Abstract
Recent molecular modeling data using collagen peptides predicted that mechanical force transmitted through intermolecular cross-links resulted in collagen triple helix unwinding. These simulations further predicted that this unwinding, referred to as triple helical microunfolding, occurred at forces well below canonical collagen damage mechanisms. Based in large part on these data, we hypothesized that mechanical loading of glycation cross-linked tendon microfibers would result in accelerated collagenolytic enzyme damage. This hypothesis is in stark contrast to reports in literature that indicated that individually mechanical loading or cross-linking each retards enzymatic degradation of collagen substrates. Using our Collagen Enzyme Mechano-Kinetic Automated Testing (CEMKAT) System we mechanically loaded collagen-rich tendon microfibers that had been chemically cross-linked with sugar and tested for degrading enzyme susceptibility. Our results indicated that cross-linked fibers were >5 times more resistant to enzymatic degradation while unloaded but became highly susceptible to enzyme cleavage when they were stretched by an applied mechanical deformation.
Keywords: Collagen, Collagenase, Tendon Microfibers, Enzyme Mechano-Kinetic Cleavage, Glycation Cross-linking
1. Introduction
Fibrillar collagens are structural proteins that assemble into a complex ordered structure of molecules cross-linked together to form an interconnected supramolecular fibril structure (Ottani et al., 2002). Collagens contribute to the mechanical properties of almost all tissues throughout the body including skin, tendon, ligament, bone, and cartilage (Bailey et al., 1998; Ottani et al., 2002).
Collagen is highly resistant to enzymatic breakdown, but is susceptible to a small number of specialized collagenolytic enzymes or collagenases. In part due to this resistance to enzymatic cleavage, collagen has a very slow turnover rate in many tissues of the body. The half-life for collagen has been reported on the order of decades in healthy tissues (Bank et al., 1999; Maroudas et al., 1992; Verzijl et al., 2000b). Due to the long protein half-life in vivo, collagen is one of the proteins that undergo spontaneous glycation and the formation of measurable amounts of Advanced Glycation End products (AGEs) during aging (Choudhary et al., 2011; Sell and Monnier, 2004; Verzijl et al., 2000a; Verzijl et al., 2000b).
Glycation, also called non-enzymatic glycosylation, is a spontaneous, nonenzymatic process in which a reducing sugar such as glucose or fructose reacts with a free amino group (e.g. lysine or arginine) to form a reactive Schiff base. The Schiff base then rearranges to form an Amadori product, which undergoes further reactions, collectively known as a Maillard reaction, to form AGEs (Aronson, 2003; Bailey et al., 1998). Of interest is that these reactions can result in stable covalent cross-links between two amine groups of amino acids such as lysine or arginine (Bailey et al., 1998; Sell and Monnier, 2004; Verzijl et al., 2002).
AGE accumulation in soft tissues is a function of tissue aging and accelerated by diabetes due to hyperglycemia (Bai et al., 1992; Freemont and Hoyland, 2007; Reddy, 2003; Reddy et al., 2002). AGE cross-linking results in changes in the mechanical properties of soft tissues, which include increased Young’s modulus, maximum failure load and toughness, while in mineralized tissue there are minimal changes in these properties after glycation (Reddy, 2003; Reddy et al., 2002). In addition AGE cross-linking has been implicated in a variety of pathological aging-related changes, including vascular (Aronson, 2003) and articular cartilage stiffening (Chen et al., 2002; Verzijl et al., 2000a) which might contribute to a arteriosclerosis and osteoarthritis, respectively.
Computational molecular modeling was previously performed using steered molecular dynamics to simulate mechanical loading of a collagen covalent cross-link (Bourne and Torzilli, 2011). These loading conditions approximated mechanical force transmitted through covalent intermolecular cross-links, such as those caused by AGEs. Computational results predicted that force transmitted via cross-links would result in local disruption and microunfolding of the collagen triple helix at approximately 350 pN (minor microunfolding) and 900 pN (major microunfolding) (Bourne and Torzilli, 2011), and well below previously described collagen failure mechanisms (Bourne and Torzilli, 2011; Buehler, 2006; Tang et al., 2010).
Due to the hierarchical structure of collagen within the extracellular matrix of tissues, tensile forces applied at the macro and micro scales are transmitted via intermolecular cross-links (Puxkandl et al., 2002). At the molecular level (nanoscale) this results in forces being transmitted across the amino acid side chains that form bridging intramolecular cross-links (Tang et al., 2010). Recent molecular modeling results now indicate that the transmission of tensile load via these cross-links can cause disruption and microunfolding of the triple helix and that these microunfolding events occur at force levels (<1,000 pN) below previously reported damage mechanisms (Bourne and Torzilli, 2011).
Based on our molecular modeling results we hypothesized that mechanical forces on cross-linked collagen substrates would accelerate enzyme degradation. We tested this by applying tensile deformation to glycation cross-linked microscale tendon fibers (fascicles of ~300 µm diameter) and quantified the rate of collagenase enzymatic degradation.
2. Results
2.1. Relative Fluorescence Measurements
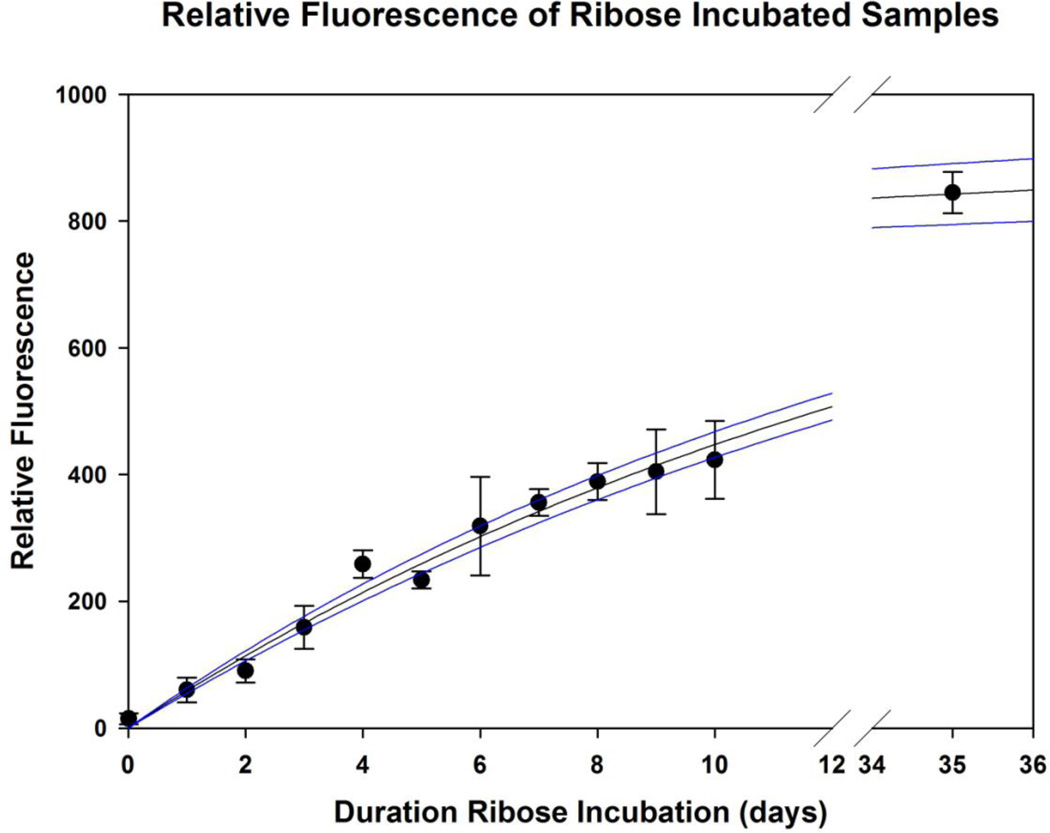
Ribose incorporation into fibers over time was assessed by fluorescence of solubilized fibers following 0 to 10 days of incubation (Verzijl et al., 2002). Maximum fluorescence was estimated from measurements of fibers incubated in ribose for 5 weeks, and data was well described by a 2 parameter exponential rise-to-max equation (R2 = 0.99) (Figure 1). 3-day and 7-day ribose incubation results in approximately 20% and 40% fluorescence relative to maximum, respectively.
Figure 1. Relative Fluorescence of Ribose Incubated Tail Tendon Samples.
Tail tendon microscale fibers were incubated in ribose for 0–35 days and relative fluorescence was measured at daily to day 10, and a final maximum was fluorescence was approximated with a measurement at day 35 (5 weeks). Data was fit with an exponential rise-to-max equation, and shown with 95% confidence bands.
2.2. Unloaded Fiber Enzyme Susceptibility Test
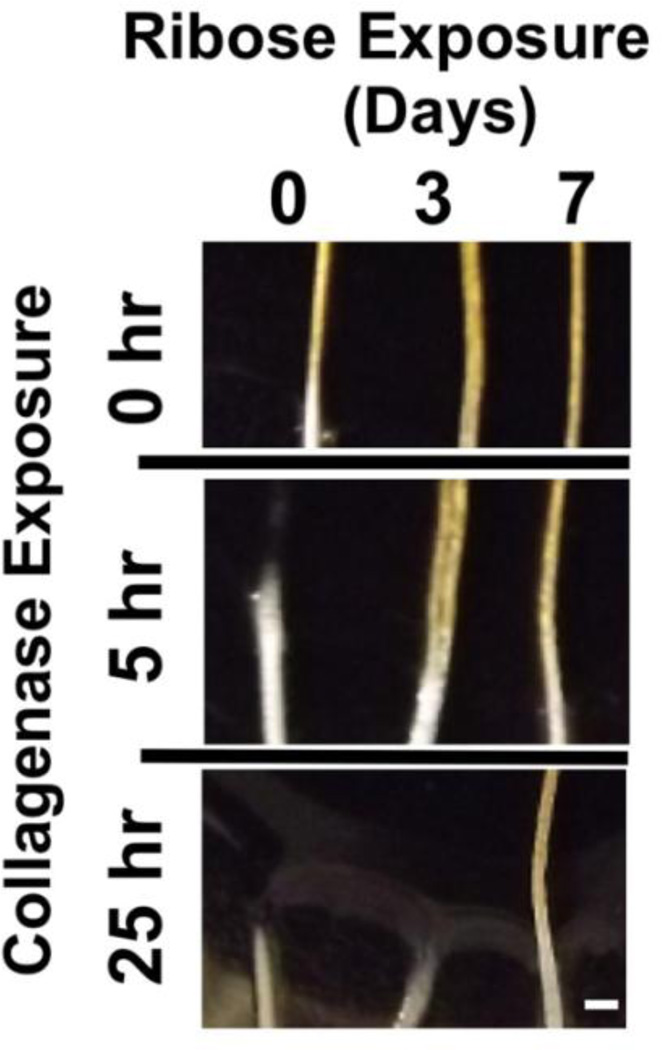
Tendon fascicles were tested for susceptibility to enzymatic degradation in an unloaded state by observing fiber digestion to dissolution over a ~24 hour period. Comparable diameter tendon microfibers were obtained from the same animal and incubated unloaded in a 1% by weight bacterial collagenase solution dissolved in Dulbecco’s PBS with calcium and magnesium. In all 3 tests, the non-glycated fiber degraded and dissolved fastest, followed by the 3-day glycated fiber. The 7-day glycated fiber showed no visible degradation after prolonged enzyme exposure.
Representative results from one experiment are shown as Figure 2. In these results the 0-day native non-glycated fiber dissolved after approximately 5 hours of exposure, while the 3-day fiber failed within 20 hours of enzyme exposure. At the conclusion of the test, the 7-day fiber was intact with no visible degradation even after more than 25 hours of enzyme exposure, and had persisted approximately five times longer in the enzyme solution than was needed to completely dissolve a native non-glycated fiber.
Figure 2. Unloaded Fiber Enzyme Susceptibility.
Fibers of comparable size from the same tail were treated with 0.2 M Ribose for 0, 3 or 7 days and then exposed to a 1% collagenase solution at room temperature. Representative images at the air – collagenase interface of the fibers were digitally recorded at the 0 hours (start) and after 5 and 25 hours of collagenase exposure; the scale bar indicates 1 mm. The native fiber (0-day, no cross-linking) was completely degraded after 5 hours of exposure to collagenase, while the cross-linked fibers were still intact. Between 5 and 25 hours of collagenase exposure the 3-day cross-linked fiber was completely degraded, while the 7-day cross-linked fiber was intact with no visible deterioration after 25 hours of exposure to collagenase.
2.3. Tensile Strain at Equilibrium
Due to the viscoelastic nature of tendon, an applied peak strain resulted in a peak stress (t=0) that underwent subsequent time-dependent decrease in stress (mechanical stress relaxation) until reaching an equilibrium state at t>>0 (Wyatt et al., 2009). Previous mechanical relaxation tests using native non-glycated rat tail tendon fibers of similar dimension had shown an unusual increase in the tensile strain during stress relaxation, with an equilibrium strain > peak strain for native tail tendon microfibers (Wyatt et al., 2009). Therefore we were interested in comparing the mechanical relaxation strain response between native and cross-linked tissue.
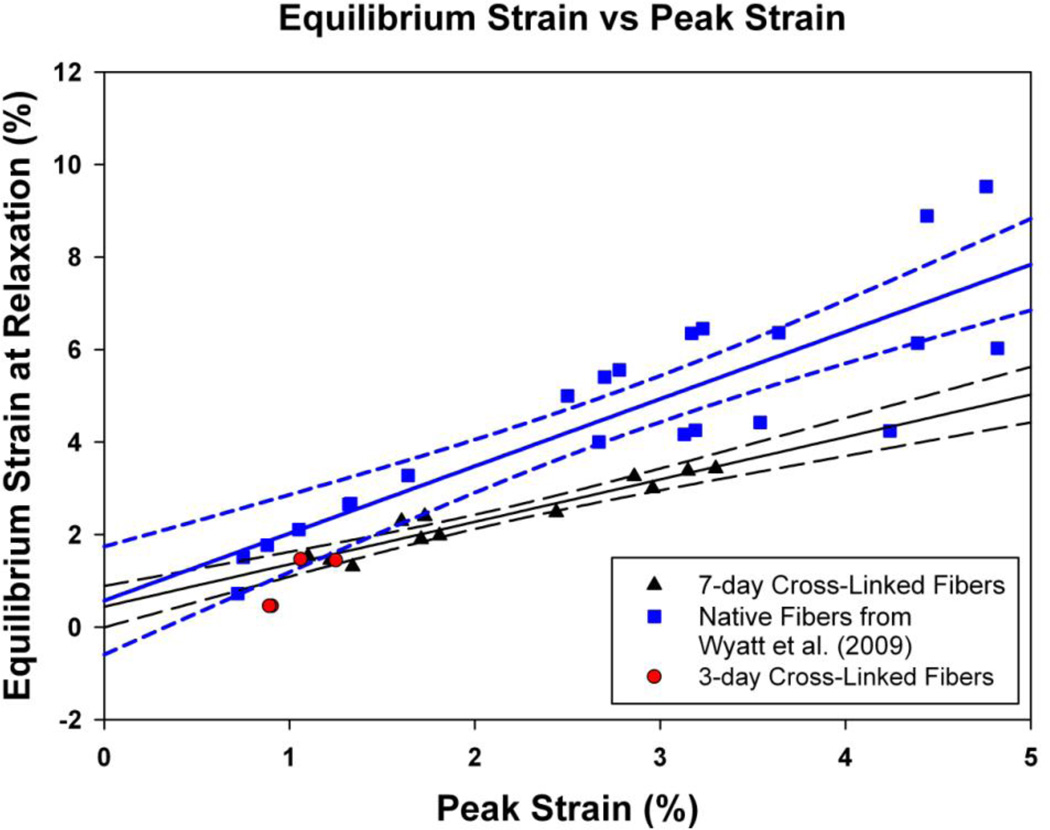
A scatter plot of the peak and equilibrium strains for the native and cross-linked fibers is shown in Figure 3. A regression analysis was performed for the native and 7-day cross-linked fibers using a linear (straight-line) fit to the equilibrium vs. peak strain data (SigmaPlot 10, Systat Software, Inc., Chicago, IL). The slope of the regression line for the native fibers was 1.45 ± 0.18 (slope ± standard error, r = 0.865, n = 23) and was different from unity (p = 0.02). The slope of 7-day cross-linked fibers was 0.92 ± 0.09 (r = 0.904, n = 12), and was not statistically different from unity (i.e., peak = equilibrium, p = 0.39). However, the regression slope of the 7-day cross-linked fibers was statistically less than the native fiber slope (one tailed t-test, p = 0.024). In addition, as to be expected the Y-intercepts (equilibrium strain at zero peak strain) for both regression fits were not statistically different from zero.
Figure 3. Equilibrium Strain vs. Peak Strain.
The peak and equilibrium strains from the mechanical relaxation tests for the 7-day glycation cross-linked fibers (black triangles) were compared to historical data for native fibers (blue squares) from our previous study (Wyatt et al., 2009). 3-day cross-linked fiber data is also shown (red circles) but excluded from regression analysis. The peak and equilibrium strains were plotted and the relationship between them was fit with a regression line model (mean regression line ± 95% confidence intervals as solid and dotted lines respectively) using SigmaPlot 10 software (Systat Software, Inc., Chicago, IL). The 7-day cross-linked fiber was not statistically different from 1 (n = 12), whereas the native fibers’ equilibrium strain was 45% greater after relaxation (n = 23).
2.4. CEMKATS Enzymatic Degradation Results
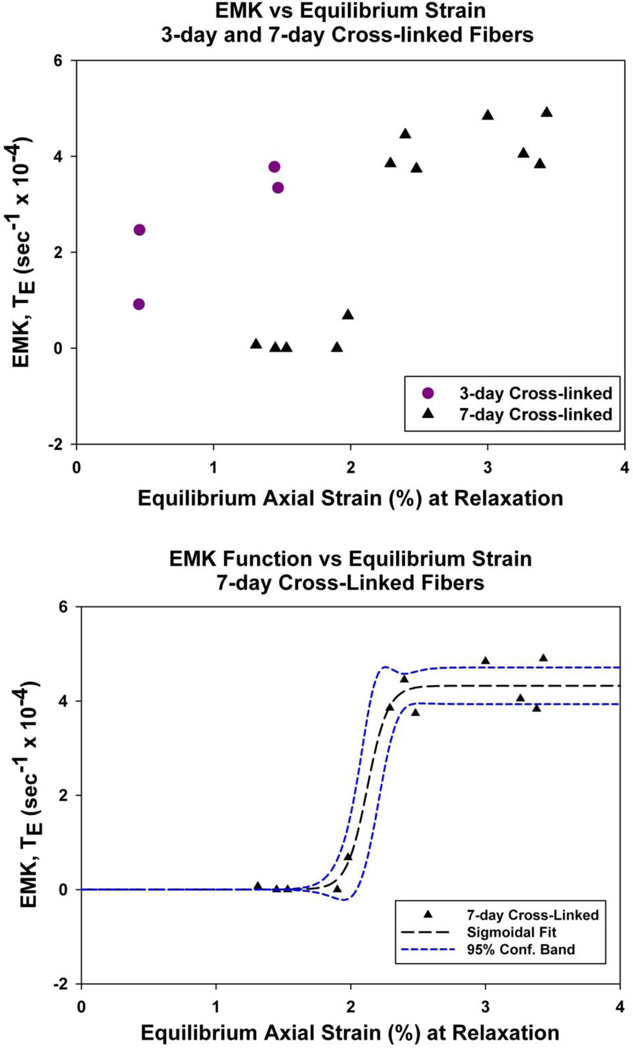
Glycation cross-linked fibers were tested for enzyme cleavage susceptibility during different amounts of applied uniaxial tensile deformation (1.3% to 3.4% equilibrium strain). In contrast with unloaded fibers’ resistance to enzyme degradation, the cross-linked tendon specimens appeared to become highly susceptible to enzymatic degradation under increasing mechanical strain. There was increasing cleavage for the ‘3-day cross-linked’ and ‘7-day cross-linked fibers’ with increasing mechanical strain, as indicated by higher EMK Function (TE) values with increasing strain (shown as a scatter plot, Figure 4).
Figure 4. EMK Function vs. Equilibrium Strain.
(Top) Both the ‘3-day cross-linked’ and ‘7-day cross-linked’ fibers showed increasing collagenase degradation with increasing strain (indicated by the increasing EMK Function, TE (ε), values).
(Bottom) The 7-day fiber data was well described with a 3-parameter sigmoidal function (r = 0.987), which indicates an “Off-to-On” transition around 2% as the axial strain increased from 0% to 3.4% (2.12 ± 0.05 % strain, coefficient ± standard error).
The plot of the EMK function vs. equilibrium axial strain for the 7-day cross-linked specimens suggests a binary “Off-to-On” cleavage response as the axial strain applied to the fibers increases from 0% to 3.4%. To statistically test for such a response the 7-day cross-linked fibers were separated into two groups, ‘high strain – easily degraded fibers’ (TE (ε) > 1) and ‘low strain – highly resistant fibers’ (TE (ε) < 1). The EMK function values for the degraded and resistant groups were TE = 4.237 ± 0.491 (mean ± standard error, n = 7) and TE = 0.15 ± 0.298 (n = 5), respectively, which were statistically different (p < 0.0001, GraphPad Software Inc., La Jolla, CA). Further, the EMK function value for the resistant group was not statistically different from zero (p > 0.3). As an alternative description, the 7-day cross-linked fiber data was fit with a 3-parameter sigmoidal function (r = 0.987, SigmaPlot 10), which indicates an “Off-to-On” cleavage transition at approximately 2% as the axial strain increased from 0% to 3.4% (transition strain = 2.12 ± 0.05%).
3. Discussion
3.1. Collagen AGE Cross-Linking
In this study, we utilized ribose to cross-link collagen as it is a well characterized reactive sugar model that exhibits a faster reaction rate than glucose, while yielding identical end products to those formed with glucose (Bai et al., 1992; Reddy et al., 2002; Tanaka et al., 1988). We selected the 3-day and 7-day incubation times with 0.2 M ribose based on glycation kinetic data from Tanaka et al. and Bai et al., which showed that incubation of 2–4 days was sufficient to cause measurable differences in fluorescence and collagen solubility due to AGE formation (Bai et al., 1992; Tanaka et al., 1988), and 7-days of ribose exposure was reported to nearly saturate the ultrastructural, fluorescent, and mechanical properties of rat tail tendon (Bai et al., 1992).
Rat-tail tendon was selected because it is composed of highly aligned collagen fibers, with lower levels of enzymatic cross-links than mature load-bearing tendons (Avery and Bailey, 2005; Eyre et al., 1984a; Eyre et al., 1984b). This tissue does undergo subsequent non-enzymatic cross-linking (e.g., glycation cross-linking) under physiologic conditions, which has been shown to alter mechanical properties during animal aging (Avery and Bailey, 2005).
Although molecular modeling of mechanical tensile loading of enzymatically cross-linked fibers is limited, available data suggest that force transmission by enzymatic cross-links would result in molecular elongation and preferential disruption of non-helical domains without causing helix microunfolding (Uzel and Buehler, 2011). Of note, these conformational changes are not expected to affect collagenase activity. In support of those predictions, rabbit patellar tendon experimental results found similar strain-dependent resistance to enzymatic degradation (Nabeshima et al., 1996) as we found using rat tail tendon. These results indicate that the presence of enzymatic cross-links do not induce degradation sensitivity during loading. Furthermore, based on the molecular modeling data reported by Uzel and Buehler (2011), in which the collagen helix remained intact during loading, we would not expect the content or type of enzymatic cross-links to significantly attenuate the glycation cross-link induced mechanical-enzymatic degradation response of other collagenous tissues.
3.2. Collagen Tensile Strain at Equilibrium
Our previous mechanical tests using untreated native rat tail tendons showed a relaxed to peak tensile strain ratio (εr/εp) after stress relaxation of approximately 1.71 ± 0.37 (εr/εp, mean ± standard deviation, n = 23), indicating that the strain increased during relaxation by 71% (Wyatt et al., 2009). Cross-linking appears to attenuate this tensile strain increase during relaxation, as we observed a smaller relaxed-to-peak tensile strain ratio of 1.15 ± 0.16 (εr/εp, n = 12) in the 7-day fibers (p < 0.0001). When we compared the results of the regression analysis (Results 2.3), the slope of the cross-linked fiber was not statistically different from unity. This suggests that the cross-linked fiber’s mechanical relaxation response behaves more like a linear elastic material, in which the applied peak strain does not change during stress relaxation (εr/εp ratio of unity). These results are in agreement recent work by Li et al., which demonstrated that AGE cross-linking reduces collagen fibril sliding (2013), and previous work by Purslow et al., which showed that strain increased during stress relaxation in part were due to structural rearrangements that were restricted by collagen–collagen intermolecular cross-links (1998). Important to note, a potential confounding factor was that the range of the applied peak strains in this study were smaller than those used in the previous study by Wyatt et al. (2009).
3.3. Collagen “Protective Effect” Paradox
Several studies showed that mechanical force applied to collagenous substrates inhibits degradation by bacterial collagenase (Huang and Yannas, 1977; Nabeshima et al., 1996; Ruberti and Hallab, 2005; Wyatt et al., 2009; Zareian et al., 2010). Nabeshima et al. discounted enzyme inhibition as a result of restricted enzyme diffusion effects while Wyatt et al. discounted inhibition by pH or osmotic effects (Nabeshima et al., 1996; Wyatt et al., 2009). In combination with results from a recent study using full length parallel single collagen molecules exposed to bacterial collagenase (Camp et al., 2011), these data suggest that inhibition can be attributed to mechanical force-deformation effects at the protein level.
In this study we utilized ribose to add glycation cross-links through AGEs to tendon fascicles and then exposed these cross-linked microscale fibers to bacterial collagenase degradation. Accumulation of collagen cross-links has been shown to increase resistance to collagenolytic digestion (Paik et al., 2006; Verzijl et al., 2002; Verzijl et al., 2000a), in agreement with these previous reports that cross-linking is protective, our unloaded fiber digestion tests (Results 2.2) showed that the ‘7-day cross-linked fibers’ are highly resistant to enzymatic degradation. However when cross-linked fibers were mechanically deformed with an applied tensile force they became increasingly susceptible to bacterial collagenase (Results 2.4). This contrasts with cross-linking and tensile deformation individually protecting collagen against enzyme cleavage.
This paradoxical combination of two protective effects combining to cancel one another is surprising. Based on the collagen microunfolding computational simulations we previously conducted using steered molecular dynamics (Bourne and Torzilli, 2011), this effect is most likely caused by the tensile forces being transmitted through the glycation cross-links and causing local microunfolding within the triple helix. In that computational study, a force was applied perpendicular to the long axis though a side chain, mimicking force transmission via cross-links, and observed to locally microunfold the triple helix at <1,000 pN of applied force (Bourne and Torzilli, 2011). Since thermal microunfolding events disrupt the collagen helix at super physiologic temperatures and induce susceptibility to proteolytic cleavage (Kuznetsova et al., 2003), we propose that mechanically-induced microunfolding is inducing a similar susceptibility to proteolytic cleavage.
Although the number of tests performed in this study were relatively small, the difference between the resistant and susceptible groups in the 7-day cross-linked data is striking. However due to both biological variability in the strain at relaxation and technical limitations preventing us from applying a predetermined strain with better then several tenths of a percent resolution, mapping the exact EMK response at additional strains would be prohibitively difficult. In addition, due to the moderately susceptible state of the 3-day fibers, where the number of AGEs was likely below the saturation level, it is unlikely that we would be able to detect a clear “Off-to-On” transition in that group. However, both the 3-day and 7-day AGE cases the response is clearly opposite from native tissue, which showed a decreasing EMK as strain increase (Wyatt et al., 2009).
We postulate that the enzyme sensitivity or cleavage transition likely occurs at lower strains in the 3-day cross-linked fibers because fewer glycation cross-links are formed than in the 7-day cross-linked fiber, with fewer cross-links resulting in more force per cross-link at the same strain. At forces below the microunfolding threshold, predicted by steered molecular dynamics to be <1,000 pN for a related collagen peptide (Bourne and Torzilli, 2011), the collagen molecule will bend but remain triple helical. Of interest, this suggests a potential tradeoff when there are different amounts of cross-links in fibers that are stretched to the same strain. For example, in lightly cross-linked tissues the involved collagen molecules are highly susceptible to removal, but their loss causes trivial changes to the overall load bearing structure. On the other hand, in moderately cross-linked tissues the stiffness is increased (more force generated for the same strain) and results in a force/cross-link ratio exceeding the helix microunfolding threshold. And in highly cross-linked tissues where the force/cross-link ratio is less than the microunfolding threshold, while further increasing axial strain will eventually exceed the microunfolding threshold.
4. Concluding Remarks
This study provides important experimental evidence supporting the mechanical force-induced microunfolding predictions previously made by us using steered molecular dynamics models (Bourne and Torzilli, 2011). More broadly these results provide new data on how the complex interplay between matrix components, matrix and tissue structure, and mechanical forces/deformations can combine to provide additional layers of biological complexity to processes such as enzyme – substrate, protein – protein, and cell – matrix interactions.
5. Experimental Procedures
5.1. Reagents
Dulbecco’s phosphate buffered saline (PBS) with calcium (CaCl2, 0.133 mg/ml) and magnesium (MgCl2, 0.1 mg/ml) and D-Ribose (99% pure) was purchased from Sigma-Aldrich (St. Lous, MO), 10x concentrated PBS from Invitrogen (Carlsbad, CA), and thymol crystals were from Fisher Chemical Company (Fair Lawn, NJ). Type II bacterial collagenase (CLS-2), purified from Clostridium histolyticum with reported activity of 245 units/mg dry weight, was purchased from Worthington Biochemical Company (Lakewood, NJ).
5.2. Rat Tail Tendon Collection and Glycation
Rat tails were collected from 6-month-old Lewis rats euthanized for unrelated studies in accordance with institutional animal care and use committee approved procedures. Tails were sectioned between the proximal caudal vertebrae and freeze-thawed twice to decellularize the tissue, then stored at −80°C until tendons were harvested. To collect tendon fibers, the tails were first thawed at room temperature for approximately an hour, then the distal tip of the tail was removed, leaving a ~100 mm length tail specimen. Microscale tendon fibers (i.e., fascicles of 342 ± 83 um diameter, mean ± standard deviation) were teased out from the distal end of the tail, immediately soaked in 15 ml of PBS and then separated into treatment groups. Tendons were either immediately stored in PBS at 4°C (0 days, no ribose exposure) or were glycated by ribose exposure.
Glycated specimens were prepared as previously described (Bai et al., 1992; Tanaka et al., 1988). Tendons were incubated for 1 to 7 days at 37°C in a 0.2 M ribose solution containing a crystal of thymol to prevent bacterial growth The specimens incubated for >3 days had the ribose solution exchanged with fresh 0.2 M ribose solution at the 3-day point. At the end of the ribose incubation, the fibers were rinsed for 5–10 minutes with PBS to remove free ribose. Specimens used for mechanical testing (glycated for 3 and 7 days), were stored in fresh PBS at 4°C.
Ribose incorporation into fibers over time was assessed by fluorescence of solubilized fibers following 0 to 10 days of incubation (Verzijl et al., 2002). Fibers were incubated in 0.2 M ribose solution (solution was exchanged with fresh ribose solution at day 3) and sample fibers were removed daily, rinsed in PBS, and then stored at 4°C until the end of the time course. Fibers were weighed, then dissolved at 60°C in a 1:20 dilution of papain (from Carica Papaya, Roche Diagnostics, Indianapolis, IN) in buffer per the commercial protocol at a concentration of 1 mg tendon per 50 uL of papain solution. Relative fluorescence was measured at ~460 nm using a spectrophotometer (340 ATTC, Tecan US, Chapel Hill, NC) as reported by Verzijl et al. (2002). Maximum fluorescence was estimated from measurements of fibers incubated in ribose for 5 weeks.
5.3. Unloaded Enzyme Susceptibility Test
In order to test for differential collagenase digestion susceptibility, fibers from the two treatment groups were incubated in bacterial collagenase at room temperature (22°C). Enzymatic digestion of the fibers was tracked by observing for visible fiber dissolution with digital images captured periodically. A needle of known diameter was included in each image and used as a reference to calculate the diameter of the fibers using ImageJ (ImageJ, U.S. NIH, Bethesda, Maryland). To control for variations in enzyme activity, fibers of comparable size from the same animal were incubated side-by-side in a single shared enzyme bath. This was repeated three times with fibers from three different animals.
5.4. Collagen Enzyme Mechano-Kinetic Automated Test System
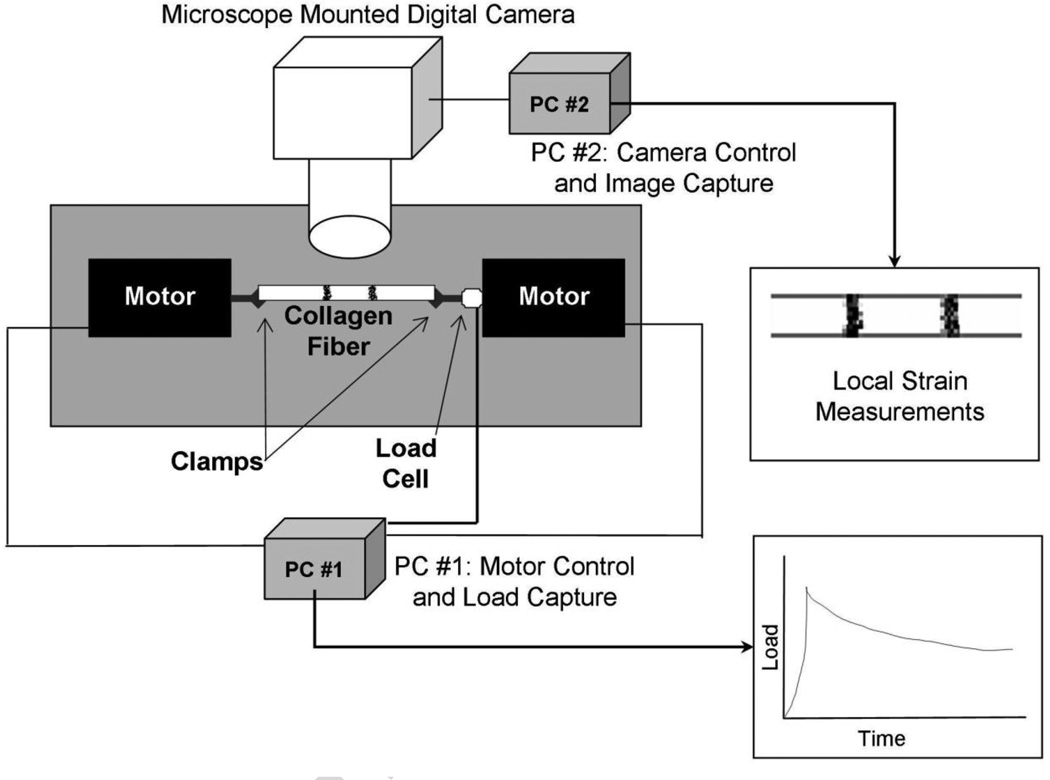
This study utilized the Collagen Enzyme Mechano-Kinetic Automated Test System (CEMKATS) originally described by Wyatt et al., with only minor changes to the previously published testing and data analysis protocol (Wyatt et al., 2009). A schematic of the test system is shown as Figure 5. To briefly describe the CEMKATS setup, a relaxation test was performed using two axially opposed computer controlled stepper motors to apply a tensile load to a fiber at a maximum velocity of 1 mm/s. When a pre-calculated strain was reached the motors were stopped to maintain the strain for the remainder of the experiment. The tensile force in the fiber was measured with a 250 gm load cell for the entirety of the test. An inverted microscope magnified inked marks on the fiber, which were recorded using a digital camera via a second computer for use in measuring local strains. Following a test the local strains in the specimen at peak load and relaxation were calculated by using NIH ImageJ software to measure the distance between the inked marks on the fiber.
Figure 5. Schematic of Collagen Enzyme Mechano-Kinetic Automated Test System (CEMKATS).
A collagen fiber is clamped at both ends and stretched using two axially opposed computer-controlled stepper motors until a pre-calculated clamp-to-clamp fiber elongation (strain) is reached. The motors then stop, fixing the clamp-to-clamp strain for the duration of the experiment. The resulting tensile load is measured using a 250 gm load cell and data acquisition computer. An inverted microscope is used to magnify two marks on the fiber while a digital camera records images using a second computer. The real-time in-situ fiber strain is determined from the distance separating the two marks on the fiber, shown as Local Strain Measurements in the figure. Figure adapted from Wyatt et al. (2009).
Small changes to the initial protocol reported by Wyatt et al. (2009) include the following; marks on the tendon were made using inkjet printer ink or industrial grade permanent marker (Sharpie™, Newell Rubbermaid, Oak Brook, IL), and the channel was etched in a block of plastic with dimensions of 26 mm wide, 1.5 mm across, and 1 mm deep. The change in channel size and material, coupled with smaller spacing between the channel and the grips, allowed us to reduce fluid drip rate and solution (i.e., PBS and collagenases) volumes used in this work.
One major revision to the previously published protocol was that the fibers in the present study were allowed to completely relax to a constant equilibrium load before adding the enzyme. This simplified data analysis as it allowed us to directly measure the decrease in load as a result of enzyme cleavage of the fiber without having to deconvolute the enzyme cleavage response from the stress-relaxation response.
As described earlier by Wyatt et al., the enzyme cleavage was described by an EMK relaxation function, TE (ε), which is a function of applied strain, ε and time t. TE was directly assessed for each applied strain by measuring the change in stress (σ, with the enzyme cleavage phase indicated with subscript e) as a function of time and normalized to the equilibrium stress (relaxation indicated with subscript r) of that fiber; the equation for TE is shown symbolically below as Eq. 1 (Wyatt et al., 2009).
| (1) |
Highlights.
Ribose cross-linking, glycation, attenuates collagenase degradation of collagen.
Tensile mechanical loading protects collagen-rich tendon microfibers.
Mechanical loading of glycation cross-linked microfibers accelerates degradation.
Supports atomistic prediction that cross-link loads may cause helix microunfolding.
Acknowledgments
Support for this investigation was provided by the Weill Cornell Graduate School of Medical Sciences and Weill Medical College's Clinical and Translational Science Center NIH-NCRR TL1RR024998 (J.W.B.) and NIH-NIAMS R21AR051636 and R01AR45748 (P.A.T.). This investigation was conducted in a facility constructed with support from Research Facilities Improvement Program grant number C06-RR12538-01 from the NIH-NCRR. We thank Sheela Damle for assistance in editing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Conflicts
A U.S. patent application related to this work has been submitted.
REFERENCES
- Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Avery NC, Bailey AJ. Enzymic and non-enzymic cross-linking mechanisms in relation to turnover of collagen: relevance to aging and exercise. Scandinavian journal of medicine & science in sports. 2005;15:231–240. doi: 10.1111/j.1600-0838.2005.00464.x. [DOI] [PubMed] [Google Scholar]
- Bai P, Phua K, Hardt T, Cernadas M, Brodsky B. Glycation alters collagen fibril organization. Connect Tissue Res. 1992;28:1–12. doi: 10.3109/03008209209014224. [DOI] [PubMed] [Google Scholar]
- Bailey AJ, Paul RG, Knott L. Mechanisms of maturation and ageing of collagen. Mech Ageing Dev. 1998;106:1–56. doi: 10.1016/s0047-6374(98)00119-5. [DOI] [PubMed] [Google Scholar]
- Bank RA, TeKoppele JM, Oostingh G, Hazleman BL, Riley GP. Lysylhydroxylation and non-reducible crosslinking of human supraspinatus tendon collagen: changes with age and in chronic rotator cuff tendinitis. Ann Rheum Dis. 1999;58:35–41. doi: 10.1136/ard.58.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourne JW, Torzilli PA. Molecular simulations predict novel collagen conformations during cross-link loading. Matrix Biol. 2011;30:356–360. doi: 10.1016/j.matbio.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buehler MJ. Atomistic and continuum modeling of mechanical properties of collagen: Elasticity, fracture, and self-assembly. J. Mater. Res. 2006;21:1947–1961. [Google Scholar]
- Camp RJ, Liles M, Beale J, Saeidi N, Flynn BP, Moore E, Murthy SK, Ruberti JW. Molecular Mechanochemistry: Low Force Switch Slows Enzymatic Cleavage of Human Type I Collagen Monomer. J Am Chem Soc. 2011 doi: 10.1021/ja110098b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AC, Temple MM, Ng DM, Verzijl N, DeGroot J, TeKoppele JM, Sah RL. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46:3212–3217. doi: 10.1002/art.10627. [DOI] [PubMed] [Google Scholar]
- Choudhary MI, Abbas G, Ali S, Shuja S, Khalid N, Khan KM, Atta ur R, Basha FZ. Substituted benzenediol Schiff bases as promising new anti-glycation agents. J Enzyme Inhib Med Chem. 2011;26:98–103. doi: 10.3109/14756361003733621. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Koob TJ, Van Ness KP. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984a;137:380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984b;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Freemont AJ, Hoyland JA. Morphology, mechanisms and pathology of musculoskeletal ageing. J Pathol. 2007;211:252–259. doi: 10.1002/path.2097. [DOI] [PubMed] [Google Scholar]
- Huang C, Yannas IV. Mechanochemical studies of enzymatic degradation of insoluble collagen fibers. J Biomed Mater Res. 1977;11:137–154. doi: 10.1002/jbm.820110113. [DOI] [PubMed] [Google Scholar]
- Kuznetsova NV, McBride DJ, Leikin S. Changes in thermal stability and microunfolding pattern of collagen helix resulting from the loss of alpha2(I) chain in osteogenesis imperfecta murine. J Mol Biol. 2003;331:191–200. doi: 10.1016/s0022-2836(03)00715-0. [DOI] [PubMed] [Google Scholar]
- Li Y, Fessel G, Georgiadis M, Snedeker JG. Advanced glycation end-products diminish tendon collagen fiber sliding. Matrix Biol. 2013;32:169–177. doi: 10.1016/j.matbio.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Maroudas A, Palla G, Gilav E. Racemization of aspartic acid in human articular cartilage. Connect Tissue Res. 1992;28:161–169. doi: 10.3109/03008209209015033. [DOI] [PubMed] [Google Scholar]
- Nabeshima Y, Grood ES, Sakurai A, Herman JH. Uniaxial tension inhibits tendon collagen degradation by collagenase in vitro. J Orthop Res. 1996;14:123–130. doi: 10.1002/jor.1100140120. [DOI] [PubMed] [Google Scholar]
- Ottani V, Martini D, Franchi M, Ruggeri A, Raspanti M. Hierarchical structures in fibrillar collagens. Micron. 2002;33:587–596. doi: 10.1016/s0968-4328(02)00033-1. [DOI] [PubMed] [Google Scholar]
- Paik DC, Saito LY, Sugirtharaj DD, Holmes JW. Nitrite-induced cross-linking alters remodeling and mechanical properties of collagenous engineered tissues. Connect Tissue Res. 2006;47:163–176. doi: 10.1080/03008200600721569. [DOI] [PubMed] [Google Scholar]
- Purslow PP, Wess TJ, Hukins DW. Collagen orientation and molecular spacing during creep and stress-relaxation in soft connective tissues. J Exp Biol. 1998;201:135–142. doi: 10.1242/jeb.201.1.135. [DOI] [PubMed] [Google Scholar]
- Puxkandl R, Zizak I, Paris O, Keckes J, Tesch W, Bernstorff S, Purslow P, Fratzl P. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Philos Trans R Soc Lond B Biol Sci. 2002;357:191–197. doi: 10.1098/rstb.2001.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy GK. Glucose-mediated in vitro glycation modulates biomechanical integrity of the soft tissues but not hard tissues. J Orthop Res. 2003;21:738–743. doi: 10.1016/S0736-0266(03)00006-8. [DOI] [PubMed] [Google Scholar]
- Reddy GK, Stehno-Bittel L, Enwemeka CS. Glycation-induced matrix stability in the rabbit achilles tendon. Arch Biochem Biophys. 2002;399:174–180. doi: 10.1006/abbi.2001.2747. [DOI] [PubMed] [Google Scholar]
- Ruberti JW, Hallab NJ. Strain-controlled enzymatic cleavage of collagen in loaded matrix. Biochem Biophys Res Commun. 2005;336:483–489. doi: 10.1016/j.bbrc.2005.08.128. [DOI] [PubMed] [Google Scholar]
- Sell DR, Monnier VM. Conversion of arginine into ornithine by advanced glycation in senescent human collagen and lens crystallins. J Biol Chem. 2004;279:54173–54184. doi: 10.1074/jbc.M408946200. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Avigad G, Eikenberry EF, Brodsky B. Isolation and partial characterization of collagen chains dimerized by sugar-derived cross-links. J Biol Chem. 1988;263:17650–17657. [PubMed] [Google Scholar]
- Tang Y, Ballarini R, Buehler MJ, Eppell SJ. Deformation micromechanisms of collagen fibrils under uniaxial tension. Journal of The Royal Society Interface. 2010;7:839–850. doi: 10.1098/rsif.2009.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzel SG, Buehler MJ. Molecular structure, mechanical behavior and failure mechanism of the C-terminal cross-link domain in type I collagen. J Mech Behav Biomed Mater. 2011;4:153–161. doi: 10.1016/j.jmbbm.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Ben ZC, Brau-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JW, Lafeber FP, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: a possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Oldehinkel E, Bank RA, Thorpe SR, Baynes JW, Bayliss MT, Bijlsma JW, Lafeber FP, Tekoppele JM. Age-related accumulation of Maillard reaction products in human articular cartilage collagen. Biochem J. 2000a;350(Pt 2):381–387. [PMC free article] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Thorpe SR, Bank RA, Shaw JN, Lyons TJ, Bijlsma JW, Lafeber FP, Baynes JW, TeKoppele JM. Effect of collagen turnover on the accumulation of advanced glycation end products. J Biol Chem. 2000b;275:39027–39031. doi: 10.1074/jbc.M006700200. [DOI] [PubMed] [Google Scholar]
- Wyatt KE, Bourne JW, Torzilli PA. Deformation-Dependent Enzyme Mechanokinetic Cleavage of Type I Collagen. J Biomech Eng. 2009;131:051004. doi: 10.1115/1.3078177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zareian R, Church KP, Saeidi N, Flynn BP, Beale JW, Ruberti JW. Probing collagen/enzyme mechanochemistry in native tissue with dynamic, enzymeinduced creep. Langmuir. 2010;26:9917–9926. doi: 10.1021/la100384e. [DOI] [PMC free article] [PubMed] [Google Scholar]