Abstract
Adolescents and young adults (AYAs, ages 15–40 years) with cancer have not experienced survival improvements to the same extent as younger and older patients. We compared changes in survival following myeloablative allogeneic hematopoietic cell transplantation (HCT) for acute lymphoblastic leukemia (ALL) among children (N=981), AYAs (N=1218) and older adults (N=469) who were transplanted over three time periods: 1990–1995, 1996–2001 and 2002–2007. Five-year survival varied inversely with age group. Survival improved over time in AYAs and paralleled that seen in children; however, overall survival did not change over time for older adults. Survival improvements were primarily related to lower rates of early treatment related mortality in the most recent era. For all cohorts, relapse rates did not change over time. A subset of 222 AYAs between the ages of 15–25 at 46 pediatric or 49 adult centers were also analyzed to describe differences by center type. In this subgroup, there were differences in transplant practices among pediatric and adult centers, although HCT outcomes did not differ by center type. Survival for AYAs undergoing myeloablative allogeneic HCT for ALL improved at a similar rate as survival for children.
Keywords: Adolescent and young adults, hematopoietic cell transplantation, allogeneic, survival
Introduction
Adolescents and young adults (AYAs, ages 15–40) with cancer are considered to be a vulnerable subgroup by the National Cancer Institute, in part because survival improvements over time have lagged behind survival improvements for older and younger patients with cancer (Bleyer A, 2007; Wood WA, 2011). AYAs with acute lymphoblastic leukemia (ALL) have garnered particular interest because of apparent survival disparities related to treatment in pediatric versus adult oncology settings. Several retrospective analyses have demonstrated superior survival for AYAs with ALL treated on pediatric protocols, such as a Children’s Cancer Group (CCG) versus Cancer and Leukemia Group B (CALGB) comparison in which 5-year event free survival and overall survival rates favored AYAs treated on pediatric studies (63% versus 34%, p<0.001 and 67% versus 46%, p<0.001 respectively) (Stock W, 2008). The reasons for these disparities are not entirely clear, though some have suggested differences in the type and amount of anti-leukemic drugs in pediatric versus adult treatment protocols (Stock W, 2010). Others have also pointed to differences in the way care is delivered to AYAs in pediatric versus adult settings, favoring improved access to care through insurance coverage (Kantarjian HM, 2009), better adherence, and a higher proportion of on-time receipt of therapy in the pediatric setting (Schafer, 2011).
In hematopoietic cell transplantation (HCT), outcomes have improved over time, in part because of improvements in supportive care and a corresponding reduction in transplant-related mortality [Gooley T, 2010; Hahn T, 2013]. However, few studies specifically addressed outcomes among AYAs undergoing HCT (Burke MJ, BBMT 2013), and it is unclear whether the benefits of improvements in supportive care have been realized equally in the vulnerable AYA population. For these reasons, we sought to determine whether outcomes for AYAs following myeloablative allogeneic HCT for ALL have improved to a similar degree as those among older and younger patients. Further, we wished to determine whether significant differences existed in care delivery characteristics associated with pediatric versus adult HCT settings for AYAs with ALL. We analyzed data reported to the Center for International Blood and Marrow Transplant Research (CIBMTR) to address these questions.
Methods
Data Source and Patients
The CIBMTR is a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on allogeneic and autologous HCTs to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Centers are required to report all consecutive transplantations and patients are followed over time, with yearly follow-up. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with the Privacy Rule (HIPAA) as a Public Health Authority and in compliance with all applicable federal regulations pertaining to the protection of human research participants as determined by continuous review of the Institutional Review Board of the NMDP.
For this study, we included patients who had received their first allogeneic HCT for ALL using either an HLA-identical sibling donor (matched sibling donor, MSD) or unrelated donor (URD) from 1990–2007 at a transplantation center in the United States. Only patients who underwent transplantation following myeloablative conditioning and were in either first or second complete remission (CR) were included in this analysis. Recipients of umbilical cord blood grafts were excluded. Patients were divided into three groups based on age at transplantation: children (<15 years), AYAs (15–40 years) and older adults (>40 years) to match the recommended NCI Progress Review Group age definition for AYAs (Adolescent and Young Adult Oncology Progress Review Group, 2006).
Outcomes and study definitions
The primary objective of this study was to compare change over time in rates of overall survival (OS), leukemia-free survival (LFS), relapse, and treatment related mortality (TRM) among children, AYAs, and older adults. For OS, death from any cause was considered an event. LFS was defined as survival in CR after HCT. Relapse was defined as leukemia recurrence. TRM was defined as death in CR. All outcomes were assessed from the date of transplantation.
The NMDP classification of HLA-matching status was used for URD recipients (well-matched, partially matched, or mismatched) (Weisdorf D, 2008). Where information was available, cytogenetic risk was classified as high-risk (t(4;11), t(9;22), t(8;14), hypodiploidy, or near triploidy, or more than 5 cytogenetic abnormalities), normal (normal cytogenetics), or other (any other abnormality) (Moorman AV, 2010; Moorman AV, 2007).
As a secondary objective, we evaluated whether the type of transplant center (adult versus pediatric) was associated with OS for a subgroup of AYAs between 15–25 years of age. We used several data sources to determine whether transplant centers were primarily adult or pediatric transplant programs. First, we used information available from the CIBMTR and the NMDP, where centers report their patients’ characteristics, including age. However, some centers with distinct adult and pediatric programs report as one center to the CIBMTR and/or NMDP. For these centers, we used data collected as part of a national CIBMTR survey to designate centers as adult versus pediatric (Navneet Majhail, personal communication). Furthermore, we also contacted each “combined center” to determine a) whether these centers performed transplants exclusively for pediatric patients, adult patients, or both; b) if both adults and children were transplanted, were there separate pediatric and adult transplant teams; and c) the age cutoff that a center used to determine whether a patient would be cared for by the adult or pediatric service. Based on information obtained from these various above listed sources, centers were classified as adult or pediatric.
Statistical methods
Summaries of patient-, disease-, and treatment-related characteristics were produced for the three age groups. The chi-square test was used to compare categorical variables, and the Kruskal-Wallis test was used for continuous variables. Univariate probabilities of OS and LFS were calculated using the Kaplan-Meier estimator (Kaplan, 1985). Probabilities of relapse and TRM were estimated using a cumulative incidence function method (Gooley T, 1999). To evaluate changes in outcomes over time, we divided the cohort into three time periods based on the year of transplantation (1990–1995–1996–2001, and 2002–2007).
Cox proportional hazards models were used to adjust for significant covariates while comparing the three age groups. All factors were examined for proportional hazards using a time-dependent covariate to appropriately model early versus later events. A backward regression model selection technique was used to identify significant covariates to be included in the models. The main effects tested in all multivariate analysis models were age and time period of transplantation. Consistent with the primary study question, potential interactions between age and time period were also examined. In addition to age and time period of transplantation, the patient and disease characteristic covariates considered in the multivariable models included gender, race/ethnicity, Karnofsky performance status, disease status, cell of origin (T vs. B-cell), cytogenetic risk, and time from diagnosis to HCT. As time from diagnosis to CR1 was confounded by disease status (CR1 vs. CR2), the two covariates were combined for multivariable analysis (CR1 vs. CR2, duration of CR1 <36 months vs. CR2, duration of CR1 ≥ 36 months, CR2, duration of CR1 unknown).
For the subgroup analysis that focused on adult versus pediatric center comparison for AYAs between 15–25 years of age, we describe the characteristics of patients transplanted at the two types of center. Univariate probabilities of OS, LFS, TRM and relapse were analyzed as described above. Because of limited number of patients, we were not able to perform multivariable analyses to study the association of center type with patient outcomes.
All computations were performed using the SAS statistical package (SAS Institute, Cary, NC). All P-values are two sided.
Results
Patient characteristics
In total, 2668 patients with ALL in CR1 or CR2 reported to the CIBMTR from 1990–2007 met the study eligibility criteria, including 981 children, 1218 AYAs, and 469 older adults (Table 1). From 1996–2007, transplant volume increased by 7% in children, 50% in AYAs, and 180% in older adults. The proportions of patients receiving peripheral blood stem cell transplants and of patients receiving HCT using well-matched URD HCT increased over time in all three age groups. The proportions of Hispanic recipients increased among children and AYAs over time (in children, 6% in 1990–1995 to 17% in 2002–2007, and in AYAs, 4% in 1990–1995 to 15% in 2002–2007), but remained unchanged in older adults (5% in 1990–1995 to 6% in 2002–2007).
Table 1.
Patient, disease and transplant characteristics for patients receiving first myeloablative allogeneic HCT for ALL
| Age group/Time period | Children (<15 years) | AYAs (15–40 years) | Older Adults (>40 years) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1990–1995 | 1996–2001 | 2002–2007 | 1990–1995 | 1996–2001 | 2002–2007 | 1990–1995 | 1996–2001 | 2002–2007 | |
| Characteristics | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| Number of patients | 267 | 343 | 371 | 309 | 362 | 547 | 60 | 106 | 303 |
| Number of centers | 44 | 59 | 57 | 75 | 103 | 118 | 34 | 49 | 77 |
| Median age at HCT (range), years | 7.2 (0.5–14.9) | 7.6 (0.5–14.9) | 8.2 (0.5–14.9) | 23.9 (15.0–39.8) | 23.7 (15.0–39.9) | 26.2 (15.0–39.9) | 44.8 (40.1–58.2) | 47.0 (40.0–61.1) | 49.1 (40.0–66.2) |
| Male | 175 (66) | 196 (57) | 230 (62) | 198 (64) | 227 (63) | 356 (65) | 37 (62) | 51 (48) | 158 (52) |
| Race/ethnicity | |||||||||
| Non-Hispanic White | 213 (80) | 242 (71) | 232 (63) | 253 (82) | 273 (75) | 298 (73) | 53 (88) | 91 (86) | 257 (85) |
| African-American | 13 (5) | 26 (8) | 28 (8) | 12 (4) | 17 (5) | 20 (4) | 0 | 6 (6) | 10 (3) |
| Asian/Pacific Islander | 12 (4) | 15 (4) | 15 (4) | 14 (5) | 19 (5) | 21 (4) | 2 (3) | 2 (2) | 7 (2) |
| Hispanic | 16 (6) | 55 (16) | 63 (17) | 12 (4) | 49 (14) | 84 (15) | 3 (5) | 5 (5) | 18 (6) |
| Other/unknown | 13 (5) | 5 (1) | 33 (9) | 18 (6) | 4 (1) | 24 (4) | 2 (3) | 2 (2) | 11 (4) |
| KPS at HCT | |||||||||
| ≥ 90 | 238 (89) | 293 (85) | 307 (83) | 240 (78) | 273 (75) | 383 (70) | 43 (72) | 75 (71) | 186 (60) |
| <90 | 27 (10) | 45 (13) | 20 (5) | 65 (21) | 84 (23) | 122 (22) | 17 (28) | 29 (27) | 94 (31) |
| Disease status at HCT | |||||||||
| CR1 | 77 (29) | 114 (33) | 127 (34) | 159 (51) | 160 (44) | 279 (51) | 47 (78) | 73 (69) | 232 (77) |
| CR2, CR1 duration <36 mos | 137 (51) | 162 (47) | 182 (49) | 104 (34) | 153 (42) | 192 (35) | 10 (17) | 29 (27) | 54 (18) |
| CR2, CR1 duration ≥ 36 mos | 37 (14) | 57 (17) | 52 (14) | 35 (11) | 42 (12) | 53 (10) | 2 (3) | 3 (3) | 13 (4) |
| CR2, CR1 duration unknown | 16 (6) | 10 (3) | 10 (3) | 11 (4) | 7 (2) | 23 (4) | 1 (2) | 1 (1) | 4 (1) |
| Time from diagnosis to HCT | |||||||||
| <6 months | 44 (16) | 75 (22) | 90 (24) | 105 (34) | 83 (23) | 180 (33) | 33 (55) | 39 (37) | 153 (50) |
| 6–12 months | 59 (22) | 55 (16) | 57 (15) | 76 (25) | 100 (28) | 133 (24) | 15 (25) | 42 (40) | 90 (30) |
| ≥ 12 months | 164 (61) | 213 (62) | 224 (60) | 128 (41) | 179 (49) | 234 (43) | 12 (20) | 25 (24) | 60 (20) |
| Cell of origin | |||||||||
| B-cell | 171 (64) | 249 (73) | 280 (75) | 155 (50) | 233 (64) | 428 (78) | 33 (55) | 72 (68) | 246 (81) |
| T-cell | 30 (11) | 31 (9) | 60 (16) | 55 (18) | 55 (15) | 83 (15) | 7 (12) | 6 (6) | 26 (9) |
| Other/unknown | 66 (25) | 63 (18) | 31 (8) | 99 (32) | 74 (20) | 36 (7) | 20 (33) | 28 (26) | 31 (10) |
| Graft type | |||||||||
| Bone marrow | 266 (100) | 319 (93) | 291 (78) | 303 (98) | 304 (84) | 209 (38) | 59 (98) | 82 (77) | 82 (27) |
| Peripheral blood | 1 (<1) | 24 (7) | 80 (22) | 6 (2) | 58 (16) | 338 (62) | 1 (2) | 24 (23) | 221 (73) |
| HLA Match | |||||||||
| HLA-identical sibling | 104 (39) | 93 (27) | 58 (16) | 203 (66) | 74 (20) | 102 (19) | 39 (65) | 38 (36) | 82 (27) |
| Unrelated, well-matched | 27 (10) | 66 (19) | 168 (45) | 27 (9) | 107 (30) | 289 (53) | 6 (10) | 17 (16) | 148 (49) |
| Unrelated, partially matched | 54 (20) | 113 (33) | 96 (26) | 28 (9) | 111 (31) | 121 (22) | 10 (17) | 33 (31) | 56 (18) |
| Unrelated, mismatched | 81 (30) | 66 (19) | 46 (12) | 49 (16) | 68 (19) | 27 (5) | 5 (8) | 17 (16) | 11 (4) |
| Conditioning | |||||||||
| TBI/Cy | 198 (74) | 285 (83) | 337 (91) | 187 (61) | 288 (80) | 398 (73) | 38 (63) | 77 (73) | 182 (60) |
| Cy/Bu | 23 (9) | 33 (10) | 4 (1) | 32 (10) | 28 (8) | 25 (5) | 7 (12) | 9 (8) | 27 (9) |
| TBI/etoposide | 18 (7) | 17 (5) | 17 (5) | 82 (27) | 35 (10) | 78 (14) | 13 (22) | 15 (14) | 58 (19) |
| TBI/other | 26 (10) | 5 (1) | 6 (2) | 7 (2) | 7 (2) | 24 (4) | 2 (3) | 4 (4) | 22 (7) |
| Other | 2 (1) | 3 (1) | 7 (2) | 1 (<1) | 4 (1) | 22 (4) | 0 | 1 (1) | 14 (5) |
| GVHD prophylaxis | |||||||||
| CsA + MTX +/− other | 122 (46) | 162 (47) | 165 (44) | 139 (45) | 188 (52) | 134 (24) | 26 (43) | 57 (54) | 71 (23) |
| FK506 + MTX +/− other | 2 (1) | 24 (7) | 101 (27) | 7 (2) | 54 (15) | 263 (48) | 1 (2) | 20 (19) | 140 (46) |
| T-cell depletion | 66 (25) | 96 (28) | 57 (16) | 46 (15) | 73 (20) | 31 (5) | 15 (25) | 14 (13) | 19 (7) |
| Other | 77 (29) | 61 (18) | 48 (19) | 117 (38) | 47 (15) | 119 (23) | 18 (30) | 15 (15) | 73 (26) |
Abbreviations: AYAs – Adolescent and young adults; KPS – Karnofsky performance status; HCT – hematopoietic cell transplantation; CR – complete remission; HLA – human leukocyte antigen; TBI – total body irradiation; Cy – cyclophosphamide; Bu – busulfan; GVHD – graft-versus-host disease; CSA – cyclosporine; MTX – methotrexate
Outcomes over time
Univariate analyses for OS, LFS, relapse, and TRM of children, AYAs and older adults over time are presented in Table 2. Survival was inversely related to age, with older patients having lower 5-year OS and LFS rates than AYAs, who in turn had lower OS and LFS rates than children, particularly in the two most recent time periods. For all time periods, higher TRM probability estimates were directly related to increasing age, with AYAs having higher 5-year TRM than children, and adults having higher TRM than AYAs. The probability of relapse was similar across cohorts for each of the time periods.
Table 2.
Unadjusted probability of outcomes by time period and age group at 5-years after HCT
| Outcome | 1990–1995 | 1996–2001 | 2002–2007 |
|---|---|---|---|
| Probability (95% CI) | Probability (95% CI) | Probability (95% CI) | |
| Overall survival | |||
| Children | 49 (43–55) | 53 (47–58) | 58 (53–63) |
| AYAs | 34 (29–40) | 34 (29–39) | 43 (39–47) |
| Older adults | 41 (29–54) | 22 (14–30) | 36 (30–41) |
| Leukemia-free survival | |||
| Children | 47 (41–53) | 33 (43–53) | 53 (48–58) |
| AYAs | 33 (28–39) | 31 (26–36) | 38 (34–43) |
| Older adults | 41 (29–55) | 19 (12–27) | 33 (28–39) |
| Relapse | |||
| Children | 23 (18–28) | 26 (21–31) | 28 (24–33) |
| AYAs | 24 (19–29) | 28 (23–33) | 31 (27–35) |
| Older adults | 4 (0–10) | 28 (20–37) | 26 (21–31) |
| Treatment-related mortality | |||
| Children | 30 (25–36) | 26 (22–31) | 19 (15–23) |
| AYAs | 43 (37–49) | 41 (36–46) | 31 (27–35) |
| Older adults | 55 (42–68) | 53 (44–63) | 41 (36–47) |
Abbreviations: AYAs – Adolescent and young adults; CI – confidence intervals
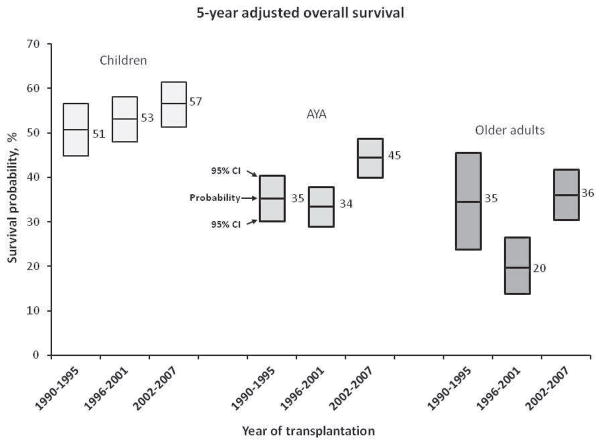
Results for multivariate analyses for OS, LFS, relapse, and TRM are shown in Table 3. After adjusting for patient and disease characteristics, older age was shown to be associated with poorer survival (hazard ratio (HR) 2.04 for older adults and 1.57 for AYAs versus children, p<0.001). No significant interactions were observed between age and time period. Figure 1 displays 5-year adjusted OS probabilities for each age group over time, highlighting that overall survival for AYAs improved and did not lag behind any survival improvements in the other age groups. Similar findings were observed for LFS and TRM, in which older patients again had inferior outcomes compared with AYAs, who in turn had inferior outcomes compared with children. Again, there was no significant interaction between age and time period.
Table 3.
Multivariate analyses for outcomes by time period and age group
| Variable | Hazard Ratio | 95% Confidence Intervals | P-value |
|---|---|---|---|
| Overall survival€ | |||
| Age group | |||
| Children | 1.00 | - | <0.001* |
| AYAs | 1.57 | 1.40–1.77 | <0.001 |
| Older adults | 2.04 | 1.75–2.39 | <0.001 |
| Year of transplantation | |||
| 1990–1995 | 1.00 | - | <0.001* |
| 1996–2001 (≤ 4 months)† | 0.82 | 0.67–0.99 | 0.04 |
| 1996–2001 (>4 months)† | 1.21 | 1.01–1.46 | 0.04 |
| 2002–2007 (≤ 4 months)† | 0.44 | 0.36–0.54 | <0.001 |
| 2002–2007 (>4 months)† | 1.12 | 0.94–1.33 | 0.22 |
| Leukemia free survival¥ | |||
| Age group | |||
| Children | 1.00 | - | <0.001* |
| AYAs | 1.50 | 1.34–1.69 | <0.001 |
| Older adults | 1.84 | 1.58–2.14 | <0.001 |
| Year of transplantation | |||
| 1990–1995 | 1.00 | - | |
| 1996–2001 (≤ 2 months)† | 0.74 | 0.58–0.94 | 0.02* |
| 1996–2001 (>2 months)† | 1.22 | 1.04–1.43 | 0.02 |
| 2002–2007 (≤ 2 months)† | 0.40 | 0.31–0.51 | <0.001 |
| 2002–2007 (>2 months)† | 1.06 | 0.91–1.24 | 0.44 |
| Relapse# | |||
| Age group | |||
| Children | 1.00 | - | <0.001* |
| AYAs (≤ 12 months)† | 1.08 | 0.89–1.32 | 0.42 |
| AYAs (>12 months)† | 2.09 | 1.59–2.75 | <0.001 |
| Older adults | 1.28 | 1.00–1.63 | 0.05 |
| Year of transplantation | |||
| 1990–1995 | 1.00 | - | 0.08* |
| 1996–2001 | 1.28 | 1.03–1.58 | 0.03 |
| 2002–2007 | 1.18 | 0.97–1.45 | 0.10 |
| Treatment related mortality | |||
| Age group | |||
| Children | 1.00 | - | <0.001* |
| AYAs | 1.66 | 1.42–1.95 | <0.001 |
| Older adults | 2.37 | 1.94–2.88 | <0.001 |
| Year of transplantation | |||
| 1990–1995 | 1.00 | - | <0.001* |
| 1996–2001 (≤ 4 months)† | 0.79 | 0.64–0.97 | 0.03 |
| 1996–2002 (>4 months)† | 1.28 | 0.96–1.71 | 0.09 |
| 2002–2007 (≤ 4 months)† | 0.42 | 0.34–0.52 | <0.001 |
| 2002–2007 (>4 months)† | 1.29 | 0.98–1.70 | 0.07 |
Overall P-value
Non-proportional hazards; hazard ratio differed by time since transplantation (e.g., ≤ 4 months or >4 months for overall survival and treatment related mortality)
Multivariable models adjusted for the following covariates: disease status, cell of origin, cytogenetic risk, and Karnofsky performance score at transplant
Multivariable models adjusted for the following covariates: disease status, cell of origin, cytogenetic risk, and Karnofsky performance score at transplant
Multivariable models adjusted for the following covariates: disease status
Multivariable models adjusted for the following covariates: cytogenetic risk, interval from diagnosis to transplant, and Karnofsky performance score at transplant
Figure 1.
5-year adjusted overall survival probabilities for each age group over time (the lines in the box represent survival probability and the ends of the box represent 95% confidence intervals)
For the entire cohort, late relapse rates (>12 months from HCT) for AYAs were higher than overall relapse rates for children (HR 2.1, p<0.001), whereas early relapse rates for AYAs were not significantly different than overall relapse rates for children (HR 1.1, p=0.42). The difference between overall relapse rates for older adults and those for children was of borderline significance (HR 1.3, p=0.05). Relapse rates following HCT were not significantly different in 2002–2007 when compared with 1990–1995 (HR 1.2, p=0.10).
An analysis of outcomes stratified by donor type for the three age cohorts in the most recent time period, 2002–2007, was also performed, with results presented in Table 4. In unadjusted outcomes, children maintained superior survival outcomes to AYAs and older adults, including recipients of both matched sibling and unrelated donor transplants. TRM was higher in AYAs and in older adults than in children for both types of transplants.
Table 4.
Unadjusted probability of outcomes by donor type and age group at 5-years after HCT for the most recent cohort (2002–2007)
| Outcome | Matched Sibling Donor | Unrelated Donor |
|---|---|---|
| Probability (95% CI) | Probability (95% CI) | |
| Overall survival | ||
| Children | 68 (56–81) | 56 (50–62) |
| AYAs | 48 (38–59) | 42 (37–47) |
| Older adults | 33 (24–46) | 37 (30–44) |
| Leukemia-free survival | ||
| Children | 61 (49–75) | 52 (46–58) |
| AYAs | 34 (34–54) | 38 (33–43) |
| Older adults | 31 (23–43) | 34 (28–41) |
| Relapse | ||
| Children | 34 (21–47) | 27 (22–32) |
| AYAs | 31 (22–41) | 31 (26–35) |
| Older adults | 29 (19–40) | 24 (18–30) |
| Treatment-related mortality | ||
| Children | 5 (1–13) | 21 (16–26) |
| AYAs | 25 (17–35) | 32 (27–37) |
| Older adults | 39 (28–50) | 42 (34–49) |
Abbreviations: AYAs – Adolescent and young adults; CI – confidence intervals
Differences in pediatric versus adult centers for AYAs
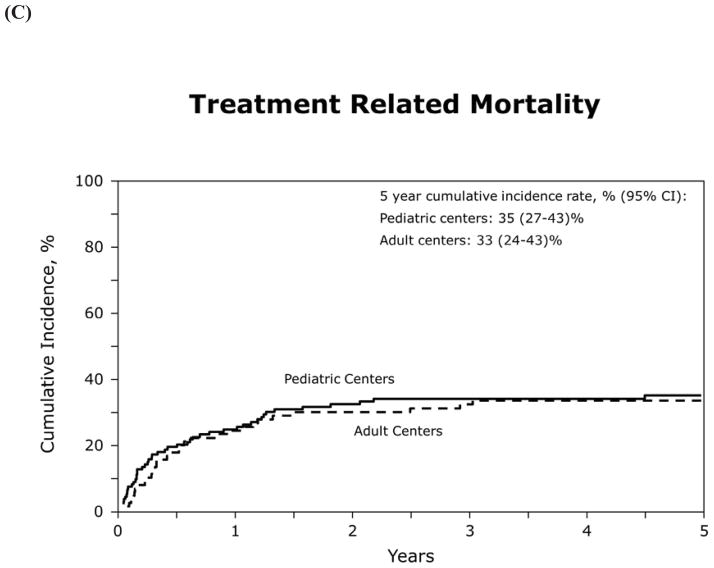
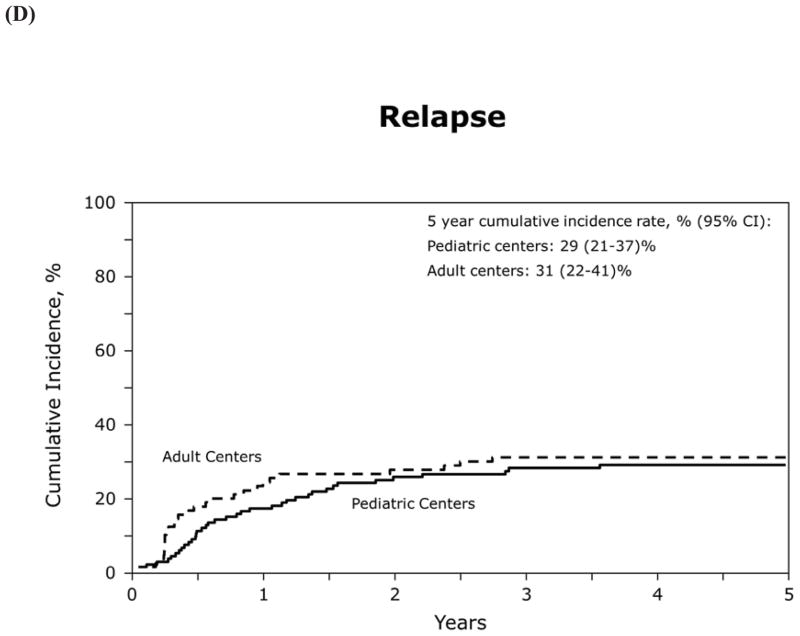
Table 5 shows transplant characteristics for 15–25 year old patients who underwent HCT at either a pediatric or adult transplant center. For this analysis there were 130 AYAs within this age group transplanted at 46 pediatric centers, and 92 AYAs transplanted at 49 adult centers. OS, LFS, relapse and TRM did not appear to differ by center type (Figure 2), but sample size precluded formal statistical comparison with adjustment for relevant patient and transplant characteristics.
Table 5.
Patient characteristics by center type (pediatric versus adult) for AYAs age 15–25 years who received a myeloablative allogeneic HCT between 2002 and 2007
| Characteristics | Pediatric Center | Adult Center | P-value |
|---|---|---|---|
| N(%) | N(%) | ||
| Number of patients | 130 | 92 | |
| Number of centers | 46 | 49 | |
| Age at HCT, years | <0.001 | ||
| 15–19 | 106 (82) | 22 (24) | |
| 20–25 | 24 (18) | 70 (76) | |
| KPS at HCT | 0.005 | ||
| ≥ 90 | 101 (78) | 61 (66) | |
| < 90 | 18 (14) | 28 (30) | |
| Disease status at HCT | 0.18 | ||
| CR1 | 46 (35) | 45 (49) | |
| CR2, CR1 duration <36 mos | 29 (35) | 36 (39) | |
| CR2, CR1 duration ≥ 36 mos | 18 (14) | 7 (8) | |
| CR2, CR1 duration unknown | 7 (5) | 4 (4) | |
| Interval from diagnosis to CR1, months | 0.003 | ||
| <1 | 54 (48) | 21 (25) | |
| 1–6 | 55 (49) | 55 (65) | |
| ≤ 6 | 4 (4) | 8 (10) | |
| Time from diagnosis to HCT, months | 0.019 | ||
| <6 | 26 (20) | 30 (33) | |
| 6–12 | 23 (18) | 22 (24) | |
| ≥ 12 | 81 (62) | 40 (43) | |
| Cytogenetic risk | 0.34 | ||
| High risk | 35 (27) | 17 (18) | |
| Normal | 33 (25) | 31 (34) | |
| Other | 35 (27) | 22 (24) | |
| Not tested/unknown | 27 (21) | 22 (24) | |
| Graft type | <0.001 | ||
| Bone Marrow | 74 (57) | 24 (26) | |
| Peripheral Blood | 56 (43) | 68 (74) | |
| HLA match | 0.11 | ||
| HLA-identical sibling | 21 (16) | 19 (21) | |
| Unrelated, well matched | 68 (52) | 49 (53) | |
| Unrelated, partially matched | 26 (20) | 22 (24) | |
| Unrelated, mismatched | 12 (9) | 1 (1) | |
| Unrelated, unknown degree of match | 3 (2) | 1 (1) | |
| Conditioning | 0.04 | ||
| TBI/Cy | 102 (78) | 63 (68) | |
| Cy/Bu | 4 (3) | 4 (4) | |
| TBI/etoposide | 15 (12) | 12 (13) | |
| TBI/other | 8 (6) | 5 (5) | |
| Other | 1 (1) | 8 (9) | |
| GVHD prophylaxis | <0.01 | ||
| CSA+ MTX +/− other | 53 (41) | 19 (21) | |
| FK506 + MTX +/− other | 35 (27) | 44 (48) | |
| T cell depletion | 21 (16) | 3 (3) | |
| Other | 21 (16) | 26 (28) |
Abbreviations: AYAs – Adolescent and young adults; KPS – Karnofsky performance status; HCT – hematopoietic cell transplantation; CR – complete remission; HLA – human leukocyte antigen; TBI – total body irradiation; Cy – cyclophosphamide; Bu – busulfan; GVHD – graft-versus-host disease; CSA – cyclosporine; MTX – methotrexate
Figure 2.
Outcomes for AYAs 15–25 years of age transplanted between 2002 and 2007
(A) overall survival
(B) leukemia-free survival
(C) treatment related mortality
(D) relapse
There were several differences between pediatric and adult centers in baseline patient characteristics and transplant techniques. AYAs of age 15–25 years at pediatric centers were more likely to have a high pre-HCT Karnofsky Performance Status (78% with KPS ≥ 90 in pediatric centers versus 66% in adult centers, p=0.005). In pediatric centers, patients had a shorter interval from diagnosis to CR1 (p=0.003) and had a longer time from diagnosis to transplant (p=0.02). AYAs transplanted at pediatric centers were more likely to receive bone marrow grafts than AYAs at adult centers (57% versus 26%, p<0.001). AYAs transplanted at pediatric centers were more likely to receive cyclosporine based graft-versus-host disease (GVHD) prophylaxis (41% versus 21%, p<0.01) and were more likely to receive cyclophosphamide/total body irradiation conditioning (78% versus 68%, p=0.04).
Discussion
Although survival improvements for AYAs with cancer in general have lagged behind children and older adults, we found that survival after transplantation for AYAs improved over time in parallel to younger patients and more favorably than older adults. The observation that survival improvements in AYAs did not lag behind other age groups is similar to findings from a recent study of outcomes following myeloablative transplantation for acute myeloid leukemia [Majhail N, 2012]. Further, although sample size precluded a formal comparison of outcomes for AYAs treated at pediatric versus adult transplant centers, in our study survival rates appeared similar despite differences in patient selection and transplantation techniques. Taken together, these data provide reassurance that AYAs with ALL seem to be benefiting from survival improvements in HCT in similar ways to their younger counterparts, and that treatment setting does not appear, at least preliminarily, to be a major determinant of outcome.
However, our study does demonstrate broader observations about the influence of increasing age upon outcomes following myeloablative transplantation for ALL. Across all time periods, children maintained a survival advantage over AYAs and older adults. Further, survival rates did not appear to improve in the older adult group over time. It appears that some of the survival improvement over time in the younger age groups was attributable to lower rates of TRM, especially in the early post-transplant period. These data are consistent with larger trends in improvements in supportive care leading to decreased TRM following allogeneic HCT in general [Gooley T, 2010; Hahn T, 2013]. In the most recent time period, TRM remained higher for AYAs and for older adults than for children, including recipients of matched sibling donor transplants. This observation highlights the continued important contribution of TRM to outcomes following myeloablative transplantation in AYAs and older adults, even in the modern transplant era. In this study, we were not able to analyze outcomes of GVHD or other potential contributors to TRM, which needs to be addressed by future research. Another important observation was the lack of reduction over time in relapse rates for any age group. This highlights the need for more research to investigate novel methods to prevent relapse in these high risk patients.
The observation that TRM is an important determinant of survival following myeloablative HCT for ALL is consistent with published data from large controlled trials. In the MRC/ECOG study, the difference in survival between the donor and no-donor groups was significant only in standard-risk patients because of the higher TRM (36%) in the high-risk patients undergoing transplantation [Goldstone AH, 2008]. In this study, risk was defined in part by age greater than or less than 35. The HOVON study did not categorize risk by age in the same way as the MRC/ECOG study, but the authors did conclude that the greatest benefit of myeloablative HCT for ALL in first CR was likely to be seen when TRM rates were less than 20% [Cornelissen JJ, 2009]. An individual patient data meta-analysis that included both of the above studies concluded that HCT for ALL in first CR was beneficial only in patients younger than 35 years of age because of higher rates of TRM in older patients [Gupta V, Blood 2013]. Within clinical trials, the reasons for differences in TRM as a function of age are not entirely known and may relate to disease-related and age-related biology.
In contrast to the above cited studies based on randomized controlled trials, our observational study also highlights significant practice variation in transplantation techniques for ALL. In pediatric versus adult treatment settings, we found differences in the characteristics of patients being transplanted and the type of conditioning regimen, stem cell source, and GVHD prophylaxis used. These are all key elements of the clinical practice of HCT. Our study was not designed to assess the impact of these differences on outcomes. While superficially these differences did not appear to impact the outcomes of 15–25 year old AYAs undergoing transplant, larger studies would be needed to confirm this observation. Whether these differences in practice patterns between pediatric and adult centers have any impact more generally on outcomes following HCT for ALL is not known. For example, characteristics of how patients come to transplant at pediatric versus adult centers (time to CR1, time from diagnosis to transplant) may impact relapse rates following transplantation, particularly if, over time, reduced intensity transplantation regimens are used with increasing frequency in adult settings. As another example, while marrow grafts are used more frequently in pediatric settings, perhaps because of the higher proportion of non-malignant diseases transplanted in pediatric centers, marrow versus peripheral blood use may affect post-transplant graft-versus-leukemia or GVHD rates in patients transplanted for ALL [Anasetti C, 2012]. Additionally, the distinction between pediatric and adult treatment programs is only one variable that impacts practice patterns [Lee SJ, 2008]. Whether outcomes are influenced by center-specific differences in transplant techniques among adult centers or among pediatric centers is also not known.
Our study does have several limitations inherent in a retrospective analysis with registry-level data. We were unable to address issues related to access to HCT, or issues related to caregiver support, financial resources, medication and supportive care adherence, or other factors that may influence outcomes following HCT for AYAs and other age groups. We were also unable to address the issue of confounding due to selection, including the possibility that differences in explicit or implicit criteria for transplantation might differ by age group. For example, it is possible that younger patients may have been more likely to have adverse prognostic features at the time of transplantation than older patients, given differences in practice patterns in the pediatric versus adult settings. Finally, systematic differences by age group in pre-transplant treatment might have affected relative outcomes among patients who were included in this analysis. Differences in pre-HCT therapy could conceivably affect TRM, as pediatric patients in our study had higher pre-HCT KPS scores than AYA and adult patients, and the relative contributions of differences in pre-HCT therapy vs host biological differences to this finding are not readily discernible with our data. Differences in pre-HCT therapy could also contribute to relapse rates. Some of the patients in the AYA group were likely treated on modified pediatric protocols and others on adult protocols, and we were not able to determine which patients were treated on which pre-HCT protocols with our available data.
Moving forward, additional studies will be needed to better understand reasons for persistent differences in late TRM in relationship to increasing patient age. The impact of conditioning regimen intensity on TRM and overall survival for comparable patients, the subject of an ongoing multicenter trial [NCT01339910], also requires clarification. A retrospective CIBMTR study of patients with Philadelphia chromosome negative ALL transplanted in first or second complete remission suggested similar age-adjusted survival after reduced intensity or full intensity conditioning [Marks DI, 2010]. In parallel, a more precise understanding of relapse risk as a function of pre-HCT “adult-like” or “pediatric-like” chemotherapy is also needed. After these issues are further clarified, individualized pre-HCT calculators of TRM and relapse risk may become possible, similar to the recent development of post-HCT calculators [Lee SJ, 2013], in turn facilitating the personalized application of transplant strategies for this disease.
In conclusion, our study shows that improvements in survival among AYAs undergoing allogeneic HCT for ALL parallel those seen among younger patients, and are more favorable than those among older adults. However, our study also demonstrates persistent survival disparities across increasing age groups that warrant further study.
Acknowledgments
CIBMTR Sources of Support:
The Center for International Blood and Marrow Transplant Research (CIBMTR) is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Conflict-of-interest disclosure: The authors have no interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bleyer A. Young adult oncology: the patients and their survival challenges. CA Cancer J Clin. 2007;57:242–255. doi: 10.3322/canjclin.57.4.242. [DOI] [PubMed] [Google Scholar]
- 2.Wood WA, Lee SJ. Malignant hematologic diseases in adolescents and young adults. Blood. 2011;117:5803–5815. doi: 10.1182/blood-2010-12-283093. [DOI] [PubMed] [Google Scholar]
- 3.Stock W, La M, Sanford B, et al. What determines the outcomes for adolescents and young adults with acute lymphoblastic leukemia treated on cooperative group protocols? A comparison of Children’s Cancer Group and Cancer and Leukemia Group B studies. Blood. 2008;112:1646–1654. doi: 10.1182/blood-2008-01-130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stock W. Adolescents and young adults with acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2010;2010:21–29. doi: 10.1182/asheducation-2010.1.21. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian HM, O’Brien S. Insurance policies in the United States may explain part of the outcome differences of adolescents and young adults with acute lymphoblastic leukemia treated on adult versus pediatric regimens. Blood. 2009;113:1861. doi: 10.1182/blood-2008-10-186718. [DOI] [PubMed] [Google Scholar]
- 6.Schafer ES, Hunger SP. Optimal therapy for acute lymphoblastic leukemia in adolescents and young adults. Nature Reviews Clinical Oncology. 2011;8:417–424. doi: 10.1038/nrclinonc.2011.77. [DOI] [PubMed] [Google Scholar]
- 7.Burke MJ, Gossai N, Wagner JE, et al. Survival differences between adolescents/young adults and children with B precursor acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2013;19:138–42. doi: 10.1016/j.bbmt.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adolescent and Young Adult Oncology Progress Review Group. Closing the gap: research and care imperatives for adolescents and young adults with cancer. Department of Health and Human Services, National Institutes of Health, National Cancer Institute, and the LiveStrong Young Adult Alliance; Bethesda, MD: 2006. NIH Publication No. 06-6067. Available at http://planning.cancer.gov/library/AYAO_PRG_Report_2006_FINAL.pdf. [Google Scholar]
- 10.Moorman AV, Ensor HM, Richards SM, et al. Prognostic effect of chromosomal abnormalities in childhood B cell precursor acute lymphoblastic leukemia: results from the UK Medical Research Council ALL 97/99 randomized trial. Lancet Onc. 2010;11:429–438. doi: 10.1016/S1470-2045(10)70066-8. [DOI] [PubMed] [Google Scholar]
- 11.Moorman AV, Harrison CJ, Buck GA, et al. Karyotype is an independent prognostic factor in adult acute lymphoblastic leukemia (ALL): analysis of cytogenetic data from patients treated on the Medical Research Council (MRC) UKALL XII/Eastern Cooperative Oncology Group (ECOG) 2993 trial. Blood. 2007;109:3189–3197. doi: 10.1182/blood-2006-10-051912. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1985;53:457–481. [Google Scholar]
- 13.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Majhail NS, Brazauskas R, Hassebroek A, et al. Outcomes of allogeneic hematopoietic cell transplantation for adolescents and young adults compared with children and older adults with acute myeloid leukemia. Biol Blood Marrow Transplant. 2012;18:861–873. doi: 10.1016/j.bbmt.2011.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gooley TA, Chien JW, Pergam SA, et al. Reduced mortality after allogeneic hematopoietic-cell transplantation. N Engl J Med. 2010;363:2091–101. doi: 10.1056/NEJMoa1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hahn T, McCarthy PL, Hassebroek A, et al. Significant improvement in survival after allogeneic hematopoietic cell transplantation during a period of significantly increased use, older recipient age, and use of unrelated donors. J Clin Oncol. 2013;31:2437–49. doi: 10.1200/JCO.2012.46.6193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993) Blood. 2008;111:1827–1833. doi: 10.1182/blood-2007-10-116582. [DOI] [PubMed] [Google Scholar]
- 18.Cornelissen JJ, van der Holt B, Verhoef GE, et al. Myeloablative allogeneic versus autologous stem cell transplantation in adult patients with acute lymphoblastic leukemia in first remission: a prospective sibling donor versus no-donor comparison. Blood. 2009;113:1375–1382. doi: 10.1182/blood-2008-07-168625. [DOI] [PubMed] [Google Scholar]
- 19.Gupta V, Richards S, Rowe J, et al. Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013;121:339–350. doi: 10.1182/blood-2012-07-445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anasetti C, Logan BR, Lee SJ, et al. Peripheral-blood stem cell versus bone marrow from unrelated donor. N Engl J Med. 2012;367:1487–1496. doi: 10.1056/NEJMoa1203517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SJ, Astigarraga CC, Eapen M, et al. Variation in supportive care practices in hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14:1231–8. doi: 10.1016/j.bbmt.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. [Accessed 11/19/2013]; http://www.clinicaltrials.gov/ct2/show/NCT01339910.
- 23.Marks DI, Wang T, Perez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2012;116:366–374. doi: 10.1182/blood-2010-01-264077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SJ, Storer B, Wang H, et al. Providing personalized prognostic information for adult leukemia survivors. Biol Blood Marrow Transplant. 2013;19:1600–1607. doi: 10.1016/j.bbmt.2013.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]