Abstract
Myeloablative therapy and autologous stem cell transplant (ASCT) is underutilized in older patients with B-cell non-Hodgkin (B-NHL) lymphoma. We hypothesized that myeloablative doses of 131I-tositumomab could be augmented by concurrent fludarabine based on preclinical data indicating synergy. Patients were ≥60 years of age, had high-risk, relapsed, or refractory B-NHL. Therapeutic infusions of 131I-tositumomab were derived from individualized organ-specific absorbed dose estimates delivering ≤27Gy to critical organs. Fludarabine was initiated 72 hours later followed by ASCT to define the maximally tolerated dose. Thirty-six patients with a median age of 65 yrs (range 60–76), 2 (range 1–9) prior regimens, and 33% with chemoresistant disease were treated on this trial. Dose limiting organs included lung (30), kidney (4), and liver (2) with a median administered 131I activity of 471 mCi (range 260–1620). Fludarabine was safely escalated to 30 mg/m2 × 7 days. Engraftment was prompt, there were no early treatment-related deaths, and 2 patients had ≥ grade 4 non-hematologic toxicities. The estimated 3 yr overall survival, progression-free survival, and non-relapse mortality were 54%, 53%, and 7%, respectively (median follow up of 3.9 yrs). Fludarabine up to 210mg/m2 can be safely delivered with myeloablative 131I-tositumomab and ASCT in older adults with B-NHL.
Introduction
Adults age 60 years and older make up the majority of the approximately 70,000 individuals newly diagnosed with non-Hodgkin lymphoma (NHL) each year in the United States.(1) Despite improved initial therapies, this group of patients is less likely to experience prolonged remissions and survival compared to younger adults.(2, 3) Though data suggest that high-dose therapy (HDT) and autologous hematopoietic stem cell transplantation (ASCT) can improve outcomes for a variety of NHL histologies, clinical data indicate that this approach is much less often employed in older adults primarily based on studies suggesting an increased risk of toxicity and treatment-related mortality (TRM).(4)
Radioimmunotherapy (RIT) given in myeloablative doses has been shown by our group and others to be able to provide effective, tolerable therapy for patients with relapsed B-cell NHL.(5–8) Based on these observations, we previously explored the use of myeloablative doses of single-agent 131I-tositumomab and ASCT in adults age ≥60 years.(9) This study demonstrated that the use of high-dose 131I-tositumomab was safe in this age group with minimal non-hematologic toxicity and long-term clinical benefit in a substantial subset of patients. However, as with other transplant modalities, relapse remained the primary cause of failure.
Efforts to improve on the outcome of high-dose RIT-based ASCT have primarily focused on the addition of agents traditionally paired with total body irradiation (TBI) such as etoposide and cyclophosphamide with these drugs given after the majority of the radionuclide has decayed or been cleared from the body.(6, 8) In contrast, preclinical data suggest that the purine analogs such as cytarabine and fludarabine optimally synergize with RIT when given concurrently with radiation exposure to target sites.(10, 11) This synergy is thought to be related to the potentially lethal incorporation of non-physiologic nucleosides during the repair of the RIT-induced single-strand DNA-breaks.(12)
Based on these preclinical data we hypothesized that a prolonged administration of therapeutic doses of fludarabine could be delivered concurrently with myeloablative doses of 131I-tositumomab with the potential to safely improve outcomes in this high-risk group of older patients. We now present the results from a phase I trial combining the maximally tolerated dose (MTD) of single agent 131I-tositumomab (27Gy) along with escalating doses and prolonged duration of administration of fludarabine. These data represent the first study of concurrent chemoradioimmunotherapy, demonstrate the feasibility of administration of chemotherapy to patients who are receiving high-energy gamma and beta irradiation, and show that up to 210mg/m2 of fludarabine can be safely added as part of an ASCT preparative regimen.
Patients and Methods
Patients
Patients with relapsed or refractory B-NHL or mantle cell lymphoma in first remission were required to be ≥60 years of age at the time of enrollment. Patients were required to have tumors expressing CD20, a serum creatinine <2.0 mg/dl, a serum bilirubin <1.5mg/dL, an expected survival of >60 days, an ECOG performance status of <2, the ability to perform self care in radiation isolation, and ≥2×106 autologous CD34 cells/kg cryopreserved. Patients were excluded if they had active systemic infection, active central nervous system lymphoma, an abnormally decreased cardiac ejection fraction, a diffusion capacity of carbon monoxide of <50% predicted, or had received >20Gy of radiotherapy to a critical normal organ (lung, liver, kidneys, spinal cord, >25% of red marrow). Documentation of <0.1% tumor contamination of the peripheral blood at the time of stem cell harvest or the collected product was also required. The institutional review boards of the Fred Hutchinson Cancer Research Center approved this protocol and all patients provided written informed consent. The protocol was registered at ClinicalTrials.gov (NCT00110071).
Biodistribution Studies
Thyroid uptake of 131I was blocked with oral potassium iodide, which was initiated 24 hr prior to RIT and continued for 30 days after the therapeutic infusion. All patients were premedicated with acetaminophen and diphenhydramine and then underwent outpatient biodistribution studies for dosimetry using tositumomab (1.7 mg/kg as a single infusion, n=3 or 485mg flat dose, n=33) labeled with 185 to 370 Mbq (5–10mCi) of 131I followed by serial quantitative planar gamma camera imaging to calculate individualized organ-specific absorbed dose estimates (cGy/MBq) based on CT-derived organ volumes as previously published.(5, 13, 14) Absorbed dose estimates were obtained by integrating the time-activity curves and applying the calculation methods recommended by the Medical Internal Radiation Dose Committee of the Society of Nuclear Medicine (Reston, VA). The overall treatment schema is shown in Figure 1.
Figure 1.
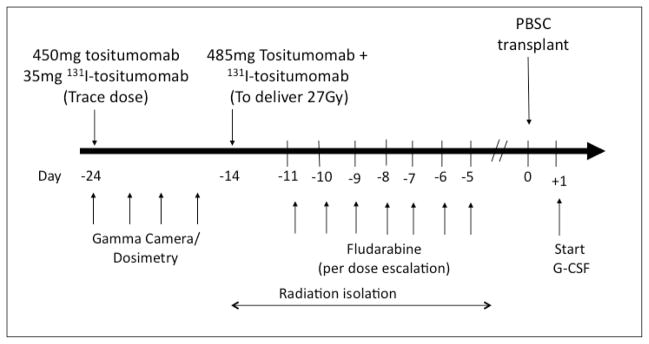
Treatment schema. Note 3 patients were received 1.7mg/kg 131I-tositumomab.
Therapeutic Antibody Infusions
Following completion of biodistribution studies, patients were admitted to lead lined radiation isolation rooms for therapeutic infusions. Patients were premedicated with ondansetron, acetaminophen, and diphenhydramine and hydrated with 5% dextrose in 0.45% sodium chloride at 200 mL/hour intravenously, starting 1 hour before therapy and continuing 48 hours after 131I-tositumomab therapy. Therapeutic infusions were given with the identical protein dose (1.7mg/mg in-house labeled or 485mg flat dose from commercially labeled product) and infusion schedule as in dosimetry, radioiodinated to deliver an estimated absorbed dose of 27 Gy to the critical normal organ receiving the highest radiation exposure. Patients remained in radiation isolation until the radiation exposure at one meter was < 0.07 mSv/h (7mR/hour).
Fludarabine Infusions
Fludarabine administration began approximately 72 hours following the therapeutic 131I-tositumomab infusion in order to minimize the juxtaposition of synergizing chemotherapy with the early post infusion period of non-specific blood-pool and normal organ radiation exposure and maximize overlap with uptake of the radioconjugate in hematolymphoid sites. Fludarabine infusions were delivered to patients while in radiation isolation following premedication with ondansetron per the dose escalation schema ranging from 50–210mg/m2.
Hematopoietic Stem Cell Transplantation and Supportive Care
Hematopoietic stem cells were infused per standard institutional practice once the radiation exposure was < 0.02 mSv/h (2mR/hr) at one meter. Filgrastim ≥5 μg/kg/day was delivered starting 1 day after HSC infusion and continued until the ANC>1000/μL × 2 days. A single 6mg dose of pegfilgrastim was allowed as an alternative. Antibiotics, blood products and other supportive care measures followed institutional standard practice.
Data Collection and Follow Up
Patients were followed up with computerized tomography at 1, 3, 6 and 12 months post ASCT and yearly thereafter. Bone marrow evaluations including cytogenetics were performed at 1 month and then annually. Response was scored using standard criteria.(15) Toxicity was measured on the Bearman transplant scale as well as the National Cancer Institute Common Toxicity Criteria scale (NCI-CTC) version 3.0.(16) Patients achieving less than a partial response (PR) from the regimen immediately preceding this therapy were categorized as having “chemoresistant” disease.
Statistical Analysis
The primary endpoint of this phase I trial was to identify the highest fludarabine dose level that would yield a dose limiting toxicity (DLT) rate of ≤25%. A DLT was defined as a grade III/IV adverse event on the Bearman transplant toxicity scale. We treated patients in cohorts of 4 and performed dose escalation if 0/4 dose DLTs were observed, maintained the dose level if 1/4 DLTs were observed, or de-escalated if ≥2/4 DLTs were observed. Additional patients beyond the requisite 4 were allowed on a dose level if the prior patients had not completed sufficient follow up to be evaluable for DLTs and they clinically required urgent transplantation. Overall and progression-free survival were estimated using the method of Kaplan-Meier.(17)
Results
Patient Characteristics
Thirty-six patients were enrolled and treated between July 2005 and May 2011. All patients who underwent biodistribution infusions went on to therapeutic infusions. Characteristics of these patients included: median age 65 yrs (range 60–76), age≥70 yrs = 7 (19%), stage III/IV = 34 (94%), median number of prior regimens = 2 (range 1–9), chemoresistant disease = 12 (33%), >1 extranodal site = 14 (39%), elevated LDH at treatment = 13 (36%), and IPI score at transplant 3–5 = 53% (Table 1). All patients had received prior rituximab and 17 (51%) had rituximab refractory disease (defined as lack of remission following or relapse within 6 months of rituximab containing regimen). Histologic subtypes included: MCL = 23 (9 in first complete remission), diffuse large B-cell = 8 (with 5 transformed from follicular lymphoma [FL]), FL=3, and marginal zone = 1, Waldenstrom’s = 1. The pre-transplant MCL international prognostic index (MIPI) scores for the 23 MCL patients included 10 low risk, 11 intermediate risk, and 2 high risk. All 8 of the DLBCL patients had either not achieved a complete remission (CR) following or experienced relapse within 12 months of rituximab-chemotherapy combinations and only 4 had chemosensitive disease going into transplant.
Table 1.
Baseline Characteristics
| Characteristic | Value |
|---|---|
| Female | 6 (17%) |
| Median age (yrs) | 65 (range 60–76) |
| Stage III/IV | 34 (94%) |
| Elevated LDH | 13 (36%) |
| >1 Extranodal Site | 14 (39%) |
| Histology | |
| Mantle Cell | 23 (64%) |
| Diffuse Large B-Cell | 8 (22%) |
| Indolent B-NHL | 5 (14%) |
| Median # Prior Regimens | 2 (range 1–9) |
| Prior rituximab | 36 (100%) |
| Rituximab refractory | 17 (47%) |
| Chemoresistant | 12 (33%) |
Therapy Delivered
131I-tositumomab
The 131I-tositumomab protein dose for dosimetry and therapy was 1.7mg/kg in 3 patients and 485mg flat dose in 33. A median activity of therapeutic 131I administered was 17.4 Gbq (471 mCi; range 9.6 to 59.9Gbq; 260–1620 mCi). The critical normal organs receiving up to a 27Gy absorbed radiation exposure were lungs (30), liver (4), and kidneys (2). Details of the absorbed radiation exposures across key organs and whole body based on delivered 131I activity are described in Table 2. The median duration of radiation isolation was 9 days (range 5 to 15 days).
Table 2.
Estimated absorbed radiation doses (supplemental)
| Organ Site | Absorbed radiation dose (cGy/mCi) | Absorbed radiation dose (Gy) | ||
|---|---|---|---|---|
| Median | Range | Median | Range | |
| Lungs | 5.36 | 1.59–10.8 | 26.9 | 14.6–28.1 |
| Liver | 3.94 | 1.27–5.72 | 18.2 | 6.5–27.9 |
| Kidney | 3.53 | 0.42–6.13 | 14.0 | 2.96–27.5 |
| Spleen | 6.08 | 1.97–31.1 | 27.8 | 10.1–207 |
| Thyroid | 4.67 | 0.66–23.6 | 26.8 | 1.88–108 |
| Brain | 0.55 | 0.28–0.89 | 2.66 | 1.33–4.67 |
| Bone Marrow | 0.86 | 0.45–1.24 | 4.06 | 2.26–17.01 |
| Total Body | 0.87 | 0.4–1.25 | 4.22 | 2.6–6.54 |
Fludarabine
Fludarabine was administered to patients while in radiation isolation according to the dose escalation schema summarized in Table 3 starting approximately 72 hours following the therapeutic 131I-tositumomab infusion in order to overlap with when lymph node retention of radioisotope typically exceeds that of non-target organs. Fludarabine was escalated from 10mg/m2 daily × 5 days (total dose 50mg/m2) to 30mg/m2 daily × 7 days (total dose 210mg/m2) without observation of a DLT.
Table 3.
Dose escalation schema and dose limiting toxicities (DLT) of High-dose (27Gy) I-131 tositumomab + fludarabine + ASCT
| Dose level | n | Fludarabine daily dose (mg/m2) | # of doses | Total dose (mg/m2) | # DLT |
|---|---|---|---|---|---|
| 0 | 7 | 10 | 5 | 50 | 0 |
| 1 | 5 | 15 | 5 | 75 | 0 |
| 2 | 5 | 20 | 5 | 100 | 0 |
| 3 | 5 | 25 | 5 | 125 | 0 |
| 4 | 6 | 30 | 5 | 150 | 0 |
| 5 | 5 | 30 | 6 | 180 | 0 |
| 6 | 3 | 30 | 7 | 210 | 0 |
ASCT
The median infused CD34 cell dose was 5.42 ×106/kg (range 2.4 to 13.2 ×106/kg) and occurred a median of 14 days (range 12 to 18 days) following the therapeutic 131I-tositumomab infusion.
Estimation of the MTD, Early Toxicity, and Engraftment
There were no treatment related deaths, no grade III/IV toxicities on the Bearman scale, and no DLTs observed. The MTD was estimated to be ≥210mg/m2 fludarabine combined with 131I-tositumomab to deliver 27Gy to critical normal organs. Twenty-five patients (69%) remained outpatients for the duration of the post transplant period after discharge from radiation isolation and did not require hospitalization for toxicity. Only 2 patients developed grade 4 non-hematologic toxicities (hypokalemia/hypophosphatemia, depression). Within the first 100 days after transplant, grade 3 non-hematologic adverse events were observed in 28 patients with the most frequent being infection (n=16; fever without neutropenia, febrile neutropenia, clostridium difficile colitis), gastrointestinal toxicity (n=10; anorexia, nausea, diarrhea), and laboratory/metabolic abnormalities (n=9, electrolyte abnormalities, hypoalbuminemia, elevated transaminases). The one early post transplant death resulted from renal failure due to ureteral obstruction from progressive tumor. The details of key non-hematologic adverse events are summarized in Table 4. There was no correlation of grade of toxicity with dose level.
Table 4.
Patients with grade 3–5 non-hematologic adverse events within 100 days of autologous stem cell transplantation regardless of attribution. (Supplemental)
| Adverse event | Grade 3 | Grade 4 | Grade 5 |
|---|---|---|---|
| Allergy/Immunology | 2 (6) | - | - |
| Cardiac General | 2 (6) | - | - |
| Constitutional Symptoms | 1 (3) | - | - |
| Dermatology/Skin | 2 (6) | - | - |
| Gastrointestinal | 10 (28) | - | - |
| Genitourinary | 1 (3) | - | 1 (3)* |
| Hemorrhage | 6 (17) | - | - |
| Hepatic | - | - | - |
| Infection/Febrile neutropenia | 16 (44) | - | - |
| Lymphatics | 1 (3) | - | - |
| Metabolic/Laboratory | 9 (24) | 1 (3) | - |
| Musculoskeletal | 1 (3) | - | - |
| Neurologic | 1 (3) | 1 (3) | - |
| Pain | 3 (8) | - | - |
| Pulmonary | 4 (11) | 0 (0) | - |
| Secondary Malignancy | - | - | - |
| Vascular | 1 (3) | - | - |
| Any adverse event | 28 (78) | 2 (6) | 1 (3)* |
Values are numbers (percentages) of patients. Adverse events were graded according to the NCI CTCAE 3.0 scale.
Renal failure and death due to tumor progression.
Expected myeloablation and grade 4 hematopoietic toxicity was observed in all patients. The median time to neutrophil (>500/μL) and platelet (>20 K/μl) engraftment was 10 days (range 8–18 days) and 12 days (range 1–>27 days) after ASCT, respectively. Seven patients required platelet transfusions beyond day 30.
Response, Overall and Progression-Free Survival
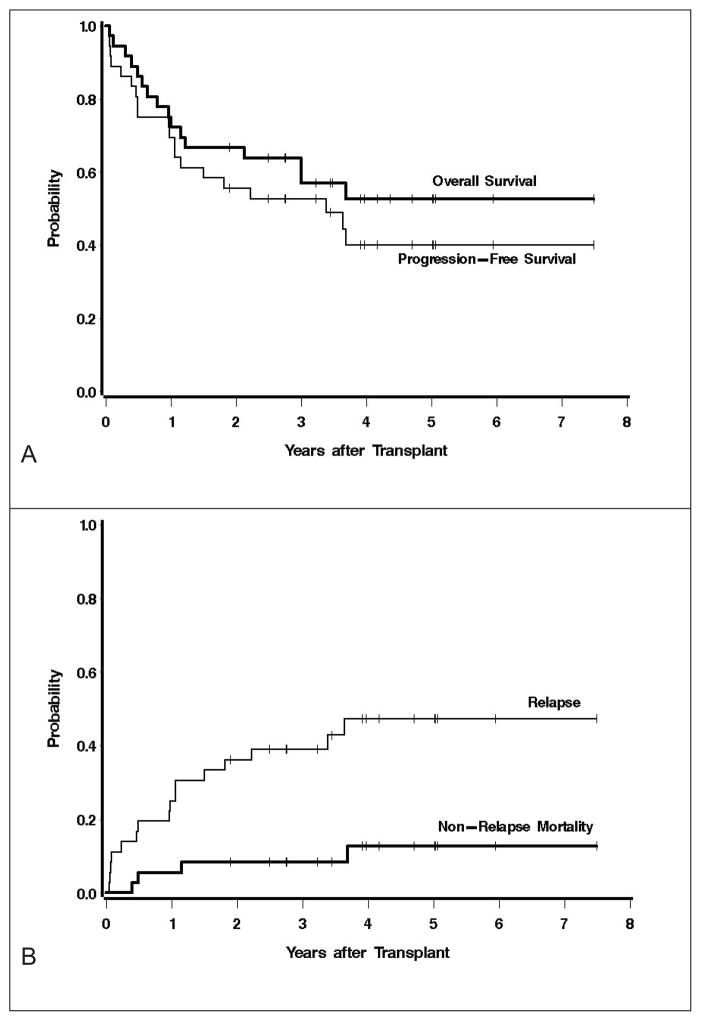
Post-transplant remission status included CR/CRu = 26 (79%), PR = 2 (6%), SD = 4 (11%), and PD = 4 (11%). Remission status by histology included MCL (19 [83%] CR, 1 [4%] PR), diffuse large B-cell lymphoma (3 [50%] CR, 0 PR), and indolent lymphoma (3CR/uCR [60%], 1PR [20%]). Twenty patients are currently alive and 16 are alive and progression-free with a median follow up of 3.9 yrs. The 4 patients with evidence of pretransplant minimal residual disease (MRD) in the marrow (4 flow +, 1 flow and PCR +) attained a MRD negative state by 30 days after transplant. The estimated 3 year overall and progression-free survival was 54% and 53% respectively (Figure 2A). The cumulative incidence of relapse and non-relapse mortality at 3 years were 41%, and 7%, respectively (Figure 2B).
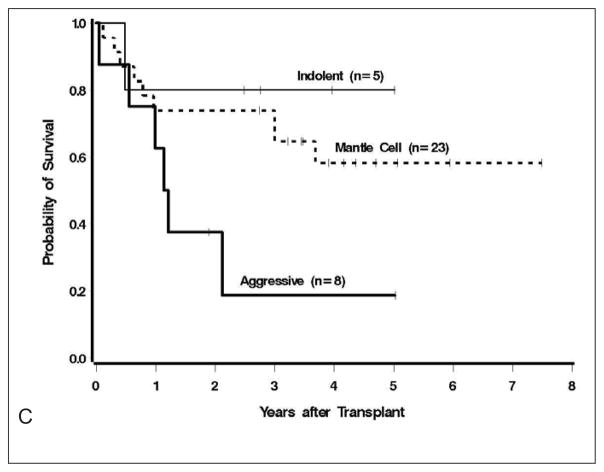
Figure 2.
A) Overall and progression free survival at median follow up of 3.9 years. B) Cumulative incidence of relapse and non-relapse mortality. C) Survival outcomes by B-cell lymphoma histology
Univariate analysis indicated that increased mortality was observed in those with ≥2 prior regimens (HR=6.80, 95% CI 1.53–30.36, p=.01) and trend to inferior survival was noted in patients with diffuse large B-cell lymphoma (HR=2.78 95% CI 0.96–8.06, p=.06, Figure 2C) and chemoresistant disease (HR=2.45, 95% CI 0.88–6.81, p=.09). In contrast there was no statistical association of inferior survival in those with more advanced age (HR=1.05, p=.45).
Delayed Effects
Three cases of AML/MDS developed following therapy including 1 with normal cytogenetics, mutated nucleophosmin, and unmutated FLT3, 1 with complex cyotogenetics in a patient harboring cytogenetic abnormalities prior to transplant, and 1 with unknown cytogenetics. One additional patient developed squamous cell carcinoma of the skin. Late non-relapse deaths also occurred in 4 patients due to pneumonia/pnuemonitis (2), unknown causes (1), and renal failure following an allogeneic transplant for MDS (1), at 5 months, 6 months, 1.1 yrs, and 3.7 yrs, respectively.
Discussion
In this manuscript, we describe the first use of concurrent chemoradioimmunotherapy in a myeloablative setting and demonstrate that up 210mg/m2 of fludarabine can be safely administered along with 131I-tositumomab to deliver ≤27Gy to critical normal organs in older adults with B-NHL. This study builds on our prior work showing that myeloablative doses of single agent 131I-tositumomab could be utilized as a safe and effective approach in older adults.(9) Since not all patients were cured with the use of our prior single-agent high-dose RIT approach and non-hematologic toxicity was minimal, we undertook a rational strategy to further optimize this regimen by adding fludarabine, an agent we demonstrated in preclinical models to best optimize the antitumor effects of RIT when compared to more traditional high-dose compounds such as cyclophosphamide and etoposide.(10, 11) We and others have previously reported the combinations of fludarabine and radioimmunotherapy, however, without the use of hematopoietic stem cell support, the ability to overlap these therapies has been limited due to myelosuppression.(18),(19, 20) Furthermore, no groups have attempted to maximize the synergistic effect by escalating the total fludarabine exposure beyond those used in standard regimens.
We were able to escalate the total dose of fludarabine to 210 mg/m2 without identifying a true MTD and did not attempt to deliver additional doses since the chemotherapy dates would have encroached upon the day of PBSC infusion. Despite the high cumulative amount of this known lymphodepleting agent increased rates of opportunistic infections, such as cytomegalovirus, were not observed and likely abrogated by the T-cell replete autologous graft. We did observe secondary myeloid disorders, though our sample size is not sufficient to determine if the rates were significantly lower than observed in other studies of fludarabine combinations and transplant.(21, 22) The 3 cases of MDS/AML following this regimen identified by prospectively collected annual bone marrow evaluations is similar to a much larger retrospectively collected data set in younger patients (median age 40 years) showing rates 8.6% at 6 years.(23) Notably, our cases included one patient with evidence of a malignant myeloid clone based on abnormal bone marrow cytogenetics prior to transplant and a second with a phenotype (FLT3 negative, NPM1 positive) not typically associated therapy-induced AML. This second patient achieved and has maintained a CR for over 4 years with cytarabine-idarubicin induction followed by high-dose cytarabine consolidation. We did, however, observe 2 late non-relapse deaths in patients with lung toxicity and infection, potentially related to their radiation-based conditioning regimen.
Efficacy estimates were not a primary endpoint of this phase I trial, thus, major conclusions should not be drawn from these data. The best results were observed in patients with mantle cell lymphoma and indolent B-cell lymphoma with the majority achieving remission durations beyond 3 years. In contrast, only 2 of 8 of patients with DLBCL a achieved progression-free survival over 2 years in our series. These results are not surprising, since all had resistance or early relapse after R-chemo, a feature clearly associated with poor outcome in similar, but younger patients.(24)
It is important to note that certain specialized expertise and infrastructure for handling high-activity gamma emitting radionuclides is required for delivering this therapy. Patients at our center were housed for a median of 9 days in standard lead lines rooms (the same rooms also utilized for 131I thyroid ablation). This prolonged radiation isolation stay clearly may add to the cost of the transplant, however, it is in contrast to the median of 19.5 inpatient days required for patients age 60 years and older undergoing BEAM conditioning for lymphoma at our center. A recent validation study from the EBMT suggests that the maximum acceptable time in the hospital for lymphoma patients undergoing autologous transplantation is 25 days, much longer than our patients experienced. (25) Our current studies focus on the therapeutic use of isotopes such as 90Y which are free of appreciable gamma emissions targeting either CD20 or CD45, further reducing the potential economic cost and broadening the applicability of this approach.(26, 27)
This study is the largest prospective autologous transplant trial designed for older patients with lymphoma and highlights the importance of developing novel conditioning regimens for adults in this age group. A recent retrospective EBMT series suggested that despite the majority of DLBCL diagnoses occurring in adults over the age of 60, only 18% of autologous transplants were performed in this age group and these patients suffered twice the rate of non-relapse mortality.(4) Similarly, in mantle cell lymphoma, where transplant evaluated in prospective trials appears to improve outcomes, most trials to-date have limited the upper age to 60 to 65 years, relegating such individuals to less intensive strategies.(28–31) In contrast, our series included 23 patients with MCL, 10 of whom were over the age of 65 years. To place these data in some context, a selection of series evaluating autologous transplant for lymphoma in older adults is provided in table 5, though caution should be employed in comparing non-randomized series of patients with varied baseline features.
Table 5.
Data from selected series of lymphoma patients undergoing high-dose therapy and autologous transplant.
| First Author/year | Median Age (range) | n | Design | Conditioning | 100-day TRM | NRM | OS | PFS |
|---|---|---|---|---|---|---|---|---|
| Gopal 2001(2) | 62 (60–68) | 53 | Retrospective | BuMelTT (45%), CY-TBI/CY-VP-16-TBI (45%) | 9.4% | 22% at 4y | 33% at 4y | 24% at 4y |
| Buadi 2006(32) | 66 (60–77) | 93 | Retrospective | BEAM (60%), BEAC (40%) | 5.4% | NA | Median 25m | Median 13m |
| Jantunen 2006(3) | 63 (60–70) | 88 | Retrospective | BEAC (56%), BEAM (39%) | 11% | 19% at 4y | 44% at 5 y | 45% at 5 yrs |
| Gopal 2007(9) | 64 (60–76) | 24 | Prospective | Anti-CD20 RIT (100%) | 0% | 4% at 2y | 59% at 3y | 51% at 3y |
| Jantunen 2008(4) | 63 (60–74) | 463 | Registry | BEAM (73%) | 4.4% | 10.8% at 3y | 60% at 3y | 51% at 3y |
| Wildes 2008(33) | 64 (60–73) | 59 | Retrospective | BEAM (100%) | 8.5% | NA | Median 48m | Median 21m |
| Elstrom 2012(34) | 71 (69–86) | 21 | Retrospective | CBV or BEAM(86%) | 19% | NA | Median 8m | Median 18m |
| Gopal 2014 (this study) | 65 (60–76) | 36 | Prospective | Anti-CD20-RIT+ fludarabine | 0% | 7% at 3y | 54% at 3y | 53% at 3y |
TRM=treatment-related mortality, NRM=non-relapse mortality, OS= overall survivial, PFS=prpgression free survival, Bu=busulfan, Mel=melphalan, TT=thiotepa, CY=cyclophosphamide, TBI=total body irradiation, BEAM=carmustine, etoposide, cytarabine, melphalan, BEAC= carmustine, etoposide, cytarabine, cyclophosphamide, Anti-CD20-RIT= I-131-tositumomab, CBV=cylcophosphamide, carmustine, etoposide
We hypothesized that both the targeted delivery and individualized pharmacokinetically-based dosing of the radioimmunoconjuate make it an ideal approach for older adults who may have increased variation in organ function for drug clearance and impaired tissue repair mechanisms. We then built on this concept by translating preclinical data indicating that nucleoside analogs may be a preferred agent to concurrently combine with myeloablative radioimmunotherapy. The results of this trial indicate that high cumulative doses of fludarabine can be safely delivered with high-dose 131I-tositumomab and more importantly strengthen the contention that arbitrary age cutoffs should not be implemented for transplant eligibility, particularly with regimens specifically designed for an older age group.
Acknowledgments
Grant support:
NCI P01CA44991, NCI R01CA076287, NCI R01 CA138720, Hutchinson Center/University of Washington Cancer Consortium Cancer Center Support Grant P30 CA015704, The Lymphoma Research Foundation, the Mary Aileen Wright and Frederick Kullman Memorial Funds, and gifts from Frank and Betty Vandermeer. AKG is a Scholar in Clinical Research of the Leukemia and Lymphoma Society. Study drug was provided by Glaxo-Smith Kline.
The authors would like to thank Lacey Hedin, Jennifer Davies, Sally Lundberg, RN, Martha Bien, Michele Wanner, NMT, Carolyn Thostenson, Shani Frayo, and the patients who courageously participated in this study.
Grant support:
NCI P01CA44991, NCI R01CA076287, NCI R01 CA138720, Hutchinson Center/University of Washington Cancer Consortium Cancer Center Support Grant P30 CA015704, The Lymphoma Research Foundation, the Mary Aileen Wright and Frederick Kullman Memorial Funds, and gifts from Frank and Betty Vandermeer. AKG is a Scholar in Clinical Research of the Leukemia and Lymphoma Society. Study drug was provided by Glaxo-Smith Kline.
Footnotes
Presented in part in oral format at the 53th annual meeting of the American Society of Hematology, San Diego, CA, December 12, 2011.
Author Contributions
Conception and Design: AKG, TAG, JGR, JMP, DRF, FRA, OWP
Data collection: AKG, DRF, JGR, RDC
Data Analysis: AKG, TAG, RDC
Data Interpretation: All authors
Manuscript writing: All authors
Approval of final manuscript: All authors
Conflict of interest:
Glaxo-Smith Kline provided study drug for this trial. DGM receives research funding from Glaxo-Smith Kline for a separate study. No other potential conflicts were identified.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA: a cancer journal for clinicians. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Gopal AK, Gooley TA, Golden JB, et al. Efficacy of high-dose therapy and autologous hematopoietic stem cell transplantation for non-Hodgkin’s lymphoma in adults 60 years of age and older. Bone Marrow Transplant. 2001;27:593–599. doi: 10.1038/sj.bmt.1702833. [DOI] [PubMed] [Google Scholar]
- 3.Jantunen E, Itala M, Juvonen E, et al. Autologous stem cell transplantation in elderly (>60 years) patients with non-Hodgkin’s lymphoma: a nation-wide analysis. Bone Marrow Transplant. 2006;37:367–372. doi: 10.1038/sj.bmt.1705266. [DOI] [PubMed] [Google Scholar]
- 4.Jantunen E, Canals C, Rambaldi A, et al. Autologous stem cell transplantation in elderly patients (> or =60 years) with diffuse large B-cell lymphoma: an analysis based on data in the European Blood and Marrow Transplantation registry. Haematologica. 2008;93:1837–1842. doi: 10.3324/haematol.13273. [DOI] [PubMed] [Google Scholar]
- 5.Press OW, Eary JF, Appelbaum FR, et al. Radiolabeled-antibody therapy of B-cell lymphoma with autologous bone marrow support [see comments] N Engl J Med. 1993;329:1219–1224. doi: 10.1056/NEJM199310213291702. [DOI] [PubMed] [Google Scholar]
- 6.Press OW, Eary JF, Gooley T, et al. A phase I/II trial of iodine-131-tositumomab (anti-CD20), etoposide, cyclophosphamide, and autologous stem cell transplantation for relapsed B-cell lymphomas. Blood. 2000;96:2934–2942. [PubMed] [Google Scholar]
- 7.Gopal AK, Gooley TA, Maloney DG, et al. High-dose radioimmunotherapy versus conventional high-dose therapy and autologous hematopoietic stem cell transplantation for relapsed follicular non-Hodgkin lymphoma: a multivariable cohort analysis. Blood. 2003;102:2351–2357. doi: 10.1182/blood-2003-02-0622. [DOI] [PubMed] [Google Scholar]
- 8.Nademanee A, Forman S, Molina A, et al. A phase 1/2 trial of high-dose yttrium-90-ibritumomab tiuxetan in combination with high-dose etoposide and cyclophosphamide followed by autologous stem cell transplantation in patients with poor-risk or relapsed non-Hodgkin lymphoma. Blood. 2005;106:2896–2902. doi: 10.1182/blood-2005-03-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopal AK, Rajendran JG, Gooley TA, et al. High-dose [131I]tositumomab (anti-CD20) radioimmunotherapy and autologous hematopoietic stem-cell transplantation for adults > or = 60 years old with relapsed or refractory B-cell lymphoma. J Clin Oncol. 2007;25:1396–1402. doi: 10.1200/JCO.2006.09.1215. [DOI] [PubMed] [Google Scholar]
- 10.Johnson TA, Press OW. Synergistic cytotoxicity of iodine-131-anti-CD20 monoclonal antibodies and chemotherapy for treatment of B-cell lymphomas. Int J Cancer. 2000;85:104–112. doi: 10.1002/(sici)1097-0215(20000101)85:1<104::aid-ijc19>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Gopal AK, Pagel JM, Rajendran JG, et al. Improving the Efficacy of Reduced Intensity Allogeneic Transplantation for Lymphoma using Radioimmunotherapy. Biol Blood Marrow Transplant. 2006;12:697–702. doi: 10.1016/j.bbmt.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 12.Begleiter A, Pugh L, Israels LG, Johnston JB. Enhanced cytotoxicity and inhibition of DNA damage repair in irradiated murine L5178Y lymphoblasts and human chronic lymphocytic leukemia cells treated with 2′-deoxycoformycin and deoxyadenosine in vitro. Cancer research. 1988;48:3981–3986. [PubMed] [Google Scholar]
- 13.Eary JF, Press OW, Badger CC, et al. Imaging and treatment of B-cell lymphoma. J Nucl Med. 1990;31:1257–1268. [PubMed] [Google Scholar]
- 14.Rajendran JG, Fisher DR, Gopal AK, Durack LD, Press OW, Eary JF. High-dose (131)I-tositumomab (anti-CD20) radioimmunotherapy for non-Hodgkin’s lymphoma: adjusting radiation absorbed dose to actual organ volumes. J Nucl Med. 2004;45:1059–1064. [PubMed] [Google Scholar]
- 15.Cheson BD, Horning SJ, Coiffier B, et al. Report of an International Workshop to Standardize Response Criteria for Non-Hodgkin’s Lymphomas. J Clin Oncol. 1999;17:1244–1253. doi: 10.1200/JCO.1999.17.4.1244. [DOI] [PubMed] [Google Scholar]
- 16.Bearman SI, Appelbaum FR, Buckner CD, et al. Regimen-related toxicity in patients undergoing bone marrow transplantation. J Clin Oncol. 1988;6:1562–1568. doi: 10.1200/JCO.1988.6.10.1562. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Leonard JP, Coleman M, Kostakoglu L, et al. Abbreviated chemotherapy with fludarabine followed by tositumomab and iodine I 131 tositumomab for untreated follicular lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:5696–5704. doi: 10.1200/JCO.2005.14.803. [DOI] [PubMed] [Google Scholar]
- 19.Bethge WA, Lange T, Meisner C, et al. Radioimmunotherapy with yttrium-90-ibritumomab tiuxetan as part of a reduced- intensity conditioning regimen for allogeneic hematopoietic cell transplantation in patients with advanced non-Hodgkin lymphoma: results of a phase 2 study. Blood. 2010;116:1795–1802. doi: 10.1182/blood-2010-02-270538. [DOI] [PubMed] [Google Scholar]
- 20.Gopal AK, Guthrie KA, Rajendran J, et al. (9)(0)Y-Ibritumomab tiuxetan, fludarabine, and TBI-based nonmyeloablative allogeneic transplantation conditioning for patients with persistent high-risk B-cell lymphoma. Blood. 2011;118:1132–1139. doi: 10.1182/blood-2010-12-324392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrison VA, Rai KR, Peterson BL, et al. Therapy-related myeloid leukemias are observed in patients with chronic lymphocytic leukemia after treatment with fludarabine and chlorambucil: results of an intergroup study, cancer and leukemia group B 9011. J Clin Oncol. 2002;20:3878–3884. doi: 10.1200/JCO.2002.08.128. [DOI] [PubMed] [Google Scholar]
- 22.Waterman J, Rybicki L, Bolwell B, et al. Fludarabine as a risk factor for poor stem cell harvest, treatment-related MDS and AML in follicular lymphoma patients after autologous hematopoietic cell transplantation. Bone marrow transplantation. 2012;47:488–493. doi: 10.1038/bmt.2011.109. [DOI] [PubMed] [Google Scholar]
- 23.Krishnan A, Bhatia S, Slovak ML, et al. Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors. Blood. 2000;95:1588–1593. [PubMed] [Google Scholar]
- 24.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4184–4190. doi: 10.1200/JCO.2010.28.1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanza F, Campioni DC, Hellmann A, et al. Individual quality assessment of autografting by probability estimation for clinical endpoints: a prospective validation study from the European group for blood and marrow transplantation. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2013;19:1670–1676. doi: 10.1016/j.bbmt.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Gopal AK, Pagel JM, Fromm JR, Wilbur S, Press OW. 131I anti-CD45 radioimmunotherapy effectively targets and treats T-cell non-Hodgkin lymphoma. Blood. 2009;113:5905–5910. doi: 10.1182/blood-2009-02-205476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gopal AK, Press OW, Wilbur SM, Maloney DG, Pagel JM. Rituximab blocks binding of radiolabeled anti-CD20 antibodies (Ab) but not radiolabeled anti-CD45 Ab. Blood. 2008;112:830–835. doi: 10.1182/blood-2008-01-132142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dreyling M, Lenz G, Hoster E, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105:2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 29.Delarue R, Haioun C, Ribrag V, et al. CHOP and DHAP plus rituximab followed by autologous stem cell transplantation in mantle cell lymphoma: a phase 2 study from the Groupe d’Etude des Lymphomes de l’Adulte. Blood. 2013;121:48–53. doi: 10.1182/blood-2011-09-370320. [DOI] [PubMed] [Google Scholar]
- 30.Geisler CH, Kolstad A, Laurell A, et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: a nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood. 2008;112:2687–2693. doi: 10.1182/blood-2008-03-147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kluin-Nelemans HC, Hoster E, Hermine O, et al. Treatment of older patients with mantle-cell lymphoma. The New England journal of medicine. 2012;367:520–531. doi: 10.1056/NEJMoa1200920. [DOI] [PubMed] [Google Scholar]
- 32.Buadi FK, Micallef IN, Ansell SM, et al. Autologous hematopoietic stem cell transplantation for older patients with relapsed non-Hodgkin’s lymphoma. Bone marrow transplantation. 2006;37:1017–1022. doi: 10.1038/sj.bmt.1705371. [DOI] [PubMed] [Google Scholar]
- 33.Wildes TM, Augustin KM, Sempek D, et al. Comorbidities, not age, impact outcomes in autologous stem cell transplant for relapsed non-Hodgkin lymphoma. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation. 2008;14:840–846. doi: 10.1016/j.bbmt.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Elstrom RL, Martin P, Hurtado Rua S, et al. Autologous stem cell transplant is feasible in very elderly patients with lymphoma and limited comorbidity. American journal of hematology. 2012;87:433–435. doi: 10.1002/ajh.23108. [DOI] [PubMed] [Google Scholar]