Abstract
OBJECTIVE
To examine the association of hospital volume and 90-day mortality after cystectomy, conditional on survival to 30 days
SUBJECTS AND METHODS
The National Cancer Database was used to evaluate 30- and 90-day mortality for 35,055 bladder cancer cases that received cystectomy at 1,118 hospitals. Patient data were aggregated into hospital volume categories based on average annual number of procedures [<10 low volume hospital (LVH), 10–19, ≥20 high volume (HVH)]. Associations between mortality and clinical, demographic and hospital characteristics were analyzed using hierarchical logistic regression models. To assess the association between hospital volume and 90-day mortality independent from shorter-term mortality, 90-day mortality conditional on 30-day survival was assessed in the multivariate modeling.
RESULTS
Unadjusted 30- and 90-day mortality rates were 2.7% and 7.2% overall, 1.9% and 5.7% among HVH, and 3.2% and 8.0% among LVH, respectively. Compared to HVH, the adjusted risks among LVH [OR (95% CI)] of 30- and 90-day mortality conditional on having survived through 30 days from the hierarchical models were 1.5 (1.3–1.9), and 1.2 (1.0–1.4), respectively.
CONCLUSION
Low hospital volume was associated with increased 30- and 90-day mortality. These data support the need for further research to better understand the relatively high mortality rates seen between 30–90 days, which are high and less variable across hospital volume strata. The stronger association between volume and 30-day mortality suggests that quality-reporting efforts should focus on shorter term outcomes.
Keywords: Bladder cancer, cystectomy, volume-outcome, 90-day mortality, conditional survival
Introduction
Cystectomy is indicated for the potentially lethal phenotype of bladder cancer (1) and has been associated with overall survival benefit compared to less aggressive management approaches.(2) Along with other complex surgical procedures (3, 4), a strong and consistent inverse association between hospital volume and short-term, in-hospital and 30-day postoperative, mortality has been observed among patients receiving cystectomy. (5–10)
Given the magnitude of cystectomy in a population with a high burden of competing risks, data suggesting substantial additional postoperative mortality out to 90 days (11–13) raise the question of whether the typical reference period of 30 days adequately captures the short-term risks, or whether the 90-day outcome may be more meaningful. Furthermore, it has been suggested that the volume/outcome association for cystectomy may extend to 90-day survival,(14) raising the question of the extent to which hospital volume may impact intermediate-term outcomes, independent of the well-established association with mortality in the earlier postoperative period. We focus on 90-day mortality conditional on survival to 30 days to assess the association between hospital volume and 90-day mortality independent from shorter-term mortality.
Subjects and Methods
The National Cancer Data Base (NCDB), a joint project of the American Cancer Society and the Commission on Cancer of the American College of Surgeons, serves as a comprehensive surveillance resource for cancer care in the United States.(15) The NCDB currently captures approximately 70% of all bladder cancers in the US, from over 1,400 facility-based cancer registries. This study was approved by the Institutional Review Board (IRB) of the University of North Carolina.
Incident bladder cancer cases diagnosed from 2004 through 2011 were queried. Selection criteria included ages ≥ 18 years, non-metastatic disease, and cystectomy procedure in the reporting facility. Total and radical cystectomies only were included; partial cystectomy procedures were excluded. Overall 30- and 90-day mortality, defined as patients who died within 30 or 90 days of the definitive surgery date, were assessed for descriptive analyses. Average annual hospital volume was determined by summing the number of cystectomies in each hospital from 2004–2011, divided by the eight year time period, excluding hospitals not accredited and reporting cases in each year between 2004–2011. Volume was calculated for individual hospitals including hospitals that are part of a network. For merged hospitals, volume was calculated for the merged entity, not individual hospitals. Hospitals missing mortality rates for 40% or more of cystectomy cases were excluded. Individual providers’ volumes could not be calculated since surgeon information is not included in the NCDB.
Co-morbidity information was derived from up to ten secondary diagnoses recorded for each patient, based on ICD-9-CM codes. The Elixhauser(16) classification, shown to be a better predictor of outcomes than the Charlson Comorbidity Index,(17, 18) was used. Reported secondary diagnoses were classified into 28 Elixhauser groups (excluding the two neoplasm groups).(19) Other measures include demographic and clinical variables, as well as median income and census region residence from 2000 U.S. Census data (based on zip codes of patient residence linked to census data). Case-mix for low versus high volume hospitals was also assessed for demographic and clinical variables.
Statistical Analysis
Thirty- and 90-day mortality was assessed overall and by hospital volume, categorized as low (<10), intermediate (10–19) and high (>20 cases/year). To examine factors associated with 90-day mortality independent of effects from shorter term mortality and to allow for between-model comparisons, conditional 90-day mortality was calculated for patients who survived beyond 30 days. For descriptive analyses, bivariate associations between 30-day and conditional 90-day mortality outcomes and demographic, clinical, and hospital variables were assessed using survey sampling methodology to account for clustering of patients within hospitals. Adjusted associations between 30- and 90-day conditional mortality and clinical, demographic, and hospital characteristics, including hospital cystectomy volume, were assessed using hierarchical logistic regression models fit using the SAS GLIMMIX procedure (SAS version 9.4, SAS Institute, Cary, NC). All models included random hospital effects to account for clustering of patients within hospitals. The model for each outcome simultaneously included all listed risk factors. Characteristics of patients in low volume hospitals (< 10 cases per year) compared to high volume hospitals (≥ 10 per year) were assessed using stepwise logistic regression. In reviewing the results, we focus on associations significant at the 0.05 level with no adjustments for multiple comparisons.
Results
In total, 38,917 patients received cystectomy in the five year period. After exclusions for incorrect hospital facility codes or hospitals not reporting cases for each of the eight years (n=1,136), cases with metastatic disease (n=1,103), and missing stage information (n=555), there were 36,123 analytic cases. In this preliminary analytic set, there were 5 hospitals with ≥40% of cases missing 30-day mortality, representing 47 cases, and 8 hospitals with ≥40% of cases missing 90-day mortality, representing 61 cases. After excluding these hospitals, all remaining hospitals had less than 40% of cases missing mortality status, with the majority of hospitals (93% and 88%, respectively, for 30-day and 90-day mortality) missing 10% or less. Excluding all other cases with missing 30- (n=1,042) or 90-day mortality (n=1,910), the final analytic cohort included 35,055 cases from 1,118 hospitals (30-day mortality) and 34,186 cases from 1,115 hospitals (cumulative 90-day mortality).
Demographic, clinical and hospital characteristics of all cases and by hospital volume are presented in Table 1. The mean age was 67.5 (interquartile range 60–76) years and 43 % were age 70 or older. The majority (51%) of procedures were performed in hospitals averaging fewer than 10 cases per year (representing 91% of hospitals), including 30% in hospitals averaging fewer than five cases per year. There were 37 hospitals with an annual volume of 20 or more, representing 32 % of all cystectomies. Lower volume hospitals had the lowest percent of cases with private health insurance (31%) and the higher percent of black cases (7%). High volume hospitals had the highest percentage of cases who received neoadjuvant chemotherapy (21%).
Table 1.
Radical Cystectomies, Patient Characteristics, 2004–2011 Patient Characteristics (N=35,055)
| Average Annual Hospital Volume, % | Patient Distributions % (n) | |||
|---|---|---|---|---|
| 0–9 (N=17,761) | 10–19 (N=5,965) | ≥20 (N=11,329) | Total (N=35,055) | |
| Age | ||||
| 18–49 | 4.9 | 5.9 | 6.6 | 5.6 (1,965) |
| 50–59 | 16.3 | 17.7 | 18.1 | 17.1 (5,993) |
| 60–69 | 30.5 | 30.5 | 32.0 | 31.0 (10,868) |
| 70–79 | 34.4 | 32.8 | 32.1 | 33.4 (11,703) |
| >=80 | 13.9 | 13.1 | 11.3 | 12.9 (4,526) |
| Sex | ||||
| Male | 75.0 | 75.0 | 76.0 | 75.3 (26,409) |
| Female | 25.0 | 25.0 | 24.0 | 24.9 (8,646) |
| Race | ||||
| White | 90.3 | 89.4 | 91.5 | 90.5 (31,728) |
| Black | 7.0 | 5.6 | 4.6 | 6.0 (2,108) |
| Other | 2.7 | 5.0 | 3.9 | 3.5 (1,219) |
| Diagnosis Years | ||||
| 2004–2007 | 49.2 | 46.3 | 44.8 | 47.3 (16,574) |
| 2008–2011 | 50.8 | 53.6 | 55.2 | 52.7 (18,481) |
| Insurance | ||||
| None, Medicaid | 5.2 | 4.8 | 4.5 | 4.9 (1,730) |
| Private, Self Pay | 30.7 | 37.4 | 36.7 | 33.8 (11,836) |
| Medicare | 57.0 | 55.5 | 55.8 | 56.4 (19,755) |
| Other, Unknown | 7.1 | 2.2 | 3.0 | 4.9 (1,339) |
| Median Income Quintiles2 | ||||
| < $28,000 | 9.1 | 8.0 | 6.7 | 8.1 (2,684) |
| $28–32,000 | 14.0 | 12.7 | 15.4 | 14.2 (4,696) |
| $33–38,000 | 19.5 | 18.3 | 20.4 | 19.6 (6,475) |
| $39–48,000 | 26.5 | 23.5 | 24.1 | 25.2 (8,322) |
| >=$49,000 | 31.0 | 37.4 | 33.4 | 32.8 (10,841) |
| Census Region3 | ||||
| New England | 6.5 | 9.1 | 3.0 | 5.8 (2,043) |
| Middle Atlantic | 11.7 | 19.0 | 18.1 | 15.0 (5,261) |
| South Atlantic | 16.4 | 19.0 | 21.0 | 18.3 (6,428) |
| East North Central | 21.6 | 15.0 | 22.7 | 20.8 (7,300) |
| East South Central | 7.9 | 7.5 | 7.2 | 7.6 (2,665) |
| West North Central | 10.0 | 4.8 | 9.1 | 8.8 (3,092) |
| West South Central | 8.6 | 9.0 | 2.6 | 6.7 (2,352) |
| Mountain | 5.7 | 2.2 | 4.6 | 4.8 (1,675) |
| Pacific | 11.4 | 14.2 | 11.2 | 11.8 (4,142) |
| Out of U.S. | 0.2 | 0.1 | 0.5 | 0.3 (97) |
| Stage | ||||
| 0–II | 46.2 | 45.8 | 47.7 | 46.6 (16,352) |
| III, T4bN0M0/AnyTN+M0 | 53.8 | 54.2 | 52.3 | 53.4 (18,703) |
| Grade | ||||
| Well or moderately Differentiated | 9.1 | 7.2 | 6.1 | 7.8 (2,735) |
| Poorly differentiated or Undifferentiated | 83.5 | 84.1 | 86.7 | 84.6 (29,673) |
| Unknown | 7.4 | 8.7 | 7.1 | 7.6 (2,647) |
| Cancer Sequence | ||||
| First Primary or Only primary | 81.2 | 80.0 | 77.4 | 79.8 (27,970) |
| Other prior primary cancer | 18.8 | 20.0 | 22.6 | 20.2 (7,085) |
| Neo-adjuvant chemotherapy | ||||
| No | 88.5 | 84.8 | 79.3 | 84.9 (29,753 |
| Yes | 11.5 | 15.2 | 20.7 | 15.1 (5,302) |
Cases with valid 30 day mortality.
Based on zip code level Income from 2000 Census. Excludes 2,037 cases with missing income.
Patient residence. Census region states include: New England: (ME,VT,NH, MA, CT,RI); Middle Atlantic (NY, PA, NJ); South Atlantic (WV,MD,DE,DC,VA,NC,SC,GA,FL); East North Central (WI,IL,MI,IN,OH); East South Central (KY,TN,MS,AL); West North Central (ND,SD,NE,KS,MN,IA,MO); West South Central (OK,AR,LA,TX); Mountain (MT,ID,WY,NV,UT,CO,AZ,NM); Pacific (WA,OR,CA,HI).
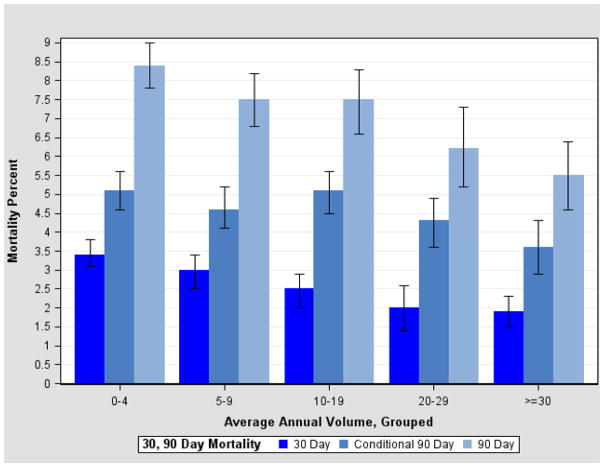
Overall unadjusted 30- and 90-day mortalities were 2.7% and 7.2%, respectively. Significant differences in 30- and cumulative 90-day mortality rates, including patients who died within 30 days, were found according to hospital volume, with decreases in mortality at both average volumes between 10–19 and 20 or higher relative to average volumes less than 10 (Figure 1). For cumulative 90-day mortality, the observed mortality rates and 95% confidence intervals were 8.0 (7.6–8.5), 7.5 (6.6–8.3) and 5.7 (5.0–6.4) for hospital volumes of less than 10, 10–19 and 20 or more, respectively (p ≤ 0.0001). Among 33,250 patients who survived beyond 30 days and for whom longer follow-up data are available, 1,519 (4.6%) died between 31 and 90 days. The vast majority of these deaths (n=1,342) occurred post discharge. The results for conditional 90-day mortality rather than cumulative 90-day mortality (data not shown) are presented to eliminate the bias from shorter-term deaths included in the 90-day cumulative outcome.
Figure 1.
Cystectomies, 2004–2008: Unadjusted 30- and 90-Day Mortality, by Average Annual Hospital Volume
Bivariate associations with 30-day and conditional 90-day mortality are presented in table 2. Volume was significantly associated with both 30-day mortality and conditional 90-day mortality although rates in the latter were similar in volumes under 20. Mortality rates increased with age, with a six percent 30-day mortality rate and a 10 percent 90-day mortality rate in patients aged 80 and over. Significantly higher 30 and 90-day mortality rates were found in blacks compared to whites, and in females compared to males for 90 day mortality only. Mortality rates were significantly higher among cases residing in lower income areas. There were no significant differences by census division, but residents in the East South Central Division had the highest rates for both 30 and 90 day mortality. Medicare recipients had higher 30- and 90-day mortality rates than cases with other insurance. Higher stage, tumor grade, and having a prior cancer were also associated with higher 30- and 90-day mortality rates. Cases diagnosed in 2008–2011 had somewhat lower 90 day mortality rates than cases diagnosed in 2004–2007. Receipt of neoadjuvant chemotherapy was associated with a lower mortality rate than no neoadjuvant chemotherapy. Mortality rates for 17 different Elixhauser comorbid conditions with significant differences are presented in table 2.
Table 2.
Unadjusted 30, 90 Day mortality Rates by selected demographic, hospital and clinical characteristics, and significant comorbid conditions
| 30-Day Mortality (N=35,055) | 90-Day Conditional Mortality1 (N=33,250) | |||
|---|---|---|---|---|
| Mortality (%), 95% Confidence Intervals | Number of Deaths/ N | Mortality (%), 95% Confidence Intervals | Number of Deaths/ N | |
| All Cases | 2.7 (2.5–2.9) | 936/35,055 | 4.6 (4.3–4.8) | 1,519/33,250 |
| Average Annual Volume2,3 | ||||
| 0–9 | 3.2 (3.0–3.5) | 574/17,761 | 4.9 (4.6–5.3) | 828/16,883 |
| 10–19 | 2.5 (2.0–2.9) | 147/5,965 | 5.1 (4.5–5.6) | 287/5,678 |
| >=20 | 1.9 (1.6–2.2) | 215/11,329 | 3.8 (3.3–4.3) | 404/10,689 |
| Diagnosis Years3 | ||||
| 2004–2007 | 2.7 (2.4–2.9) | 444/16,574 | 4.8 (4.4–5.2) | 771/16,031 |
| 2008–2011 | 2.7 (2.4–2.9) | 492/18,481 | 4.3 (4.0–4.7) | 748/17,219 |
| Hospital Category2,3 | ||||
| Community | 3.4 (2.4–4.4) | 57/1,674 | 5.2 (4.0–6.4) | 83/1,601 |
| Comprehensive Community | 3.3 (3.0–3.6) | 354/10,752 | 5.1 (4.6–5.5) | 519/10,212 |
| Academic | 2.3 (2.0–2.6) | 456/19,837 | 4.3 (3.9–4.7) | 805/18,778 |
| Other | 2.5 (1.9–3.1) | 69/2,792 | 4.2 (3.4–5.1) | 112/2,659 |
| Age2,3 | ||||
| 18–49 | 1.0 (0.6–1.5) | 20/1,965 | 2.8 (2.1–3.5) | 53/1,885 |
| 50–59 | 1.1 (0.8–1.3) | 63/5,993 | 2.0 (1.6–2.4) | 116/5,783 |
| 60–69 | 1.8 (1.5–2.0) | 192/10,868 | 3.1 (2.7–3.5) | 322/10,413 |
| 70–79 | 3.3 (2.9–3.6) | 383/11,703 | 5.7 (5.2–6.2) | 628/11,036 |
| >=80 | 6.1 (5.4–6.9) | 278/4,526 | 9.7 (8.8–10.6) | 400/4,133 |
| Sex3 | ||||
| Male | 2.7 (2.4–2.9) | 702/26,409 | 4.3 (4.0–4.6) | 1,079/25,031 |
| Female | 2.7 (2.3–3.1) | 234/8,646 | 5.4 (4.8–6.0) | 440/8,219 |
| Race2,3 | ||||
| White | 2.6 (2.4–3.9) | 840/31,728 | 4.6 (4.3–4.8) | 1,374/30,133 |
| Black | 3.4 (2.6–4.2) | 72/2,108 | 5.5 (4.4–6.6) | 108/1,968 |
| Other | 2.0 (1.2–2.7) | 24/1,219 | 3.2 (2.2–4.3) | 37/1,149 |
| Insurance2,3 | ||||
| None/Medicaid | 1.7 (1.1–2.3) | 29/1,730 | 4.8 (3.7–5.9) | 79/1,635 |
| Private | 1.3 (1.1–1.5) | 154/11,836 | 2.8 (2.5–3.1) | 317/11,381 |
| Medicare | 3.6 (3.3–3.9) | 710/19,755 | 5.6 (5.3–6.0) | 1,050/18,589 |
| Other, Unknown | 2.5 (1.8–3.2) | 43/1,734 | 4.4 (3.3–5.6) | 73/1,645 |
| Median Income Quintiles2,3.4 | ||||
| < $28,000 (Lowest Quintle) | 3.6 (2.8–4.4) | 96/2,684 | 5.4 (4.5–6.3) | 136/2,517 |
| >=$28,000 | 2.6 (2.4–2.8) | 840/32,371 | 4.5 (4.2–4.8) | 1,383/30,733 |
| Census Region5 | ||||
| New England | 2.5 (1.8–3.2) | 51/2,043 | 4.6 (3.7–5.6) | 91/1,962 |
| Middle Atlantic | 2.5 (2.1–3.0) | 133/5,261 | 4.7 (3.8–5.6) | 234/4,985 |
| South Atlantic | 2.6 (2.1–3.1) | 166/6,428 | 4.9 (4.3–5.5) | 297/6,068 |
| East North Central | 3.3 (2.7–3.9) | 240/7,300 | 4.2 (3.7–4.8) | 291/6,894 |
| East South Central | 3.0 (2.4–3.6) | 80/2,665 | 5.4 (4.6–6.2) | 137/2,530 |
| West North Central | 2.4 (1.8–3.1) | 75/3,092 | 4.1 (3.1–5.0) | 120/2,951 |
| West South Central | 2.6 (1.8–3.4) | 62/2,352 | 4.7 (3.7–5.7) | 105/2,232 |
| Mountain | 2.5 (1.5–3.5) | 42/1,675 | 4.3 (3.1–5.4) | 68/1,592 |
| Pacific | 2.1 (1.7–2.5) | 87/4,142 | 4.4 (3.6–5.2) | 174/3,959 |
| Stage2,3 | ||||
| 0–II | 2.1 (1.8–2.3) | 337/16,352 | 2.2 (2.0–2.5) | 349/15,515 |
| III,T4bN0M0/N+M0 | 3.2 (2.9–3.5) | 599/18,703 | 6.6 (6.2–7.0) | 1,170/17,735 |
| Grade | ||||
| Well or moderately differentiated | 2.5 (1.9–3.1) | 69/2,735 | 3.8 (3.0–4.6) | 100/2,618 |
| Poorly differentiated or undifferentiated | 2.7 (2.5–2.9) | 805/29,673 | 4.7 (4.4–5.0) | 1,320/28,140 |
| Unknown | 2.3 (1.8–2.9) | 62/2,647 | 4.0 (3.1–4.8) | 99/2,492 |
| Prior Cancers2,3 | ||||
| None | 2.5 (2.3–2.7) | 693/27,970 | 4.3 (4.0–4.6) | 1,133/26,595 |
| One or more | 3.4 (3.0–3.9) | 243/7,085 | 5.8 (5.2–6.4) | 386/6,655 |
| Cystectomy Type3 | ||||
| Total | 3.5 (2.5–4.6) | 48/1,356 | 4.0 (2.8–5.1) | 51/1,285 |
| With reconstruction | 2.6 (2.4–2.9) | 698/26,384 | 4.4 (4.1–4.6) | 1,094/25,075 |
| With Pelvic exenteration | 2.6 (2.2–3.0) | 190/7,315 | 5.4 (4.8–6.1) | 374/6,890 |
| Neo-adjuvant Chemo2,3 | ||||
| Yes | 1.6 (1.2–2.0) | 85/5,302 | 3.1 (2.6–3.7) | 155/4,975 |
| No | 2.9 (2.6–3.1) | 851/29,753 | 4.8 (4.5–5.1) | 1,364/28,275 |
| Elixhauser Comorbid conditions | ||||
| Congestive Heart Failure2,3 | ||||
| No | 2.5 (2.3–2.7) | 861/34,071 | 4.4 (4.1–4.7) | 1,417/32,351 |
| Yes | 7.6 (5.9–9.4) | 75/984 | 11.3 (9.3–13.4) | 102/899 |
| Cardiac Arrythmias2,3 | ||||
| No | 2.5 (2.3–2.7) | 805/32,288 | 4.3 (4.0–4.6) | 1,324/30,685 |
| Yes | 4.7 (3.9–5.6) | 131/2,767 | 7.6 (6.5–8.8) | 195/2,565 |
| Vascular Disease2 | ||||
| No | 2.7 (2.4–2.9) | 928/34,938 | 4.6 (4.3–4.8) | 1,512/33,148 |
| Yes | 6.8 (2.3–11.3) | 8/117 | 6.9 (1.9–11.8) | 7/102 |
| Peripheral Vascular Disease3 | ||||
| No | 2.6 (2.4–2.9) | 896/33,893 | 4.5 (4.2–4.8) | 1,452/32,151 |
| Yes | 3.4 (2.3–4.6) | 40/1,162 | 6.1 (4.7–7.5) | 67/1,099 |
| Hypertension3 | ||||
| No | 2.8 (2.6–3.1) | 618/21,855 | 4.8 (4.5–5.1) | 998/20,715 |
| Yes | 2.4 (2.1–2.7) | 318/13,200 | 4.2 (3.7–4.6) | 521/12,535 |
| Other Neurologic Disorders2,3 | ||||
| No | 2.6 (2.4–2.8) | 900/34,593 | 4.5 (4.2–4.8) | 1,484/32,844 |
| Yes | 7.8 (5.3–10.2) | 36/462 | 8.6 (5.8–11.4) | 35/406 |
| Chronic Pulmonary Disease2,3 | ||||
| No | 2.5 (2.3–2.7) | 762/30,463 | 4.4 (4.1–4.7) | 1,286/28,935 |
| Yes | 3.8 (3.2–4.3) | 174/4,592 | 5.4 (4.8–6.0) | 233/4,315 |
| Diabetes, Uncomplicated2,3 | ||||
| No | 2.6 (2.4–2.8) | 791/30,466 | 4.4 (4.1–5.7) | 1,282/28,914 |
| Yes | 3.2 (2.7–3.6) | 145/4,444 | 5.5 (4.8–6.1) | 237/4,336 |
| Diabetes, Complicated2 | ||||
| No | 2.6 (2.4–2.9) | 920/34,746 | 4.6 (4.3–4.9) | 1,507/32,694 |
| Yes | 5.2 (2.9–7.5) | 16/309 | 4.2 (2.0–6.4) | 12/286 |
| Renal Failure2,3 | ||||
| No | 2.6 (2.4–2.8) | 906/34,657 | 4.5 (4.2–4.8) | 1,480/32,889 |
| Yes | 7.5 (4.9–10.1) | 30/398 | 10.8 (7.6–14.0) | 39/361 |
| Liver Disease2 | ||||
| No | 2.7 (2.4–2.9) | 926/34,870 | 4.6 (4.3–4.8) | 1,508/33,079 |
| Yes | 5.4 (2.3–8.5) | 10/185 | 6.4 (2.6–10.2) | 11/171 |
| Coagulopathy2,3 | ||||
| No | 2.6 (2.4–2.8) | 905/34,566 | 4.5 (4.2–4.8) | 1,485/32,808 |
| Yes | 6.3 (4.1–8.6) | 31/489 | 7.7 (5.2–10.2) | 34/442 |
| Obesity3 | ||||
| No | 2.7 (2.5–2.9) | 919/34,128 | 4.6 (4.3–4.9) | 1,495/32,372 |
| Yes | 1.8 (0.9–2.7) | 17/927 | 2.7 (1.8–3.7) | 24/878 |
| Weight Loss2,3 | ||||
| No | 2.6 (2.4–2.8) | 884/34,086 | 4.7 (4.1–4.6) | 1,415/32,357 |
| Yes | 5.4 (3.9–6.9) | 52/964 | 11.6 (9.7–13.6) | 104/893 |
| Fluid, Electrolyte Disorders2,3 | ||||
| No | 2.5 (2.3–2.7) | 792/31,978 | 4.3 (4.1–4.6) | 1,315/30,380 |
| Yes | 4.7 (3.9–5.5) | 144/3,077 | 7.1 (6.2–8.0) | 204/2,870 |
| Blood Loss Anemias2 | ||||
| No | 2.6 (2.4–2.9) | 916/34,578 | 4.6 (4.3–4.8) | 1,498/32,800 |
| Yes | 4.2 (2.4–6.0) | 20/477 | 4.7 (2.8–6.5) | 21/450 |
| Psychoses3 | ||||
| No | 2.7 (2.5–2.9) | 931/34,740 | 4.5 (4.3–4.8) | 1,497/32,945 |
| Yes | 1.6 (0.2–3.0) | 5/315 | 7.2 (4.1–10.3) | 22/305 |
90-day mortality excludes deaths within first 30 days.
p < =.05, 30-day mortality
p < =.05, 90-day mortality.
Based on 2000 Census data income of patient zip code. 2,037 cases with missing income classifed included in top quartile group.
Excludes 97 cases living outside the U.S. Census region states include: New England: (ME,VT,NH, MA, CT,RI); Middle Atlantic (NY, PA, NJ); South Atlantic (WV,MD,DE,DC,VA,NC,SC,GA,FL); East North Central (WI,IL,MI,IN,OH); East South Central (KY,TN,MS,AL); West North Central (ND,SD,NE,KS,MN,IA,MO); West South Central (OK,AR,LA,TX); Mountain (MT,ID,WY,NV,UT,CO,AZ,NM); Pacific (WA,OR,CA,HI).
Results from the hierarchical models for 30- and 90-day mortality are included in Table 3. Adjusted odds ratios show that low volume hospitals had a significantly elevated odds ratio of 1.5 (95% CI 1.3–1.9) of 30-day mortality compared to high volume hospitals, but the association was attenuated for conditional 90-day mortality (estimated OR 1.2, 95% CI 1.0 – 1.4). Additionally, for both 30- and 90-day mortality, significant associations were observed for age, race, stage, insurance type, and income. Several comorbid conditions were also significantly associated with both 30 and 90 day mortality.
Table 3.
Adjusted Odds Ratios1 for 30- and 90-Day Mortality
| 30-day Mortality2 | 90-day Conditional Mortality3 | |
|---|---|---|
| Odds Ratio, 95% Confidence Limits | Odds Ratio, 95% Confidence Limits | |
| Hospital Average Annual Volume | ||
| >= 20 | 1.0 (Reference) | 1.0 (Reference) |
| 10–19 | 1.2 (1.0–1.6) | 1.3 (1.1–1.5) |
| 0–9 | 1.5 (1.3–1.9) | 1.2 (1.0–1.4) |
| Elixhauser Group | ||
| Congestive Heart Failure | 1.8 (1.4–2.3) | 1.7 (1.4–2.1) |
| Cardiac Arrhythmias | 1.3 (1.1–1.6) | 1.3 (1.1–1.6) |
| Hypertension | 0.7 (0.6–0.9) | 0.8 (0.7–0.9) |
| Other Neurologic Disorders | 2.5 (1.8–3.6) | 1.6 (1.1–2.3) |
| Chronic Pulmonary Disease5 | 1.3 (1.1–1.6) | 1.1 (0.9–1.3) |
| Diabetes, Uncomplicated4 | 1.2 (1.0–1.4) | 1.2 (1.1–1.4) |
| Renal Failure | 1.8 (1.2–2.7) | 1.7 (1.2–2.4) |
| Liver Disease5 | 3.0 (1.6–5.9) | 1.8 (1.0–3.5) |
| Coagulopathy5 | 1.8 (1.2–2.7) | 1.3 (0.9–1.9) |
| Weight Loss | 1.4 (1.0–1.9) | 2.1 (1.7–2.7) |
| Fluid and Electrolyte Disorders | 1.4 (1.1–1.7) | 1.2 (1.0–1.4) |
| Age Group | ||
| 18–49 (Reference) | 1.0 (Reference) | 1.0 (Reference) |
| 50–59 | 1.0 (0.6–1.7) | 0.8 (0.6–1.1) |
| 60–69 | 1.5 (0.9–2.4) | 1.2 (0.9–1.6) |
| 70–79 | 2.3 (1.4–3.8) | 2.1 (1.5–2.8) |
| >=80 | 4.1 (2.6–6.7) | 3.4 (2.4–4.7) |
| Race | ||
| White | 1.0 (Reference) | 1.0 (Reference) |
| Black | 1.4 (1.1–1.8) | 1.2 (1.0–1.5) |
| Other/Unknown | 0.9 (0.6–1.3) | 0.7 (0.5–1.0) |
| Median Income5 | ||
| >=$28,000 | 1.0 (Reference) | 1.0 (Reference) |
| <$28,000 | 1.3 (1.0–1.6) | 1.1 (0.9–1.4) |
| Insurance | ||
| Private | 1.0 (Reference) | 1.0 (Reference) |
| None, Medicaid | 1.2 (0.8–1.8) | 1.7 (1.3–2.2) |
| Medicare | 1.5 (1.2–1.9) | 1.1 (0.9–1.3) |
| Other, Unknown | 1.5 (1.0–2.1) | 1.3 (1.0–1.7) |
| Stage1 | ||
| 0–II | 1.0 (Reference) | 1.0 (Reference) |
| III,T4bN0M0/N+M0 | 1.4 (1.2–1.6) | 2.8 (2.5–3.2) |
| Prior Cancers4 | ||
| None | 1.0 (Reference) | 1.0 (Reference) |
| One or more | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) |
| Neoadjuvant Chemotherapy5 | ||
| Yes | 1.0 (Reference) | 1.0 (Reference) |
| No | 1.3 (1.0–1.6) | 1.1 (0.9–1.3) |
| Diagnosis Years4 | ||
| 2008–2011 | 1.0 (Reference) | 1.0 (Reference) |
| 2004–2007 | 1.0 (0.9–1.1) | 1.1 (1.0–1.3) |
Variables significant at p <= .05, unless otherwise indicated. Non-significant variables also included in the model: sex, tumor grade.
N=35, 055, 936 deaths
Conditional on having survived through 30 days; N=33,250, 1,519 Deaths, excludes 936 cases who died within 30 days,
Not significant 30 day mortality (p> .05).
Not significant 90 day conditional mortality (p>.05).
Based on 2000 Census data income of patient zip code. 2,037 cases with missing income classifed included in top quartile groups.
Table 4 presents results comparing patients in low- to intermediate and high-volume hospitals. Older patients, those of black race, and patients with non-private insurance were more likely to be seen in low volume hospitals than younger patients, non-black patients, and those with private insurance, respectively. Selected comorbid conditions were also more prevalent in low volume hospitals.
Table 4.
Variables1 Associated with having cystectomy in a low volume2 compared to intermediate and high volume hospitals
| Odds Ratio (95% Confidence Interval)3 | |
|---|---|
| Age Group | |
| 18–49 | 1.00 (Reference) |
| 50–59 | 1.2 (1.1–1.4) |
| 60–69 | 1.3 (1.2–1.5) |
| 70–79 | 1.5 (1.3–1.6) |
| >=80 | 1.6 (1.4–1.8) |
| Race | |
| White | 1.0 (Reference) |
| Black | 1.3 (1.2–1.5) |
| Other/UK | 0.6 (0.5–0.7) |
| Insurance | |
| Private | 1.00 (Reference) |
| None, Medicaid | 1.3 (1.2–1.5) |
| Medicare | 1.0 (1.0–1.1) |
| Other, Unknown | 3.0 (2.7–3.4) |
| Neoadjuvant Chemotherapy | |
| No | 1.0 (Reference) |
| Yes | 0.6 (0.5–0.6) |
| Cancer Sequence | |
| First Primary or Only Primary | |
| Other Primary Prior Cancer | 0.8 (0.7–0.8) |
| Grade | |
| Low | 1.0 (Reference) |
| High | 0.7 (0.7–0.8) |
| Missing | 0.7 (0.7–0.8) |
| Elixhauser Comorbid Conditions4 | |
| Congestive Heart Failure | 1.3 (1.1–1.4) |
| Cardiac Arrthymias | 0.9 (0.8–0.9) |
| Chronic Pulmonary Disease | 1.2 (1.2–1.3) |
| Diabetes, Uncomplicated | 1.1 (1.0–1.2) |
| Renal Failure | 1.4 (1.1–1.7) |
| Obesity | 0.9 (0.8–1.0) |
| Fluid, Electrolyte Disorders | 1.2 (1.1–1.3) |
| Blood Loss Anemia | 2.1 (1.7–2.5) |
| Deficiency Anemias | 1.5 (1.4–1.7) |
| Alcohol Abuse | 1.3 (1.1–1.6) |
| Depression | 0.9( 0.8–1.0) |
Variables significant at p < .05 in a stepwise logistic regression model.
Low volume defined as less than 10 cystectomies per year.
N=35,055;17,761 cases in low volume hospitals.
Condition present compared to absence of condition.
Discussion
Cystectomy is the reference standard for the management of muscle-invasive bladder cancer, a potentially lethal phenotype.(1) The demanding extirpative and reconstructive requirements classify this procedure, along with pancreatectomy and esophagectomy,(3, 4) among the high-risk, complex cancer operations, for which evidence supports an inverse association between facility volume and short-term mortality.(5–11) Recent reports suggest this phenomenon may also extend to longer term survival outcomes.(20, 21) Our analysis of a large, contemporary, multicenter cohort in the United States adds to the body of literature supporting an independent inverse association between facility-level cystectomy volume and 30-day postoperative mortality, and demonstrates a similar, albeit attenuated association with 90-day mortality. This study is the first to document a statistically significant association between hospital volume and cumulative 90-day mortality after cystectomy. However, recognizing the inherent potential bias of 90-day mortality rates from shorter-term deaths, we sought to examine the independent effect of hospital volume on intermediate-term mortality beyond the 30-day window by separately examining 90-day mortality conditional on survival beyond 30 days. Conditional survival estimates have been suggested to provide a more dynamic and clinically meaningful lens through which to examine long-term survival estimates(22); however, this approach has had limited application to shorter-term outcomes. Interestingly, the rates of conditional 90-day mortality so defined were relatively high, and, while associated with hospital volume, the magnitude of this association was somewhat less than that observed for the 30-day outcome. Though cystectomy has been associated with benefits in longer term overall survival relative to less aggressive treatments,(2) these data underscore the potential for substantial risks of short-term mortality, even among high-volume centers, which in turn provide potential targets for quality improvement efforts.
The first notable finding of this study is the relatively high (7.2%) overall 90-day postoperative mortality following cystectomy. This is in line with results (6.7–8.2%) from several recent studies (12–14) though greater than that reported in an analysis of SEER data (3.9%).(23) The discrepant results with the latter study may be accounted for by a higher proportion of partial cystectomy and lower proportions of patients in the oldest age groups as compared to the present cohort. In support of our findings, a previous examination reporting a non-significant association between hospital volume and 90-day cumulative mortality in a single U.S. state(14) reported similar unadjusted rates by high-, medium- and low-volume hospitals (5.4, 6.9, and 8.4%, respectively). In this context, it is noteworthy that approximately 51% of procedures in our nationally representative sample took place in low volume hospitals, representing approximately 91% of the facilities. Our findings underscore relatively high early-term mortality risks, particularly in certain subgroups of patients, including those receiving care at low volume centers. These data support further study of patient-level factors associated with short-term risks (24) of the different treatment options to enhance the informed consent process and shared decision making. The observation of poorer access to high volume hospitals and 90-day survival among low income patients, blacks, and the elderly raises concerns regarding racial and socioeconomic disparities in outcome consistent with previous findings (25) which warrant further study.
Our findings of substantial conditional 90 day mortality, even among the relatively higher hospital volume strata, underscore the need to elucidate the root causes of postoperative mortality in this time frame to inform the development of strategies to optimize outcomes. The concept of “failure to rescue” (i.e., case fatality among patients with complications) (26) has recently re-emerged as a stronger mediator of differences between low- and high-30-day-mortality hospitals as compared to differences in the absolute incidence of complications, which are in general comparatively small.(27) The relevance of this concept to cystectomy is supported by a study with similar findings for esophagectomy and pancreatectomy.(28) A more recent study examined this concept in the specific context of cystectomy, finding reduced odds of failure to rescue among higher volume hospitals.(29) Delayed complications are common after cystectomy, with one high volume center reporting 54% of patients experiencing grade 2–5 complications, 57% of which occurred between discharge and 90 days.(30) The high number of 30–90 day deaths in our data, even among high volume hospitals, warrants further investigation for potentially actionable targets for quality improvement.
An important overarching question relates to the mechanisms underlying the observed association between hospital volume and short-term mortality outcomes. Prior cystectomy studies demonstrated differences in hospital staffing and capacity,(31) including more intensive nurse staffing in particular,(9, 32) and utilization of specific processes of care (11) to be mediators of the volume effect with respect to 30-day mortality. These data are not currently available in the NCDB; however, future work in other datasets should examine the effects of these variables on 90-day mortality. Additional factors including coordination of care, access and/or distance to the treating facility and the management of late postoperative complications may be important possible mediators of 90-day mortality worthy of future study.
Although the volume-outcome phenomenon has been previously described for cystectomy, this has not been a target for volume-based referral by the Leapfrog Group or other policymakers, as have pancreatectomy and esophagectomy. Bladder cancer care has, however, been designated among Blue Cross’ Blue Distinction Centers’ “rare and complex cancers” initiative, distinguishing approximately 90 centers in the US on the basis of multidisciplinary management and minimum volume thresholds (11 cases per year for cystectomy).(33) Our threshold for low-volume hospitals is consistent with this policy. Additionally, our finding of the extension of the volume-outcome association to 90 days adds additional support to efforts assisting consumers in identifying higher volume centers. That said, the relatively wider variation in 30-day outcomes between hospital volume strata suggests that this earlier time point may represent a more meaningful and feasible target for performance feedback. Despite the lack of formal regulatory or legislative mandates, regionalization and concentration of cystectomy procedures to high volume centers is already underway.(4, 6, 34) Our results are consistent with this, with the proportion of cases occurring in very low volume (<5 cases per year) hospitals declining from 42% to 33% over the study period as well as the fact that 27/1178 hospitals accounted for over 20% of the procedures.
This study has several notable strengths. The NCDB provides a large sample with strong generalizability to the US population.(15) In contrast to prior literature describing volume-outcome relationships to in-hospital or 30-day postoperative mortality(3, 5–8, 10, 13), this dataset allowed us to ascertain 90-day mortality, which may more meaningfully capture the intermediate-term risks. This study also has several important limitations. The NCDB does not collect individual surgeon data so we are unable to draw inferences with regard to surgeon volume and the primary outcome—the importance of which, independent of facility volume, is somewhat unclear.(8, 20, 21, 35) ICD-9 coding for comorbidities provides no detail on illness severity, and we have no data on other nutritional and functional status variables that may contribute to short-term survival.(24) We do not have information on cause of death, limiting our ability to make inferences regarding the observed poorer 30- and 90-day survival among patients with advanced stage disease; while this may represent rapid disease progression, we believe this more likely reflects the complexity of surgery and potentially associated higher short-term risks in these cases. While improved 90-day survival among patients who received neoadjuvant chemotherapy, an underutilized (36) adjunct to cystectomy, obviously reflects selection bias, these findings may nevertheless assuage concerns regarding the potential for poorer short-term outcomes with chemotherapy.
Conclusions
Hospital volume is inversely associated with 90-day mortality after cystectomy; however this association is largely driven by variation in shorter-term outcomes. The results of this study point to the need for further study of potentially actionable root causes of mortality in the window from 30 to 90 days postoperative, where mortality rates are relatively high across hospital volume strata.
Acknowledgments
Funding: Drs. Nielsen and Milowsky’s contributions were supported by the University Cancer Research Fund at the UNC Lineberger Comprehensive Cancer Center, and Drs. Nielsen and Weaver’s contributions were supported by the National Institutes of Health (1KL2RR025746 and UL1RR025747, respectively). Drs. Mallin and Winchester and Messrs. Palis and Stewart were supported by the American College of Surgeons.
Footnotes
Conflicts of Interest: None
This research was presented at the Society of Urologic Oncology 2012 Annual Meeting, November 29, 2012, Bethesda, Maryland
References
- 1.Clark P. NCCN Guidelines for Bladder Cancer. NCCN Clinical Practice Guidelines in Oncology. 2012 [cited 2012 April 24]; Version 2.2012:[Available from: http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
- 2.Gore JL, Litwin MS, Lai J, Yano EM, Madison R, Setodji C, et al. Use of radical cystectomy for patients with invasive bladder cancer. J Natl Cancer Inst. 2009;102(11):802–11. doi: 10.1093/jnci/djq121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birkmeyer JD, Siewers AE, Finlayson EV, Stukel TA, Lucas FL, Batista I, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 4.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364(22):2128–37. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hollenbeck BK, Dunn RL, Miller DC, Daignault S, Taub DA, Wei JT. Volume-based referral for cancer surgery: informing the debate. J Clin Oncol. 2007;25(1):91–6. doi: 10.1200/JCO.2006.07.2454. [DOI] [PubMed] [Google Scholar]
- 6.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Montie JE, Wei JT. The regionalization of radical cystectomy to specific medical centers. J Urol. 2005;174(4 Pt 1):1385–9. doi: 10.1097/01.ju.0000173632.58991.a7. discussion 1389. [DOI] [PubMed] [Google Scholar]
- 7.Smaldone MC, Simhan J, Kutikov A, Canter DJ, Starkey R, Zhu F, et al. Trends in regionalization of radical cystectomy in three large northeastern states from 1996 to 2009. Urol Oncol. 2012 doi: 10.1016/j.urolonc.2012.04.018. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Konety BR, Dhawan V, Allareddy V, Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. 2005;173(5):1695–700. doi: 10.1097/01.ju.0000154638.61621.03. [DOI] [PubMed] [Google Scholar]
- 9.Elting LS, Pettaway C, Bekele BN, Grossman HB, Cooksley C, Avritscher EB, et al. Correlation between annual volume of cystectomy, professional staffing, and outcomes: a statewide, population-based study. Cancer. 2005;104(5):975–84. doi: 10.1002/cncr.21273. [DOI] [PubMed] [Google Scholar]
- 10.Barbieri CE, Lee B, Cookson MS, Bingham J, Clark PE, Smith JA, Jr, et al. Association of procedure volume with radical cystectomy outcomes in a nationwide database. J Urol. 2007;178(4 Pt 1):1418–21. doi: 10.1016/j.juro.2007.05.156. discussion 1421–2. [DOI] [PubMed] [Google Scholar]
- 11.Hollenbeck BK, Wei Y, Birkmeyer JD. Volume, process of care, and operative mortality for cystectomy for bladder cancer. Urology. 2007;69(5):871–5. doi: 10.1016/j.urology.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damhuis RA, Wijnhoven BP, Plaisier PW, Kirkels WJ, Kranse R, van Lanschot JJ. Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg. 2012;99(8):1149–54. doi: 10.1002/bjs.8813. [DOI] [PubMed] [Google Scholar]
- 13.Hollenbeck BK, Miller DC, Taub DA, Dunn RL, Khuri SF, Henderson WG, et al. The effects of adjusting for case mix on mortality and length of stay following radical cystectomy. J Urol. 2006;176(4 Pt 1):1363–8. doi: 10.1016/j.juro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Porter MP, Gore JL, Wright JL. Hospital volume and 90-day mortality risk after radical cystectomy: a population-based cohort study. World J Urol. 2011;29(1):73–7. doi: 10.1007/s00345-010-0626-3. [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90. doi: 10.1245/s10434-007-9747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Lieffers JR, Baracos VE, Winget M, Fassbender K. A comparison of Charlson and Elixhauser comorbidity measures to predict colorectal cancer survival using administrative health data. Cancer. 2011;117(9):1957–65. doi: 10.1002/cncr.25653. [DOI] [PubMed] [Google Scholar]
- 18.Southern DA, Quan H, Ghali WA. Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–60. doi: 10.1097/01.mlr.0000118861.56848.ee. [DOI] [PubMed] [Google Scholar]
- 19.van Walraven C, Austin PC, Jennings A, Quan H, Forster AJ. A modification of the Elixhauser comorbidity measures into a point system for hospital death using administrative data. Med Care. 2009;47(6):626–33. doi: 10.1097/MLR.0b013e31819432e5. [DOI] [PubMed] [Google Scholar]
- 20.Morgan TM, Barocas DA, Keegan KA, Cookson MS, Chang SS, Ni S, et al. Volume outcomes of cystectomy--is it the surgeon or the setting? J Urol. 2012;188(6):2139–44. doi: 10.1016/j.juro.2012.08.042. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni GS, Urbach DR, Austin PC, Fleshner NE, Laupacis A. Higher surgeon and hospital volume improves long-term survival after radical cystectomy. Cancer. 2013 doi: 10.1002/cncr.28235. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 22.Zabor EC, Gonen M, Chapman PB, Panageas KS. Dynamic prognostication using conditional survival estimates. Cancer. 2013 doi: 10.1002/cncr.28273. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Isbarn H, Jeldres C, Zini L, Perrotte P, Baillargeon-Gagne S, Capitanio U, et al. A population based assessment of perioperative mortality after cystectomy for bladder cancer. J Urol. 2009;182(1):70–7. doi: 10.1016/j.juro.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 24.Morgan TM, Keegan KA, Barocas DA, Ruhotina N, Phillips SE, Chang SS, et al. Predicting the probability of 90-day survival of elderly patients with bladder cancer treated with radical cystectomy. J Urol. 186(3):829–34. doi: 10.1016/j.juro.2011.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu JH, Zingmond DS, McGory ML, SooHoo NF, Ettner SL, Brook RH, et al. Disparities in the utilization of high-volume hospitals for complex surgery. JAMA. 2006;296(16):1973–80. doi: 10.1001/jama.296.16.1973. [DOI] [PubMed] [Google Scholar]
- 26.Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Med Care. 1992;30(7):615–29. doi: 10.1097/00005650-199207000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361(14):1368–75. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 28.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 49(12):1076–81. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 29.Trinh VQ, Trinh QD, Tian Z, Hu JC, Shariat SF, Perotte P, Karakiewicz PI, Sun M. In-hospital mortality and failure-to-rescue rates after radical cystectomy. BJU Int. 2013;112(2):E20–7. doi: 10.1111/bju.12214. [DOI] [PubMed] [Google Scholar]
- 30.Donat SM, Shabsigh A, Savage C, Cronin AM, Bochner BH, Dalbagni G, et al. Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol. 2009;55(1):177–85. doi: 10.1016/j.eururo.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 31.Hollenbeck BK, Daignault S, Dunn RL, Gilbert S, Weizer AZ, Miller DC. Getting under the hood of the volume-outcome relationship for radical cystectomy. J Urol. 2007;177(6):2095–9. doi: 10.1016/j.juro.2007.01.153. discussion 2099. [DOI] [PubMed] [Google Scholar]
- 32.Friese CR, Lake ET, Aiken LH, Silber JH, Sochalski J. Hospital nurse practice environments and outcomes for surgical oncology patients. Health Serv Res. 2008;43(4):1145–63. doi: 10.1111/j.1475-6773.2007.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.BCBS. Blue Distinction Centers for Complex and Rare Cancers Program Selection Criteria for 2008/2009 Designations. 2009 [cited July 20, 2012]; Available from: http://www.bcbs.com/why-bcbs/blue-distinction/blue-distinction-complex-and-rare/CRC_MidLevel-Criteria_101309.pdf.
- 34.Cooperberg MR, Modak S, Konety BR. Trends in regionalization of inpatient care for urological malignancies, 1988 to 2002. J Urol. 2007;178(5):2103–8. doi: 10.1016/j.juro.2007.07.040. discussion 2108. [DOI] [PubMed] [Google Scholar]
- 35.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349(22):2117–27. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 36.David KA, Milowsky MI, Ritchey J, Carroll PR, Nanus DM. Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol. 2007;178(2):451–4. doi: 10.1016/j.juro.2007.03.101. [DOI] [PubMed] [Google Scholar]