Abstract
PURPOSE
To better understand the combined effects of pre-transplant, transplant, and post-transplant factors in determining risks of serious cardiovascular disease following hematopoietic cell transplantation (HCT).
METHODS
Hospitalizations and deaths associated with serious cardiovascular outcomes were identified among 1,379 Washington State residents who received HCT (57% allogeneic; 43% autologous) at a single center from 1985–2005, survived ≥2 years, and followed through 2008. Using a nested-case-cohort design, relationships (hazard ratios, HR) between potential risk factors and outcomes were examined among affected survivors and a randomly selected sub-cohort (n=509).
RESULTS
After 7.0 years median follow-up (range 2.0–23.7), the 10-year cumulative incidence of ischemic heart disease, cardiomyopathy, stroke, and all-cause cardiovascular death was 3.8%, 6.0%, 3.5%, and 3.7%, respectively. In multivariable analysis, increased pre-transplant anthracyclines was associated with cardiomyopathy. Active chronic graft vs. host disease was associated with cardiovascular death (HR 4.0, 95% CI 1.1–14.7); risk was otherwise similar between autologous vs. allogeneic HCT recipients. Independent of therapeutic exposures, pre-transplant smoking, hypertension, dyslipidemia, diabetes, and obesity conferred additional risk of all outcomes except stroke (HR ≥1.5 for each additional risk factor, p<0.03). Hypertension and dyslipidemia at one year with persistence of these conditions two or more years following HCT also were associated with independent risks of multiple outcomes.
CONCLUSION
Hematopoietic cell transplant survivors with pre-existing or newly developed and persistent cardiovascular risk factors remain at greater risk of subsequent serious cardiovascular disease compared with other survivors, independent of chemo- and radiotherapy exposures. These survivors should receive appropriate follow-up and be considered for primary intervention.
INTRODUCTION
More than 60,000 patients receive some form of allogeneic or autologous hematopoietic cell transplantation (HCT) annually worldwide(1). Although chronic graft versus host disease (GVHD) and disease recurrence remain the leading causes of mortality in long-term HCT survivors(2–4), investigators have recognized the increased risk of long-term cardiovascular and other morbidities in HCT survivors compared with the general population(5–11). Although many HCT recipients receive chemo- and radiotherapies that affect cardiovascular health before HCT, few studies have examined the influence of pre-transplant exposures in combination with transplant-related factors(12–14).
The goal of this nested case-cohort study was to measure the relative contributions of selected pre-transplant therapeutic exposures and known cardiovascular risk factors (obesity, hypertension, dyslipidemia, diabetes, smoking) in combination with transplant and posttransplant exposures in determining subsequent risk of ischemic heart disease, cardiomyopathy/heart failure, stroke, and all-cause cardiovascular death among ≥2-year HCT survivors. Specifically, we wanted to investigate the importance of early manifestations of posttransplant obesity, hypertension, dyslipidemia, and diabetes. This information would inform the development of more appropriate screening and intervention among HCT survivors, including earlier identification of at-risk patients(15;16).
METHODS
Patient Population and Outcomes Ascertainment
The original cohort and method for outcomes ascertainment have been described previously(9). Briefly, eligible HCT recipients were Washington State residents treated at the Fred Hutchinson Cancer Research Center (FHCRC) from 1985–2005 and alive ≥2 years post-HCT (n=1,405). FHCRC is a National Cancer Institute-designated comprehensive cancer center and the only accredited institution that performs allogeneic HCT in Washington State. Study procedures were approved by the institutional review boards at FHCRC and the Washington State Department of Health. After excluding residents who emigrated out-of-state within 2 years after HCT (n=19) and those who withdrew consent for future research (n=7), 1,379 survivors were available for analysis.
Primary outcomes (Table S1) were ischemic heart disease (acute myocardial infarct, coronary artery bypass/angioplasty or related therapeutic interventions, atherosclerotic heart disease, and angina/chronic ischemic heart disease), cardiomyopathy/heart failure (including need for heart transplant/assist device), stroke (cerebrovascular accident, intracranial hemorrhage, transient ischemic attack, brain/neck endarterecteomy/angioplasty or related interventions), and any cardiovascular death occurring ≥2 years after the index HCT, as ascertained by the state hospital discharge registry and the state death registry through December 31, 2008. The hospital discharge registry included all discharges from non-Federal facilities state-wide, with up to 9 diagnosis and 5 procedure codes (International Classification of Diseases-9th revision, ICD-9) per hospitalization. The death registry records the primary and up to 6 contributing causes of death (ICD-10) and includes deaths of Washington residents who die in neighboring states. FHCRC records, including health surveys mailed annually to all HCT survivors, also were reviewed since cardiomyopathy/heart failure may present less acutely and not be associated with hospitalization compared with our other outcomes of interest. This review identified 3 additional residents with clinically documented heart failure not ascertained by administrative data. Persons could experience more than one outcome, but only the first occurrence for any given outcome was counted.
Nested Case-Cohort Study
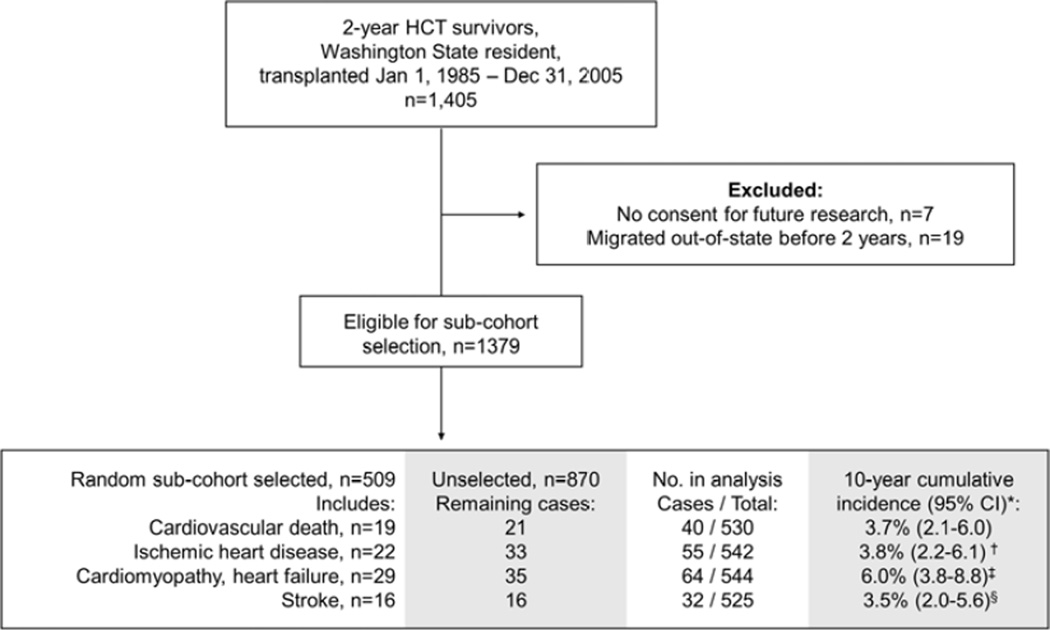
Given limited resources and the detailed medical record review required for exposure assessment, we used a nested case-cohort study design. In contrast to a nested case-control study, this design allows for analyses of multiple outcomes while using only a representative subset of the entire cohort without need to select different comparison groups for each outcome(17). A subset of the cohort (n=509 out of 1,379) was randomly selected to provide approximately 10 comparison subjects per case; comparison subjects do not need to be selected on the basis of any a priori matching criteria in a case-cohort study(17). In the analysis of each outcome, we used this randomly selected sub-cohort plus all additional subjects within the overall cohort who developed the specific outcome of interest. Thus, the numbers available for analysis varied for different outcomes, ranging from 525 for stroke to 544 for cardiomyopathy (Figure 1). Overall, records of 629 survivors were reviewed in detail. Subjects with a history of ischemic heart disease (n=11), cardiomyopathy (n=9), or stroke (n=5) before HCT were excluded from the analysis of each respective outcome.
FIGURE 1.
CONSORT diagram outlining the analytic population used for the nested case-cohort study.
*Based on sub-cohort and excludes individuals with history of ischemic heart disease (n=11†), cardiomyopathy (n=9‡), and stroke (n=5§) prior to HCT
Exposure Ascertainment
Pre-transplant exposures ascertained from medical records included anthracyclines, radiotherapy, smoking history (never, any, and current), and baseline medication use for hypertension, dyslipidemia, or diabetes. Anthracycline doses were converted to the equivalent doxorubicin dose: daunorubicin*0.83, epirubicin*0.67, idarubicin*5, and mitoxantrone*4(18). Radiotherapy records were abstracted and exposures classified by the body area exposed: brain, chest, and abdomen/pelvis. Fields that spanned multiple body areas, e.g. spine (chest, and/or abdomen/pelvis depending on extent), had all relevant areas coded as exposed.
Self-reported race/ethnicity, clinically-recorded body mass index (BMI) at the time of transplant, and selected transplant-related exposures (donor type, stem cell source, and conditioning regimen including total body or lymphoid irradiation) were obtained from the FHCRC transplant database. BMI was categorized as <18.5, 18.5–24, 25–29, and ≥30 kg/m2, corresponding to underweight, normal, overweight, and obese, respectively. For subjects age 2–20 years, we used corresponding categories based on age- and sex-standardized BMI percentiles(19). All radiotherapy records were reviewed (by EC and KW) and the cumulative average cardiac dose from all pre-transplant and transplant-related exposures was estimated based on available treatment records and comparison to sex and body size matched recreated 3-dimensional radiation plan models(20).
Post-transplant exposures such as duration of systemic immunosuppressive therapy for chronic GVHD, BMI at 1-year post-HCT, and use of medications for hypertension, dyslipidemia, and diabetes throughout the entire post-HCT period were abstracted from the medical records. We also examined available total cholesterol, triglyceride, and glucose values one year after HCT among patients who returned to FHCRC for follow-up; these are typically requested to be fasting.
Statistical Analysis
Chi-square and Wilcoxon rank sum tests were used to test for differences among proportions and non-normally distributed continuous outcomes. We used multivariable proportional hazards models to calculate hazard ratios (HRs) and 95% confidence intervals (CI) for each of the 4 outcomes, starting two years after HCT. Primary models adjusted for demographic characteristics (sex, age at and year of HCT, and non-Hispanic White race/ethnicity vs. other); anthracycline dose (none, <200, 200–299, ≥300 mg/m2); cardiac radiation dose including total body irradiation (TBI; <1, 1–19, ≥20 Gy); any radiotherapy exclusive of TBI to the brain or abdomen/pelvis (yes/no); TBI dose separately (<10/≥10 Gy); donor type (autologous, related allogeneic, unrelated allogeneic); chronic GVHD (none, active, resolved); and any relapse of one’s primary disease following HCT (yes/no). Relapse and chronic GVHD were modeled as separate time-dependent covariates. In lieu of cardiac radiotherapy dose, any chest radiotherapy other than TBI also was examined with similar results.
Secondary models examined any additional influence of the following pre-HCT factors added to the primary models: BMI at time of HCT (normal/underweight, overweight, obese), current smoking (yes/no), and medications for hypertension, dyslipidemia, or diabetes (each yes/no). The influence of BMI one year post-transplant also was examined, along with post-transplant use of medications for hypertension, dyslipidemia, and/or diabetes as time-dependent covariates. Given our case-cohort study design, we used robust variance estimators for all models(21). For the 4 outcomes we examined, we only counted the first occurrence of that specific outcome; follow-up was otherwise censored at December 31, 2008, or when patients migrated out-of-state. Death from causes other than the specific outcome being examined was treated as a competing risk event(22). Given the possibility of introducing bias and losing precision with overly adjusted models, we also examined simpler models with at least 5 events per covariate, with overall similar results(23). Analyses were performed using Stata, version 12 (StataCorp, College Station, TX).
RESULTS
Baseline demographic and HCT-related characteristics were similar between the full study cohort and the randomly selected sub-cohort (Table 1). Among the sub-cohort, the median age at time of HCT was 40 years (range 0–69) with median follow-up 7.0 years (range 2.0–23.7). After accounting for out-of-state migration ≥2-years post-HCT (n=17) and death (n=144), 348 subjects (68.4%) remained alive as of 12/31/2008. Most sub-cohort subjects were treated for hematologic malignancies (80.6%), and most had received prior alkylating agents (54.5%) or anthracyclines (70.2%), although few had received any radiotherapy before HCT (e.g. 10.4% prior chest exposure). Most received an allogeneic transplant (57.2%), with 60.1% requiring subsequent systemic immunosuppressive therapy for chronic GVHD. Nearly half (48.9%) received some form of TBI-based conditioning for HCT; all TBI doses were fractionated. Inclusive of TBI, 8.5% of the sub-cohort received an average of 1–9 Gy to the heart, 45.2% received 10–19 Gy, 2.6% received 20–29 Gy, and 2.0% received ≥30 Gy.
TABLE 1.
Demographic and treatment characteristics of study cohort and randomly selected sub-cohort.
| Characteristic, n (%)* | Cohort n=509 |
Sub-Cohort n=509 |
P- value |
||
|---|---|---|---|---|---|
| Female | 653 | (47.4) | 238 | (46.8) | 0.84 |
| Age at transplant, years | 0.79 | ||||
| <20 | 302 | (21.9) | 110 | (21.6) | |
| 20–39 | 360 | (26.1) | 144 | (28.3) | |
| 40–59 | 604 | (43.8) | 217 | (42.6) | |
| ≥60 | 113 | (8.2) | 38 | (7.5) | |
| Year of transplant | 0.27 | ||||
| 1985–1994 | 365 | (26.5) | 150 | (29.5) | |
| 1995–2000 | 450 | (32.6) | 170 | (33.4) | |
| 2001–2005 | 564 | (40.9) | 189 | (37.1) | |
| Race/ethnicity | 0.85 | ||||
| White, non-Hispanic | 1,161 | (84.2) | 436 | (85.7) | |
| Black | 30 | (2.2) | 11 | (2.2) | |
| Hispanic | 64 | (4.6) | 23 | (4.5) | |
| Asian / Pacific Islander | 39 | (2.8) | 9 | (1.8) | |
| Other | 19 | (1.4) | 8 | (1.6) | |
| Unknown | 66 | (4.8) | 22 | (4.3) | |
| Underlying diagnosis | 0.63 | ||||
| Acute leukemia, myelodysplastic syndrome | 443 | (32.1) | 184 | (36.1) | |
| Chronic leukemia | 206 | (14.9) | 67 | (13.2) | |
| Lymphoma | 305 | (22.1) | 106 | (20.8) | |
| Multiple myeloma | 169 | (12.3) | 53 | (10.4) | |
| Breast cancer | 77 | (5.6) | 34 | (6.7) | |
| Neuroblastoma | 50 | (3.6) | 18 | (3.5) | |
| Other solid tumor | 35 | (2.5) | 9 | (1.8) | |
| Non-malignant blood disorder | 51 | (3.7) | 22 | (4.3) | |
| Immune/metabolic disease | 25 | (1.8) | 7 | (1.4) | |
| Multiple/systemic sclerosis | 18 | (1.3) | 9 | (1.8) | |
| Pre-transplant exposures† | |||||
| Alkylating agent | - | 277 | (54.5) | ||
| Anthracycline, mg/m2 | |||||
| None | - | 151 | (29.8) | ||
| <200 | - | 124 | (24.5) | ||
| 200–299 | - | 80 | (15.8) | ||
| ≥300 | - | 151 | (29.8) | ||
| Radiotherapy | |||||
| Brain | - | 18 | (3.5) | ||
| Neck | - | 35 | (6.9) | ||
| Spine | - | 24 | (4.7) | ||
| Chest | - | 53 | (10.4) | ||
| Abdomen/pelvis | - | 41 | (8.1) | ||
| Prior total body/lymphoid | - | 3 | (0.6) | ||
| Stem cell donor | 0.70 | ||||
| Autologous | 599 | (43.4) | 218 | (42.8) | |
| Related allogeneic | 491 | (35.6) | 191 | (37.5) | |
| Unrelated allogeneic | 289 | (21.0) | 100 | (19.6) | |
| Stem cell source‡ | 0.53 | ||||
| Marrow | 584 | (42.3) | 220 | (43.2) | |
| Peripheral blood | 819 | (59.4) | 297 | (58.3) | |
| Cord blood | 12 | (0.9) | 2 | (0.4) | |
| Transplant conditioning regimen§ | |||||
| No total body irradiation (TBI) | 736 | (53.4) | 260 | (51.1) | 0.54 |
| TBI, 1–9 Gy | 102 | (7.4) | 35 | (6.9) | |
| TBI, ≥10Gy | 541 | (39.2) | 214 | (42.0) | |
| Cyclophosphamide/TBI | 413 | (29.9) | 165 | (32.4) | 0.30 |
| Busulfan/cyclophosphamide | 263 | (19.1) | 84 | (16.5) | 0.20 |
| Chronic graft vs. host disease | 483 | (35.0) | 175 | (34.4) | 0.79 |
| Resolved at last follow-up†, ** | - | 113 | (22.2) | ||
| Post-transplant relapse | 359 | (26.0) | 146 | (28.7) | 0.25 |
| Relapse within 2 yrs of transplant | 217 | (15.7) | 86 | (16.9) | |
| Died, any cause** | 353 | (25.6) | 146 | (28.7) | 0.18 |
Proportions shown based on those without missing data.
Only determined for sub-cohort.
Exceeds totals because some individuals had mixed products as part of single or tandem transplants.
Categories are not mutually exclusive.
As of December 31, 2008.
Between time of transplant and one year later, rates of obesity and use of lipid-lowering medications remained stable (22.9% vs. 21.0% and 3.0% vs. 2.8%, respectively; Table 2), but use of antihypertensives and diabetes medications was much more frequent at 1-year post-HCT (6.7% vs. 19.6% and 4.1% vs. 12.9%). The proportions of survivors on hypertension and lipid-lowering medications increased further by time of last follow-up, although 34 of 65 patients on diabetes medications at 1-year post-HCT had discontinued treatment by time of last follow-up. Rates of chronic GVHD did not differ significantly among subjects who discontinued treatment for hypertension, dyslipidemia, and diabetes between one year and last follow-up compared with those who remained on treatment (data not shown).
TABLE 2.
Distribution of pre- and post-transplant cardiovascular risk factors among sub-cohort (n=509*).
| Trait, n (%) | Pre-transplant | 1-yr post-transplant | Last follow-up | |||
|---|---|---|---|---|---|---|
| History of tobacco use† | 171 | (33.8) | - | - | ||
| Current user | 49 | (9.7) | - | - | ||
| Body mass index‡, kg/m2 | ||||||
| Underweight, <18.5 | 11 | (2.2) | 21 | (4.3) | - | |
| Normal, 18.5–24 | 206 | (41.4) | 224 | (46.2) | - | |
| Overweight, 25–29 | 167 | (33.5) | 138 | (28.5) | - | |
| Obese, ≥30 | 114 | (22.9) | 102 | (21.0) | - | |
| Hypertension treatment | 34 | (6.7) | 98 | (19.6) | 119 | (23.9) |
| On treatment at prior time point, still on | - | 28 | (5.6) | 63 | (12.7) | |
| Off treatment at prior time point, now on | - | 70 | (14.0) | 56 | (11.2) | |
| On treatment at prior time point, now off | - | 6 | (1.2) | 34 | (6.8) | |
| Dyslipidemia treatment | 15 | (3.0) | 14 | (2.8) | 79 | (15.8) |
| On treatment at prior time point, still on | - | 7 | (1.4) | 13 | (2.6) | |
| Off treatment at prior time point, now on | - | 7 | (1.4) | 64 | (12.8) | |
| On treatment at prior time point, now off | - | 8 | (1.6) | 1 | (0.2) | |
| Diabetes treatment | 21 | (4.1) | 65 | (12.9) | 54 | (10.7) |
| On treatment at prior time point, still on | - | 21 | (4.2) | 31 | (6.2) | |
| Off treatment at prior time point, now on | - | 44 | (8.7) | 23 | (4.6) | |
| On treatment at prior time point, now off | - | 0 | 34 | (6.7) | ||
Percentages based on those with data among the sub-cohort (depending on the risk factor and time point, n=498 to 509).
Only assessed pre-transplant.
Limited to those age ≥2 years. Values for survivors ages 2–20 years based on equivalent age- and sex-specific percentiles. Only assessed pre-transplant and 1-yr post-transplant.
Among survivors with data available one year after HCT, 10.8% (29 of 268) had glucose ≥126 mg/dl, and the proportions with total cholesterol ≥240 mg/dl and triglyceride ≥150 mg/dl were even greater (28.2% and 58.0%, respectively; n=241). The proportions were similar after excluding patients taking diabetes and/or lipid-lowering medications: 8.6%, 28.5%, and 57.8%, respectively. Overall, subjects with laboratory data were more likely to have been transplanted in more recent years, and to reflect the population more likely to return to the transplant center for an in-person evaluation (allogeneic vs. autologous HCT, and among allogeneic recipients, those with a history of chronic GVHD).
Overall, 135 individuals (9.8%) experienced at least one of the serious cardiovascular complications ascertained by this study. The 10-year cumulative incidence of serious cardiovascular disease associated with hospitalization/death ranged from 3.5% (stroke) to 6.0% (cardiomyopathy; Figure 1). The median time from HCT to onset of these complications was 5.0 years (range 2.0–20.7).
In multivariable analyses, increased anthracycline dose was associated with cardiomyopathy (Table 3). Radiotherapy, including cardiac radiation, was not significantly associated with any adverse outcome although estimates were imprecise. Among transplant-related exposures, having active chronic GVHD was associated with an increased risk of cardiovascular death (HR 4.0, 95% CI 1.1–14.7) although the relative hazard was attenuated after GVHD resolution. Nine of the 12 cardiovascular deaths among individuals with active chronic GVHD listed codes consistent with underlying/chronic heart disease. Finally, any history of original disease relapse after HCT was associated with a significantly increased risk of all cardiovascular complications except ischemic heart disease, suggesting that relapse may be acting as a surrogate marker for additional cardiotoxic exposures.
TABLE 3.
Relative hazards of cardiovascular outcomes associated with pre-transplant and transplant-related therapeutic exposures.*
| Exposure | Hazard ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cardiovascular death |
Ischemic heart disease |
Cardiomyopathy /heart failure |
Stroke | |||||
| Anthracycline†, mg/m2 | ||||||||
| None | 1.0 | (ref) | 1.0 | (ref) | 1.0 | (ref) | 1.0 | (ref) |
| <200 | 2.2 | (0.7–7.6) | 1.0 | (0.4–2.5) | 2.3 | (0.8–6.8) | 1.6 | (0.5–4.6) |
| 200–299 | 3.6 | (0.9–14.1) | 1.2 | (0.3–4.1) | 3.3 | (0.8–13.2) | 0.9 | (0.2–4.4) |
| ≤300 | 2.8 | (0.9–8.8) | 0.8 | (0.3–.2) | 4.5 | (1.5–13.3) | 2.2 | (0.7–7.2) |
| Brain radiotherapy vs. none‡ | 1.1 | (0.1–9.8) | - | 0.7 | (0.1–6.2) | 0.7 | (0.1–4.2) | |
| Abdomen radiotherapy vs. none‡ | 2.7 | (0.9–8.3) | 1.8 | (0.7–4.5) | 0.8 | (0.3–2.4) | 0.9 | (0.2–3.3) |
| Cardiac radiotherapy dose, Gy§ | ||||||||
| <1 | 1.0 | (ref) | 1.0 | (ref) | 1.0 | (ref) | 1.0 | (ref) |
| 1–19 | 0.3 | (0.1–1.3) | 0.6 | (0.2–1.7) | 0.9 | (0.4–2.1) | 0.6 | (0.2–2.1) |
| ≥20 | 1.4 | (0.4–5.3) | 1.3 | (0.3–5.0) | 1.3 | (0.4–4.7) | 0.5 | (0.1–5.1) |
| Total body irradiation ≥10 Gy vs. less | 1.4 | (0.3–6.9) | 1.7 | (0.5–5.3) | 1.2 | (0.5–2.8) | 1.0 | (0.2–3.6) |
| Allogeneic vs. autologous donor | 1.9 | (0.5–7.7) | 0.6 | (0.2–2.2) | 1.2 | (0.4–3.4) | 1.3 | (0.3–5.5) |
| Chronic graft vs. host disease | ||||||||
| None | 1.0 | (ref) | 1.0 | (ref) | 1.0 | (ref) | 1.0 | (ref) |
| Active | 4.0 | (1.1–14.7) | 2.6 | (0.7–8.9) | 1.1 | (0.4–3.1) | 1.7 | (0.4–7.0) |
| Resolved | 1.2 | (0.3–4.8) | 2.2 | (0.8–6.1) | 1.4 | (0.5–3.9) | 1.4 | (0.4–5.2) |
| Original disease relapse vs. none | 4.3 | (1.9–9.6) | 1.5 | (0.7–.2) | 3.2 | (1.7–6.0) | 5.9 | (2.4–14.7) |
Estimates reflect adjustment for exposures shown plus sex, age at and year of transplant, and race/ethnicity.
In terms of doxorubicin equivalent dose.
Brain and abdomen (including pelvis) fields exclusive of total body irradiation.
Inclusive of any total body irradiation.
Increased HRs were observed for most pre-transplant cardiovascular risk factors (smoking, obesity, hypertension, diabetes) although individual confidence intervals did not meet statistical significance (Table 4). However, having an increasing number of these adverse risk factors pre-transplant was associated with all subsequent cardiovascular complications (HR ≥1.5 for each additional risk factor, p<0.03) except stroke. Estimates were similar even if smoking status was not counted.
TABLE 4.
Relative hazards of cardiovascular outcomes associated with presence of pre- and post-transplant cardiovascular risk factors.
| Risk factor | Hazard ratio (95% CI) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Cardiovascular death |
Ischemic heart disease |
Cardiomyopathy /heart failure |
Stroke | |||||
| Pre-transplant* | ||||||||
| Current smoking vs. none | 2.7 | (0.8–9.2) | 2.3 | (0.95–5.4) | 1.8 | (0.6–5.1) | 0.4 | (0.04–3.8) |
| Obese vs. normal/underweight | 1.5 | (0.6–4.0) | 2.0 | (0.8–4.9) | 1.2 | (0.5–2.9) | 1.5 | (0.5–4.3) |
| Hypertension treatment vs. none | 0.8 | (0.1–4.0) | 2.3 | (0.6–8.9) | 1.5 | (0.6–3.7) | 4.8 | (1.1–21.0) |
| Dyslipidemia treatment vs. none | 3.8 | (1.0–14.4) | 1.8 | (0.4–8.1) | 3.2 | (0.7–14.9) | 1.4 | (0.2–11.2) |
| Diabetes treatment vs. none | 3.3 | (0.6–7.4) | 2.7 | (0.8–0.0) | 1.5 | (0.5–4.8) | 0.6 | (0.1–6.1) |
| Risk associated with presence of each additional risk factor† | 1.8 | (1.2–2.8) | 2.1 | (1.4–3.1) | 7.5 | (1.1–2.2) | 1.3 | (0.7–2.2) |
| 1-yr post-transplant* | ||||||||
| Obese vs. normal/underweight | 1.7 | (0.6–4.8) | 2.0 | (0.8–4.9) | 0.6 | (0.2–1.5) | 1.4 | (0.5–3.6) |
| Hypertension treatment vs. none | 1.9 | (0.6–5.8) | 2.2 | (0.7–6.6) | 3.8 | (1.4–8.6) | 2.2 | (0.9–5.8) |
| Dyslipidemia treatment vs. none | 2.0 | (0.3–16.3) | 3.6 | (0.7–18.0) | 1.0 | (0.1–8.3) | 7.4 | (1.2–47.0) |
| Diabetes treatment vs. none | 1.3 | (0.4–4.2) | 1.2 | (0.4–3.4) | 1.4 | (0.5–3.8) | 0.1 | (0.02–0.8) |
| Risk associated with presence of each additional risk factor‡ | 1.6 | (0.97–2.6) | 1.7 | (1.1–2.7) | 1.2 | (0.8–1.8) | 1.2 | (0.7–2.2) |
| If restricted to those with risk factor also present at last follow-up‡ | 1.9 | (1.1–3.1) | 2.2 | (1.4–3.7) | 1.3 | (0.9–2.0) | 1.7 | (0.98–2.9) |
| ≥2-yr post-transplant§ | ||||||||
| Hypertension treatment vs. none | 1.4 | (0.5–3.9) | 3.6 | (1.7–7.6) | 2.8 | (1.3–5.7) | 1.8 | (0.8–4.0) |
| Dyslipidemia treatment vs. none | 2.1 | (0.8–5.5) | 1.1 | (0.4–3.0) | 1.8 | (0.8–4.4) | 1.9 | (0.6–5.4) |
| Diabetes treatment vs. none | 3.5 | (1.3–9.6) | 2.8 | (1.0–7.9) | 1.5 | (0.5–4.6) | 0.9 | (0.2–3.3) |
Models included factors examined in Table 3, plus concurrent adjustment of factors listed below.
Trend test for 0, 1,2, and ≥3 risk factors including tobacco use, obesity, hypertension, dyslipidemia and diabetes.
Trend test for 0, 1,2, and ≥3 risk factors including obesity, hypertension, dyslipidemia and diabetes.
Models included all factors examined in Table 3, plus concurrent adjustment of factors listed below as time-dependent variables.
Having an increased number of these risk factors at 1-year remained predictive of subsequent ischemic heart disease (HR 1.7, 95% CI 1.1–2.7; Table 4). Compared with pre-transplant estimates, HRs for multiple risk factors at 1-year associated with cardiovascular death and cardiomyopathy became attenuated. However, when only subjects who had persistent conditions (i.e. present both at 1-year and at last follow-up) were classified as exposed, the magnitude of resulting associations was increased, more similar to the effect of having these conditions before HCT (Table S2).
Since most survivors with abnormal glucose, cholesterol, and triglyceride values one year after HCT did not appear to be receiving targeted treatment, we also examined whether subjects who developed subsequent adverse cardiovascular complications would have different values compared with those who were unaffected and alive at last follow-up (Table S3). Excluding subjects receiving concurrent lipid-lowering or diabetes medications, those who developed cardiovascular disease generally had greater total cholesterol and triglyceride median values. This was most marked for ischemic heart disease (triglyceride 280 vs. 160 mg/dL [3.16 vs. 1.81 mmol/L], p=0.03; cholesterol 213 vs. 195 mg/dL [5.52 vs. 5.05 mmol/L], p=0.06).
Hypertension and diabetes at ≥2-years post-HCT remained important predictors of subsequent cardiovascular disease (Table 4). Hypertension was significantly associated with increased risks of ischemic heart disease and cardiomyopathy (HRs ≥2.8, p<0.01) whereas diabetes was associated with an increased risk of all-cause cardiovascular mortality and ischemic heart disease (HRs ≥2.8, p<0.05). Risk estimates for dyslipidemia were not statistically significant, although they generally indicated increased risk. In these time-dependent models, we did not observe any secular effect associated with any of our outcomes, except for a decreased risk of cardiovascular death among patients transplanted in later years (HR 0.3–0.4 for 1990–1999 and 2000–2005 compared with pre-1990, p<0.05).
DISCUSSION
Our prior analysis compared the incidence rates of major cardiovascular complications among this cohort of greater than 1,300 HCT survivors with a matched general population sample from the same geographic region(9). In that study we found that HCT survivors had a significantly greater cumulative incidence of cardiovascular complications including conditions such as hypertension, dyslipidemia, and diabetes. That study was limited by lack of information on pre-transplant treatment and the temporal relationship of hypertension, dyslipidemia, and diabetes on subsequent risk of cardiovascular disease. We extended that work here by additionally considering important therapeutic and non-therapeutic exposures along the entire treatment continuum, including detailed ascertainment of chronic GVHD, hypertension, dyslipidemia, and diabetes status over time. Our results underscore the important continued influence of conventional risk factors on subsequent cardiovascular disease among heavily-treated HCT survivors, and provide further insight into subsets of survivors who may benefit from additional intervention and prevention strategies.
Among pre-transplant therapeutic exposures, our results confirm the known association between greater anthracycline dose and cardiomyopathy(12;24;25). Similar to some other studies of HCT survivors, no associations between cardiac radiotherapy and subsequent cardiomyopathy(12;24) or other cardiovascular complications(5;7) were observed. These results contrast with the well-documented dose-response associations reported in other studies of HCT and non-transplant cancer survivors(13;14;26–28). However, most irradiated survivors in our study received relatively low cardiac exposure (<5% of our sub-cohort received ≥20 Gy to the heart) and our median follow-up also was relatively short. Both factors likely limited our ability to detect significant differences.
There was no difference in risk of cardiovascular outcomes between autologous or allogeneic recipients after accounting for other treatment exposures and chronic GVHD status. However, having active chronic GVHD was associated with an increased risk of cardiovascular death. Chronic GVHD, as a chronic inflammatory condition has been hypothesized to contribute to atherosclerotic disease(11;29), and importantly, immunosuppressants used to treat GVHD (e.g. glucocorticoids, calcineurin inhibitors, sirolimus) often have cardiovascular side effects including hypertension, insulin resistance, renal dysfunction, and dyslipidemia(30). In other studies, even after accounting for effects of active chronic GVHD and/or its treatment, allogeneic HCT survivors still appear to have an increased risk of developing hypertension, dyslipidemia, and diabetes which predispose towards more serious cardiovascular disease compared with other cancer survivors or the general population(6;11;14;31–34).
However, data from our study and others have shown that although these cardiovascular risk factors develop frequently after HCT, especially following allogeneic transplant, it appears that many HCT survivors do not receive specific treatment for these conditions(32;35;36). Our reliance on medication status to define cardiovascular risk factors in this study likely underestimates the true prevalence of these conditions. This may be because clinicians believe that these conditions often improve within 2 years among some transplant recipients, especially after patients are tapered off chronic immunosuppression agents (e.g. corticosteroids, calcineurin inhibitors, sirolimus) which not infrequently have hypertension, glucose-intolerance, and dyslipidemia as medication side-effects(37). This may also explain why our estimates examining the influence of these risk factors (as classified by medication status) at 1-year post-transplant tended to be less strongly associated with future morbidity compared with the presence of risk factors before transplantation or at ≥2 years. Although we did not observe a significant secular effect for most of the outcomes we studied, practice patterns with respect to the monitoring and treatment of cardiovascular risk factors have likely evolved to some degree over the 20+ years covered by this study(36).
Persistence of these cardiovascular risk factors for longer periods is clearly important. Long-term HCT survivors who develop serious cardiovascular complications have been shown to be more likely to be smokers, and/or have hypertension, dyslipidemia, or diabetes at last follow-up(7;9;11;13;24). The combined effect of cardiotoxic cancer treatment exposures and subsequent development of conventional cardiovascular risk factors may be more than additive(38). However, our study was not sufficiently large enough to reliably assess these interaction effects. Our results and those of others also demonstrate that survivors with preexisting cardiovascular conditions are at greater risk(5;13;37). Greater cumulative steroid exposure also has been identified as a risk factor for persistent diabetes beyond 2 years(37). Since the median interval between transplant and a more serious complication was only 5 years among our cohort of ≥2 year survivors, the window of opportunity for intervention and control of cardiovascular risk factors may be limited. As it remains difficult for clinicians to easily predict in advance which patients with cardiovascular risk factors present one year after HCT will have persistent conditions, more aggressive screening and treatment early on may be indicated. Prospective studies also are needed to determine whether standard interventions provide the same level of risk reduction as seen in the general population(36;39).
Ascertainment of late adverse effects many years after HCT poses a challenge because many survivors may no longer communicate with, or receive care at their original transplant center(40). Our use of administrative datasets minimized the influence of these response and survival biases. Although our results are based on patients transplanted at one center, the broad range of years covered, ascertainment of outcomes from state registries similar in design to those available in many other states, and consideration of multiple exposures similar to those received by HCT patients elsewhere should permit generalization of our relative risk estimates. Prevalence estimates should be interpreted in the context that regional differences in cardiovascular health have been well-described, with Washington State in general having average indices of cardiovascular health compared with the nation at-large(41). Although our numbers of each specific cardiovascular outcome were not large in absolute terms, limiting our power to detect smaller effects, to our knowledge this study still represents the one of the largest compilation of serious cardiovascular events among HCT survivors published to date.
In general, the specificity of hospitalization administrative databases is typically >90% although sensitivity may be more variable when compared with chart review(42–48). Sensitivity for serious acute illness such as stroke and myocardial infarction typically diagnosed in the hospital setting is >70%(42–44;47), while sensitivity for diagnoses that may present less acutely such as heart failure can be more variable(44–46;48). We addressed this latter limitation in part by searching for and including additional clinical heart failure cases (n=3) identified in institutional records. Importantly, misclassification of individuals with cardiovascular disease not requiring hospitalization as “unaffected” would be expected to bias our results towards the null.
Other factors not examined in our study such as iron overload, diet, and physical inactivity may also influence subsequent cardiovascular health. Given the relatively young age of the average transplant recipient, awareness of and surveillance for premature cardiovascular disease may be more easily overlooked(16;49). Efforts to promote smoking cessation and a healthy lifestyle should be strongly encouraged, as these behaviors have been shown to influence subsequent cardiovascular morbidity even among HCT survivors(11). Although our results indicate that patients with cardiovascular risk factors merit special attention, particularly those with pre-existing and newly developed and persistent conditions, the optimal intervention strategy remains an active area for investigation.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank the Washington State Department of Health for providing data access, and Gary Schoch and William O’Brien for their assistance in linking the analytic data sets. Results presented in part at the Tandem BMT Meeting, Honolulu, HI, on February 21, 2011.
FUNDING
American Society for Blood and Marrow Transplantation Pfizer New Investigator Award (EJC), Leukemia and Lymphoma Society Special Fellowship in Clinical Research (EJC), the Seattle Children’s Research Institute, and the National Cancer Institute (CA15704, CA18029, CA151775, CA167451). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflicts of interests, including no competing financial interests.
REFERENCES
- 1.Pasquini MC, Wang Z. [accessed December 27, 2013];Current use and outcome of hematopoietic stem cell transplantation: CIBMTR Summary Slides. 2012 http://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/.
- 2.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia S, Robison LL, Francisco L, et al. Late mortality in survivors of autologous hematopoietic-cell transplantation: report from the Bone Marrow Transplant Survivor Study. Blood. 2005;105:4215–4222. doi: 10.1182/blood-2005-01-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatia S, Francisco L, Carter A, et al. Late mortality after allogeneic hematopoietic cell transplantation and functional status of long-term survivors: report from the Bone Marrow Transplant Survivor Study. Blood. 2007;110:3784–3792. doi: 10.1182/blood-2007-03-082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tichelli A, Bucher C, Rovo A, et al. Premature cardiovascular disease after allogeneic hematopoietic stem-cell transplantation. Blood. 2007;110:3463–3471. doi: 10.1182/blood-2006-10-054080. [DOI] [PubMed] [Google Scholar]
- 6.Baker KS, Ness KK, Steinberger J, et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: a report from the bone marrow transplantation survivor study. Blood. 2007;109:1765–1772. doi: 10.1182/blood-2006-05-022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tichelli A, Passweg J, Wojcik D, et al. Late cardiovascular events after allogeneic hematopoietic stem cell transplantation: a retrospective multicenter study of the Late Effects Working Party of the European Group for Blood and Marrow Transplantation. Haematologica. 2008;93:1203–1210. doi: 10.3324/haematol.12949. [DOI] [PubMed] [Google Scholar]
- 8.Sun CL, Francisco L, Kawashima T, et al. Prevalence and predictors of chronic health conditions after hematopoietic cell transplantation: a report from the Bone Marrow Transplant Survivor Study. Blood. 2010;116:3129–3139. doi: 10.1182/blood-2009-06-229369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chow EJ, Mueller BA, Baker KS, et al. Cardiovascular hospitalizations and mortality among recipients of hematopoietic stem cell transplantation. Ann Intern Med. 2011;155:21–32. doi: 10.7326/0003-4819-155-1-201107050-00004. [DOI] [PubMed] [Google Scholar]
- 10.Khera N, Storer B, Flowers ME, et al. Nonmalignant late effects and compromised functional status in survivors of hematopoietic cell transplantation. J Clin Oncol. 2012;30:71–77. doi: 10.1200/JCO.2011.38.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chow EJ, Baker KS, Lee SJ, et al. Influence of conventional cardiovascular risk factors and lifestyle characteristics on cardiovascular disease after hematopoietic cell transplantation. J Clin Oncol. 2014;32:191–198. doi: 10.1200/JCO.2013.52.6582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armenian SH, Sun CL, Francisco L, et al. Late congestive heart failure after hematopoietic cell transplantation. J Clin Oncol. 2008;26:5537–5543. doi: 10.1200/JCO.2008.17.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armenian SH, Sun CL, Mills G, et al. Predictors of late cardiovascular complications in survivors of hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2010;16:1138–1144. doi: 10.1016/j.bbmt.2010.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Armenian SH, Sun CL, Vase T, et al. Cardiovascular risk factors in hematopoietic cell transplantation survivors: role in development of subsequent cardiovascular disease. Blood. 2012;120:4505–4512. doi: 10.1182/blood-2012-06-437178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rovo A, Tichelli A. Cardiovascular complications in long-term survivors after allogeneic hematopoietic stem cell transplantation. Semin Hematol. 2012;49:25–34. doi: 10.1053/j.seminhematol.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 16.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prentice RL. On the design of synthetic case-control studies. Biometrics. 1986;42:301–310. [PubMed] [Google Scholar]
- 18.Le Deley MC, Leblanc T, Shamsaldin A, et al. Risk of secondary leukemia after a solid tumor in childhood according to the dose of epipodophyllotoxins and anthracyclines: a case-control study by the Societe Francaise d'Oncologie Pediatrique. J Clin Oncol. 2003;21:1074–1081. doi: 10.1200/JCO.2003.04.100. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention. [accessed December 27, 2013];Growth Charts. http://www.cdc.gov/growthcharts/
- 20.Ng A, Nguyen TN, Moseley JL, et al. Navigator channel adaptation to reconstruct three dimensional heart volumes from two dimensional radiotherapy planning data. BMC Med Phys. 2012;12:1. doi: 10.1186/1756-6649-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barlow WE. Robust variance estimation for the case-cohort design. Biometrics. 1994;50:1064–1072. [PubMed] [Google Scholar]
- 22.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol. 2007;165:710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- 24.Armenian SH, Sun CL, Shannon T, et al. Incidence and predictors of congestive heart failure after autologous hematopoietic cell transplantation. Blood. 2011;118:6023–6029. doi: 10.1182/blood-2011-06-358226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dalen EC, van der Pal HJ, Caron HN, et al. Different dosage schedules for reducing cardiotoxicity in cancer patients receiving anthracycline chemotherapy. Cochrane Database Syst Rev. 2009:CD005008. doi: 10.1002/14651858.CD005008.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Shapiro CL, Hardenbergh PH, Gelman R, et al. Cardiac effects of adjuvant doxorubicin and radiation therapy in breast cancer patients. J Clin Oncol. 1998;16:3493–3501. doi: 10.1200/JCO.1998.16.11.3493. [DOI] [PubMed] [Google Scholar]
- 27.Hull MC, Morris CG, Pepine CJ, et al. Valvular dysfunction and carotid, subclavian, and coronary artery disease in survivors of hodgkin lymphoma treated with radiation therapy. JAMA. 2003;290:2831–2837. doi: 10.1001/jama.290.21.2831. [DOI] [PubMed] [Google Scholar]
- 28.Tukenova M, Guibout C, Oberlin O, et al. Role of cancer treatment in long-term overall and cardiovascular mortality after childhood cancer. J Clin Oncol. 2010;28:1308–1315. doi: 10.1200/JCO.2008.20.2267. [DOI] [PubMed] [Google Scholar]
- 29.Biedermann BC. Vascular endothelium and graft-versus-host disease. Best Pract Res Clin Haematol. 2008;21:129–138. doi: 10.1016/j.beha.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Chao NJ, Sullivan KM. Pharmacologic prevention of acute graft versus host disease. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas' Hematopoietic Cell Transplantation. 4th ed. West Sussex: Wiley-Blackwell; 2008. pp. 1257–1274. [Google Scholar]
- 31.Annaloro C, Usardi P, Airaghi L, et al. Prevalence of metabolic syndrome in long-term survivors of hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:797–804. doi: 10.1038/sj.bmt.1705972. [DOI] [PubMed] [Google Scholar]
- 32.Majhail NS, Flowers ME, Ness KK, et al. High prevalence of metabolic syndrome after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2009;43:49–54. doi: 10.1038/bmt.2008.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chow EJ, Simmons JH, Roth CL, et al. Increased cardiometabolic traits in pediatric survivors of acute lymphoblastic leukemia treated with total body irradiation. Biol Blood Marrow Transplant. 2010;16:1674–1681. doi: 10.1016/j.bbmt.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rovo A, Daikeler T, Halter J, et al. Late altered organ function in very long-term survivors after allogeneic hematopoietic stem cell transplantation: a paired comparison with their HLA-identical sibling donor. Haematologica. 2011;96:150–155. doi: 10.3324/haematol.2010.030874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee SJ, Seaborn T, Mao FJ, et al. Frequency of abnormal findings detected by comprehensive clinical evaluation at 1 year after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:416–420. doi: 10.1016/j.bbmt.2008.12.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blaser BW, Kim HT, Alyea EP, et al. Hyperlipidemia and statin use after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:575–583. doi: 10.1016/j.bbmt.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majhail NS, Challa TR, Mulrooney DA, et al. Hypertension and diabetes mellitus in adult and pediatric survivors of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1100–1107. doi: 10.1016/j.bbmt.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffith ML, Savani BN, Boord JB. Dyslipidemia after allogeneic hematopoietic stem cell transplantation: evaluation and management. Blood. 2010;116:1197–1204. doi: 10.1182/blood-2010-03-276576. [DOI] [PubMed] [Google Scholar]
- 40.Shankar SM, Carter A, Sun CL, et al. Health care utilization by adult long-term survivors of hematopoietic cell transplant: report from the Bone Marrow Transplant Survivor Study. Cancer Epidemiol Biomarkers Prev. 2007;16:834–839. doi: 10.1158/1055-9965.EPI-06-0714. [DOI] [PubMed] [Google Scholar]
- 41.Fang J, Yang Q, Hong Y, et al. Status of cardiovascular health among adult Americans in the 50 States and the District of Columbia 2009. J Am Heart Assoc. 2012;1:e005371. doi: 10.1161/JAHA.112.005371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tirschwell DL, Longstreth WT. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470. doi: 10.1161/01.str.0000032240.28636.bd. [DOI] [PubMed] [Google Scholar]
- 43.Kiyota Y, Schneeweiss S, Glynn RJ, et al. Accuracy of Medicare claims-based diagnosis of acute myocardial infarction: estimating positive predictive value on the basis of review of hospital records. Am Heart J. 2004;148:99–104. doi: 10.1016/j.ahj.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 44.Quan H, Parsons GA, Ghali WA. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care. 2004;42:801–809. doi: 10.1097/01.mlr.0000132391.59713.0d. [DOI] [PubMed] [Google Scholar]
- 45.Powell H, Lim LL, Heller RF. Accuracy of administrative data to assess comorbidity in patients with heart disease. an Australian perspective. J Clin Epidemiol. 2001;54:687–693. doi: 10.1016/s0895-4356(00)00364-4. [DOI] [PubMed] [Google Scholar]
- 46.Lee DS, Donovan L, Austin PC, et al. Comparison of coding of heart failure and comorbidities in administrative and clinical data for use in outcomes research. Med Care. 2005;43:182–188. doi: 10.1097/00005650-200502000-00012. [DOI] [PubMed] [Google Scholar]
- 47.Ives DG, Samuel P, Psaty BM, et al. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. J Am Geriatr Soc. 2009;57:133–139. doi: 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saydah SH, Geiss LS, Tierney E, et al. Review of the performance of methods to identify diabetes cases among vital statistics, administrative, and survey data. Ann Epidemiol. 2004;14:507–516. doi: 10.1016/j.annepidem.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Shankar SM, Marina N, Hudson MM, et al. Monitoring for cardiovascular disease in survivors of childhood cancer: report from the Cardiovascular Disease Task Force of the Children's Oncology Group. Pediatrics. 2008;121:e387–e396. doi: 10.1542/peds.2007-0575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.