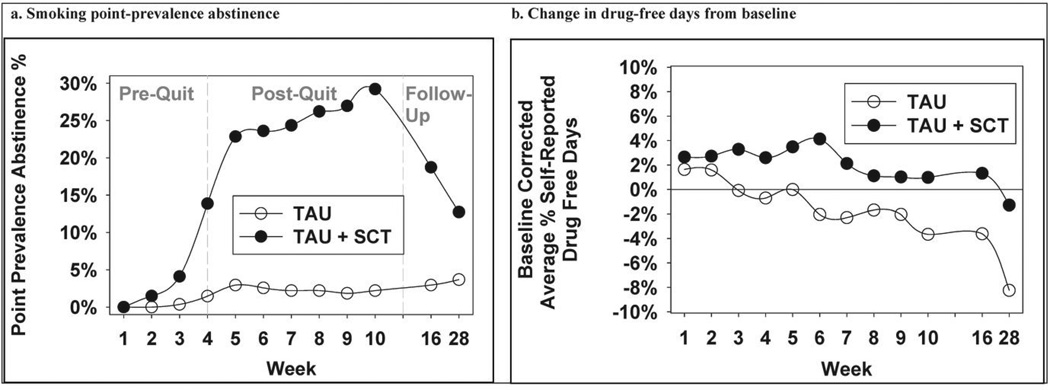
Point-prevalence abstinence (PPA) rates were significantly higher in the TAU+SCT, compared to TAU, group (Figure 2a) at week 10, (25.5% vs. 2.2%; X2(1)=44.69, p<0.0001; odds ratio=18.23 [95% CI: 7.78–42.69]), 3-month follow-up (19.1% vs. 3.0%; X2(1)=26.73, p<0.0001; odds ratio=7.58 [95% CI: 3.52–16.32]), and 6-month follow-up (13.1% vs. 3.7%; X2(1)=13.00, p=0.0003; odds ratio=3.81[95% CI:1.84–7.88]).
Figure 2.
Nicotine and drug-use outcomes as a function of treatment group and time
Other SUD outcomes
Other SUD outcomes included drug-abstinence, treatment attendance, and drug-free days. The treatment groups did not differ significantly on the weekly proportion of drug-abstinent participants during active treatment with non-significant treatment (X2(1)=1.80, p=.18) and treatment-by-week interaction (X2(1)=1.55, p=.21) effects. There was a similar lack of treatment effect on drug-abstinence at 3-month (X2(1)=1.35, p=.25) and 6-month (X2(1)=1.23, p=.27) follow-ups. There were no significant treatment effects for SUD treatment attendance during the active treatment phase, with non-significant treatment (X2(1)=0.03, p=.86) and treatment-by-week interaction (X2(1)=1.52, p=.22) effects.
Figure 2b displays baseline-corrected drug-free days as a function of treatment and time. TAU+SCT, relative to TAU, participants tended to have better outcomes with 10-week, 3-month, and 6-month changes in drug-free days from baseline being 0.5% vs. −3.3%, 1.1% vs. −3.3%, and −1.3% vs. −7.6%, respectively. Statistically, there was a trend toward a significant treatment-by-week interaction effect (X2(1)=3.61, p=.058) during treatment, no significant treatment effect at 3-month follow-up (X2(1)=2.44, p=.12) and a significant treatment effect at 6-month follow-up (X2(1)=4.09, p=.04). The Cohen's d for the 6-month effect is .22 [95% CI: 0.03–0.41], which is a small effect.
Safety outcomes
The occurrence of treatment emergent adverse events (TEAEs) was significantly higher in the TAU+SCT, relative to TAU, group (Table 3). The AEs occurring at a rate of 5% or more in the TAU+SCT group, and at a significantly higher rate than in the TAU group were dry mouth, nausea, headache, anxiety, insomnia, and throat irritation. Twenty-three participants experienced a treatment emergent serious adverse event (SAE), with no significant difference between arms (Table 3). Four participants experienced a medication-related SAE, with one experiencing suicidal ideation and chest pain, two experiencing suicidal ideation, and one experiencing panic attacks.
Table 3.
Summary of Treatment Emergent Adverse Events by MedDRA®a preferred term
| TEAEb | TAU+SCT (n=267) | TAU (n=271) | P Value |
|---|---|---|---|
| Any TEAEs, n (%) | 195 (73.0) | 157 (57.9) | 0.0002 |
| TEAEs related to study medication, n (%)c | 128 (47.9) | NA | |
| Discontinued bupropion XL due to TEAEs, n (%) | 13 (4.9) | NA | |
| Discontinued nicotine inhaler due to TEAEs, n (%) | 7 (2.6) | NA | |
| Most frequent TEAEs, n (%)d | |||
| Psychiatric disorders | |||
| Insomnia | 21 (7.9) | 3 (1.1) | 0.0001 |
| Anxiety | 20 (7.5) | 5 (1.8) | 0.0019 |
| Gastrointestinal disorders | |||
| Nausea | 18 (6.7) | 7 (2.6) | 0.0220 |
| Dry mouth | 14 (5.2) | 0 (0.0) | 0.0001 |
| Headache | 34 (12.7) | 13 (4.8) | 0.0011 |
| Throat irritation | 16 (6.0) | 0 (0.0) | < 0.0001 |
| Any Serious TEAE, n (%) | 14 (5.2) | 9 (3.3) | 0.2704 |
| Serious TEAE related to study medication , n (%)c | 4 (1.5) | NA | |
| Suicidal ideation | 3 | NA | |
| Panic attacks | 1 | NA | |
| Chest pain | 1 | NA | |
| Serious TEAE unrelated to study medication , n (%) | 11 (4.1) | 9 (3.3) | 0.6244 |
| Cardiac disorders | |||
| Angina pectoris | 0 | 1 | |
| Myocardial infarction | 0 | 1 | |
| Eye disorders: Vision blurred | 1 | 0 | |
| Gastrointestinal disorders | |||
| Abdominal pain upper | 0 | 1 | |
| Constipation | 0 | 1 | |
| Pancreatitis | 0 | 1 | |
| General disorders/administration site conditions | |||
| Death | 1 | 0 | |
| Drug withdrawal syndrome | 1 | 0 | |
| Non-cardiac chest pain | 1 | 0 | |
| Infections and infestations | |||
| Appendicitis | 0 | 1 | |
| Pyelonephritis | 1 | 0 | |
| Sepsis | 1 | 0 | |
| Sinusitis | 1 | 0 | |
| Injury: Tibia fracture | 1 | 0 | |
| Metabolism and nutrition disorder: Dehydration | 0 | 1 | |
| Musculoskeletal and connective tissue disorder: Exostosis | 1 | 0 | |
| Psychiatric disorders | |||
| Depression | 1 | 0 | |
| Suicidal ideation | 0 | 1 | |
| Suicidal attempt | 0 | 1 | |
| Respiratory, thoracic, and mediastinal disorders: Asthma | 0 | 1 | |
| Vascular disorders: Hematoma | 1 | 0 | |
Note.
MedDRA® the Medical Dictionary for Regulatory Activities terminology is the international medical terminology developed under the auspices of the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH). MedDRA® is a registered trademark of the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA);
TEAE = Treatment Emergent Adverse Event, defined as a new illness, or an exacerbation of a pre-existing condition, with onset date post-randomization;
TEAE rated as possibly, probably or definitely related to treatment;
Reported by >5% of TAU+SCT group and at a statistically significant (p<.05) greater rate than by the TAU group.
DISCUSSION
This trial is the first to evaluate the impact of concurrently providing smoking-cessation and SUD treatment to cocaine- and/or methamphetamine-dependent patients. We had predicted that TAU+SCT, relative to TAU, would significantly improve stimulant-use outcomes. The results from ITT analyses indicate that stimulant use during active treatment and follow-up did not differ significantly between the treatment groups. This suggests that providing smoking-cessation treatment to cocaine- and/or methamphetamine-dependent patients in outpatient SUD treatment does not affect stimulant-use outcomes. Alternatively, the findings might reflect a ceiling effect given the relatively low rate of stimulant use (i.e., participants averaged 77.6% weekly abstinence during treatment). A secondary objective of the trial was to evaluate the impact of TAU+SCT, relative to TAU, on other drug-abuse outcomes. The results suggest that there were no significant treatment effects for drug-abstinence but that the TAU+SCT, relative to TAU, participants evidenced better outcomes for drug-free days, with a trend for a significant treatment difference during active treatment, and a significant difference at 6-month follow-up. This finding is consistent with past research which has found that concurrent smoking cessation and SUD treatment for alcohol use disorders can enhance abstinence from substance use.6,27 The results from this trial also revealed that SUD treatment attendance did not differ significantly between the TAU+SCT and TAU participants. This finding is in contrast to a past smoking-cessation trial which found that, in non-methadone-maintenance sites, there was a significant decrease in SUD treatment attendance in the SCT, relative to TAU, participants.18 Given the larger sample-size and site diversity of the present study, the present finding should help reassure community treatment providers who worry that providing smoking-cessation treatment concurrently with SUD treatment might increase treatment drop out.
Another objective of the present trial was to evaluate the efficacy of TAU+SCT, relative to TAU, in improving smoking-abstinence outcomes. The results suggest that TAU+SCT significantly improved smoking-abstinence outcomes for cocaine- and/or methamphetamine-dependent participants in outpatient SUD treatment as indicated by the odds ratio of 18.23 for TAU+SCT, compared to TAU, for end-of-treatment point prevalence abstinence (PPA). Still, the PPA rate of 25.5% for TAU+SCT was somewhat modest and lower than rates of approximately 35% obtained in smoking-cessation trials completed with non-substance abusing populations.28–30 The present end-of-treatment PPA rate does, however, compare favorably with PPA rates from smoking-cessation trials completed with alcohol-dependent patients.31,32 Smoking-cessation trials typically include longer-term follow-up assessments. The final follow-up assessment for the present trial was at 6 months post-smoking quit for which the PPA rate was 13.1% for the TAU+SCT participants. As with the end of treatment PPA rate, this is lower than the 6-month PPA rate of approximately 26% reported for bupropion in past trials with non-substance abusing populations,30,33 but is comparable to 6-month PPA rates from trials with alcohol-dependent smokers.31,32 Moreover, the odds ratio of 3.81 for TAU+SCT, compared to TAU, for 6-month follow-up suggests that smoking-cessation outcomes over longer-term follow-up can be significantly improved in stimulant-dependent patients in outpatient SUD treatment.
The present study had several strengths. First, this trial was conducted at 12 sites, which enhances the generalizability of the results, and included a relatively large sample of stimulant-dependent participants. Another study strength is that it was conducted with individuals seeking treatment at SUD treatment programs and, thus, the results are likely generalizable to individuals in treatment for stimulant-dependence disorders.34 Other strengths include the high retention rate and strong adherence with smoking cessation counseling and taking bupropion as prescribed. Thus, while adherence with the nicotine inhaler was poor, the TAU+SCT participants received important elements of effective smoking-cessation treatment. A limitation of the present study was the use of a more intensive smoking cessation intervention, comprised of two medications and two psychosocial treatments, than could be implemented by many SUD treatment programs outside the context of a clinical trial. Thus, the smoking interventions implemented in SUD treatment programs may have less of an impact on smoking behavior than the SCT provided in the present trial. Another limitation was the relatively high rate of stimulant-abstinence and, thus, the lack of significant effect of TAU+SCT, relative to TAU, on stimulant-abstinence may reflect a ceiling effect. A final limitation was the lack of a biomarker for medication adherence and, thus, the reported adherence rates for bupropion likely reflect upper limit estimates.
In conclusion, the present results demonstrate that providing smoking-cessation treatment to cocaine- and/or methamphetamine-dependent patients in outpatient SUD treatment had no effect on stimulant-use outcomes, significantly improved smoking-abstinence outcomes, and did not significantly impact treatment attendance. TAU+SCT, relative to TAU, participants had significantly better outcomes for drug-free days at 6-month follow-up. These results are consistent with findings from research studying the impact of smoking-cessation treatment in other SUD populations6,35 and add to a growing literature demonstrating that concurrent smoking-cessation and SUD treatment can significantly improve smoking-abstinence outcomes and do not negatively impact non-nicotine outcomes.
Clinical Points.
The prevalence of smoking in cocaine- and/or methamphetamine-dependent patients is ≥ 75%.
Smoking-cessation treatment can significantly increase smoking abstinence in these patients.
Intensive smoking-cessation treatment will not worsen, and may enhance, abstinence from non-nicotine substance use.
Podcast for CTN-0046 Main Outcome paper.
PODCAST OPPORTUNITY:
- To reach a broader audience, please write a short summary (no more than 250 words) of your findings to spotlight your work in the JCP Publisher's Podcast. The tone of the summary should be conversational and light, compared to your scholarly article. This summary will be recorded by the journal's publisher and will (1) appear as a link beside your article in the online Table of Contents and (2) be included in the entire issue's podcast.
The JCP is finding that these audio files are quite popular with their readers, who increasingly use audio to direct them to works of interest.
- Upload your summary separately from your manuscript by selecting the file type "Additional Information File" and labeling the file "Podcast. "
Cigarette smoking accounts for 443,000 deaths annually in the United States. The smoking prevalence rate in the general population is approximately 20% while it is estimated to be over 75% in cocaine- and methamphetamine-dependent patients. Despite the pervasiveness and deadly consequences of smoking in addicted individuals, smoking-cessation treatment is typically not provided in substance use disorder treatment programs. This is due, in part, to some concerns that concurrent treatment of nicotine and stimulant dependence might be overwhelming for patients and, thus, might negatively impact both treatment attendance and stimulant use outcomes. The present randomized controlled trial, conducted with 538 cocaine- and/or methamphetamine-dependent patients, evaluated the impact of concurrent substance use disorder and nicotine-dependence treatment for stimulant-dependent patients. Participants were recruited from 12 outpatient substance use disorder treatment programs. All participants received substance use disorder treatment as usually provided at the participating site. Participants assigned to concurrent treatment also received smoking-cessation treatment consisting of extended-release (XL) bupropion, the nicotine inhaler, weekly individual smoking cessation counseling and contingency management for smoking abstinence. The study results revealed no significant treatment effects on stimulant-use outcomes, on drug-abstinence, or on treatment attendance. Participants receiving concurrent treatment had significantly better outcomes for drug-free days at 6-month follow-up. Participants receiving concurrent treatment achieved smoking abstinence at a significantly higher rate compared to participants receiving only standard substance use disorder treatment. These results suggest that providing smoking-cessation treatment to illicit stimulant-dependent patients in outpatient SUD treatment will not worsen, and may improve, non-nicotine substance use outcomes.
Acknowledgements
The participating sites were: ADAPT, Inc., Roseburg, OR; Addiction Medicine Services, Pittsburgh, PA; Behavioral Health Services of Pickens County, Pickens, SC; Dorchester Alcohol and Drug Commission, Summerville, SC; Gateway Community Services, Jacksonville, FL; Gibson Recovery, Cape Girardeau, MO; La Frontera Center, Inc., Tucson, AZ; Lexington/Richland Alcohol & Drug Abuse Council, Columbia, SC; Maryhaven, Inc., Columbus, OH; Matrix Institute on Addictions, Rancho Cucamonga, CA; Nexus Recovery, Inc., Dallas, TX; Tarzana Treatment Centers, Tarzana CA.
Funding/Support: This study was supported by the following grants from the National Institute on Drug Abuse: U10-DA013732 to University of Cincinnati (Dr. Winhusen); U10-DA020036 to University of Pittsburgh (Dr. Daley), U10-DA013720 to University of Miami School of Medicine (Drs. Szapocznik and Metsch); U10-DA013045 to University of California Los Angeles (Dr. Ling); U10-DA013727 to Medical University of South Carolina (Dr. Brady); U10- DA020024 to University of Texas Southwestern Medical Center (Dr. Trivedi); U10-DA015815 to University of California San Francisco (Drs. Sorensen and McCarty).
Footnotes
Financial Disclosures: The authors report no financial disclosures.
References
- 1.Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses--United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. [PubMed] [Google Scholar]
- 2.Schroeder SA. A 51-year-old woman with bipolar disorder who wants to quit smoking. JAMA. 2009;301(5):522–531. doi: 10.1001/jama.2008.982. [DOI] [PubMed] [Google Scholar]
- 3.Hurt RD, Offord KP, Croghan IT, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275(14):1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 4.Ziedonis DM, Guydish J, Williams J, et al. Barriers and solutions to addressing tobacco dependence in addiction treatment programs. Alcohol Res Health. 2006;29(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 5.Prochaska JJ. Failure to treat tobacco use in mental health and addiction treatment settings: a form of harm reduction? Drug Alcohol Depen. 2010;110(3):177–182. doi: 10.1016/j.drugalcdep.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prochaska JJ, Delucchi K, Hall SM. A meta-analysis of smoking cessation interventions with individuals in substance abuse treatment or recovery. J Consult Clin Psychol. 2004;72(6):1144–1156. doi: 10.1037/0022-006X.72.6.1144. [DOI] [PubMed] [Google Scholar]
- 7.Budney AJ, Higgins ST, Hughes JR, Bickel WK. Nicotine and caffeine use in cocainedependent individuals. J Subst Abuse. 1993;5(2):117–130. doi: 10.1016/0899-3289(93)90056-h. [DOI] [PubMed] [Google Scholar]
- 8.Sees KL, Clark HW. When to begin smoking cessation in substance-abusers. J Subst Abuse Treat. 1993;10(2):189–195. doi: 10.1016/0740-5472(93)90044-3. [DOI] [PubMed] [Google Scholar]
- 9.Gorelick DA, Simmons MS, Carriero N, Tashkin DP. Characteristics of smoked drug use among cocaine smokers. Am J Addiction. 1997;6(3):237–245. [PubMed] [Google Scholar]
- 10.Grant KM, Kelley SS, Agrawal S, et al. Methamphetamine use in rural Midwesterners. Am J Addict. 2007 Mar-Apr;16(2):79–84. doi: 10.1080/10550490601184159. [DOI] [PubMed] [Google Scholar]
- 11.Weinberger AH, Sofuoglu M. The impact of cigarette smoking on stimulant addiction. Am J Drug Alcohol Abuse. 2009;35(1):12–17. doi: 10.1080/00952990802326280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roll JM, Higgins ST, Tidey J. Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharm. 1997;5(3):263–268. doi: 10.1037//1064-1297.5.3.263. [DOI] [PubMed] [Google Scholar]
- 13.Nemeth-Coslett R, Henningfield J, Katz J, Goldberg S. Effect of cocaine on rate of cigarette-smoking. Pharmacol Biochem Behav. 1986;25(1):303–303. [Google Scholar]
- 14.Roll JM, Higgins ST, Budney AJ, et al. A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug Alcohol Depend. 1996;40(3):195–201. doi: 10.1016/0376-8716(96)01219-7. [DOI] [PubMed] [Google Scholar]
- 15.Reid MS, Mickalian JD, Delucchi KL, Berger SP. A nicotine antagonist, mecamylamine, reduces cue-induced cocaine craving in cocaine-dependent subjects. Neuropsychopharmacology. 1999;20(3):297–307. doi: 10.1016/S0893-133X(98)00076-1. [DOI] [PubMed] [Google Scholar]
- 16.Reid MS, Mickalian JD, Delucchi KL, et al. An acute dose of nicotine enhances cueinduced cocaine craving. Drug Alcohol Depend. 1998;49(2):95–104. doi: 10.1016/s0376-8716(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 17.Shoptaw S, Rotheram-Fuller E, Yang X, et al. Smoking cessation in methadone maintenance. Addiction. 2002;97(10):1317–1328. doi: 10.1046/j.1360-0443.2002.00221.x. discussion 1325. [DOI] [PubMed] [Google Scholar]
- 18.Reid MS, Fallon B, Sonne S, et al. Smoking cessation treatment in community-based substance abuse rehabilitation programs. J Subst Abuse Treat. 2008;35(1):68–77. doi: 10.1016/j.jsat.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Ebbert JO, Croghan IT, Sood A, et al. Varenicline and bupropion sustained-release combination therapy for smoking cessation. Nicotine Tob Res. 2009;11(3):234–239. doi: 10.1093/ntr/ntn031. [DOI] [PubMed] [Google Scholar]
- 20.Fiore MC, Jaen CR, Baker TB, et al. A clinical practice guideline for treating tobacco use and dependence: 2008 update - a US Public Health Service report. Am J Prev Med. 2008;35(2):158–176. doi: 10.1016/j.amepre.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winhusen T, Stitzer M, Woody G, et al. Design considerations for a study to evaluate the impact of smoking cessation treatment on stimulant use outcomes in stimulant-dependent individuals. Contemp Clin Trials. 2012;33(1):197–205. doi: 10.1016/j.cct.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Croghan IT, Trautman JA, Winhusen T, et al. Tobacco dependence counseling in a randomized multisite clinical trial. Contemp Clin Trials. 2012;33(4):576–582. doi: 10.1016/j.cct.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sobell LC, Sobell MB. Timeline follow-back - a technique for assessing self-reported alcohol-consumption. In: Allen J, editor. Measuring Alcohol Consumption. Totowa, NJ: Humana Press; 1992. pp. 41–72. [Google Scholar]
- 24.Fals-Stewart W, O'Farrell TJ, Freitas TT, et al. The timeline followback reports of psychoactive substance use by drug-abusing patients: psychometric properties. J Consult Clin Psychol. 2000;68(1):134–144. doi: 10.1037//0022-006x.68.1.134. [DOI] [PubMed] [Google Scholar]
- 25.Hurt RD, Krook JE, Croghan IT, et al. Nicotine patch therapy based on smoking rate followed by bupropion for prevention of relapse to smoking. J Clin Oncol. 2003;21(5):914–920. doi: 10.1200/JCO.2003.08.160. [DOI] [PubMed] [Google Scholar]
- 26.Nakagawa S. A farewell to Bonferroni: the problems of low statistical power and publication bias. Beha. Ecol. 2004;15(6):1044–1045. [Google Scholar]
- 27.Bobo JK, McIlvain HE, Lando HA, et al. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction. 1998;93(6):877–887. doi: 10.1046/j.1360-0443.1998.9368779.x. [DOI] [PubMed] [Google Scholar]
- 28.Croghan IT, Hurt RD, Dakhil SR, et al. Randomized comparison of a nicotine inhaler and bupropion for smoking cessation and relapse prevention. Mayo Clin Proc. 2007;82(2):186–195. doi: 10.4065/82.2.186. [DOI] [PubMed] [Google Scholar]
- 29.Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 30.Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 31.Kalman D, Herz L, Monti P, et al. Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: results from a randomized, double-blind, placebo-controlled study. Drug Alcohol Depend. 2011;118(2–3):111–118. doi: 10.1016/j.drugalcdep.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant KM, Kelley SS, Smith LM, et al. Bupropion and nicotine patch as smoking cessation aids in alcoholics. Alcohol. 2007;41(5):381–391. doi: 10.1016/j.alcohol.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hurt RD, Sachs DP, Glover ED, et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N Engl J Med. 1997;337(17):1195–1202. doi: 10.1056/NEJM199710233371703. [DOI] [PubMed] [Google Scholar]
- 34.Winhusen T, Winstanley EL, Somoza E, Brigham G. The potential impact of recruitment method on sample characteristics and treatment outcomes in a psychosocial trial for women with co-occurring substance use disorder and PTSD. Drug Alcohol Depend. 2012;120(1–3):225–228. doi: 10.1016/j.drugalcdep.2011.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baca CT, Yahne CE. Smoking cessation during substance abuse treatment: what you need to know. J Subst Abuse Treat. 2009;36(2):205–219. doi: 10.1016/j.jsat.2008.06.003. [DOI] [PubMed] [Google Scholar]