Abstract
Lithium (Li) continues to be a standard small compound used for the treatment of neurological disorders. Besides neuronal cells, Li is also known to affect immune cell function. In spite of its clinical use, potential mechanisms by which Li modulates immune cells, especially macrophages and its clinical relevance in bipolar patients are not well understood. Here, we provide an overview of the literature with regard to Li’s effects on monocytes and macrophages. We have also included some of our results showing that Li differentially modulates chemokine gene expression in the absence and presence of Toll-like receptor-4 stimulation in a human macrophage model. Given that Li has a wide range of intracellular targets both in macrophages as well as in other cell types, more studies are needed to further understand the mechanistic basis of Li’s effect in neurological and other inflammatory diseases. These studies could undoubtedly identify new therapeutic targets for treating such diseases.
Introduction
Macrophages play pivotal role in regulating acute and chronic inflammatory processes in the human body through secretion of various cytokines, chemokines and growth factors (Murray and Wynn, 2011; Sica and Mantovani, 2012). Toll-like receptors (TLRs) present on macrophages and other cells are major sensors of foreign microbial components and tissue breakdown products (Moresco et al., 2011). Upon sensing these molecules, TLRs initiate a series of downstream signaling events that drive cellular responses including the production of cytokines, chemokines, and other inflammatory mediators. Central to the macrophage function is the expression of chemokines that aid in chemo-attracting immune cells to the site of injury. Production of these chemokines in general is crucial for effective induction and resolution of disease processes. Because of this function, chemokines have received a great deal of attention in neurological disorders since it is now widely accepted that inflammation is an underpinning factor in the pathogenesis of many neurological diseases including bipolar disorders (Leonard, 2007; Dantzer et al., 2008). Immune cells and the neuronal system integrate their signals with each other for homeostasis. Therefore, it is not surprising that many drugs that modulate the neuronal system have also been shown to regulate immune function. One such compound is Lithium (Li) that is currently used in clinical practice for treatment of bipolar disorders in humans.
Li is a monovalent cation used clinically as a mood stabilizer and for treatment of bipolar disorders (Chiu and Chuang, 2010). Although earlier studies suggested that GSK3β is one of the targets for Li, more recent studies have suggested a range of functions for Li including regulation of receptor signaling to modulation of ion channels. In addition to its mood stabilizing effects, Li has long been known to cause leukocytosis (Shopsin et al., 1971; Tisman et al., 1973; Watanabe et al., 1974; Balon and Berchou, 1986; Gualtieri et al., 1986). In addition to these hematopoietic effects, studies have also examined the role of Li in modulating inflammation in different immune cell models (Shenkman et al., 1978; Shenkman et al., 1980; Kleinerman et al., 1989; Beyaert et al., 1991, 1992; Kucharz et al., 1993; Maes et al., 1999; Merendino et al., 2000a; Chiu and Chuang, 2010; Nahman et al., 2012). In this manuscript we provide a brief account of the immunomodulatory role of Li in immune cells, especially macrophages in the context of inflammatory diseases. We hope that this perspective will provide a fresh outlook of this old drug that has been used in clinical practice for more than 5 decades in the treatment of manic episodes.
Modulation of cytokines and chemokines by Lithium
Several studies have examined the role of Li on inflammatory cytokine production by macrophages and other immune cells. In the late 1970s, Shenkman et al (Shenkman et al., 1978; Shenkman et al., 1980) showed that human lymphocytes and macrophages respond to Li in vitro and that Li enhances several indices of cellular immunity at therapeutic concentrations. They showed that Li is capable of inducing lymphocyte proliferation (in response to mitogens) as well as rosette formation and increase macrophage phagocytosis. Klienerman et al (Kleinerman et al., 1989) later showed that human monocytes (from normal donors) are capable of producing TNFα but not IL-1 in response to Li treatment. They showed that these effects of Li are related to Li’s effect on TNFα transcription. In another study using mouse macrophages, we recently demonstrated that macrophages indeed are capable of producing TNFα in response to Li treatment (Hull et al., 2013). We further showed that this response to Li is significantly enhanced in the presence of TLR2 and TLR3 ligands. Effect of Li on monocyte/macrophage TNFα production was proposed by Kleinerman et al (Kleinerman et al., 1989) to be related to elevated white blood cell counts observed in patients on Li therapy (Murphy et al., 1971). Interestingly in a study comparing monocyte response between breast cancer patients and healthy donors, Li was shown to stimulate less TNFα from breast cancer patients compared to healthy donors and this effect was specific for TNFα and not IL-6 (Arena et al., 1997). In addition to these cytokines, Li has been shown to also induce IL-2 production from T-lymphocytes (Kucharz et al., 1993) as well as able to induce several pro-inflammatory and anti-inflammatory cytokines from whole blood treatments (Maes et al., 1999). In another study (Merendino et al., 2000b) IL-15 production was analyzed from monocyte cultures obtained from breast cancer patients and healthy donors. LiCl treatment in the absence and presence of LPS significantly induced IL-15 production by monocytes mainly from non-metastatic breast cancer patient group. This effect of Li on IL-15 (a hematopoiesis promoting and antitumor cytokine) was proposed as a means to counteract the immunosuppression state of cancer patients.
In contrast to these “inducing” effects on cytokines, LiCl pretreatment of primary glial cells, inhibits LPS-induced secretion of TNF-α, IL1-β, PGE2 and NO (Nahman et al., 2012). Similar effect was also observed in primary bone marrow derived macrophages (Zhang et al., 2009). More recently, LiCl treatment was shown to attenuate LPS-, polyinosinic-polycytidylic acid-, and Sendai virus-induced IFN-beta production and IFN regulatory factor 3 activation in macrophages and these effects were shown to be independent of GSK3β inhibition. Importantly, in this study the in vitro effects were further confirmed in vivo in a mouse model (Wang et al., 2013).
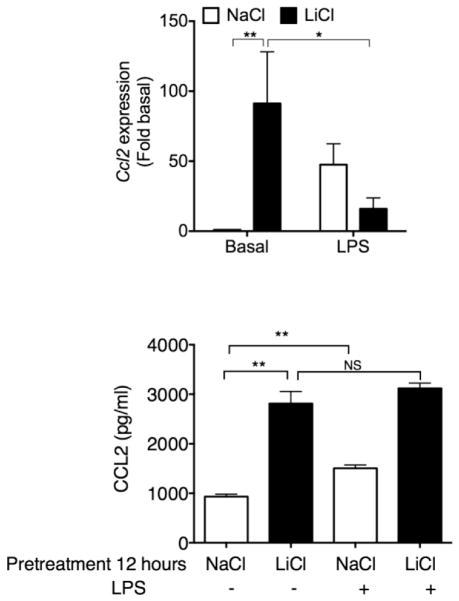
Even though several studies have examined the effects of Li on major cytokines such as TNFα and IL-6 in macrophages, very little is known in terms Li’s effects on the regulation of chemokine gene expression in human macrophages. Given the important role of chemokines in mediating immune cell chemotaxis as well as the role of TLRs in regulating immune cell activation, we recently performed some studies using a human macrophage model to understand the role of Li on chemokine gene expression in the presence and absence of TLR activation. In particular we chose to examine the cross talk between Li and TLR4. Toll-like receptor-4 is activated by microbial components (lipopolysaccharide from gram negative bacteria) as well as by some cell breakdown products (heat shock proteins, HMGB1 etc) and is well known for its role in many inflammatory disorders (Moresco et al., 2011). We first examined the effects of Li chloride (LiCl) on the expression levels of different chemokines in THP1 differentiated human macrophages in the absence and presence of lipopolysaccharide (LPS), a TLR4 agonist. Interestingly, LiCl treatment by itself significantly induced Ccl2 mRNA expression (Fig 1) but did not affect either Ccl1 or Ccl5 (data not shown). Using a more selective GSK3β inhibitor (SB216763) we ruled out the role of GSK3β on LiCl-induced Ccl2 expression (data not shown). Although LPS induced expression of all the chemokines tested (Fig 1 and data not shown), LiCl pretreatment caused a marked decrease in LPS-induced Ccl2 expression. This effect was selective for Ccl2 since LiCl pretreatment did not affect the other chemokines (data not shown). We then assessed CCL2 levels in the culture supernatants. Both LiCl and LPS were able to independently induce CCL2 production as measured in the culture supernatants (Fig 1). Even though LPS induced CCL2 production (measured in the culture supernatant), LPS treatment of LiCl pre-treated cells did not cause any further increase. However, there was no decrease in CCL2 levels (similar to mRNA expression) likely because LiCl pretreatment itself induced maximal secretion into the culture supernatants prior to LPS treatment and further LPS treatment likely was not able to decrease this level in the culture supernatant. Note that the secreted CCL2 in the cell culture supernatant is also likely quite stable (Leemasawatdigul and Gappa-Fahlenkamp, 2011) with in the time points that we examined. Taken together, our results demonstrate differential effects of LiCl on Ccl2 expression in the absence and presence of LPS and further suggest that these observations are likely independent of its effects on GSK3β. A similar scenario was noted recently in a study investigating the effect of LiCl on differentiation and maturation of monocyte-derived dendritic cells (Liu et al., 2011). The authors showed that although LiCl substantially enhanced the expression of CD86 expression and secretion of a number of proinflammatory cytokines during differentiation, LiCl suppressed these functions during LPS-induced maturation of dendritic cells. These authors proposed that the actions of LiCl during differentiation and maturation are quite different. Together, these and other results suggest that LiCl can either induce or inhibit inflammatory cytokine and chemokine production but the effect however, is quite context dependent. An effect similar to that of ours was also shown for LPS-induced IL-8 from human monocytes (Merendino et al., 2000a). Given the plasticity and heterogeneity of macrophages (Sica and Mantovani, 2012), one could envision that the different types of macrophages could potentially respond differently to LiCl. Although many of these studies described above have utilized concentrations that are therapeutically acceptable (based on plasma Li concentration), more studies are needed to systematically determine whether Li plays an anti-inflammatory or pro-inflammatory role in the different macrophage subtypes and whether these effects on inflammatory cytokines are differentially regulated in the presence of other stimuli such as TLR activators.
Fig 1. Effect of Li chloride (LiCl) on Ccl2 expression and production in the absence (basal) or presence of lipopolysaccharide (LPS) in human macrophages.
THP-1X-blue monocytes (Anur et al., 2012; Tang et al., 2012; Waisberg et al., 2012) were differentiated into macrophages by incubating with 50 ng/ml of phorbol 12-myristate 13-acetate (PMA) (Sigma Aldrich) as described before (Vuletic et al., 2011). Cells were washed with PBS and allowed to rest 24 hours prior to stimulation. Differentiated macrophages were pretreated with NaCl (30 mM), or LiCl (30 mM) for 12 hours followed by either no treatment or treatment with lipopolysaccharide (2 ng/ml) for another 9 hours (for RNA) or 18 hours (for ELISA). After treatment, RNA was extracted and mRNA levels determined using quantitative real-time RT-PCR as described before (Packiriswamy et al., 2013; Sharma et al., 2013). Supernatants were analyzed for CCL2 levels using ELISA from eBiosciences Inc. as described before (Irwin et al., 2013; Lee et al., 2013). *p<0.05; **p<0.01. N = 3–10.
Intracellular targets of Lithium in macrophages and other immune cells
As elegantly described in other review articles (Chiu and Chuang, 2010), Li has a number of effects in addition to its effect on GSK3β activity. Not all of the Li targets have been characterized in macrophages or in other immune cells. Li’s effect on GSK3β was found to be crucial in the activation of STAT3 in primary astrocytes as well as macrophages and primary mouse glial cells (Beurel and Jope, 2008). These authors showed using other complementary approaches that GSK3β is important in STAT3 activation. Other studies have shown that the effect of selective GSK3β inhibitors and LiCl are not consistent with GSK3β inhibition with certain effects of LiCl (Zhang et al., 2009; Hull et al., 2013). For example, we showed that while LiCl activates TNFα production, inhibition of GSK3β with SB216763 did not elicit the same response. Although we did not identify the molecular target, another group showed that in macrophages NFκB1p105 is one of the targets (Zhang et al., 2009). LiCl has also been used as an adenylate cyclase inhibitor in immune cells and its effects on this enzyme was proposed earlier as an immune adjuvant especially in immune deficiency conditions of excessive cAMP levels (Shenkman et al., 1980).
Studies have also shown that Li can inhibit inositol monophosphatase (IMPase) thereby decreasing free inositol and IP3 levels in the cells. Because of its role in macrophage apoptosis (Zhang et al., 2009), De Meyer et al (De Meyer et al., 2011) examined the role of LiCl in inhibiting IMPase activity and consequently its effect on macrophage apoptosis. LiCl at therapeutic concentration was able to inhibit IMPase activity in macrophages and therefore induce apoptosis of these cells without affecting vascular smooth muscle cells or endothelial cells. This effect of Li was proposed to be important in preventing atherosclerotic plaque destabilization. More recently, Wang et al have also shown that Li can suppress TANK-binding kinase 1 kinase activity in vitro suggesting another target of LiCl in macrophages (Wang et al., 2013).
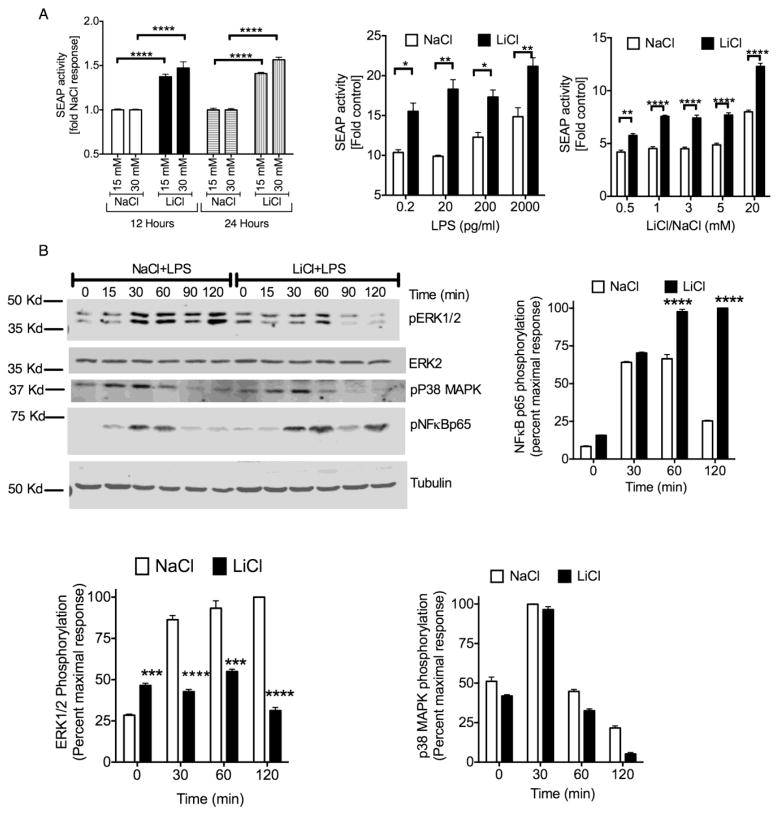
To understand the mechanism by which LiCl enhanced Ccl2 expression in human macrophages, we examined the effect of LiCl on NFκB/AP-1 reporter activity (Secreted Embryonic Alkaline Phosphatase, SEAP activity) in human macrophages. Interestingly, LiCl by itself induced significant activation of SEAP activity (Fig 2A). Similar to LiCl, LPS treatment in the absence of LiCl also induced a concentration dependent increase in SEAP activity. This effect however was further enhanced in the presence of LiCl and was concentration dependent (Fig 2A). These results suggest that the negative effect of LiCl that we observed on LPS-induced Ccl2 expression (Fig 1) is likely not mediated via NFκB/AP-1 activity since LiCl enhanced LPS-induced SEAP activity.
Fig 2. Effect of Li chloride (LiCl) on NFκB and MAPK signaling in the absence and presence of lipopolysaccharide (LPS) in human macrophages.
THP-1X-blue macrophages were pretreated with indicated concentrations of NaCl, or LiCl for 12 hrs and 24 hrs. For dose response with LPS, cells were pretreated with NaCl (30 mM), or LiCl (30 mM), for 12 hours followed by different concentrations of LPS as indicated for another 24 hours. For dose response with LiCl, cells were pretreated with NaCl, or LiCl with the indicated concentrations for 12 hours followed by LPS treatment (2 ng/ml) for another 24 hours. Secretion of SEAP (secreted alkaline phosphatase) was measured using plate reader assay as described before (Anur et al., 2012; Tang et al., 2012; Waisberg et al., 2012). *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. N=3. (B). Western blots were performed as described before (Patial et al., 2011b; Patial et al., 2011a; Hull et al., 2013). For this, THP-1X-blue macrophages were pretreated with NaCl (30 mM) or LiCl (30 mM) for 12 hours followed by LPS (2 ng/ml) for the indicated time points. Cell lysates were then subjected to Western blot analysis for the indicated proteins. Blots were scanned and quantified using Licor Odyssey. pERK1/2, pNFκB, and pP38 were normalized for loading using ERK2 or tubulin. Representative blots are shown in the left middle column. N=3. **p<0.01; ***p<0.001; ****p<0.0001.
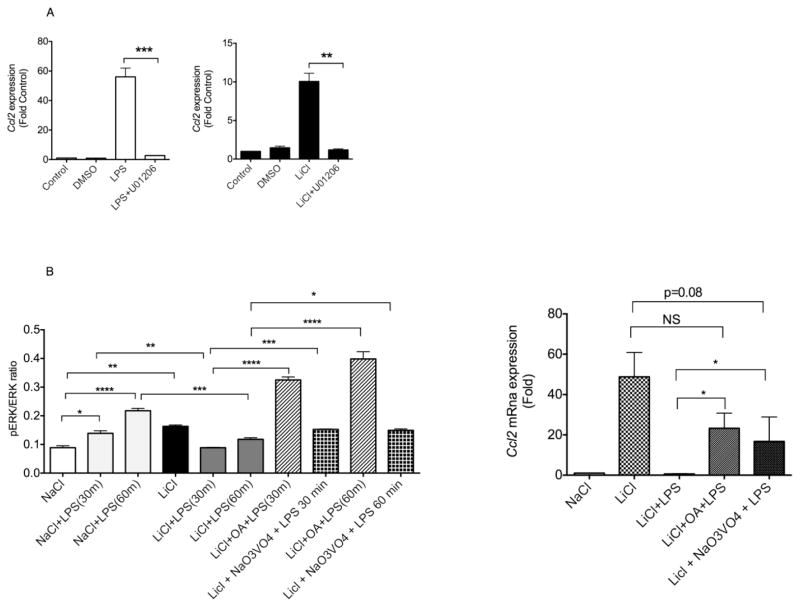
To further examine the mechanisms of LiCl’s effect, we determined the phosphorylation status of the MAPK and NFκB pathways. Consistent with the SEAP activity, LiCl pretreatment significantly enhanced LPS-induced NFκBp65 phosphorylation (Fig 2B). Since LPS-induced p38 and JNK activation were not affected by LiCl pretreatment (Fig 2B and data not shown), the enhanced SEAP activity was likely due to enhanced NFκB activation. Compared to these effects, LiCl significantly enhanced ERK1/2 phosphorylation in the absence of LPS (Fig 2B). However, pretreatment with LiCl markedly inhibited LPS-induced ERK1/2 phosphorylation (Fig 2B). Because the effect of LiCl on the ERK pathway appeared to be similar to that of Ccl2 expression, we reasoned that LiCl might affect Ccl2 expression via regulation of the ERK pathway. We first confirmed that LPS- and LiCl-induced Ccl2 expression is ERK-dependent. Pretreating the cells with U01206, a selective ERK inhibitor blocked Ccl2 expression induced by LPS and LiCl (Fig 3A). Using actinomycin-D our results further showed that LPS- and LiCl-induced Ccl2 expression is transcriptionally regulated (data not shown).
Fig 3. Effect of MAPK and phosphatase inhibitors on Li’s effect on LPS-induced Ccl2 mRNA expression in human macrophages.
(A). Cells were treated with vehicle or U01206 (10 μM) for 30 min followed by LPS (2 ng/ml) or LiCl (30 mM) for 12 hours. RNA was extracted and Ccl2 mRNA expression determined by real time Q-RT-PCR. (B). THP-1X-blue macrophages were pretreated with vehicle, okadaic acid (OA, 100 nM) or sodium orthovanadate (Na3VO4) for 30 min followed by treatment with NaCl (30 mM), or LiCl (30 mM) for 12 hours. These treatments were followed without or with LPS (2 ng/ml) for 30/60 min (for pERK) or 12 hours (for Ccl2 mRNA expression). PhosphoERK levels and Ccl2 mRNA expression were determined as described in Fig 1 and 2. N=3. *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001.
To further determine the mechanism by which pERK is negatively regulated by LiCl in the presence of LPS, we examined the phosphorylation of status of MEK1/2 (immediate upstream activator of ERK1/2). Interestingly, LiCl pretreatment enhanced LPS-induced MEK1/2 phosphorylation unlike ERK (data not shown) suggesting that Li’s effect on LPS-induced ERK likely occurs below MEK1/2 and potentially via a protein that could directly affect ERK. Therefore, we hypothesized that LiCl may activate a MAPK phosphatase in the presence of LPS which then could dephosphorylate ERK1/2. MAP kinase phosphatases in general are divided into dual specific, tyrosine-specific or serine/threonine-specific phosphatases depending on which residues on ERK they dephosphorylate (Roskoski, 2012). Among these phosphatases, previous studies have shown that okadaic acid- and vanadate-sensitive phosphatases are potential targets of LiCl (Zhen et al., 2002; Tsuji et al., 2003). To test whether these phosphatases could play a role in our system, we pretreated the cells with okadaic acid and sodium orthovanadate followed by treatments with LiCl and LPS. As expected LiCl pretreatment significantly inhibited LPS-induced ERK phosphorylation (Fig 3B). Interestingly, pretreatment with okadaic acid and sodium orthovanadate reversed LiCl’s effect on LPS-induced ERK phosphorylation (Fig 3B). To confirm that the effect of LiCl on the okadaic acid- and vanadate-sensitive phosphatases is indeed the reason for its effects on Ccl2 expression, we pretreated cells with okadaic acid or vanadate prior to treatment with LiCl+LPS and examined Ccl2 mRNA expression. Consistent with the effects on ERK phosphorylation, our results show that pretreatment of okadaic acid or sodium orthovanadate reversed LiCl’s negative effect on LPS-induced Ccl2 expression (Fig 3B). Together, our results demonstrate that LiCl by itself enhances Ccl2 expression via activation of the ERK pathway whereas in the presence of LPS signaling, LiCl induces phosphatases that decrease ERK phosphorylation and consequently decrease Ccl2 expression. Although the identity of this phosphatase is unknown, our results suggest that the phosphatase(s) (that target MAPK) could be additional targets of Li in macrophages. Because CCL2 has been shown to be important in many physiological processes including regulation of immune cell infiltration in the CNS (Savarin-Vuaillat and Ransohoff, 2007; Conductier et al., 2010; Lehto et al., 2010) understanding the mechanisms of how CCL2 production is regulated, especially by therapeutic compounds such as Li is important. A recent study by Liu et al showed that LiCl-mediated effects on dendritic cells involve PI3K/AKT, MEK/ERK, GSK-3β and PPARγ pathways and that these specific signaling pathways appear to be important for regulation of distinct features of dendritic cell differentiation and maturation (Liu et al., 2011). Given the multiple biological roles of macrophages, it would be of interest to mechanistically understand which selective signaling pathway modulated by LiCl targets which cellular functions of macrophages. This could lead to further identification of drug targets to selectively modulate these cellular functions of macrophages in inflammatory diseases.
Effects of Li in inflammatory disease models
Even though Li has multiple effects, its role in inhibition of GSK3β has been used to test whether it would serve as a good therapeutic target. De Sarno et al showed that Li is effective in both preventing and suppressing experimental autoimmune encephalomyelitis (De Sarno et al., 2008). In addition to neurological diseases, Li has also been used in animal models that are typically considered to be inflammatory disorders. In this regard, Wang et al showed that LiCl treatment of endotoxemic mice decreases acute renal failure as well as mortality. They further showed that in that model, LiCl is able to inhibit LPS-induced TNFα as well as CCL5/RANTES in vivo (Wang et al., 2009). Based on some in vitro studies, LiCl has also been proposed to be protective in arthritis (Hui et al., 2010). LiCl was shown to significantly decrease pro-inflammatory cytokine-induced collagen release from bovine cartilage independent of GSK3β. Studies have also shown that LiCl treatment of NZB/W mice leads to reduced immune mediated damage in renal failure and therefore possibly extending the life span of the animal (Hart et al., 1994). As described before, LiCl has also been proposed to be beneficial in atherosclerosis and has been used in rabbit model to reduce macrophage load in atherosclerotic plaques (De Meyer et al., 2011). A similar effect was found in a mouse model of atherosclerosis using high fat diet in ApoE knockout mice (Choi et al., 2010). Recent studies have also shown that LiCl decreases the severity of corneal disease (induced by Pseudomonas aeruginosa) by reducing inflammatory response and bacterial load. In this context, LiCl can differentially regulate anti-inflammatory and pro-inflammatory cytokines and promote macrophage and neutrophil apoptosis. These studies suggest a protective role for LiCl in Pseudomonas aeruginosa-induced keratitis (Chen et al., 2013). In contrast to these protective effects, Li has been linked to psoriasis in patients on Li therapy. In an in vivo study using mouse model, Beyaert et al (Beyaert et al., 1992) demonstrated that Li in combination with TNFα is able to induce IL-6. Since TNFα and IL-6 have been linked to triggering or aggravation of psoriasis in patients that are under Li treatment, they proposed that Li’s effect on IL-6 production in the presence of TNFα might be important in the context of psoriasis. Together, these in vivo studies suggest that we are only scratching the surface in understanding the potential usefulness or effects of Li in inflammatory diseases. More studies are needed to extensively understand Li’s therapeutic effect in these various diseases and to relate the effects of Li on macrophages and other immune cells to that of the disease development.
Conclusions
While the intended target of Li is based on its neuroprotective and neurotrophic effects, this clinically used drug certainly has effects outside of the neuronal system. Although it is difficult to yet conclude with confidence that these extra-neuronal effects especially the effects on macrophage biology has clinical relevance in patients under Li treatment, in vivo studies in animal models and in vitro studies using human and mouse macrophages indicate that Li can modulate macrophage biology and inflammatory disease. Thus a clearly important and crucial question is whether this effect of Li has clinical relevance in patients being treated with Li. In an interesting clinical study comparing monocytes from bipolar patients without and with Li treatment, Knijff et al found that Li treatment of patients restored the ratio of IL1β to IL6 production that was dysregulated in the bipolar patients without Li treatment (Knijff et al., 2007). Interestingly, ex vivo treatment did not restore this phenotype, suggesting that one needs to be careful in interpreting ex vivo or in vitro studies. Based on the various studies on Li, it is clear that we still do not completely understand the mechanistic basis for the in vivo and clinical effects of Li in macrophages. It should be noted however, that an equally important question coming out of these studies on Li in macrophage biology is whether any of these pathways identified can be used to selectively target disease processes. For example, a recent study showed that LiCl can inhibit IMPase in macrophages and therefore induce apoptosis in atherosclerotic plaques thereby stabilizing the plaque. This suggests that IMPase inhibitor could be effective in preventing plaque destabilization (De Meyer et al., 2011), suggesting a potential target in atherosclerosis. Thus, we believe that while Li likely has modulatory effects in patients due to its effect on monocytes/macrophages, the fact that this old drug can modulate inflammatory disease pathogenesis in animal models suggests that understanding the mechanisms by which Li modulates macrophage biology is crucial. This in future could identify therapeutic targets in the treatment of inflammatory diseases.
Acknowledgments
We gratefully acknowledge the support from NIH (grants HL095637, AR055726 and AR056680 to N.P.).
Footnotes
Authors declare no conflict of interest.
References
- Anur P, Yates J, Garbati MR, Vanderwerf S, Keeble W, Rathbun K, Hays LE, Tyner JW, Svahn J, Cappelli E, Dufour C, Bagby GC. p38 MAPK inhibition suppresses the TLR-hypersensitive phenotype in FANCC- and FANCA-deficient mononuclear phagocytes. Blood. 2012;119:1992–2002. doi: 10.1182/blood-2011-06-354647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena A, Capozza AB, Orlando ME, Curro F, Losi E, Chillemi S, Mesiti M, Merendino RA. In vitro effects of lithium chloride on TNF alpha and IL-6 production by monocytes from breast cancer patients. Journal of chemotherapy (Florence, Italy) 1997;9:219–226. doi: 10.1179/joc.1997.9.3.219. [DOI] [PubMed] [Google Scholar]
- Balon R, Berchou R. Hematologic side effects of psychotropic drugs. Psychosomatics. 1986;27:119–120. 125–117. doi: 10.1016/S0033-3182(86)72722-9. [DOI] [PubMed] [Google Scholar]
- Beurel E, Jope RS. Differential regulation of STAT family members by glycogen synthase kinase-3. The Journal of biological chemistry. 2008;283:21934–21944. doi: 10.1074/jbc.M802481200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyaert R, Schulze-Osthoff K, Van Roy F, Fiers W. Lithium chloride potentiates tumor necrosis factor-induced and interleukin 1-induced cytokine and cytokine receptor expression. Cytokine. 1991;3:284–291. doi: 10.1016/1043-4666(91)90496-z. [DOI] [PubMed] [Google Scholar]
- Beyaert R, Schulze-Osthoff K, Van Roy F, Fiers W. Synergistic induction of interleukin-6 by tumor necrosis factor and lithium chloride in mice: possible role in the triggering and exacerbation of psoriasis by lithium treatment. European journal of immunology. 1992;22:2181–2184. doi: 10.1002/eji.1830220835. [DOI] [PubMed] [Google Scholar]
- Chen K, Wu Y, Zhu M, Deng Q, Nie X, Li M, Wu M, Huang X. Lithium chloride promotes host resistance against Pseudomonas aeruginosa keratitis. Molecular vision. 2013;19:1502–1514. [PMC free article] [PubMed] [Google Scholar]
- Chiu CT, Chuang DM. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacology & therapeutics. 2010;128:281–304. doi: 10.1016/j.pharmthera.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SE, Jang HJ, Kang Y, Jung JG, Han SJ, Kim HJ, Kim DJ, Lee KW. Atherosclerosis induced by a high-fat diet is alleviated by lithium chloride via reduction of VCAM expression in ApoE-deficient mice. Vascular pharmacology. 2010;53:264–272. doi: 10.1016/j.vph.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. Journal of neuroimmunology. 2010 doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Meyer I, Martinet W, Van Hove CE, Schrijvers DM, Hoymans VY, Van Vaeck L, Fransen P, Bult H, De Meyer GR. Inhibition of inositol monophosphatase by lithium chloride induces selective macrophage apoptosis in atherosclerotic plaques. British journal of pharmacology. 2011;162:1410–1423. doi: 10.1111/j.1476-5381.2010.01152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sarno P, Axtell RC, Raman C, Roth KA, Alessi DR, Jope RS. Lithium prevents and ameliorates experimental autoimmune encephalomyelitis. Journal of immunology. 2008;181:338–345. doi: 10.4049/jimmunol.181.1.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gualtieri RJ, Berne RM, McGrath HE, Huster WJ, Quesenberry PJ. Effect of adenine nucleotides on granulopoiesis and lithium-induced granulocytosis in long-term bone marrow cultures. Experimental hematology. 1986;14:689–695. [PubMed] [Google Scholar]
- Hart DA, Done SJ, Benediktsson H, Lenz SP. Partial characterization of the enhanced survival of female NZB/W mice treated with lithium chloride. International journal of immunopharmacology. 1994;16:825–833. doi: 10.1016/0192-0561(94)90056-6. [DOI] [PubMed] [Google Scholar]
- Hui W, Litherland GJ, Jefferson M, Barter MJ, Elias MS, Cawston TE, Rowan AD, Young DA. Lithium protects cartilage from cytokine-mediated degradation by reducing collagen-degrading MMP production via inhibition of the P38 mitogen-activated protein kinase pathway. Rheumatology. 2010;49:2043–2053. doi: 10.1093/rheumatology/keq217. [DOI] [PubMed] [Google Scholar]
- Hull M, Lee E, Lee T, Anand N, Lalone V, Parameswaran N. Lithium chloride induces TNFalpha in mouse macrophages via MEK-ERK-dependent pathway. Journal of cellular biochemistry. 2013 doi: 10.1002/jcb.24634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin R, Lee T, Young VB, Parameswaran N, McCabe LR. Colitis-induced bone loss is gender dependent and associated with increased inflammation. Inflammatory bowel diseases. 2013;19:1586–1597. doi: 10.1097/MIB.0b013e318289e17b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinerman ES, Knowles RD, Blick MB, Zwelling LA. Lithium chloride stimulates human monocytes to secrete tumor necrosis factor/cachectin. Journal of leukocyte biology. 1989;46:484–492. doi: 10.1002/jlb.46.5.484. [DOI] [PubMed] [Google Scholar]
- Knijff EM, Breunis MN, Kupka RW, de Wit HJ, Ruwhof C, Akkerhuis GW, Nolen WA, Drexhage HA. An imbalance in the production of IL-1beta and IL-6 by monocytes of bipolar patients: restoration by lithium treatment. Bipolar disorders. 2007;9:743–753. doi: 10.1111/j.1399-5618.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- Kucharz EJ, Sierakowski SJ, Goodwin JS. Lithium in vitro enhances interleukin-2 production by T cells from patients with systemic lupus erythematosus. Immunopharmacology and immunotoxicology. 1993;15:515–523. doi: 10.3109/08923979309019728. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee E, Irwin R, Lucas PC, McCabe LR, Parameswaran N. beta-Arrestin-1 deficiency protects mice from experimental colitis. The American journal of pathology. 2013;182:1114–1123. doi: 10.1016/j.ajpath.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leemasawatdigul K, Gappa-Fahlenkamp H. Effect of storage conditions on the stability of recombinant human MCP-1/CCL2. Biologicals: journal of the International Association of Biological Standardization. 2011;39:29–32. doi: 10.1016/j.biologicals.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto SM, Niskanen L, Herzig KH, Tolmunen T, Huotari A, Viinamaki H, Koivumaa-Honkanen H, Honkalampi K, Ruotsalainen H, Hintikka J. Serum chemokine levels in major depressive disorder. Psychoneuroendocrinology. 2010;35:226–232. doi: 10.1016/j.psyneuen.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Leonard BE. Inflammation, depression and dementia: are they connected? Neurochemical research. 2007;32:1749–1756. doi: 10.1007/s11064-007-9385-y. [DOI] [PubMed] [Google Scholar]
- Liu KJ, Lee YL, Yang YY, Shih NY, Ho CC, Wu YC, Huang TS, Huang MC, Liu HC, Shen WW, Leu SJ. Modulation of the development of human monocyte-derived dendritic cells by lithium chloride. Journal of cellular physiology. 2011;226:424–433. doi: 10.1002/jcp.22348. [DOI] [PubMed] [Google Scholar]
- Maes M, Song C, Lin AH, Pioli R, Kenis G, Kubera M, Bosmans E. In vitro immunoregulatory effects of lithium in healthy volunteers. Psychopharmacology. 1999;143:401–407. doi: 10.1007/s002130050965. [DOI] [PubMed] [Google Scholar]
- Merendino RA, Arena A, Gangemi S, Ruello A, Losi E, Bene A, D’Ambrosio FP. In vitro interleukin-8 production by monocytes treated with lithium chloride from breast cancer patients. Tumori. 2000a;86:149–152. doi: 10.1177/030089160008600208. [DOI] [PubMed] [Google Scholar]
- Merendino RA, Arena A, Gangemi S, Ruello A, Losi E, Bene A, Valenti A, D’Ambrosio FP. In vitro effect of lithium chloride on interleukin-15 production by monocytes from IL-breast cancer patients. Journal of chemotherapy (Florence, Italy) 2000b;12:252–257. doi: 10.1179/joc.2000.12.3.252. [DOI] [PubMed] [Google Scholar]
- Moresco EM, LaVine D, Beutler B. Toll-like receptors. Current biology: CB. 2011;21:R488–493. doi: 10.1016/j.cub.2011.05.039. [DOI] [PubMed] [Google Scholar]
- Murphy DL, Goodwin FK, Bunney WE., Jr Leukocytosis during lithium treatment. The American journal of psychiatry. 1971;127:1559–1561. doi: 10.1176/ajp.127.11.1559. [DOI] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature reviews Immunology. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahman S, Belmaker RH, Azab AN. Effects of lithium on lipopolysaccharide-induced inflammation in rat primary glia cells. Innate immunity. 2012;18:447–458. doi: 10.1177/1753425911421512. [DOI] [PubMed] [Google Scholar]
- Packiriswamy N, Lee T, Raghavendra PB, Durairaj H, Wang H, Parameswaran N. G-protein-coupled receptor kinase-5 mediates inflammation but does not regulate cellular infiltration or bacterial load in a polymicrobial sepsis model in mice. Journal of innate immunity. 2013;5:401–413. doi: 10.1159/000347002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Shahi S, Saini Y, Lee T, Packiriswamy N, Appledorn DM, Lapres JJ, Amalfitano A, Parameswaran N. G-protein coupled receptor kinase 5 mediates lipopolysaccharide-induced NFkappaB activation in primary macrophages and modulates inflammation in vivo in mice. Journal of cellular physiology. 2011a;226:1323–1333. doi: 10.1002/jcp.22460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patial S, Saini Y, Parvataneni S, Appledorn DM, Dorn GW, 2nd, Lapres JJ, Amalfitano A, Senagore P, Parameswaran N. Myeloid-specific GPCR kinase-2 negatively regulates NF-kappaB1p105-ERK pathway and limits endotoxemic shock in mice. Journal of cellular physiology. 2011b;226:627–637. doi: 10.1002/jcp.22384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskoski R., Jr ERK1/2 MAP kinases: structure, function, and regulation. Pharmacological research: the official journal of the Italian Pharmacological Society. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma D, Malik A, Lee E, Britton RA, Parameswaran N. Gene dosage-dependent negative regulatory role of beta-arrestin-2 in polymicrobial infection-induced inflammation. Infection and immunity. 2013;81:3035–3044. doi: 10.1128/IAI.00653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenkman L, Borkowsky W, Shopsin B. Lithium as an immunologic adjuvant. Medical hypotheses. 1980;6:1–6. doi: 10.1016/0306-9877(80)90025-0. [DOI] [PubMed] [Google Scholar]
- Shenkman L, Borkowsky W, Holzman RS, Shopsin B. Enhancement of lymphocyte and macrophage function in vitro by lithium chloride. Clinical immunology and immunopathology. 1978;10:187–192. doi: 10.1016/0090-1229(78)90026-0. [DOI] [PubMed] [Google Scholar]
- Shopsin B, Friedmann R, Gershon S. Lithium and leukocytosis. Clinical pharmacology and therapeutics. 1971;12:923–928. doi: 10.1002/cpt1971126923. [DOI] [PubMed] [Google Scholar]
- Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. The Journal of clinical investigation. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Sharma A, Jen R, Hirschfeld AF, Chilvers MA, Lavoie PM, Turvey SE. Inflammasome-mediated IL-1beta production in humans with cystic fibrosis. PloS one. 2012;7:e37689. doi: 10.1371/journal.pone.0037689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisman G, Herbert V, Rosenblatt S. Evidence that lithium induces human granulocyte proliferation: elevated serum vitamin B 12 binding capacity in vivo and granulocyte colony proliferation in vitro. British journal of haematology. 1973;24:767–771. doi: 10.1111/j.1365-2141.1973.tb01704.x. [DOI] [PubMed] [Google Scholar]
- Tsuji S, Morinobu S, Tanaka K, Kawano K, Yamawaki S. Lithium, but not valproate, induces the serine/threonine phosphatase activity of protein phosphatase 2A in the rat brain, without affecting its expression. Journal of neural transmission. 2003;110:413–425. doi: 10.1007/s00702-002-0798-0. [DOI] [PubMed] [Google Scholar]
- Vuletic S, Dong W, Wolfbauer G, Tang C, Albers JJ. PLTP regulates STAT3 and NFkappaB in differentiated THP1 cells and human monocyte-derived macrophages. Biochimica et biophysica acta. 2011;1813:1917–1924. doi: 10.1016/j.bbamcr.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisberg M, Cerqueira GC, Yager SB, Francischetti IM, Lu J, Gera N, Srinivasan P, Miura K, Rada B, Lukszo J, Barbian KD, Leto TL, Porcella SF, Narum DL, El-Sayed N, Miller LH, Pierce SK. Plasmodium falciparum merozoite surface protein 1 blocks the proinflammatory protein S100P. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:5429–5434. doi: 10.1073/pnas.1202689109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang L, Zhao X, Zhang M, Zhao W, Gao C. Lithium Attenuates IFN-beta Production and Antiviral Response via Inhibition of TANK-Binding Kinase 1 Kinase Activity. Journal of immunology. 2013 doi: 10.4049/jimmunol.1203142. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang WC, Wang CY, Tsai CC, Chen CL, Chang YT, Kai JI, Lin CF. Inhibiting glycogen synthase kinase-3 reduces endotoxaemic acute renal failure by down-regulating inflammation and renal cell apoptosis. British journal of pharmacology. 2009;157:1004–1013. doi: 10.1111/j.1476-5381.2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Taguchi K, Nakashima Y, Ebara T, Iguchi K. Leukocytosis during lithium treatment and its correlation to serum lithium level. Folia psychiatrica et neurologica japonica. 1974;28:161–165. doi: 10.1111/j.1440-1819.1974.tb02298.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Jin W, Zhou X, Yu J, Lee AJ, Sun SC. Deregulation of Tpl2 and NF-kappaB signaling and induction of macrophage apoptosis by the anti-depressant drug lithium. Cellular signalling. 2009;21:559–566. doi: 10.1016/j.cellsig.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen X, Torres C, Friedman E. Lithium regulates protein tyrosine phosphatase activity in vitro and in vivo. Psychopharmacology. 2002;162:379–384. doi: 10.1007/s00213-002-1126-y. [DOI] [PubMed] [Google Scholar]