Abstract
Beclin 1 has a well-established role in regulating autophagy, a cellular degradation pathway. Although the yeast ortholog of beclin 1 (Atg6/Vps30) was discovered to also regulate vacuolar protein sorting nearly 30 years ago, the varied functions of beclin 1 in mammalian cells are only beginning to be sorted out. We recently described a role for beclin 1 in regulating recycling of phagocytic receptors in microglia, a function analogous to that of its yeast ortholog. Microglia lacking beclin 1 have a reduced phagocytic capacity, which impairs clearance of amyloid β (Aβ) in a mouse model of Alzheimer’s Disease (AD). Here we summarize these findings and discuss the implications for beclin 1-regulated receptor recycling in neurodegenerative disease.
Keywords: Beclin 1, phosphatidylinositol 3-phosphate, PI3P, phosphatidylinositol 3-kinase, PI3K, VPS35, retromer, receptor recycling, phagocytosis, Alzheimer’s Disease, neurodegeneration
Beclin 1: a multi-functional protein
Beclin 1, originally identified in mammals as a Bcl-2 interacting protein (Liang et al. 1998), plays a role in several key cellular pathways including autophagy and protein sorting, and has been implicated in such diverse processes as development, innate immunity, tumor suppression, and neuroprotection (Deretic and Levine 2009; Salminen et al. 2013; Yue et al. 2003). While the role of beclin 1 in autophagy is well established, its other functions are less widely appreciated. Beclin 1 is a 52 kDa protein containing a BH3 domain, a coiled-coil domain, and a C-terminal evolutionarily conserved domain. These domains mediate protein-protein interactions which allows beclin 1 to act as a scaffold to bring together a variety of binding partners (He and Levine 2010). It is a core component of the type III phosphatidylinositol-3-kinase (PI3K) complex, along with the PI3K Vps34 and its regulatory protein kinase Vps15 (Itakura et al. 2008; Kihara et al. 2001). This complex generates phosphatidylinositol-3-phosphate (PI3P) in vesicle membranes, an important phospholipid involved in membrane trafficking. Through its interaction with additional binding partners (discussed below), beclin 1 localizes PI3P production to specific membrane compartments. It is this function that allows beclin 1 to regulate the membrane trafficking events in numerous cellular pathways.
Beclin 1 regulates autophagy initiation
Autophagy is a bulk degradation pathway by which cytoplasmic cargo is engulfed in double-membrane vesicles called autophagosomes. Autophagosomes then fuse with lysosomes, resulting in cargo degradation. Autophagy is induced under a variety of stressful conditions including nutrient starvation, oxidative stress, and neuronal excitotoxicity. This process serves to clear long-lived proteins, aggregated protein, and damaged organelles, such as mitochondria (Murrow and Debnath 2013; Shacka et al. 2007). The molecular machinery that orchestrates the sequential steps of vesicle initiation, elongation, fusion and degradation in autophagy was originally defined in yeast (Tsukada 1993). There are now more than 30 known autophagy related genes (i.e, Atg, also known as Apg), many of which are conserved from yeast to mammals (Yang and Klionsky 2010).
Beclin 1 helps initiate autophagy by promoting vesicle nucleation (Suzuki et al. 2001). Cells lacking Atg6, the yeast ortholog of beclin 1, fail to accumulate autophagic vesicles under nutrient starvation (Kametaka 1998). This function is highly conserved, as loss of beclin 1 orthologs in plants (Fujiki et al. 2007), C. elegans (Meléndez et al. 2003), and mammals, such as mice and human cells, inhibits autophagy (Liang et al. 1999a; Yue et al. 2003). Beclin 1 serves to localize PI3P production at nascent autophagosomes through its interaction with Atg14 (Obara et al. 2006; Sun et al. 2008). Disrupting the interaction between beclin 1 and Atg14 is sufficient to inhibit autophagy, indicating that this local generation of PI3P is critical for autophagy initiation. A more in-depth discussion of the beclin 1 interactome and its role in autophagy can be found in He and Levine 2010.
The role of beclin 1 in autophagy is thought to be important for a number of diseaserelated processes. Autophagy plays a fundamental role in immunity by sequestering and degrading intracellular pathogens (Deretic and Levine 2009). For example, Beclin 1 protects mouse neurons from infection with Sindbis virus (Liang et al. 1998), and numerous pathogens express proteins that target beclin 1 to inhibit their clearance by autophagy (Deretic and Levine 2009). Beclin 1-mediated autophagy is also involved in tumor suppression. Beclin 1 haploinsufficiency is associated with many human cancers, particularly breast cancers (Liang et al. 1999b), and mice deficient for beclin 1 develop tumors at a higher rate than their wild type littermates (Yue et al. 2003).
Autophagy is also implicated in neuronal homeostasis. Deletion of various autophagy proteins results in progressive neurodegeneration, featuring the accumulation of ubiquitinpositive inclusions and neuronal apoptosis (Hara et al. 2006; Komatsu et al. 2006). Furthermore, autophagic vesicles accumulate in AD, indicating a perturbation in this pathway (Nixon et al. 2005). Studies from our lab revealed that levels of beclin 1 are decreased in AD brain tissue. When amyloid precursor protein (APP) overexpressing mice were crossed with beclin 1 deficient mice, we observed enhanced Aβ deposition and synaptic loss, two key pathological features of the disease (Pickford et al. 2008). Indeed, knockdown of beclin 1 in cultured neurons increases levels of both total APP and Aβ (Jaeger et al. 2010; Tian et al. 2011). Conversely, increasing beclin 1 levels protects neurons in mouse models of both AD and Parkinson’s Disease (Pickford et al. 2008; Spencer et al. 2009). These data suggest an important role for beclin 1-mediated functions in protecting against neurodegeneration.
Beclin 1-mediated protein sorting: not just for yeast?
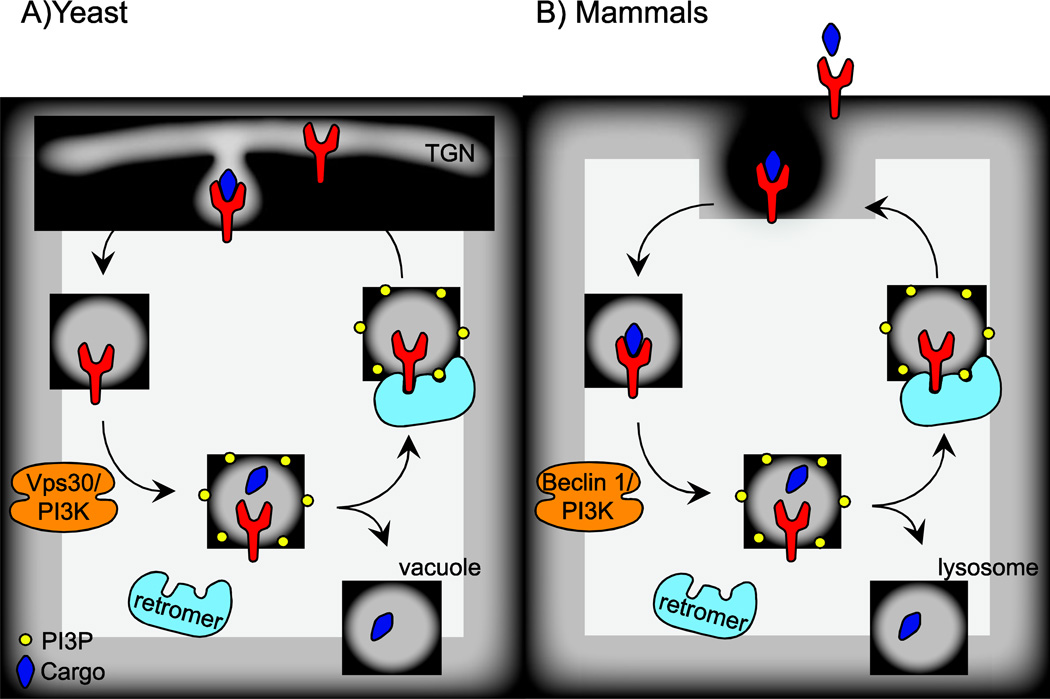
The yeast ortholog of beclin 1 was first described as a regulator of vacuolar protein sorting. Lysosomes (or vacuoles in yeast) contain acid hydrolases that degrade vesicular cargo. These hydrolases are synthesized in the secretory pathway and are sorted away from material that is delivered to the cell surface at the trans-Golgi network (TGN). Briefly, hydrolases bind to receptors in the TGN and are packaged into endosomes. As the endosomes acidify, the hydrolases disengage from their receptors and are delivered to the lysosome where they are processed to mature forms. The receptors are then recycled back to the TGN for further rounds of sorting (Fig 1a). In the absence of functional vacuolar protein sorting, hydrolases are secreted in an immature form. The machinery involved in vacuolar protein sorting (Vps) was defined in two seminal studies in yeast (Robinson et al. 1988; Rothman and Stevens 1986). Mutant libraries were screened for cells that secrete immature forms of the hydrolases carboxypeptidase Y (CPY), proteinase A and proteinase B, and more than 50 genes involved in vacuolar protein sorting were identified. Subsequent studies investigating the central mechanisms of vacuolar protein sorting determined that a PI3K complex containing Vps30 (Atg6/beclin 1), Vps34, Vps15, and Vps38, is required for PI3P formation on sorting endosomes (Kihara et al. 2001; Schu et al. 1993). Vps30/beclin 1 interacts with both Vps38 and Atg14 through its coiled-coil domain, forming mutually exclusive complexes with these two proteins. This interaction therefore acts as a switch, specifying whether beclin 1 and the PI3K complex will participate in autophagy (via Atg14) or protein sorting (via Vps38). Generation of PI3P in the membrane of the sorting endosome recruits the retromer complex, composed of Vps35, Vps29, Vps26, and a pair of sorting nexins. The retromer complex binds both PI3P and the hydrolase receptor, and sorts the receptor back to the TGN (Burda 2002; Paravicini et al. 1992; Reddy and Seaman 2001; Seaman et al. 1997).
Figure 1. Beclin 1 recruits the retromer complex to recycle receptors.
a) In yeast, hydrolase receptors such as Vps10, bind their cargo in the TGN for delivery to the vacuole. Vps30, the yeast ortholog of beclin 1, and the PI3K complex (containing Vps34 and Vps15) generates PI3P on the endosomal membrane, which recruits the retromer. While the cargo traffics to the vacuole, the retromer recycles the receptor back to the TGN for further rounds of sorting. b) In mammals, cell surface receptors such as the microglial scavenger receptor CD36 bind extracellular cargo and are endocytosed. Beclin 1 and the PI3K rapidly recruit the retromer to the endosome via PI3P production. The retromer then sorts the receptor to a recycling endosome and ultimately back to the cell surface. Meanwhile, cargo is delivered to the lysosome for degradation.
Mammalian cells employ a similar system to deliver hydrolases, such as the cathepsins, to the lysosome. Hydrolases are modified by the addition of mannose-6-phosphate (M6P) residues in the TGN. Receptors for M6P then sort these hydrolases to the lysosome and are recycled in a retromer-dependent manner for additional rounds of sorting (Kornfeld and Mellman 1989; Seaman 2004). Beclin 1, however, is not required for proper lysosomal hydrolase sorting in mammals, as cells lacking beclin 1 contain mature cathepsin D in the lysosome (Furuya et al. 2005). Furthermore, expression of human beclin 1 in a Δvps30 yeast strain rescues the autophagy defect in these cells, but not the missorting of CPY (Liang et al. 1999a), suggesting that mammalian beclin 1 is not required for vacuolar or lysosomal protein sorting. This may reflect an inability of mammalian beclin 1 to interact with the protein-sorting specific PI3K complex component Vps38. Until recently, these data were collectively held as evidence that beclin 1 does not function in protein sorting in mammalian cells. Work from our lab, described below, challenges this idea, and indicates that beclin 1 does indeed play a role in protein sorting in mammalian cells, recycling receptors, not between the lysosome and TGN, but rather at the cell surface.
Intersecting roles of beclin-mediated vesicle trafficking and protein sorting in phagocytosis
Phagocytosis is the process by which extracellular material is engulfed and degraded by cells, namely immune cells such as macrophages and microglia. As such it plays a key role in eliminating pathogens and controlling inflammation by clearing extracellular debris. Once internalized, phagosomes enter the endosomal trafficking system and eventually fuse with lysosomes, a feature phagocytosis shares with autophagy. A key difference is that phagocytosis engulfs extracellular material in single-membrane vesicles, while autophagy engulfs intracellular material in double-membrane vesicles.
Despite these differences, there seems to be considerable crosstalk and shared machinery between these pathways. The microtubule associated protein light chain 3 (LC3) is lipidated and conjugated to membranes during autophagy initiation and has been extensively used as a specific marker for autophagosomes. Recently, however, LC3-associated phagocytosis, or LAP, has been described based on the colocalization of LC3 with phagocytosed yeast, bacteria, and apoptotic cells (Li et al. 2013; Martinez et al. 2011; Sanjuan et al. 2007). LC3 is likely conjugated directly to phagosomes, as fusion between phagosomes and autophagosomes has not been observed. LAP is dependent on Atg5 and Atg7, both part of the LC3-conjugation machinery, as well as the PI3K Vps34 and beclin 1. In fact, the appearance of LC3 on phagosomes follows a similar sequence of events as that seen at the early autophagosome: association of beclin 1, PI3P production, and finally LC3-membrane association (Sanjuan et al. 2007; Suzuki et al. 2007). The beclin 1-PI3K complex is also recruited to phagosomes containing the microbial sensor SLAM, which in turn recruits NOX2 to regulate radical oxygen production and bacterial killing (Berger et al. 2012). In addition to ROS production, PI3P likely plays a role in phagosome maturation (Vieira et al. 2001), though the precise mechanisms by which PI3P regulates this process are not fully understood.
Phagocytosis represents an intriguing crossroads for the two main functions of beclin 1: regulation of vesicle-mediated degradation and protein sorting, through its role in PI3P generation. Phagocytic receptors, like many cell surface receptors, are recycled back to the surface after ligand binding and uptake. Previous work in C. elegans demonstrated a role for retromer in phagocytosis (Chen et al. 2010) as well as a requirement for the beclin 1 homolog bec-1 in apoptotic cell clearance and recycling of the retromer cargo MIG-14/Wntless (Ruck et al. 2011). In mammalian cells, beclin 1 knockdown inhibits apoptotic cell engulfment (Konishi et al. 2012; Qu et al. 2007), and the retromer clearly plays a role in trafficking of receptors at the cell surface (Feinstein et al. 2011; Temkin et al. 2011a; Vergés et al. 2004). However, these data did not clearly establish a role for beclin 1-mediated retromer recruitment in phagocytic receptor recycling in invertebrates or in mammalian cells.
Decreased Beclin 1 levels in Alzheimer Disease alters microglial activation
Alzheimer Disease is an age-dependent neurodegenerative disorder resulting in loss of cognitive function. It is characterized by aberrant accumulation of the APP-derived amyloid β peptide in extracellular plaques, as well as by hyperphosphorylation of the microtubule-associated protein tau and its accumulation in intracellular tangles. Microglial activation and neuroinflammation are other prominent features of the disease. Microglia are capable of binding aggregated Aβ via various receptors, including the type A and type B scavenger receptors. The binding of Aβ by microglia initiates phagocytosis and promotes the degradation of Aβ (Wilkinson and El Khoury 2012). Microglia are often observed surrounding plaques in the brains of patients, as well as in AD mouse models (Bolmont et al. 2008; Meyer-Luehmann et al. 2008; Mrak 2012). While microglial activation early in disease progression may help to prevent Aβ accumulation, recent evidence suggests that altered microglial function may drive pathogenesis at later stages of disease (Czirr and Wyss-Coray 2012; Griciuc et al. 2013; Guerreiro et al. 2013; Jonsson et al. 2013).
Several years ago, our lab observed that levels of beclin 1 are decreased in cortical brain extracts of AD patients. Mimicking this reduction, heterozygous beclin 1 deficiency in APP mice resulted in not only synaptic loss and enhanced Aβ deposition, but also increased microglial activation (Pickford et al. 2008). In our most recent work, we hypothesized that loss of beclin 1, in addition to inhibiting autophagic clearance of Aβ within neurons, may also impair microglial phagocytosis, further contributing to Aβ accumulation (Lucin et al. 2013). Using both the microglial cell line BV2 infected with control or beclin 1 shRNA lentivirus and primary microglia from heterozygous beclin 1 deficient mice, we observed that loss of beclin 1 decreased phagocytosis of latex beads by flow cytometry and live-cell imaging. Furthermore, BV2 cells with decreased beclin 1 levels cleared less Aβ when cultured on brain slices from APP transgenic mice as measured by immunohistochemistry and ELISA. Additionally, amyloid fibrils injected into mouse frontal cortex were cleared more efficiently in wild type than in beclin 1 deficient mice. Together these results suggest that beclin 1 regulates phagocytosis, and that impaired microglial phagocytosis may contribute to Aβ accumulation in AD.
Intriguingly, in our time-lapse experiments using beclin 1 deficient microglia, we observed the greatest impairment of phagocytosis at later time points. This suggested that beclin 1 is not essential for initiating phagocytosis, but rather for sustaining phagocytosis. Given that phagocytic receptors recycle back to the cell surface after ligand binding, and that beclin 1 regulates receptor recycling in lower organisms, we hypothesized that beclin 1 might be regulating recycling of phagocytic receptors at the cell surface. We measured recycling of the scavenger receptor CD36, which is known to bind Aβ (El Khoury et al. 2003), and found that beclin 1 depletion resulted in impaired recycling of this receptor in both BV2 cells and primary microglia. Beclin 1-mediated receptor recycling in microglia, like that initially described in yeast, relies on PI3K activity and retromer recruitment. Knockdown of beclin 1 impairs PI3P production and Vps35 recruitment to phagosomes, although we observed no change in phagosomal pH suggesting phagosomal maturation was not affected.
We next investigated whether beclin 1-mediated impairments of the retromer were responsible for the phagocytic receptor recycling defects we observed. Knockdown of the retromer component Vps35 directly inhibited receptor recycling and phagocytosis, while overexpression of Vps35 in the context of beclin 1 knockdown rescued defects in these processes. These data indicate that the same sequence of events, involved in receptor recycling between the TGN and vacuole in yeast, namely PI3P production by the beclin1-PI3K complex and subsequent retromer recruitment, occur in mammals to regulate recycling of receptors at the plasma membrane (Fig 1b). Importantly, loss of beclin 1 in both BV2 cells and in brain tissue from AD patients is accompanied by decreases in components of the retromer complex, indicating microglial phagocytosis is likely impaired in AD, possibly driving further Aβ accumulation and inflammation.
Our results clearly indicate a role for beclin 1-mediated protein sorting at the plasma membrane in mammalian cells. It is likely, however, that beclin 1 regulates only a subset of cell surface receptors. In fact, previous work found no effect of beclin 1 knockdown on the downregulation of the epidermal growth factor receptor (Zeng et al. 2006). Furthermore, there is evidence of both retromer-dependent (Temkin et al. 2011b) and retromer-independent (Nisar et al. 2010) receptor recycling at the plasma membrane. These studies, however, did not examine the role of beclin 1 in receptor recycling. Future studies will need to identify the repertoire of cell surface receptors that beclin 1 regulates and determine if beclin 1 and the retromer always act in concert to regulate recycling.
Implications for beclin 1-mediated protein sorting in neurodegeneration
Loss of beclin 1 in AD appears to have three separate consequences: impairment of phagocytosis, autophagy, and protein sorting, all of which can contribute to pathogenesis. Altered protein sorting has recently been implicated in AD. Amyloid precursor protein binds to members of the Vps10p receptor family, such as SorLA, sortillin, and SorCs, which mediates APP trafficking through the endosomal system (Spoelgen et al. 2006). The Vps10 receptor family proteins are well-known retromer cargo and SorLA has been shown to interact directly with the retromer (Fjorback et al. 2012). As processing of APP into Aβ fragments by β-secretase occurs within endosomes, factors that alter APP trafficking and prolong the time it spends within these compartments can enhance Aβ production. Variants in both SorLA and SorCs have been linked to AD, indicating a potential role for impaired altered protein sorting in pathogenesis (Lane et al. 2010; Liang et al. 2009; Rogaeva et al. 2007). Overexpression of SorLA in cell culture reduces Aβ production, while levels of this protein are decreased in AD brain, suggesting that loss of SorLA may impair APP trafficking and enhance Aβ production in AD (Andersen et al. 2005). Alterations in the retromer itself have also been implicated in AD. Vps35, which can regulate APP processing, is decreased in disease-relevant brain regions in AD (Small et al. 2005). Deletion of retromer components in both fly and mouse models enhances Aβ production, similar to our observations in beclin 1 deficient mice (Muhammad et al. 2008).
Given the relationship between the retromer and PI3P, one would expect that altering PI3P levels would have similar effects on APP trafficking and Aβ production as well. Indeed, inhibition of PI3P production increases the localization of APP in endosomes and increases Aβ levels. Moreover, levels of PI3P are decreased in AD brain tissue, suggesting yet another step in the protein sorting process may be impaired (Morel et al. 2013). Even in the absence of exogenous APP expression, conditional deletion of the type III PI3 kinase in sensory neurons is sufficient to cause rapid neurodegeneration (Zhou et al. 2010). Taken together, these data indicate that alterations in any of the machinery involved in beclin 1-mediated protein sorting (beclin 1, the PI3K, or the retromer) can contribute to Aβ accumulation and neurodegeneration.
Data linking variants of Vps35 to Parkinson’s Disease (Vilariño-Güell et al. 2011; Zimprich et al. 2011) may indicate a broader role of altered protein sorting in neurodegeneration. Cells express many plasma membrane receptors that mediate their interaction with the environment and alterations in the profile of these receptors may drastically alter cell function. Microglia constantly survey their microenvironment in the resting state, and can adopt one of two activation states: the classical, pro-inflammatory M1 state, and the alternative, phagocytic or anti-inflammatory M2 state. Changes in surface expression of microglial receptors could alter the ability of microglia to sense their environment and become activated. It could also potentially shift the balance between activation states, driving microglia into a more pro- or anti-inflammatory state.
If similar receptor recycling mechanisms exist in neurons, beclin 1 deficiency could have broad implications. Neurons express a wide variety of neurotransmitter receptors, ion channels, and growth factor receptors, all of which contribute to neuronal function and health. Changes in surface expression of any of these may drastically alter neural function and could potentially contribute to neurodegeneration. The consequences of altered protein sorting in the brain will depend on the repertoire of receptors that beclin 1 and the retromer regulate. It will therefore be important to characterize the subset of receptors whose surface expression is modulated by beclin 1 and the retromer. Steinberg et al. recently published a mass spectrometry-based screen of receptors whose surface expression changes between cells with and without Vps35 knockdown (Steinberg et al. 2013). This study found changes in surface expression of 152 receptors upon Vps35 knockdown, including the glucose transporter GLUT1, several receptors in from the tumor necrosis factor (TNFR) family, and the interleukin-6 receptor β (IL6RB). These results must be validated and expanded to include different cell types. Future studies must also profile the changes in surface expression of receptors in both neurons and microglia from AD patients compared to healthy individuals so that we may better understand the full impact of altered protein sorting in disease.
Conclusions
Our recent work demonstrates a role for beclin 1 in regulating sorting of cell surface receptors in mammalian cells. Decreased levels of beclin 1 in microglia inhibit recycling of the phagocytic receptor CD36 and impair phagocytosis. Beclin 1, like its yeast ortholog Vps30, promotes PI3P production at specific membranes (i.e. the phagosome during phagocytosis) and recruits the retromer complex to promote receptor recycling. Reduced beclin 1 levels in AD likely contribute to neurodegeneration in part by impairing microglial clearance of Aβ. Uncovering the set of receptors regulated by beclin 1-mediated protein sorting may help identify additional mechanisms underlying neurodegeneration and offer new potential targets for therapy.
Footnotes
The authors declare that they have no conflict of interest.
References
- Andersen OM, Reiche J, Schmidt V, Gotthardt M, Spoelgen R, Behlke J, von Arnim CaF, Breiderhoff T, Jansen P, Wu X, Bales KR, Cappai R, Masters CL, Gliemann J, Mufson EJ, Hyman BT, Paul SM, Nykjaer A, Willnow TE. Neuronal sorting protein-related receptor sorLA/LR11 regulates processing of the amyloid precursor protein. Proc Natl Acad Sci U S A. 2005;102:13461–13466. doi: 10.1073/pnas.0503689102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger S, Romero X, Ma C, Guoxing W, Faubian W, Liao G, Compeer E, Keszei M, Rameh L, Wang N. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat Immunol. 2012;11:920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolmont T, Haiss F, Eicke D, Radde R, Mathis Ca, Klunk WE, Kohsaka S, Jucker M, Calhoun ME. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda P. Retromer function in endosome-to-Golgi retrograde transport is regulated by the yeast Vps34 PtdIns 3-kinase. J Cell Sci. 2002;115:3889–3900. doi: 10.1242/jcs.00090. [DOI] [PubMed] [Google Scholar]
- Chen D, Xiao H, Zhang K, Wang B, Gao Z, Jian Y, Qi X, Sun J, Miao L, Yang C. Retromer is required for apoptotic cell clearance by phagocytic receptor recycling. Science. 2010;327:1261–1264. doi: 10.1126/science.1184840. [DOI] [PubMed] [Google Scholar]
- Czirr E, Wyss-coray T. Review series The immunology of neurodegeneration. 2012 doi: 10.1172/JCI58656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein TN, Wehbi VL, Ardura Ja, Wheeler DS, Ferrandon S, Gardella TJ, Vilardaga J-P. Retromer terminates the generation of cAMP by internalized PTH receptors. Nat Chem Biol. 2011;7:278–284. doi: 10.1038/nchembio.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjorback AW, Seaman M, Gustafsen C, Mehmedbasic A, Gokool S, Wu C, Militz D, Schmidt V, Madsen P, Nyengaard JR, Willnow TE, Christensen EI, Mobley WB, Nykjær A, Andersen OM. Retromer binds the FANSHY sorting motif in SorLA to regulate amyloid precursor protein sorting and processing. J Neurosci. 2012;32:1467–1480. doi: 10.1523/JNEUROSCI.2272-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiki Y, Yoshimoto K, Ohsumi Y. An Arabidopsis homolog of yeast ATG6/VPS30 is essential for pollen germination. Plant Physiol. 2007;143:1132–1139. doi: 10.1104/pp.106.093864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya N, Yu J, Byfield M, Pattingre S, Levine B. The Evolutionarily Conserved Domain of Beclin 1 is Required for Vps34 binding, Autophagy and Tumor Supressor function. Autophagy. 2005;1:46–52. doi: 10.4161/auto.1.1.1542. [DOI] [PubMed] [Google Scholar]
- Griciuc A, Serrano-Pozo A, Parrado AR, Lesinski AN, Asselin CN, Mullin K, Hooli B, Choi SH, Hyman BT, Tanzi RE. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, Carrasquillo M, Rogaeva E, Majounie E, Cruchaga C, Sassi C, Kauwe JSK, Younkin S, Hazrati L, Collinge J, Pocock J, Lashley T, Williams J, Lambert J-C, Amouyel P, Goate A, Rademakers R, Morgan K, Powell J, St George-Hyslop P, Singleton A, Hardy J. TREM2 variants in Alzheimer’s disease. N Engl J Med. 2013;368:117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara T, Nakamura K, Matsui M, Yamamoto A, Nakahara Y, Suzuki-Migishima R, Yokoyama M, Mishima K, Saito I, Okano H, Mizushima N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- He C, Levine B. The Beclin 1 interactome. Curr Opin Cell Biol. 2010;22:140–149. doi: 10.1016/j.ceb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itakura E, Kishi C, Inoue K, Mizushima N. Beclin 1 Forms Two Distinct Phosphatidylinositol 3-Kinase Complexes with Mammalian Atg14 and UVRAG. Mol Biol Cell. 2008;19:5360–5372. doi: 10.1091/mbc.E08-01-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger Pa, Pickford F, Sun C-H, Lucin KM, Masliah E, Wyss-Coray T. Regulation of amyloid precursor protein processing by the Beclin 1 complex. PLoS One. 2010;5:e11102. doi: 10.1371/journal.pone.0011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, Bjornsson S, Huttenlocher J, Levey AI, Lah JJ, Rujescu D, Hampel H, Giegling I, Andreassen Oa, Engedal K, Ulstein I, Djurovic S, Ibrahim-Verbaas C, Hofman A, Ikram MA, van Duijn CM, Thorsteinsdottir U, Kong A, Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N Engl J Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kametaka S. Apg14p and Apg6/Vps30p Form a Protein Complex Essential for Autophagy in the Yeast, Saccharomyces cerevisiae. J Biol Chem. 1998;273:22284–22291. doi: 10.1074/jbc.273.35.22284. [DOI] [PubMed] [Google Scholar]
- El Khoury JB, Moore KJ, Means TK, Leung J, Terada K, Toft M, Freeman MW, Luster AD. CD36 mediates the innate host response to beta-amyloid. J Exp Med. 2003;197:1657–1666. doi: 10.1084/jem.20021546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara a, Noda T, Ishihara N, Ohsumi Y. Two distinct Vps34 phosphatidylinositol 3-kinase complexes function in autophagy and carboxypeptidase Y sorting in Saccharomyces cerevisiae. J Cell Biol. 2001;152:519–530. doi: 10.1083/jcb.152.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- Konishi A, Arakawa S, Yue Z, Shimizu S. Involvement of Beclin 1 in engulfment of apoptotic cells. J Biol Chem. 2012;287:13919–13929. doi: 10.1074/jbc.M112.348375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Lane RF, Raines SM, Steele JW, Ehrlich ME, Lah Ja, Small Sa, Tanzi RE, Attie AD, Gandy S. Diabetes-associated SorCS1 regulates Alzheimer’s amyloid-beta metabolism: evidence for involvement of SorL1 and the retromer complex. J Neurosci. 2010;30:13110–13115. doi: 10.1523/JNEUROSCI.3872-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Prescott M, Adler B, Boyce JD, Devenish RJ. Beclin 1 is required for starvation-enhanced, but not rapamycin-enhanced, LC3-associated phagocytosis of Burkholderia pseudomallei in RAW 264.7 cells. Infect Immun. 2013;81:271–277. doi: 10.1128/IAI.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Slifer M, Martin ER, Schnetz-boutaud N, Anderson B, Züchner S, Gwirtsman H, Gilbert JR, Pericak-vance MA, Haines JL. Genomic convergence to identify candidate genes for Alzheimer disease on chromosome 10. Hum Mutat. 2009;30:463–471. doi: 10.1002/humu.20953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999a;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999b;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- Liang XH, Kleeman LK, Jiang HH, Goldman JE, Berry G, Herman B, Levine B, Jiang HUIHUI, Gordon G. Protection against Fatal Sindbis Virus Encephalitis by Beclin, a Novel Bcl-2-Interacting Protein Protection against Fatal Sindbis Virus Encephalitis by Beclin, a Novel Bcl-2-Interacting Protein. J Virol. 1998;72:8586–8596. doi: 10.1128/jvi.72.11.8586-8596.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucin KM, O’Brien CE, Bieri G, Czirr E, Mosher KI, Abbey RJ, Mastroeni DF, Rogers J, Spencer B, Masliah E, Wyss-Coray T. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer’s disease. Neuron. 2013;79:873–886. doi: 10.1016/j.neuron.2013.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez J, Almendinger J, Oberst A, Ness R, Dillon CP, Fitzgerald P, Hengartner MO, Green DR. (LC3) -associated phagocytosis is required for the efficient clearance of dead cells. 2011 doi: 10.1073/pnas.1113421108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Meléndez A, Tallóczy Z, Seaman M, Eskelinen E-L, Hall DH, Levine B. Autophagy genes are essential for dauer development and life-span extension in C elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, Dall’Armi C, Simoes S, Point Du Jour KS, McCabe BD, Small Sa, Di Paolo G. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat Commun. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrak RE. Microglia in Alzheimer brain: a neuropathological perspective. Int J Alzheimers Dis. 2012;2012:165021. doi: 10.1155/2012/165021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhammad A, Flores I, Zhang H, Yu R, Staniszewski A, Planel E, Herman M, Ho L, Kreber R, Honig LS, Ganetzky B, Duff K, Arancio O, Small Sa. Retromer deficiency observed in Alzheimer’s disease causes hippocampal dysfunction, neurodegeneration, and Abeta accumulation. Proc Natl Acad Sci U S A. 2008;105:7327–7332. doi: 10.1073/pnas.0802545105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrow L, Debnath J. Autophagy as a stress-response and quality-control mechanism: implications for cell injury and human disease. Annu Rev Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisar S, Kelly E, Cullen PJ, Mundell SJ. Regulation of P2Y1 receptor traffic by sorting Nexin 1 is retromer independent. Traffic. 2010;11:508–519. doi: 10.1111/j.1600-0854.2010.01035.x. [DOI] [PubMed] [Google Scholar]
- Nixon Ra, Wegiel J, Kumar A, Yu WH, Peterhoff C, Cataldo A, Cuervo AM. Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. J Neuropathol Exp Neurol. 2005;64:113–122. doi: 10.1093/jnen/64.2.113. [DOI] [PubMed] [Google Scholar]
- Obara K, Sekito T, Ohsumi Y. Assortment of Phosphatidylinositol 3-Kinase Complexes — Atg14p Directs Association of Complex I to the Pre-autophagosomal Structure in Saccharomyces cerevisiae. Mol Biol Cell. 2006;17:1527–1539. doi: 10.1091/mbc.E05-09-0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravicini G, Horazdovsky BF, Emr SD. Alternative pathways for the sorting of soluble vacuolar proteins in yeast: a vps35 null mutant missorts and secretes only a subset of vacuolar hydrolases. Mol Biol Cell. 1992;3:415–427. doi: 10.1091/mbc.3.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickford F, Masliah E, Britschgi M, Lucin K, Narasimhan R, Jaeger PA, Small S, Spencer B, Rockenstein E, Levine B, Wyss-coray T. The autophagy-related protein beclin 1 shows reduced expression in early Alzheimer disease and regulates amyloid β accumulation in mice. J Clin Invest. 2008;118:2190–2199. doi: 10.1172/JCI33585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu X, Zou Z, Sun Q, Luby-Phelps K, Cheng P, Hogan RN, Gilpin C, Levine B. Autophagy gene-dependent clearance of apoptotic cells during embryonic development. Cell. 2007;128:931–946. doi: 10.1016/j.cell.2006.12.044. [DOI] [PubMed] [Google Scholar]
- Reddy JV, Seaman MN. Vps26p, a component of retromer, directs the interactions of Vps35p in endosome-to-Golgi retrieval. Mol Biol Cell. 2001;12:3242–3256. doi: 10.1091/mbc.12.10.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JS, Klionsky DJ, Banta LM, Emr SD. Protein Sorting in Saccharomyces cerevisiae : Isolation of Mutants Defective in the Delivery and Processing of Multiple Vacuolar Hydrolases. Mol Cell Biol. 1988;8:4936–4948. doi: 10.1128/mcb.8.11.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogaeva E, Meng Y, Lee JH, Gu Y, Kawarai T, Zou F, Katayama T, Baldwin CT, Cheng R, Hasegawa H, Chen F, Shibata N, Lunetta KL, Pardossi-Piquard R, Bohm C, Wakutani Y, Cupples LA, Cuenco KT, Green RC, Pinessi L, Rainero I, Sorbi S, Bruni A, Duara R, Friedland RP, Inzelberg R, Hampe W, Bujo H, Song Y-Q, Andersen OM, Willnow TE, Graff-Radford N, Petersen RC, Dickson D, Der SD, Fraser PE, Schmitt-Ulms G, Younkin S, Mayeux R, Farrer La, St George-Hyslop P. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JH, Stevens TH. Protein sorting in yeast: mutants defective in vacuole biogenesis mislocalize vacuolar proteins into the late secretory pathway. Cell. 1986;47:1041–1051. doi: 10.1016/0092-8674(86)90819-6. [DOI] [PubMed] [Google Scholar]
- Ruck A, Attonito J, Garces KT, Núnez L, Palmisano NJ, Rubel Z, Bai Z, Nguyen KCQ, Sun L, Grant BD, Hall DH, Meléndez A. The Atg6/Vps30/Beclin 1 ortholog BEC-1 mediates endocytic retrograde transport in addition to autophagy in C elegans. Autophagy. 2011;7:386–400. doi: 10.4161/auto.7.4.14391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Kaarniranta K, Kauppinen A, Ojala J, Haapasalo A, Soininen H, Hiltunen M. Impaired autophagy and APP processing in Alzheimer’s disease: The potential role of Beclin 1 interactome. Prog Neurobiol. 2013;106–107:33–54. doi: 10.1016/j.pneurobio.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Sanjuan Ma, Dillon CP, Tait SWG, Moshiach S, Dorsey F, Connell S, Komatsu M, Tanaka K, Cleveland JL, Withoff S, Green DR. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature. 2007;450:1253–1257. doi: 10.1038/nature06421. [DOI] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Seaman MN, Marcusson EG, Cereghino JL, Emr SD. Endosome to Golgi retrieval of the vacuolar protein sorting receptor, Vps10p, requires the function of the VPS29, VPS30, and VPS35 gene products. J Cell Biol. 1997;137:79–92. doi: 10.1083/jcb.137.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seaman MNJ. Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol. 2004;165:111–122. doi: 10.1083/jcb.200312034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacka JJ, Lu J, Xie Z-L, Uchiyama Y, Roth Ka, Zhang J. Kainic acid induces early and transient autophagic stress in mouse hippocampus. Neurosci Lett. 2007;414:57–60. doi: 10.1016/j.neulet.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small Sa, Kent K, Pierce A, Leung C, Kang MS, Okada H, Honig L, Vonsattel J-P, Kim T-W. Model-guided microarray implicates the retromer complex in Alzheimer’s disease. Ann Neurol. 2005;58:909–919. doi: 10.1002/ana.20667. [DOI] [PubMed] [Google Scholar]
- Spencer B, Potkar R, Trejo M, Rockenstein E, Patrick C, Gindi R, Adame A, Wyss-Coray T, Masliah E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoelgen R, von Arnim CaF, Thomas AV, Peltan ID, Koker M, Deng A, Irizarry MC, Andersen OM, Willnow TE, Hyman BT. Interaction of the cytosolic domains of sorLA/LR11 with the amyloid precursor protein (APP) and beta-secretase beta-site APP-cleaving enzyme. J Neurosci. 2006;26:418–428. doi: 10.1523/JNEUROSCI.3882-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavaré JM, Cullen PJ. A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol. 2013;15:461–471. doi: 10.1038/ncb2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q, Fan W, Chen K, Ding X, Chen S, Zhong Q. Identification of Barkor as a mammalian autophagy-specific factor for Beclin 1 and class III phosphatidylinositol 3-kinase. Proc Natl Acad Sci U S A. 2008;105:19211–19216. doi: 10.1073/pnas.0810452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kirisako T, Kamada Y, Mizushima N, Noda T, Ohsumi Y. The pre-autophagosomal structure organized by concerted functions of APG genes is essential for autophagosome formation. EMBO J. 2001;20:5971–5981. doi: 10.1093/emboj/20.21.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Kubota Y, Sekito T, Ohsumi Y. Hierarchy of Atg proteins in pre-autophagosomal structure organization. Genes Cells. 2007;12:209–218. doi: 10.1111/j.1365-2443.2007.01050.x. [DOI] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jager S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011a;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin P, Lauffer B, Jäger S, Cimermancic P, Krogan NJ, von Zastrow M. SNX27 mediates retromer tubule entry and endosome-to-plasma membrane trafficking of signalling receptors. Nat Cell Biol. 2011b;13:715–721. doi: 10.1038/ncb2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Bustos V, Flajolet M, Greengard P. A small-molecule enhancer of autophagy decreases levels of Abeta and APP-CTF via Atg5-dependent autophagy pathway. FASEB J. 2011;25:1934–1942. doi: 10.1096/fj.10-175158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada M. Isolation and charact zation of autophagy-defective mutants of. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-e. [DOI] [PubMed] [Google Scholar]
- Vergés M, Luton F, Gruber C, Tiemann F, Reinders LG, Huang L, Burlingame AL, Haft CR, Mostov KE. The mammalian retromer regulates transcytosis of the polymeric immunoglobulin receptor. Nat Cell Biol. 2004;6:763–769. doi: 10.1038/ncb1153. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber a, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilariño-Güell C, Wider C, Ross Oa, Dachsel JC, Kachergus JM, Lincoln SJ, Soto-Ortolaza AI, Cobb Sa, Wilhoite GJ, Bacon Ja, Behrouz B, Melrose HL, Hentati E, Puschmann A, Evans DM, Conibear E, Wasserman WW, Aasly JO, Burkhard PR, Djaldetti R, Ghika J, Hentati F, Krygowska-Wajs A, Lynch T, Melamed E, Rajput A, Rajput AH, Solida A, Wu R-M, Uitti RJ, Wszolek ZK, Vingerhoets F, Farrer MJ. VPS35 mutations in Parkinson disease. Am J Hum Genet. 2011;89:162–167. doi: 10.1016/j.ajhg.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson K, El Khoury J. Microglial scavenger receptors and their roles in the pathogenesis of Alzheimer’s disease. Int J Alzheimers Dis. 2012;2012:489456. doi: 10.1155/2012/489456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Klionsky DJ. Mammalian autophagy: core molecular machinery and signaling regulation. Curr Opin Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Overmeyer JH, Maltese Wa. Functional specificity of the mammalian Beclin-Vps34 PI 3-kinase complex in macroautophagy versus endocytosis and lysosomal enzyme trafficking. J Cell Sci. 2006;119:259–270. doi: 10.1242/jcs.02735. [DOI] [PubMed] [Google Scholar]
- Zhou X, Wang L, Hasegawa H, Amin P, Han B, Kaneko S, He Y. Deletion of PIK3C3 / Vps34 in sensory neurons causes rapid neurodegeneration by disrupting the endosomal but not the autophagic pathway. PNAS. 2010;107:9424–9429. doi: 10.1073/pnas.0914725107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pagès A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brücke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]