Abstract
Discoidin domain receptors, DDR1 and DDR2, lie at the intersection of two large receptor families, namely the extracellular matrix and tyrosine kinase receptors. As such, DDRs are uniquely positioned to function as sensors for extracellular matrix and to regulate a wide range of cell functions from migration and proliferation to cytokine secretion and extracellular matrix homeostasis/remodeling. While activation of DDRs by extracellular matrix collagens is required for normal development and tissue homeostasis, aberrant activation of these receptors following injury or in disease is detrimental. The availability of mice lacking DDRs has enabled us to identify key roles played by these receptors in disease initiation and progression. DDR1 promotes inflammation in atherosclerosis, lung fibrosis and kidney injury, while DDR2 contributes to osteoarthritis. Furthermore, both DDRs have been implicated in cancer progression. Yet the mechanisms whereby DDRs contribute to diseases progression are poorly understood. In this review we highlight the mechanisms whereby DDRs regulate two important processes, namely inflammation and tissue fibrosis. In addition, we discuss the challenges of targeting DDRs in disease. Selective targeting of these receptors requires understanding of how they interact with and are activated by extracellular matrix, and whether their cellular function is dependent on or independent of receptor kinase activity.
Keywords: matrix, signaling, fibrosis, cancer, kidney, inflammation
DDRs structure and expression
DDR1 and DDR2 are tyrosine kinase receptors composed of an extracellular Discoidin (DS) homology domain which encompasses the collagens binding site, a DS-like domain which contributes to collagen-induced receptor activation, an extracellular juxtamembrane region which contains N- and O-glycosylation sites and matrix metalloproteinase cleavage sites. There is a transmembrane domain which mediates collagen-independent receptor dimerization, a large intracellular juxtamembrane region which contains phosphorylatable tyrosines that may serve as docking sites for DDR binding proteins, and an intracellular tyrosine kinase domain with homology to Trk and insulin receptor family members (Carafoli and Hohenester, 2013; Carafoli et al., 2012; Fu et al., 2013a; Fu et al., 2013b; Lemeer et al., 2012; Noordeen et al., 2006; Perez et al., 1994). DDR2 has only 1 isoform, while DDR1 exists in 5 different isoforms (DDR1a–e) which are generated through alternative splicing (Alves et al., 2001). DDR1a–c encode functional receptor tyrosine kinases with DDR1b and DDR1c carrying an additional 37 amino acids within the intracellular juxtamembrane region. DDR1c has 6 additional amino acids in the kinase domain which make it the longest DDR1 isoform. DDR1d and DDR1e lack kinase activity.
DDRs are widely expressed during development and in adulthood. In solid tissues, DDR1 expression is restricted to epithelial cells, while expression of DDR2 is restricted to mesenchymal cells (Alves et al., 1995). Analysis of DDR1 isoforms in mouse embryonic and adult tissues revealed that DDR1b is the major isoform (Perez et al., 1996). However, DDR1a is found in high levels in breast and glioma tumor cell lines (Perez et al., 1996; Ram et al., 2006). In cells of hematopoietic origin no detectable baseline expression of DDR1 is observed (Alves et al., 1995). Freshly isolated peripheral blood mononuclear cells express low level of DDR1 mRNA and undetectable protein levels; however increased DDR1 (mainly DDR1a) expression is evident when these cells are either cultured in complete medium or stimulated with cytokines (Kamohara et al., 2001). DDR1 expression is also evident in cultured megakaryocyte (Abbonante et al., 2013) and in germinal centre B cells expressing EBV-encoded latent membrane protein-1 (Cader et al., 2013). In contrast, DDR2 is expressed constitutively in immature dendritic cells and is upregulated in mature DCs following treatment with tumor necrosis factor alpha (Lee et al., 2007).
Collagen-induced DDR activation
A key feature of DDRs is their ability to bind both fibrillar and non-fibrillar collagens (Shrivastava et al., 1997; Vogel et al., 1997). DDR1 and DDR2 recognize the GVMGVO (O, hydroxyproline) motif within fibrillar collagen I–III and V [(Konitsiotis et al., 2008; Xu et al., 2011a) and reviewed in (Leitinger, 2011)]. Additional binding sites for DDR2 have been mapped on collagens II and III, although peptides encompassing these sites do not activate DDR2 (Xu et al., 2011a). DDR1 and DDR2 display distinct specificity for network forming collagens with DDR1 only binding to collagen IV (Vogel et al., 1997; Xu et al., 2011a) and DDR2 only binding to collagen X (Leitinger and Kwan, 2006). The amino acids in DDR1 and DDR2 that are critical for collagen binding are located within the DS domain and are well-conserved: Trp53 (Trp52 in DDR2), Thr57 (Thr56 in DDR2), Arg105 and Glu113 in both DDR1 and DDR2 (Abdulhussein et al., 2004; Carafoli et al., 2009; Leitinger, 2003). The collagen IV binding site in DDR1 has been mapped to 5 amino acid residues within the receptor DS domain (Xu et al., 2011a); however the sequence(s) of the DDR1 biding sites on collagen IV are not well characterized. Although DDR2 can interact with collagen X, its DS domain is not sufficient for the binding (Leitinger and Kwan, 2006). Interestingly, DDR binding sites on fibrillar collagens map to residues which are distinct from the ones recognized by other matrix receptors (e.g., integrins) so that simultaneous binding and signaling from both DDRs and integrins can be achieved.
Although a single DS domain of DDR1 and DDR2 contains the collagen binding site, high affinity binding to collagen requires DS domain dimerization (Leitinger, 2003). DDRs exist in dimers that form in a ligand independent manner through interactions mediated by their transmembrane domains (Noordeen et al., 2006). Thus, collagen interacts with pre-formed DDR dimers and upon binding induces dimer oligomerization and conformational changes that result in receptor activation.
Unlike the interaction of soluble growth factors with receptor tyrosine kinases, collagen binding to DDRs induces a slow receptor tyrosine autophosphorylation that requires hours to reach full activation and can persist up to 18 hours. Upon collagen binding DDR1 undergoes internalization at a faster rate than receptor autophosphorylation (Mihai et al., 2009) and it has been proposed that DDR1 phosphorylation occurs in the endocytic vesicles (Fu et al., 2013b). In addition to tyrosine autophosphorylation, maximal receptor activation may require the involvement of additional tyrosine kinases or inhibition of tyrosine phosphatases. In this context, Src activates both DDR1 and DDR2 (Dejmek et al., 2003; Ikeda et al., 2002) and Src-mediated DDR2 phosphorylation is required for full receptor kinase activity (Yang et al., 2005). Furthermore, treatment of cells expressing DDR1 or DDR2 with tyrosine phosphatase inhibitors increases DDR tyrosine phosphorylation in a ligand-independent manner (Alves et al., 1995). Despite these studies, there are still outstanding questions regarding mechanisms of DDR activation that need to be answered. How DDRs are activated by collagens is still poorly understood and whether DDR activation requires a combination of internalization into endocytic vesicles and phosphorylation by additional tyrosine kinases or inactivation of tyrosine phosphatases is unknown. Furthermore the identity of the tyrosine phosphatases that negatively regulate DDR1 is not clear, although analysis with the PhosphoMotif finder program revealed that some of the tyrosine residues in DDR1 are putative substrates for tyrosine phosphatases such as TCPTP, PTP1b, and SHP1 (Borza and Pozzi, unpublished).
DDR tyrosine phosphorylation
Upon collagen binding DDRs become phosphorylated on tyrosine residues and can activate various downstream signaling pathways. Since mutations of the critical residues within kinase domain, Lys618 in DDR1a or Thr664 in DDR2, effectively block collagen-induced DDR1 or DDR2 phosphorylation, it is likely that DDR phosphorylation is due to autophosphorylation (Vogel et al., 2000) (Olaso et al., 2001). Residues Tyr792, Tyr796 and Tyr797 in DDR1 and Tyr736, Tyr740 and Tyr741 in DDR2 are located within the kinase activation loop, a region of 20–35 residues which starts with the conserved residues Asp-Phe-Gly (DFG) motif and contains tyrosine residues which upon phosphorylation regulate the kinase catalytic activity. Thus, these tyrosine residues are predicted to be critical for the receptor activation (Perez et al., 1994). Quantitative mass spectrometry-based phosphoproteomic analysis of collagen-stimulated DDR2 expressing cells showed that Tyr736 and Tyr740 become phosphorylated from 8 to 24 hours after collagen binding (Iwai et al., 2013b), which is in agreement with the activation kinetics of this receptor (Vogel et al., 1997). Moreover, in vitro kinase assays with DDR2 showed that Tyr740 and Tyr741 are only phosphorylated when DDR2 reaches the maximal kinase activity (Iwai et al., 2013b). In regard to DDR1, a phosphotyrosine profile of non-small cell lung cancer (NSCLC) tumors and cell lines revealed that DDR1 is phosphorylated in lung tumors and that phosphorylation occurs at the activation loop (Rikova et al., 2007). Similarly, analysis of pervanadate-treated DDR1-expressing cells with antibodies directed against phosphotyrosines within the activation loop indicated that these tyrosines are indeed phosphorylated (Lemeer et al., 2012). Moreover, proteomic analysis of pervanadate-treated DDR1-expressing cells revealed that Tyr484, Tyr513 and Tyr521 within the intracellular juxtamembrane region are phosphorylated (Lemeer et al., 2012). Moreover, Tyr792 in DDR1 is phosphorylated in response to collagen (Borza and Pozzi unpublished, Phospho-DDR1 (Tyr792) Antibody #11994 Cell Signaling). Interestingly, Tyr481 within the cytoplasmic juxtamembrane region of DDR2 is constitutively phosphorylated and does not follow the kinetic of receptor activation (Iwai et al., 2013b). Overall these studies demonstrate that phosphorylation of tyrosine residues within the DDR kinase domain activation loop is an indicator of receptor activation. Both receptors contain additional tyrosine residues in the cytoplasmic domain that can be phosphorylated and serve as docking sites for adaptor molecules. For instance, analysis for DDR1-expressing cells showed that DDR1 activation by collagen results in binding of ShcA to Tyr513, SHP-2 to Tyr703, Tyr796 and Tyr740, and the p85 subunit of PI3K to Tyr881 (Koo et al., 2006; L'Hote C et al., 2002; Vogel et al., 1997; Wang et al., 2006). These interactions were confirmed and additional binding proteins such as RasGAP, SHIP1, SHIP2, STATs and SRC family kinases were identified using proteomics approaches (Lemeer et al., 2012). Phosphoproteomic analysis of DDR2-expressing cells identified several candidate downstream signaling proteins including SHP-2, Nck1, Lyn, SHIP-2 following collagen activation (Iwai et al., 2013b).
DDR-mediated signaling
DDRs initiate signaling pathways in a context and cell type-dependent manner. For instance, DDR1 was reported to activate ERK in vascular smooth muscle cells (Lu et al., 2011), to inhibit ERK in mesangial cells (Curat and Vogel, 2002), and to have no effect on ERK activation in T47D breast cancer cells (L'Hote C et al., 2002). Furthermore DDR1 was reported to both promote and inhibit epithelial-to-mesenchymal transition (EMT) in a ligand- and cell type-dependent manner. In pancreatic cancer cells, DDR1, together with integrin α2β1, promotes cell scattering on collagen I and increases the mesenchymal marker N-cadherin (Shintani et al., 2008). By contrast, DDR1 inhibits integrin α2β1-mediated migration and spreading and stabilizes the epithelial marker E-cadherin in Madin-Darby canine kidney (MDCK) cells (Eswaramoorthy et al., 2010; Wang et al., 2006; Yeh et al., 2009).
Of interest, some DDR1-mediated cell functions are independent of collagen binding or receptor kinase activity. For instance, DDR1 was reported to support collective cell migration by decreasing the activity of actomyosin at cell-cell contacts. This function was dependent on the DDR1 carboxyl terminus which contains a PDZ binding motif and interacts with the cell polarity protein Par3/Par6 complex (Hidalgo-Carcedo et al., 2011).
Like DDR1, DDR2 was also shown to promote EMT. In this context, EMT in MDCK cells is accompanied by increased DDR2 expression (Maeyama et al., 2008); TGF-β promotes DDR2 expression, and collagen I-induced EMT is prevented by downregulating DDR2 expression (Walsh et al., 2011). Furthermore, collagen I-mediated DDR2 activation increases the stability of the EMT driver SNAIL1 and promotes breast cancer cells invasion in vitro and increased metastasis in vivo (Zhang et al., 2013a).
Thus, DDRs can interact with multiple proteins and these interactions result in complex signaling processes that vary between cell types and can be ligand or receptor kinase activity dependent and independent.
DDRs cross-talk with receptors and growth factors
In addition to mediating direct collagen-dependent signaling, DDRs can also modulate signaling pathways initiated by other matrix receptors (e.g., integrins), cytokines (e.g., TGF-β) and transmembrane receptors (e.g., insulin receptor and Notch1). Cross-talk between DDRs and integrin is complex and influences multiple processes including cell adhesion and differentiation. DDR1 can both potentiate and inhibit integrin-mediated signaling. DDR1 cooperates with integrin α2β1 in maintaining mouse embryonic stem cells undifferentiated via activation of selective collagen-DDR and collagen-integrin mediated signaling pathways that ultimately converge to the self-renewal controlling molecule Bim-1 (Suh and Han, 2011). Moreover, overexpression of DDR1 or DDR2 in cells expressing the collagen binding receptors integrins α1β1 and α2β1, results in enhanced integrin-mediated adhesion to collagen due to increased integrin activation rather than increased integrin expression levels (Xu et al., 2012). In contrast to these findings, DDR1 has been shown to counteract integrin-mediated signaling and promote epithelial cells differentiation (Yeh et al., 2012). In MDCK cells, for example, integrin β1 promotes cell dedifferentiation by downregulating E-cadherin, while DDR1 promotes cell differentiation by increasing membrane stability of E-cadherin (Yeh et al., 2012). Thus DDR1-integrin cross-talk is highly dependent on the type of integrins the cells express and the cell type.
DDRs can also modulate signaling initiated by growth factors. Cross-talk between DDR1 and TGF-β is critical for proper growth and patterning of mammary gland in mice. In this context, TGF-β negatively regulates ductal extension and lateral branching in the mammary gland by promoting Wnt5a expression and DDR1 phosphorylation (Roarty and Serra, 2007). Wnt5a acts as an upstream regulator of DDR1 promoting collagen-induced DDR1 phosphorylation in human mammary epithelial cells. In addition, levels of Wnt5a are directly associated to increased cell adhesion and reduced cell migration on collagen (Jonsson and Andersson, 2001), suggesting that Wnt5a might control two important cell functions by regulating the phosphorylation and activation of DDR1.
Recently, cross-talk between DDR2 and the insulin receptor and between Notch1 and DDR1 was proposed. Stimulation of cells with collagen I and insulin promotes Tyr740 as well as total tyrosine phosphorylation of DDR2 receptor to a greater extent than the phosphorylation stimulated by collagen I alone (Iwai et al., 2013a). Finally, it has been proposed that collagen-stimulated DDR1 promotes survival of cancer cells by binding to and activating Notch1 thus promoting the activation of the two transcription factors Hes1 and Hey2 (Kim et al., 2011).
In conclusion, cross-talk of DDRs with various receptors is critical for the regulation of cell survival, migration, and differentiation in development as well as in pathological conditions (Figure 1).
Figure 1.
Crosstalk between DDRs and transmembrane receptors and/or soluble factors can regulate various processes, including cell differentiation, adhesion, motility, survival as well as potentiate DDR phosphorylation and activation.
DDR function in development
The generation of global DDR1- and DDR2-null mice has contributed significantly to the understanding of the role of these two receptors in development. Global deletion of DDR1 or DDR2 does not impair embryonic development, although DDR1-null and DDR2-null mice present with a wide range of defects. DDR1 ablation in mice results in reduced calcification of the fibula bone, defects in mammary gland morphogenesis which result in a lactation impairment, and reproduction defects due to the inability of blastocysts to implant properly in the uterine wall (Vogel et al., 2001). DDR-2 deletion in mice results in reduced bone growth due to reduced chondrocyte proliferation as well as impaired dermal wound healing due to reduced fibroblast proliferation (Olaso et al., 2002). Interestingly, Smallie, a spontaneous mutation that causes dwarfism in mice was shown to be the result of DDR2 deletion (Kano et al., 2008) and mutations of DDR2 have been found in patients with spondylo-meta-epiphyseal dysplasia, a rare human genetic disorder characterized by disproportionate short stature and bone abnormalities (Ali et al., 2010).
All together, these findings indicate that DDR signaling is required for normal skeletal development, mammary gland branching morphogenesis, and blastocyst implantation (Figure 2).
Figure 2.
DDR expression and/or activation plays a role in both physiological (e.g., development) and pathological (e.g., cancer, inflammation, fibrosis) conditions by controlling key cellular processes, including protease production, cytokine secretion, cell migration, immune cell recruitment, and matrix production.
DDRs in disease
Despite some of the developmental defects found in DDR-null mice, these mice have been valuable in understating the role of these receptors in various diseases, including cancer, atherosclerosis, lung and liver fibrosis, renal injury, and osteoarthritis (Figure 2).
Cancer
The observation that mutations and altered expression of DDRs are found in many types of cancers suggests that these receptors might be involved in the development and progression of cancer. The picture is complicated by the fact that DDRs can act as pro-tumorigenic and anti-tumorigenic receptors and their effect is highly dependent on the type and stage of cancer. Since the contribution of DDRs in cancer has been extensively reviewed by Valiathan and colleagues (Valiathan et al., 2012), we will only highlight a few recently published studies pointing to DDRs as positive modulators of cancer progression. Zhang and colleagues have recently reported that DDR2 is expressed in 71% of invasive ductal breast cancer and that 5% of the invasive breast tumors had amplified copy number of the DDR2 and were associated with worse survival rate. As some DDR2 expressing tumors are also SNAIL1 positive, the authors postulate that DDR2 might sustain EMT and in turn tumor invasion by stabilizing post-transcriptionally SNAL1 protein levels (Zhang et al., 2013a). However, a significant proportion of DDR2-positive breast tumors were SNAIL1 negative, suggesting that additional mechanisms may be responsible for DDR2-mediated metastasis. Consistent with a pro-tumorigenic action of DDR2, DDR2-nll mice show reduced primary tumor-associated angiogenesis and reduced lung colonization following tail vein injection of melanoma cells (Zhang et al., 2013b). Furthermore, a recent study of DDR1 and DDR2 expression in breast cancer patients showed a six fold increase of DDR2 in tumor vs. normal tissue, while DDR1 expression was decreased in tumor tissues (Ren et al., 2013). By contrast, a study on non-small cell lung cancer indicated that DDR1 expression increases and may contribute to progression of NSCLC (Miao et al., 2013).
Atherosclerosis
The first evidence that DDR1 contributes to atherosclerosis comes from the finding that the carotid arteries of DDR1-null mice have a lower cross-sectional area of the neointima after mechanical injury than wild-type mice. In this model, DDR1-null mice also have a significant decrease in collagen deposition in the injured arteries (Hou et al., 2001). The role of DDR1 as a positive regulator of atherosclerosis was confirmed in the low-density lipoprotein receptor (Ldlr)-deficient mice, an established model of atherosclerosis. Consistent with the mechanical injury model, DDR1−/−;Ldlr−/− mice showed a significant reduction in atherosclerotic lesion area in the descending aorta when compared to Ldlr−/− mice. However, unlike the mechanical injury model, the DDR1−/−;Ldlr−/− mice showed a significant increase in fibrillar collagens within the plaques at early stages of diseases (Franco et al., 2008). This is consistent with the observation that loss of DDR1 increases mRNA levels of fibrillar collagens I and III in atherosclerotic plaques and smooth muscle cells isolated from carotid arteries (Franco et al., 2010; Franco et al., 2008). Although counter-intuitive, the increased matrix accumulation observed in the atherosclerotic plaques of Ldlr−/− mice with vessel wall specific-deleted DDR 1 may play a beneficial effect by stabilizing the plaques and make it less susceptible to rupture (Franco et al., 2010).
In addition to controlling matrix production, DDR1 could contribute to atherosclerosis by exerting a pro-inflammatory effect. Analysis of the cellular composition of the atherosclerotic plaque in DDR1−/−;Ldlr−/− mice revealed a significant reduction in the number of infiltrating macrophages compared to Ldlr−/− mice (Franco et al., 2008). The pro-inflammatory role for DDR1 in atherosclerosis was also confirmed by the finding that deletion of DDR1 selectively in bone marrow cells resulted in a significant reduction in atherosclerosis in the descending aorta (Franco et al., 2009). Furthermore, macrophages from DDR1-deficient mice have decreased adhesion to and invasion through collagen IV at baseline or in response to monocyte chemoattractant protein-1 (Franco et al., 2009).
Overall, these studies suggest that DDR1 contributes to atherosclerosis by increasing the number of macrophages and decreasing extracellular matrix deposition. Thus, targeting DDR1 may be beneficial in the treatment of atherosclerosis.
Osteoarthritis
The findings that DDR2 expression increases in the cartilage of patients with osteoarthritis (OA) and in mouse models of OA (Xu et al., 2007) and that DDR2 expression correlates with the degree of cartilage damage in human knee joints (Sunk et al., 2007) clearly indicate that DDR2 contribute to this disease. DDR2 contribution to OA is primarily due to DDR2 kinase activity-induced expression of the matrix metalloproteinase (MMP) 13, the major MMP responsible for collagen II degradation (Xu et al., 2005). A recent study, however, showed that DDR2 overexpression alone is not sufficient to induce MMP13 and that DDR2 has to be activated by collagen II, a process normally prevented by an intact pericellular matrix. However, in response to stimuli such as biomechanical stress DDR2 activation does occur and this could lead to DDR2-mediated OA (Xu et al., 2011b). Interestingly, DDR2 heterozygote mice, which express reduced levels of DDR2, have attenuated OA (Xu et al., 2010), suggesting that reducing DDR2 levels and/or function may be beneficial in the treatment of OA. In contrast to DDR2, DDR1 has a protective role in OA, since DDR-1-null mice show a high incidence of OA in the temporomandibular joint (Schminke et al., 2013). Interestingly, the levels of DDR2 were increased in chondrocytes isolated from the temporomandibular joint of DDR1-null mice which suggests that increased DDR2, rather than loss of DDR1 expression, may contribute to the OA phenotype observed in DDR1-null mice. All together, these findings suggest that DDR2 contributes to disease progression in OA and targeting DDR2 either with specific tyrosine kinase inhibitors or with compounds able to prevent binding to collagen II may be a valid tool to prevent and/or ameliorate OA.
Fibrosis
The finding that the expression of DDRs, mainly DDR1, is up regulated in fibrotic diseases has initiated a series of studies aimed to determine how these receptors contribute to initiation and progression of fibrosis. DDR1-deficient mice show reduced bleomycin-induced pulmonary injury characterized by reduced collagen and tenascin-C levels (Avivi-Green et al., 2006). Two possible reasons for the decreased fibrotic response in DDR1-null mice are decreased inflammation, with reduced CD3-positive lymphocytes and F4/80-positive cells infiltrating the lungs, and decreased activation of the p38 MAPK, a kinase involved in lung fibrosis. Consistent with these findings, p38 MAPK inhibitors reduce lung inflammation and fibrosis in the bleomycin mouse model (Underwood et al., 2000).
A pro-fibrotic and pro-inflammatory role for DDR1 was also demonstrated in mouse models of kidney injury. DDR1 expression is elevated in patients with lupus nephritis and Goodpasture's syndrome as well as in a mouse model of crescentic glomerulonephritis (Kerroch et al., 2012). Similarly, DDR1 expression increases in the glomeruli of rats that have undergone partial renal ablation (Lee et al., 2004) and in tubules of mice undergone unilateral ureteral obstruction (Guerrot et al., 2011). Although these findings suggest that DDR1 plays a role in renal injury, it is unclear whether upregulation of DDR1 counteracts or contributes to the disease. The finding that older DDR1-null mice show focal swelling of the glomerular basement membrane (GBM) and mild proteinuria (Gross et al., 2004) suggests that DDR1 might play a protective role in the maintenance of glomerular homeostasis. Interestingly, a similar mild defect in the GBM was described for older integrin α2-null mice (Girgert et al., 2010), yet the integrin α2-null mice have reduced glomerulosclerosis and reduced proteinuria following glomerular injury (Borza et al., 2012). Detailed analysis of DDR1-null mice in several mouse models of kidney injury indicated that compared to wild type mice DDR1-null mice have improved renal function, reduced fibrosis and reduced inflammation. In this context, DDR1-null mice are protected from angiotensin II-mediated proteinuria, glomerular fibrosis, and inflammation (Guerrot et al., 2011), and show reduced collagen deposition, tubular macrophage infiltration and pro-inflammatory cytokine levels following unilateral ureteral obstruction (Guerrot et al., 2011). Moreover, Alport mice (a model of chronic kidney fibrosis) crossed onto the DDR1-null mice have reduced renal fibrosis and inflammation due to reduced TGF-β-mediated signaling and reduced levels of the pro-inflammatory cytokine IL6 (Gross et al., 2010). Finally, DDR1-null mice have increased survival and improved renal function in a model of crescentic glomerulonephritis induced by injection of alloimmune sheep nephrotoxic serum (Kerroch et al., 2012). Similar to the findings in atherosclerosis and lung fibrosis, DDR1-null mice show reduced macrophage infiltration following kidney injury clearly supporting the pro-inflammatory role of DDR1. Moreover, like in atherosclerosis models, DDR1-null macrophages fail to migrate in response to monocyte chemoattractant protein-1, suggesting that DDR1 contributes to kidney damage and fibrosis by primarily promoting inflammatory responses.
The contribution of DDR2 to fibrosis has been studied in the context of liver fibrosis. Deletion of DDR2 aggravates hepatic fibrosis following chronic administration of carbon tetrachloride (Olaso et al., 2011). In this model, DDR2-null mice show increased collagen deposition and hepatic stellate cell density. Although this study suggests that DDR2 plays a beneficial effects in negatively regulating liver fibrosis, it seems to contradict a previous finding indicating that DDR2 promotes proliferation and invasion of hepatic stellate cell in vitro (Olaso et al., 2002). All together, the pro- and anti-fibrotic actions reported for both DDR1 and DDR2 make them attractive, yet challenging targets for anti-fibrotic therapy.
Inhibiting DDR: what we know and what we should know
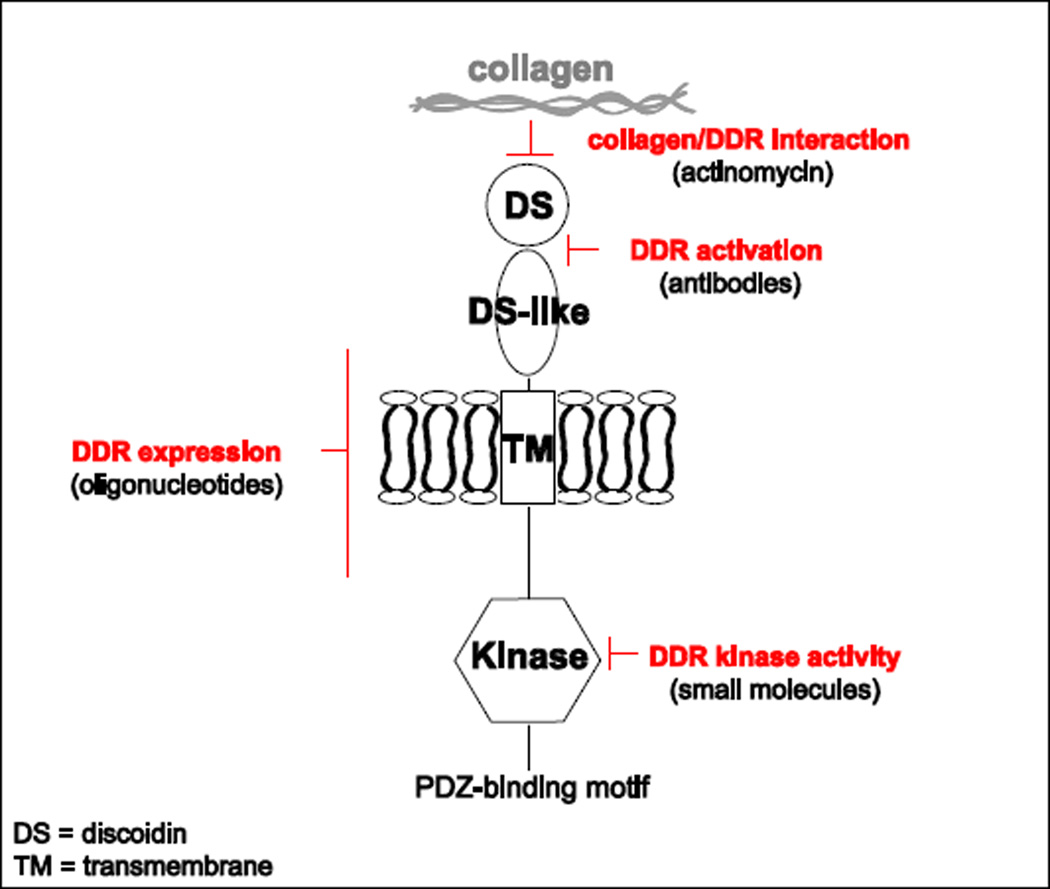
The contribution of DDRs to fibrotic diseases and cancer progression indicates that blocking these receptors might represent a promising therapeutic strategy. A plausible approach is to prevent DDR expression (Figure 3), as DDRs levels increase significantly in disease. In this regard, in glomerulonephritis, DDR1 expression increases up to 17 fold and reducing by 50% DDR1 expression using antisense oligonucleotides, results in improved renal function similar to that seen in DDR1-null mice (Kerroch et al., 2012). Furthermore, reduction of DDR2 expression in DDR2-haploinsufficient (Ddr2+/−) mice reduces the severity of osteoarthritis both in genetic models of osteoarthritis and in surgical destabilization of the medial meniscus (Xu et al., 2010). Other strategies to prevent DDR-mediated function could be aimed to block the interaction of DDRs with collagen or to sterically block the conformational change required for receptor activation (Figure 3). For instance, Actinomycin D inhibits collagen I-mediated activation of DDR2 in cell cultures without affecting the activation of other receptor tyrosine kinases (Siddiqui et al., 2009) and monoclonal antibodies to the extracellular domain of DDR1, specifically to the DS-like domain, inhibit collagen-induced DDR1 activation without affecting collagen binding (Carafoli et al., 2012).
Figure 3.
Illustration of how expression and/or activation of DDRs can be targeted in disease. For details see main text.
Another appealing alternative is to target the DDR tyrosine kinase activity in order to block DDR-mediated downstream signaling (Figure 3). Protein tyrosine kinases (PTKs) catalyze the transfer of the γ-phosphate of ATP to the hydroxyl group of tyrosine residues on various protein substrates. Tyrosine phosphorylation induces conformational changes which result in increased catalytic activity and/or generation of docking sites for other proteins to bind, thus amplifying signaling pathways (Lemmon and Schlessinger, 2010; Taylor and Kornev, 2011). Because uncontrolled activation of PTKs has been associated with cancer, inflammatory and fibrotic diseases, the identification of small molecule compounds that inhibit their activity has been pursued intensely in the last decade [reviewed in (Dar and Shokat, 2011; Endicott et al., 2012; Liu et al., 2013; Smyth and Collins, 2009; Zhang et al., 2009)]. Most of the kinase inhibitors to date are ATP competitive and can be classified as type I (if the inhibitor targets the catalytically competent conformation of the kinase with the activation loop in the DFG-IN conformation) or as type II (if the inhibitor targets the inactive conformation of the kinase with activation loop in the DFG-OUT conformation). Type II inhibitors tend to be more selective because the inactive DFG-OUT kinase conformation allows additional interactions between the inhibitor and specific, not-well-conserved exposed hydrophobic sites within the kinase domain. On the other hand, type I inhibitors tend to be promiscuous, because they tend to target well-conserved active kinase binding sites. However type I inhibitors have the advantage of inhibiting kinases that have acquired mutations resistant to type II inhibitors (Tokarski et al., 2006). Another class of inhibitors, type III inhibitors, do not target the ATP binding site, but rather allosteric sites that regulate kinase activation (Taylor and Kornev, 2011).
Three kinase inhibitors dasatinib (type I), imatinib (type II) and nilotinib (type II), identified initially as inhibitors of tyrosine kinase BCR-ABL, were found to target DDRs in a chemical proteomic profiling study (Bantscheff et al., 2007; Hantschel et al., 2008). These tyrosine kinase inhibitors block DDR1 and DDR2 kinase activity in biochemical assays and prevent collagen-mediated DDR tyrosine autophosphorylation in cells overexpressing these two receptors (Day et al., 2008). Although dasatinib inhibits DDR1 at very low concentrations, it is not selective for DDR1, thus increasing the chances of off-target effects when used in vivo and limiting the analysis of DDR1-mediated specific signaling.
Recently, two studies reported the identification of novel and selective DDR1 inhibitors. Screening of a library of approximately 2000 compounds originally designed to inhibit BCR-ABL and other RTKs allowed the identification of pyrazolopyrimidine derivatives as selective DDR1 inhibitors. In particular, two of these pyrazolopyrimidine derivatives have high affinity for DDR1 but not for other 455 kinases tested, and inhibit the proliferation of cancer cells expressing high levels of DDR1 (Gao et al., 2013). However, whether these derivatives are type I or type II inhibitors remains to be determined. A second study, aimed to identify selective type II DDR1 inhibitors, identified DDR1-IN-1 as a potent DDR1 inhibitor able to inhibit DDR1 autophosphorylation in cells at concentrations in the micromolar range (Kim et al., 2013). A crystal structure of the DDR1 kinase domain together with DDR1-IN-1 confirmed that this is a type II inhibitor, and mutagenesis studies revealed that a mutation in the hinge region of DDR1 close to the gate-keeper residue confers resistance to this type II inhibitor (Kim et al., 2013). Thus, this mutant may be useful in establishing off-targets effects of DDR1 type II inhibitors in a cellular context. Although promising, DDR1-IN-1 did not significantly block proliferation of DDR1-expressing cancer cell lines, suggesting that blocking DDR1 kinase activity alone may not be sufficient to inhibit and/or halt unwanted cell proliferation (Kim et al., 2013). Nevertheless, the identification of selective DDR inhibitors is expected to advance our understanding of DDR-mediated physiological and pathological effects in vivo and to provide new tools for the treatment of DDR-mediated diseases.
Conclusions
DDRs are unique tyrosine kinase receptors that function as extracellular matrix receptors. Accumulating evidence indicates that DDRs play a role in development and contribute to various diseases, including cancer, atherosclerosis, osteoarthritis, and fibrosis. Despite the progress made in assessing the role of DDRs in both physiological and pathological conditions, a detailed understanding of the molecular mechanisms whereby DDRs control such conditions is lacking. Yet, such understanding is critical for developing selective DDR inhibitors that may result in novel and safe therapies for the treatment of a broad range of diseases.
Highlights.
We review the mechanisms whereby DDRs regulate disease processes.
Understanding how DDRs participate in diseases is critical for DDR targeting.
We discuss the challenges of targeting DDRs in disease.
DDRs may represent ideal targets for the treatment of a broad range of diseases.
Acknowledgments
We would like to thank Roy Zent for critically reading the manuscript.
This work was supported by the VA Merit Review 1I01BX002025 (AP) and the National Institutes of Health Grants R01-CA162433 (AP), R01-DK095761 (AP).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbonante V, Gruppi C, Rubel D, Gross O, Moratti R, Balduini A. Discoidin domain receptor 1 protein is a novel modulator of megakaryocyte-collagen interactions. J Biol Chem. 2013;288:16738–16746. doi: 10.1074/jbc.M112.431528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF. Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor. J Biol Chem. 2004;279:31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- Ali BR, Xu H, Akawi NA, John A, Karuvantevida NS, Langer R, Al-Gazali L, Leitinger B. Trafficking defects and loss of ligand binding are the underlying causes of all reported DDR2 missense mutations found in SMED-SL patients. Hum Mol Genet. 2010;19:2239–2250. doi: 10.1093/hmg/ddq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF. Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines. Faseb J. 2001;15:1321–1323. doi: 10.1096/fj.00-0626fje. [DOI] [PubMed] [Google Scholar]
- Alves F, Vogel W, Mossie K, Millauer B, Hofler H, Ullrich A. Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer. Oncogene. 1995;10:609–618. [PubMed] [Google Scholar]
- Avivi-Green C, Singal M, Vogel WF. Discoidin domain receptor 1-deficient mice are resistant to bleomycin-induced lung fibrosis. Am J Respir Crit Care Med. 2006;174:420–427. doi: 10.1164/rccm.200603-333OC. [DOI] [PubMed] [Google Scholar]
- Bantscheff M, Eberhard D, Abraham Y, Bastuck S, Boesche M, Hobson S, Mathieson T, Perrin J, Raida M, Rau C, Reader V, Sweetman G, Bauer A, Bouwmeester T, Hopf C, Kruse U, Neubauer G, Ramsden N, Rick J, Kuster B, Drewes G. Quantitative chemical proteomics reveals mechanisms of action of clinical ABL kinase inhibitors. Nat Biotechnol. 2007;25:1035–1044. doi: 10.1038/nbt1328. [DOI] [PubMed] [Google Scholar]
- Borza CM, Su Y, Chen X, Yu L, Mont S, Chetyrkin S, Voziyan P, Hudson BG, Billings PC, Jo H, Bennett JS, Degrado WF, Eckes B, Zent R, Pozzi A. Inhibition of integrin alpha2beta1 ameliorates glomerular injury. J Am Soc Nephrol. 2012;23:1027–1038. doi: 10.1681/ASN.2011040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cader FZ, Vockerodt M, Bose S, Nagy E, Brundler MA, Kearns P, Murray PG. The EBV oncogene LMP1 protects lymphoma cells from cell death through the collagen-mediated activation of DDR1. Blood. 2013 doi: 10.1182/blood-2013-04-499004. [DOI] [PubMed] [Google Scholar]
- Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, Leitinger B, Hohenester E. Crystallographic insight into collagen recognition by discoidin domain receptor 2. Structure. 2009;17:1573–1581. doi: 10.1016/j.str.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli F, Hohenester E. Collagen recognition and transmembrane signalling by discoidin domain receptors. Biochim Biophys Acta. 2013;1834:2187–2194. doi: 10.1016/j.bbapap.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carafoli F, Mayer MC, Shiraishi K, Pecheva MA, Chan LY, Nan R, Leitinger B, Hohenester E. Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling. Structure. 2012;20:688–697. doi: 10.1016/j.str.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curat CA, Vogel WF. Discoidin domain receptor 1 controls growth and adhesion of mesangial cells. J Am Soc Nephrol. 2002;13:2648–2656. doi: 10.1097/01.asn.0000032419.13208.0c. [DOI] [PubMed] [Google Scholar]
- Dar AC, Shokat KM. The evolution of protein kinase inhibitors from antagonists to agonists of cellular signaling. Annu Rev Biochem. 2011;80:769–795. doi: 10.1146/annurev-biochem-090308-173656. [DOI] [PubMed] [Google Scholar]
- Day E, Waters B, Spiegel K, Alnadaf T, Manley PW, Buchdunger E, Walker C, Jarai G. Inhibition of collagen-induced discoidin domain receptor 1 and 2 activation by imatinib, nilotinib and dasatinib. Eur J Pharmacol. 2008;599:44–53. doi: 10.1016/j.ejphar.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Dejmek J, Dib K, Jonsson M, Andersson T. Wnt-5a and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion. Int J Cancer. 2003;103:344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- Endicott JA, Noble ME, Johnson LN. The structural basis for control of eukaryotic protein kinases. Annu Rev Biochem. 2012;81:587–613. doi: 10.1146/annurev-biochem-052410-090317. [DOI] [PubMed] [Google Scholar]
- Eswaramoorthy R, Wang CK, Chen WC, Tang MJ, Ho ML, Hwang CC, Wang HM, Wang CZ. DDR1 regulates the stabilization of cell surface E-cadherin and E-cadherin-mediated cell aggregation. J Cell Physiol. 2010;224:387–397. doi: 10.1002/jcp.22134. [DOI] [PubMed] [Google Scholar]
- Franco C, Ahmad PJ, Hou G, Wong E, Bendeck MP. Increased cell and matrix accumulation during atherogenesis in mice with vessel wall-specific deletion of discoidin domain receptor 1. Circ Res. 2010;106:1775–1783. doi: 10.1161/CIRCRESAHA.109.213637. [DOI] [PubMed] [Google Scholar]
- Franco C, Britto K, Wong E, Hou G, Zhu SN, Chen M, Cybulsky MI, Bendeck MP. Discoidin domain receptor 1 on bone marrow-derived cells promotes macrophage accumulation during atherogenesis. Circ Res. 2009;105:1141–1148. doi: 10.1161/CIRCRESAHA.109.207357. [DOI] [PubMed] [Google Scholar]
- Franco C, Hou G, Ahmad PJ, Fu EY, Koh L, Vogel WF, Bendeck MP. Discoidin domain receptor 1 (ddr1) deletion decreases atherosclerosis by accelerating matrix accumulation and reducing inflammation in low-density lipoprotein receptor-deficient mice. Circ Res. 2008;102:1202–1211. doi: 10.1161/CIRCRESAHA.107.170662. [DOI] [PubMed] [Google Scholar]
- Fu HL, Sohail A, Valiathan RR, Wasinski BD, Kumarasiri M, Mahasenan KV, Bernardo MM, Tokmina-Roszyk D, Fields GB, Mobashery S, Fridman R. Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J Biol Chem. 2013a;288:12114–12129. doi: 10.1074/jbc.M112.409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu HL, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, Mahasenan KV, Mobashery S, Huang P, Agarwal G, Fridman R. Discoidin domain receptors: unique receptor tyrosine kinases in collagen-mediated signaling. J Biol Chem. 2013b;288:7430–7437. doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Duan L, Luo J, Zhang L, Lu X, Zhang Y, Zhang Z, Tu Z, Xu Y, Ren X, Ding K. Discovery and optimization of 3-(2-(Pyrazolo[1,5-a]pyrimidin-6-yl)ethynyl)benzamides as novel selective and orally bioavailable discoidin domain receptor 1 (DDR1) inhibitors. J Med Chem. 2013;56:3281–3295. doi: 10.1021/jm301824k. [DOI] [PubMed] [Google Scholar]
- Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Muller GA, Gross O. Integrin alpha2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair. 2010;3:19. doi: 10.1186/1755-1536-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF. DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int. 2004;66:102–111. doi: 10.1111/j.1523-1755.2004.00712.x. [DOI] [PubMed] [Google Scholar]
- Gross O, Girgert R, Beirowski B, Kretzler M, Kang HG, Kruegel J, Miosge N, Busse AC, Segerer S, Vogel WF, Muller GA, Weber M. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010;29:346–356. doi: 10.1016/j.matbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Guerrot D, Kerroch M, Placier S, Vandermeersch S, Trivin C, Mael-Ainin M, Chatziantoniou C, Dussaule JC. Discoidin domain receptor 1 is a major mediator of inflammation and fibrosis in obstructive nephropathy. Am J Pathol. 2011;179:83–91. doi: 10.1016/j.ajpath.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantschel O, Rix U, Superti-Furga G. Target spectrum of the BCR-ABL inhibitors imatinib, nilotinib and dasatinib. Leuk Lymphoma. 2008;49:615–619. doi: 10.1080/10428190801896103. [DOI] [PubMed] [Google Scholar]
- Hidalgo-Carcedo C, Hooper S, Chaudhry SI, Williamson P, Harrington K, Leitinger B, Sahai E. Collective cell migration requires suppression of actomyosin at cell-cell contacts mediated by DDR1 and the cell polarity regulators Par3 and Par6. Nat Cell Biol. 2011;13:49–58. doi: 10.1038/ncb2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou G, Vogel W, Bendeck MP. The discoidin domain receptor tyrosine kinase DDR1 in arterial wound repair. J Clin Invest. 2001;107:727–735. doi: 10.1172/JCI10720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ, Labrador P, Klein R, Lovett D, Yancopoulos GD, Friedman SL, Lin HC. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem. 2002;277:19206–19212. doi: 10.1074/jbc.M201078200. [DOI] [PubMed] [Google Scholar]
- Iwai LK, Chang F, Huang PH. Phosphoproteomic analysis identifies insulin enhancement of discoidin domain receptor 2 phosphorylation. Cell adhesion & migration. 2013a;7:161–164. doi: 10.4161/cam.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai LK, Payne LS, Luczynski MT, Chang F, Xu H, Clinton RW, Paul A, Esposito EA, Gridley S, Leitinger B, Naegle KM, Huang PH. Phosphoproteomics of collagen receptor networks reveals SHP-2 phosphorylation downstream of wild-type DDR2 and its lung cancer mutants. Biochem J. 2013b;454:501–513. doi: 10.1042/BJ20121750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson M, Andersson T. Repression of Wnt-5a impairs DDR1 phosphorylation and modifies adhesion and migration of mammary cells. J Cell Sci. 2001;114:2043–2053. doi: 10.1242/jcs.114.11.2043. [DOI] [PubMed] [Google Scholar]
- Kamohara H, Yamashiro S, Galligan C, Yoshimura T. Discoidin domain receptor 1 isoform-a (DDR1alpha) promotes migration of leukocytes in three-dimensional collagen lattices. Faseb J. 2001;15:2724–2726. doi: 10.1096/fj.01-0359fje. [DOI] [PubMed] [Google Scholar]
- Kano K, Marin de Evsikova C, Young J, Wnek C, Maddatu TP, Nishina PM, Naggert JK. A novel dwarfism with gonadal dysfunction due to loss-of-function allele of the collagen receptor gene, Ddr2, in the mouse. Mol Endocrinol. 2008;22:1866–1880. doi: 10.1210/me.2007-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerroch M, Guerrot D, Vandermeersch S, Placier S, Mesnard L, Jouanneau C, Rondeau E, Ronco P, Boffa JJ, Chatziantoniou C, Dussaule JC. Genetic inhibition of discoidin domain receptor 1 protects mice against crescentic glomerulonephritis. Faseb J. 2012;26:4079–4091. doi: 10.1096/fj.11-194902. [DOI] [PubMed] [Google Scholar]
- Kim HG, Hwang SY, Aaronson SA, Mandinova A, Lee SW. DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation. J Biol Chem. 2011;286:17672–17681. doi: 10.1074/jbc.M111.236612. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim HG, Tan L, Weisberg EL, Liu F, Canning P, Choi HG, Ezell SA, Wu H, Zhao Z, Wang J, Mandinova A, Griffin JD, Bullock AN, Liu Q, Lee SW, Gray NS. Discovery of a Potent and Selective DDR1 Receptor Tyrosine Kinase Inhibitor. ACS Chem Biol. 2013 doi: 10.1021/cb400430t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B. Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen. J Biol Chem. 2008;283:6861–6868. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- Koo DH, McFadden C, Huang Y, Abdulhussein R, Friese-Hamim M, Vogel WF. Pinpointing phosphotyrosine-dependent interactions downstream of the collagen receptor DDR1. FEBS Lett. 2006;580:15–22. doi: 10.1016/j.febslet.2005.11.035. [DOI] [PubMed] [Google Scholar]
- L'Hote CG, Thomas PH, Ganesan TS. Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation. Faseb J. 2002;16:234–236. doi: 10.1096/fj.01-0414fje. [DOI] [PubMed] [Google Scholar]
- Lee JE, Kang CS, Guan XY, Kim BT, Kim SH, Lee YM, Moon WS, Kim DK. Discoidin domain receptor 2 is involved in the activation of bone marrow-derived dendritic cells caused by type I collagen. Biochem Biophys Res Commun. 2007;352:244–250. doi: 10.1016/j.bbrc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- Lee R, Eidman KE, Kren SM, Hostetter TH, Segal Y. Localization of discoidin domain receptors in rat kidney. Nephron Exp Nephrol. 2004;97:e62–e70. doi: 10.1159/000078407. [DOI] [PubMed] [Google Scholar]
- Leitinger B. Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2. J Biol Chem. 2003;278:16761–16769. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- Leitinger B. Transmembrane collagen receptors. Annu Rev Cell Dev Biol. 2011;27:265–290. doi: 10.1146/annurev-cellbio-092910-154013. [DOI] [PubMed] [Google Scholar]
- Leitinger B, Kwan AP. The discoidin domain receptor DDR2 is a receptor for type X collagen. Matrix Biol. 2006;25:355–364. doi: 10.1016/j.matbio.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Lemeer S, Bluwstein A, Wu Z, Leberfinger J, Muller K, Kramer K, Kuster B. Phosphotyrosine mediated protein interactions of the discoidin domain receptor 1. Journal of proteomics. 2012;75:3465–3477. doi: 10.1016/j.jprot.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2010;141:1117–1134. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Sabnis Y, Zhao Z, Zhang T, Buhrlage SJ, Jones LH, Gray NS. Developing irreversible inhibitors of the protein kinase cysteinome. Chem Biol. 2013;20:146–159. doi: 10.1016/j.chembiol.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KK, Trcka D, Bendeck MP. Collagen stimulates discoidin domain receptor 1-mediated migration of smooth muscle cells through Src. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2011;20:71–76. doi: 10.1016/j.carpath.2009.12.006. [DOI] [PubMed] [Google Scholar]
- Maeyama M, Koga H, Selvendiran K, Yanagimoto C, Hanada S, Taniguchi E, Kawaguchi T, Harada M, Ueno T, Sata M. Switching in discoid domain receptor expressions in SLUG-induced epithelial-mesenchymal transition. Cancer. 2008;113:2823–2831. doi: 10.1002/cncr.23900. [DOI] [PubMed] [Google Scholar]
- Miao L, Zhu S, Wang Y, Li Y, Ding J, Dai J, Cai H, Zhang D, Song Y. Discoidin domain receptor 1 is associated with poor prognosis of non-small cell lung cancer and promotes cell invasion via epithelial-to-mesenchymal transition. Med Oncol. 2013;30:626. doi: 10.1007/s12032-013-0626-4. [DOI] [PubMed] [Google Scholar]
- Mihai C, Chotani M, Elton TS, Agarwal G. Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy. J Mol Biol. 2009;385:432–445. doi: 10.1016/j.jmb.2008.10.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B. A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1. J Biol Chem. 2006;281:22744–22751. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- Olaso E, Arteta B, Benedicto A, Crende O, Friedman SL. Loss of discoidin domain receptor 2 promotes hepatic fibrosis after chronic carbon tetrachloride through altered paracrine interactions between hepatic stellate cells and liver-associated macrophages. Am J Pathol. 2011;179:2894–2904. doi: 10.1016/j.ajpath.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL. DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells. J Clin Invest. 2001;108:1369–1378. doi: 10.1172/JCI12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaso E, Labrador JP, Wang L, Ikeda K, Eng FJ, Klein R, Lovett DH, Lin HC, Friedman SL. Discoidin domain receptor 2 regulates fibroblast proliferation and migration through the extracellular matrix in association with transcriptional activation of matrix metalloproteinase-2. J Biol Chem. 2002;277:3606–3613. doi: 10.1074/jbc.M107571200. [DOI] [PubMed] [Google Scholar]
- Perez JL, Jing SQ, Wong TW. Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines. Oncogene. 1996;12:1469–1477. [PubMed] [Google Scholar]
- Perez JL, Shen X, Finkernagel S, Sciorra L, Jenkins NA, Gilbert DJ, Copeland NG, Wong TW. Identification and chromosomal mapping of a receptor tyrosine kinase with a putative phospholipid binding sequence in its ectodomain. Oncogene. 1994;9:211–219. [PubMed] [Google Scholar]
- Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U. Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2. J Neurooncol. 2006;76:239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- Ren T, Zhang J, Liu X, Yao L. Increased expression of discoidin domain receptor 2 (DDR2): a novel independent prognostic marker of worse outcome in breast cancer patients. Med Oncol. 2013;30:397. doi: 10.1007/s12032-012-0397-3. [DOI] [PubMed] [Google Scholar]
- Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, Lee K, Reeves C, Li Y, Hu Y, Tan Z, Stokes M, Sullivan L, Mitchell J, Wetzel R, Macneill J, Ren JM, Yuan J, Bakalarski CE, Villen J, Kornhauser JM, Smith B, Li D, Zhou X, Gygi SP, Gu TL, Polakiewicz RD, Rush J, Comb MJ. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell. 2007;131:1190–1203. doi: 10.1016/j.cell.2007.11.025. [DOI] [PubMed] [Google Scholar]
- Roarty K, Serra R. Wnt5a is required for proper mammary gland development and TGF-beta-mediated inhibition of ductal growth. Development. 2007;134:3929–3939. doi: 10.1242/dev.008250. [DOI] [PubMed] [Google Scholar]
- Schminke B, Muhammad H, Bode C, Sadowski B, Gerter R, Gersdorff N, Burgers R, Monsonego-Ornan E, Rosen V, Miosge N. A discoidin domain receptor 1 knock-out mouse as a novel model for osteoarthritis of the temporomandibular joint. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1436-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani Y, Fukumoto Y, Chaika N, Svoboda R, Wheelock MJ, Johnson KR. Collagen I-mediated up-regulation of N-cadherin requires cooperative signals from integrins and discoidin domain receptor 1. J Cell Biol. 2008;180:1277–1289. doi: 10.1083/jcb.200708137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD. An orphan receptor tyrosine kinase family whose members serve as nonintegrin collagen receptors. Mol Cell. 1997;1:25–34. doi: 10.1016/s1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- Siddiqui K, Kim GW, Lee DH, Shin HR, Yang EG, Lee NT, Yang BS. Actinomycin D identified as an inhibitor of discoidin domain receptor 2 interaction with collagen through an insect cell based screening of a drug compound library. Biol Pharm Bull. 2009;32:136–141. doi: 10.1248/bpb.32.136. [DOI] [PubMed] [Google Scholar]
- Smyth LA, Collins I. Measuring and interpreting the selectivity of protein kinase inhibitors. Journal of chemical biology. 2009;2:131–151. doi: 10.1007/s12154-009-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh HN, Han HJ. Collagen I regulates the self-renewal of mouse embryonic stem cells through alpha2beta1 integrin- and DDR1-dependent Bmi-1. J Cell Physiol. 2011;226:3422–3432. doi: 10.1002/jcp.22697. [DOI] [PubMed] [Google Scholar]
- Sunk IG, Bobacz K, Hofstaetter JG, Amoyo L, Soleiman A, Smolen J, Xu L, Li Y. Increased expression of discoidin domain receptor 2 is linked to the degree of cartilage damage in human knee joints: a potential role in osteoarthritis pathogenesis. Arthritis Rheum. 2007;56:3685–3692. doi: 10.1002/art.22970. [DOI] [PubMed] [Google Scholar]
- Taylor SS, Kornev AP. Protein kinases: evolution of dynamic regulatory proteins. Trends Biochem Sci. 2011;36:65–77. doi: 10.1016/j.tibs.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokarski JS, Newitt JA, Chang CY, Cheng JD, Wittekind M, Kiefer SE, Kish K, Lee FY, Borzillerri R, Lombardo LJ, Xie D, Zhang Y, Klei HE. The structure of Dasatinib (BMS-354825) bound to activated ABL kinase domain elucidates its inhibitory activity against imatinib-resistant ABL mutants. Cancer Res. 2006;66:5790–5797. doi: 10.1158/0008-5472.CAN-05-4187. [DOI] [PubMed] [Google Scholar]
- Underwood DC, Osborn RR, Bochnowicz S, Webb EF, Rieman DJ, Lee JC, Romanic AM, Adams JL, Hay DW, Griswold DE. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L895–L902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- Valiathan RR, Marco M, Leitinger B, Kleer CG, Fridman R. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev. 2012;31:295–321. doi: 10.1007/s10555-012-9346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W, Brakebusch C, Fassler R, Alves F, Ruggiero F, Pawson T. Discoidin domain receptor 1 is activated independently of beta(1) integrin. J Biol Chem. 2000;275:5779–5784. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- Vogel W, Gish GD, Alves F, Pawson T. The discoidin domain receptor tyrosine kinases are activated by collagen. Mol Cell. 1997;1:13–23. doi: 10.1016/s1097-2765(00)80003-9. [DOI] [PubMed] [Google Scholar]
- Vogel WF, Aszodi A, Alves F, Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol Cell Biol. 2001;21:2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LA, Nawshad A, Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biol. 2011;30:243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CZ, Su HW, Hsu YC, Shen MR, Tang MJ. A discoidin domain receptor 1/SHP-2 signaling complex inhibits alpha2beta1-integrin-mediated signal transducers and activators of transcription 1/3 activation and cell migration. Mol Biol Cell. 2006;17:2839–2852. doi: 10.1091/mbc.E05-11-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Bihan D, Chang F, Huang PH, Farndale RW, Leitinger B. Discoidin domain receptors promote alpha1beta1- and alpha2beta1-integrin mediated cell adhesion to collagen by enhancing integrin activation. PLoS ONE. 2012;7:e52209. doi: 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B. Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1. Matrix Biol. 2011a;30:16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Peng H, Glasson S, Lee PL, Hu K, Ijiri K, Olsen BR, Goldring MB, Li Y. Increased expression of the collagen receptor discoidin domain receptor 2 in articular cartilage as a key event in the pathogenesis of osteoarthritis. Arthritis Rheum. 2007;56:2663–2673. doi: 10.1002/art.22761. [DOI] [PubMed] [Google Scholar]
- Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- Xu L, Polur I, Servais JM, Hsieh S, Lee PL, Goldring MB, Li Y. Intact pericellular matrix of articular cartilage is required for unactivated discoidin domain receptor 2 in the mouse model. Am J Pathol. 2011b;179:1338–1346. doi: 10.1016/j.ajpath.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Servais J, Polur I, Kim D, Lee PL, Chung K, Li Y. Attenuation of osteoarthritis progression by reduction of discoidin domain receptor 2 in mice. Arthritis Rheum. 2010;62:2736–2744. doi: 10.1002/art.27582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang K, Kim JH, Kim HJ, Park IS, Kim IY, Yang BS. Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation. J Biol Chem. 2005;280:39058–39066. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- Yeh YC, Lin HH, Tang MJ. A tale of two collagen receptors, integrin beta1 and discoidin domain receptor 1, in epithelial cell differentiation. Am J Physiol Cell Physiol. 2012;303:C1207–C1217. doi: 10.1152/ajpcell.00253.2012. [DOI] [PubMed] [Google Scholar]
- Yeh YC, Wang CZ, Tang MJ. Discoidin domain receptor 1 activation suppresses alpha2beta1 integrin-dependent cell spreading through inhibition of Cdc42 activity. J Cell Physiol. 2009;218:146–156. doi: 10.1002/jcp.21578. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang PL, Gray NS. Targeting cancer with small molecule kinase inhibitors. Nat Rev Cancer. 2009;9:28–39. doi: 10.1038/nrc2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD. The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis. Nat Cell Biol. 2013a;15:677–687. doi: 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Bu X, Zhao H, Yu J, Wang Y, Li D, Zhu C, Zhu T, Ren T, Liu X, Yao L, Su J. A host deficiency of discoidin domain receptor 2 (DDR2) inhibits both tumor angiogenesis and metastasis. J Pathol. 2013b doi: 10.1002/path.4311. [DOI] [PubMed] [Google Scholar]