Abstract
Objective
To explore the relationship between medroxyprogesterone acetate (MPA) pharmacokinetic (PK) parameter estimates and weight gain.
Study design
Prospective study of adolescents (N=40; age 12 – 21) initiating DMPA. PK parameters were calculated: maximum MPA concentration (Cmax, ng/mL), time to Cmax (Tmax, days), and elimination rate constant (ng/mL/day). Optimal PK cut points were determined for predicting BMI increase ≥10%.
Results
Cmax <2.88 ng/mL and elimination rate constant <0.021 ng/mL/day were associated (p<0.05) with BMI increase ≥10%. Elimination rate constant was most predictive of weight gain.
Conclusions
Pharmacokinetic evaluation may help identify adolescents at risk of excessive DMPA-associated weight gain.
Keywords: contraception, pharmacokinetics, adolescence, Depo Provera, weight
Introduction
Weight gain is highly variable among adolescents on depot medroxyprogesterone acetate (DMPA) [1–3]. Literature describing DMPA disposition reveals wide inter-individual variability in circulating medroxyprogesterone acetate (MPA) concentrations [4–7]. This wide variability has not been explained by number of prior DMPA injections, body mass index (BMI), or racial/ethnic background [5].
To our knowledge, the relationship between MPA pharmacokinetic (PK) measures and clinical side effects such as weight gain has not been examined. The objective of this pilot study was to explore the relationship between MPA PK parameter estimates and weight gain among adolescent females initiating DMPA.
Methods
Subjects consisted of healthy, post-menarcheal females, ages 12–21, initiating DMPA at two urban, outpatient Adolescent Medicine Clinics between December 2007 and September 2011. Exclusion criteria included: 1) DMPA use within the past 12 months; 2) pregnancy within the past 6 months; 3) other hormonal contraceptive use within the past 3 months; 4) chronic disease known to affect weight (e.g. diabetes); 5) use of medications known to affect weight (e.g. daily corticosteroids); and 6) need for confidential contraceptive care if <18 years.
Written informed consent was obtained from subjects ≥18 years and from a parent/legal guardian of subjects <18. Minor subjects provided written informed assent. The study protocol was approved by the Institutional Review Boards at participating institutions.
Study visits were conducted at baseline (within 1 week prior to the first DMPA injection); every 4 weeks during the first 12-week dosing interval; and at 12, 24, and 48 weeks at time of subsequent DMPA injections. Of 62 patients approached, 45 enrolled, and 40 had sufficient data for PK analyses. 39 and 31 returned at 24 and 48 weeks, respectively. No significant differences were found between study completers and non-completers. All patients received DMPA 150mg intramuscularly every 12 weeks.
Subject demographics and medical history were collected at baseline. Gynecologic age was calculated as the difference between chronologic and menarcheal age. Update interviews were conducted at subsequent visits to assess for changes in health or medications. At baseline, 12, 24, and 48 weeks, weight and height were measured. BMI was calculated as weight (kg)/height (m2).
Blood was obtained at all study visits. In order to maximize available data for PK analyses and minimize number of blood draws, a sparse sampling strategy (population PK approach) was employed. Subjects were randomly assigned to one of four groups: blood draws at weeks 1, 5, and 9; blood draws at weeks 2, 6, and 10; blood draws at weeks 3, 7, and 11; or blood draws at weeks 4, 8, and 12. Serum samples were analyzed for MPA by liquid chromatography-mass spectrometry using Multiple Reaction Monitoring.
The main outcome measure was annualized percentage change in BMI dichotomized into two groups: 1) excessive weight gainers (BMI increase ≥10%); and 2) non-excessive weight gainers (BMI increase <10%). Patient demographics, weight measures, and PK parameter estimates were described for all subjects and by weight gain group.
A composite, population, time concentration curve was generated using all available MPA concentrations across the first 12-week interval. Individual PK parameter estimates (maximum MPA concentration (Cmax, ng/mL), time to Cmax (Tmax, days), and elimination rate constant (ng/mL/day)) were then calculated and compared across weight gain groups using Student’s t-test. Optimal cut scores for individual PK parameter estimates were determined using Receiver Operating Characteristics (ROC) analysis. Backwards stepwise logistic regression was performed to investigate the independent relationship between individual PK parameter estimates and weight gain group. P-values are two-sided and unadjusted for multiple comparisons. Data analyses were performed using SAS (version 9.2, SAS Institute, Cary, NC).
Results
Fifteen subjects (37.5%) self-identified as white non-Hispanic, 14 (35.0%) as black non-Hispanic, and 11 (27.5%) as Hispanic. Mean chronologic and gynecologic age were 16.2 and 4.2 years, respectively. The majority of subjects had regular menstrual cycles (93.3%) and no prior pregnancy (91.1%). Excessive weight gain (BMI gain ≥10%) was observed in 11 (27.5%) subjects. Excessive and non-excessive weight gainers did not significantly differ at baseline with regards to mean chronologic/gynecologic age, race, or BMI.
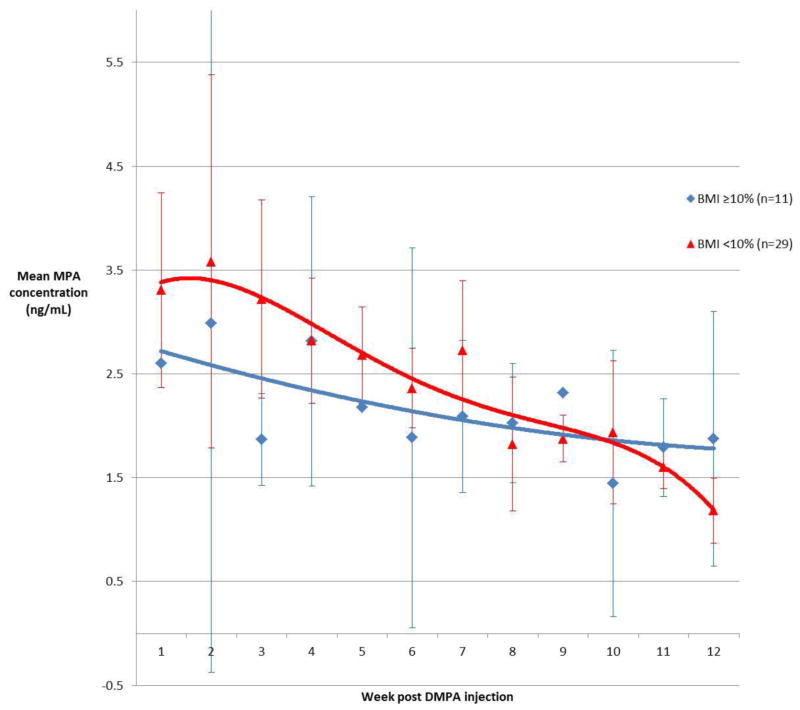
Composite serum concentration time curves, constructed from MPA values during the first 12-week interval, support a relationship between weight gain and PK parameter estimates (Figure 1). Subjects with excessive weight gain demonstrated a lower Cmax and a flatter concentration curve than subjects with non-excessive weight gain.
Figure 1.
Composite MPA concentration time curves (95% confidence intervals)
In unadjusted, bivariate analyses, significant (p<0.05) associations were found between weight gain group and two PK parameter estimate cut points (Table 1). Cmax <2.88 ng/mL had a sensitivity of 0.73 and a 1-specificity of 0.38 for identifying subjects with excessive weight gain. Elimination rate constant <0.021 ng/mL/day had a sensitivity of 1.00 and a 1-specificity of 0.64 for identifying subjects with excessive weight gain. Although not significant, excessive weight gainers had, on average, a longer Tmax (38.9 vs. 30.4 days, respectively). In backwards stepwise logistic regression (data not shown), only elimination rate constant <0.021 ng/mL/day remained a significant predictor (p=0.05) of an annualized BMI increase ≥10%.
Table 1.
Medroxyprogesterone acetate pharmacokinetic parameters estimates by annualized percentage increase in body mass index
| Pharmacokinetic Parameter Estimate | BMI gain ≥10% (n=11) |
BMI gain <10% (n=29) |
p-valuea |
|---|---|---|---|
|
| |||
| Cmaxb | 0.145 | ||
| Mean (SD) | 2.61 (1.04) | 3.23 (1.21) | |
| Median | 2.52 | 2.92 | |
| Min - Max | 1.27 – 4.70 | 1.57 – 6.12 | |
|
| |||
| Cmax <2.88 | 0.049 | ||
| n(%) | 8 (72.7) | 11 (37.9) | |
|
| |||
| Elimination Rate Constantc,d | 0.196 | ||
| Mean (SD) | 0.011 (0.006) | 0.019 (0.017) | |
| Median | 0.009 | 0.016 | |
| Min - Max | 0.003 – 0.021 | 0.002 – 0.076 | |
|
| |||
| Elimination Rate Constant <0.021 | 0.047 | ||
| n(%) | 8 (100.0) | 18 (64.3) | |
|
| |||
| Tmaxe | 0.320 | ||
| Mean (SD) | 38.9 (28.81) | 30.4 (21.88) | |
| Median | 28 | 27 | |
| Min - Max | 7 – 87 | 6 – 87 | |
Unadjusted Student’s t-test analyses p-value
Maximum serum medroxyprogesterone acetate concentration (ng/mL)
Natural logarithm medroxyprogesterone acetate elimination rate constant (ng/mL/day)
Three subjects with BMI gain ≥10% and one subject with BMI gain <10% had no post-peak concentration data and were missing from the elimination rate constant determination
Time to Cmax (days)
Discussion
We found that flatter MPA concentration time curves, i.e. those with lower Cmax and more gradual slope, were related to higher BMI gains. Lower Cmax coupled with slower elimination rate likely results in increased area under the curve (AUC), a surrogate marker for drug exposure. Higher AUC and correspondingly higher MPA exposure might explain greater weight gain.
Interpretation of our data is limited by small sample size. Our sparse sampling strategy (employed to improve recruitment and compliance in adolescents) limited our ability to fully characterize each subject’s concentration time curve. Although given intramuscularly, DMPA injection site (gluteal or deltoid) was not standardized.
Despite these limitations, the current study provides a new framework for evaluating DMPA-associated side effects as prior studies found inconsistent relationships between clinical characteristics and weight gain [2,3,8,9]. Our data suggest that PK evaluation may assist in the identification of patients at risk of excessive weight gain. Future studies with larger sample sizes or standard PK methods should be conducted to determine optimal PK predictors of DMPA-associated side effects and to better characterize the etiology of MPA PK variability.
Acknowledgments
Funded by the National Institutes of Health (NIH) Award Number Grant K23RR024029. This project was also made possible by the National Center for Research Resources Grant UL1RR024989, which is now the Clinical and Translational Science Collaborative of Cleveland, Award Number Grant UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS). Additional support was received from The Ohio State University Center for Clinical and Translational Science, NCATS Award Number Grant UL1TR000090. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NCATS or the NIH.
The authors thank Mary Jo Day, LPN and Kandice Roush, BSN for coordinating and conducting study visits and Dr. Barbara Cromer for her invaluable mentorship.
Footnotes
Dr. Bonny had full access to all the data in the study and accepts full responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Bonny and Reed. Acquisition of data: Bonny, Lange, and Rogers. Analysis and interpretation of data: Bonny, Lange, Rogers, Gothard, and Reed. Drafting of the manuscript: Bonny. Critical revision of the manuscript for important intellectual content: Lange, Rogers, Gothard, and Reed.
Conflict of Interest/Financial Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Arch Pediatr Adolesc Med. 2006;160:40–5. doi: 10.1001/archpedi.160.1.40. [DOI] [PubMed] [Google Scholar]
- 2.Risser WL, Gefter LR, Barratt MS, Risser JM. Weight change in adolescents who used hormonal contraception. J Adolesc Health. 1999;24:433–6. doi: 10.1016/s1054-139x(98)00151-7. [DOI] [PubMed] [Google Scholar]
- 3.Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. J Pediatr Adolesc Gynecol. 2002;15:79–82. doi: 10.1016/s1083-3188(01)00147-4. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz A, Hirol M, Stanczyk FZ, Goebelsmann U, Mishell DR. Serum medroxyprogesterone acetate (MPA) concentrations and ovarian function following intramuscular injection of depo-MPA. J Clin Endocrinol Metab. 1977;44:32–8. doi: 10.1210/jcem-44-1-32. [DOI] [PubMed] [Google Scholar]
- 5.Smit J, Botha J, McFadyen L, Beksinska M. Serum medroxyprogesterone acetate levels in new and repeat users of depot medroxyprogesterone acetate at the end of the dosing interval. Contraception. 2004;69:3–7. doi: 10.1016/j.contraception.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Koetsawang S, Shrimanker K, Fotherby K. Blood levels of medroxyprogesterone acetate after multiple injections of depoprovera or cycloprovera. Contraception. 1979;20:1–4. doi: 10.1016/0010-7824(79)90038-6. [DOI] [PubMed] [Google Scholar]
- 7.Jeppsson S, Johansson Medroxyprogesterone acetate, estradiol, FSH and LH in peripheral blood after intramuscular administration of Depo-ProveraR to women. Contraception. 1976;14:461–69. doi: 10.1016/s0010-7824(76)80060-1. [DOI] [PubMed] [Google Scholar]
- 8.Polaneczky M, Liblanc M. Long-term depot medroxyprogesterone acetate (Depo Provera) use in inner-city adolescents. J Adolesc Health. 1998;23:81–8. doi: 10.1016/s1054-139x(98)00014-7. [DOI] [PubMed] [Google Scholar]
- 9.O’Dell CM, Forke CM, Polaneczky MM, Sondheimer SJ, Slap GB. Depot medroxyprogesterone acetate or oral contraception in postpartum adolescents. Obstet Gynecol. 1998;91:609–14. doi: 10.1016/s0029-7844(97)00710-2. [DOI] [PubMed] [Google Scholar]