Abstract
Recent advances in multiparametric magnetic resonance imaging (MRI) have enabled image-guided detection of prostate cancer. Fusion of MRI with real-time ultrasound (US) allows the information from MRI to be used to direct biopsy needles under US guidance in an office-based procedure. Fusion can be performed either cognitively or electronically, using a fusion device. Fusion devices allow superimposition (coregistration) of stored MRI images on real-time US images; areas of suspicion found on MRI can then serve as targets during US-guided biopsy. Currently available fusion devices use a variety of technologies to perform coregistration: robotic tracking via a mechanical arm with built-in encoders (Artemis/Eigen, BioJet/Geoscan); electromagnetic tracking (UroNav/Philips-Invivo, Hi-RVS/Hitachi); or tracking with a 3D US probe (Urostation/Koelis). Targeted fusion biopsy has been shown to identify more clinically significant cancers and fewer insignificant cancers than conventional biopsy. Fusion biopsy appears to be a major advancement over conventional biopsy because it allows (1) direct targeting of suspicious areas not seen on US and (2) follow-up biopsy of specific cancerous sites in men undergoing active surveillance.
Keywords: Prostatic neoplasms, Magnetic resonance imaging, Ultrasonography, Biopsy
1. Introduction
Performed more than 1 million times annually in Medicare recipients, prostate biopsy diagnoses approximately 240,000 new cases of cancer each year [1-3]. The conventional biopsy method under transrectal ultrasound (TRUS) guidance was introduced in the late 1980s [2-6]. The current standard of 12 systematic cores is widely employed [7-9]. However, this technique is limited both by overdetection of low-grade, microfocal “cancers” of little clinical significance and underdetection of large, clinically significant cancers [10-14].
To compensate for the limitations of a 12-core systematic biopsy, more extensive saturation protocols have been described that increase cancer detection but also lead to increased morbidity and detection of more indolent cancers[15,16]. A more rational approach to cancer diagnosis than simply taking more blind biopsy cores is to improve the quality of each biopsy using real-time targeting and documentation of individual biopsy location [17,18].
Although some prostate cancers are visible on US and can be targeted by experienced ultrasonographers [19,20], most biopsies are performed targeting only the various sections of the prostate. Currently, prostate tumors are not often seen on US. Thus, to date, neither targeting of cancers nor tracking the spatial location of cancers within the prostate is a part of prostate biopsy routine.
Improving biopsy procedure would require imaging of cancer and accurate biopsy needle placement into those cancers. Magnetic resonance imaging (MRI), when performed using multiparametric protocols, offers greatly improved imaging of prostate cancer [21-23]. Several methods have been employed to use MRI in guiding biopsy detection of prostate cancer. The most direct method involves “in-bore” MR-guided biopsy [24,25]. This method, used in certain centers and in specific patient populations, is expensive and time consuming, requires MRI-compatible equipment, often a general anesthetic, and is not available in most hospitals. Therefore, in-bore biopsy is unlikely to replace conventional prostate biopsy.
This article focuses on MR-US fusion, a method that uses MRI to improve the accuracy of prostate biopsy in an office-based procedure. It describes the factors required to perform fusion biopsy accurately, the available technologies, and the implications of a switch from conventional blind biopsy to image-targeted biopsy.
1.1. Interpretation and reporting of multiparametric MRI
For MR-US fusion to be beneficial, the MR images must be interpreted and the findings communicated accurately. Interpretation of prostate MRI requires substantial radiologic expertise; nearly all recent publications demonstrating the benefit of prostate MRI come from centers of excellence. The value of prostate MRI when interpreted by inexperienced radiologists is yet to be proven. Discordance between the level of suspicion on MRI reported by the radiologist and subsequent biopsy findings suggests either mistargeting or incorrect MRI interpretation.
Prostate MRI was first reported 30 years ago [26-29]. Much of the early work in optimization was aimed at staging rather than detection. An early comparison of MRI and surgical pathology revealed an accuracy of 78% for detecting extraprostatic extension [30,31]. The use of MRI in men with prior negative biopsy results was first described in 1999, and the first published attempts at MRI-guided biopsy using an in-bore transperineal approach appeared a year later [18,32,33]. Beginning with these studies, different methods for describing suspicion levels emerged as prostate MRI was increasingly being used for detection rather than staging. This became increasingly important as prostate MRI branched out to include diffusion and perfusion imaging in addition to spectroscopic and T2-weighted imaging [1-6]. The National Institutes of Health group used a binary descriptor for each parameter (normal or abnormal) with the overall suspicion given as the number of abnormal parameters (0–4) [7-9]. The use of a rating scale with values from 1 to 5 for each of the parameters was common at many sites, with some assigning a sum or average or weighted average as the overall suspicion level[10-14]. Other sites would use one of the parameters (e.g., diffusion) to identify the region of highest suspicion and then find a corresponding abnormality on other pulse sequences [15,16]. A review of many of these methods is given in an article, which also discusses the relative merits of each and proposes a method for development and validation of a scoring method [17,18].
Given the heterogeneity in the methods by which suspicion is ascribed, many of the authors of the afore-mentioned article, as part of the European Society of Uroradiology, developed guidelines for the performance and reporting of prostate MRI for detection of cancer[19,20]. The goal was to set minimum performance standards and to provide a uniform method for describing MRI findings. As the quantitative parameters on MRI are highly dependent on a number of factors including field strength, gradient performance, scan parameters, and coil positioning, the descriptions of each parameter were qualitative. Additionally, these were based on expert opinion rather than multi-institutional evidence. However, they serve an important role in seeding the idea of reporting standards for MRI. This was echoed in the International Working Group’s Standards of Reporting for MRI-targeted Biopsy Studies (START) of the Prostate, which recommended, among other things, explicit description of the reporting and scoring method of MRI for biopsy targeting[21-23].
1.2. MR-US fusion prostate biopsy
MR-US fusion allows the information from MRI to be used to direct biopsy needles under US guidance. It combines the superior sensitivity of MRI for targeting suspicious lesions with the practicality and familiarity of TRUS. It can be performed using either cognitive fusion[24,25] or with a fusion device [26-29]. Both methods have been used effectively.
1.2.1. Cognitive fusion
Using cognitive fusion, the US operator first views a suspicious lesion on MRI, imagines its location within the prostate, and then attempts to identify and biopsy the same location using real-time US. Cognitive fusion is quick and requires no additional equipment beyond that used in conventional TRUS biopsy. The results of more than 20 studies employing cognitive fusion were recently detailed in a comprehensive review of targeted biopsy of lesions defined on MRI [30,31]. The disadvantages of cognitive fusion lie in the potential for human error when attempting to mentally fuse the MRI with TRUS while aiming for cancers that are often <1 cm in diameter and the inability to track the location of each biopsy site. Cognitive fusion has been performed with success in expert hands but the extent to which this skill is transferable to practicing urologists has not been evaluated.
1.2.2. Fusion biopsy device
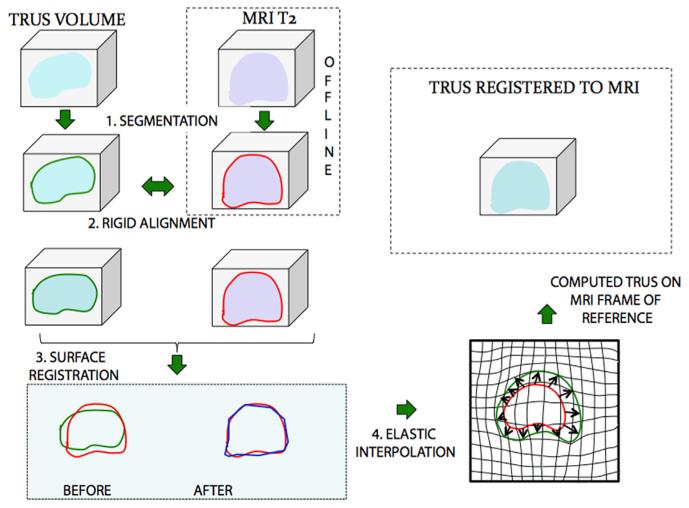
With a fusion device, the stored MRI and real-time US are superimposed (coregistered) using computer software to enable targeted biopsy. Although real-time TRUS is 2-dimensional (D), the tracking system of such a device during TRUS allows computer-assisted construction of a 3D representation of the prostate using individual US images. The fusion results in creation of a 3D model of the prostate that integrates both MRI and real-time US (Fig. 1) and allows the urologist to target lesions seen only on the MRI scan. Aiming and tracking of biopsy sites occurs on the reconstructed 3D model. Accuracy of targeted biopsy relies heavily upon the ability to closely match (register) the 3D models from MRI and US. This is made difficult by changes in gland location and deformation. Registration can be either rigid or elastic.
Fig. 1.
Schematic of the process of MR-US fusion. A 3D model of the prostate created from preprocedure MRI scans is registered and elastically fused to a model created from real-time ultrasound. The fused model is then used for biopsy targeting. Reprinted with permission from Nataranjan et al. [39]. (Color version of figure is available online.)
Rigid registration involves alignment of the MRI and US 3D models by simple rotation or magnification or both. Precise and accurate registration can be accomplished if the 2 models are identical in morphology and scale. However, the 3D shape of the prostate at biopsy is not identical to that at the time of preoperative MRI [18,32,33]. Owing to motion of both the patient and the prostate [34-36], and deformation from pressure from the TRUS probe, it is helpful to have real-time feedback and the ability to modify registration.
Elastic image fusion is more sophisticated than rigid fusion and attempts to compensate for changes in prostate shape or position between the preprocedure MRI and that during the biopsy procedure [37]. It allows for real-time “elastic” deformation of the preoperative MR image to mirror changes in real-time 3D TRUS imaging [18]. The ideal system would constantly update the 3D planning model to match real-time TRUS, keeping up with changes in prostate position and shape, and thereby preserving accuracy during the procedure. Efforts to develop a system that enables such continuous and automatic correction are ongoing.
Several different methods currently in use enable MR-US fusion for prostate biopsy. Those approved by the FDA are highlighted in Table. Existing devices use electromagnetic sensors, optical sensors, or a robotic arm with encoders for tracking the TRUS probe, or they employ a real-time 3D TRUS probe [18]. When compared with cognitive fusion, the use of a fusion device automates the complex process of superimposing MRI and US and therefore may make the results reported by experts achievable by all urologists. These devices also track the location of all biopsy cores, thereby enabling rebiopsy of prior cancerous sites in men on active surveillance. A brief description of the variety of strategies for device-based MR-US fusion follows.
Table.
MR-US fusion devices approved by the US Food and Drug Administration. Reprinted with permission from Marks et al. [50]
| Manufacturer/trade name |
US image acquisition | Biopsy route | Tracking mechanism | Year of FDA approval |
Comments |
|---|---|---|---|---|---|
| Philips/UroNav | Manual US sweep from base to apex |
Transrectal | External magnetic field generator |
2005 | Prospective targeting, integrated with existing ultrasound device, freehand manipulation |
| Eigen/Artemis | Manual rotation along fixed axis |
Transrectal | Mechanical arm with encoders |
2008 | Prospective targeting, stabilized TRUS probe |
| Koelis/Urostation | Automatic US probe rotation, 3 different volumes elastically registered |
Transrectal | Real-time TRUS-TRUS registration |
2010 | Retrospective targeting, real time elastic registration |
| Hitachi/HI-RVS (real-time virtual sonography) |
Real-time biplanar TRUS | Transrectal or transperineal |
External magnetic field generator |
2010 | Prospective targeting, integrated with existing ultrasound device |
| BioJet/Jetsoft/ GeoScan |
Manual US sweep in sagittal | Transrectal or transperineal |
Mechanical arm with encoders; uses stepper |
2012 | Prospective targeting, rigid registration |
1.3. Robotic tracking with encoders
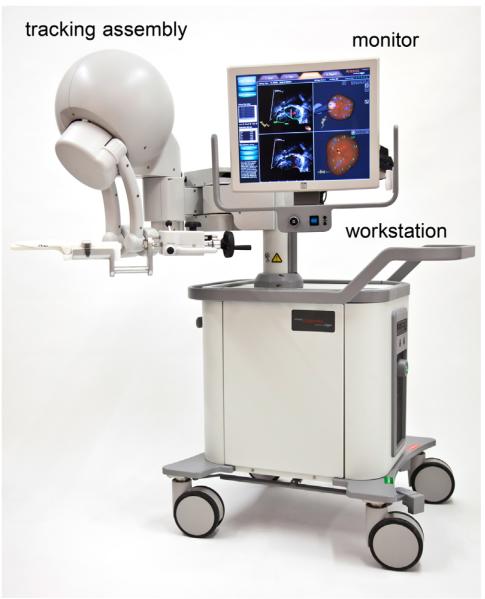
With the Artemis Device (Eigen, Grass Valley, CA), the location of the TRUS probe in space is tracked by direct attachment to a robotic arm (Fig. 2) [38]. This permits reconstruction of the 2D US into a 3D model, which is then elastically fused with the preprocedure MRI scan. The device enables office-based transrectal prostate biopsy of lesions detected on MRI and tracks the location of both targeted and systematic biopsy cores (Fig. 3). Biopsy site tracking enables subsequent rebiopsy of specific cancer-containing sites. At the University of California, Los Angeles (UCLA), the success of rebiopsy of 74 prior positive sites in men on active surveillance was dependent on the length of cancer on the initial biopsy and if the site was associated with a visible lesion on MRI. When the initial cancer core length (CCL) was ≥4 mm, 71% of sites contained cancer on repeat biopsy. When cancerous sites were found in an MRI target, cancer was found on 61% of repeat biopsies vs. 29% of those from systematic sites (unpublished data).
Fig. 2.
Artemis MR-US fusion device including a touch-screen monitor, digital video processor and hard drive, and tracking arm that attaches to a standard transrectal US probe (Eigen, Grass Valley, CA). (Color version of figure is available online.)
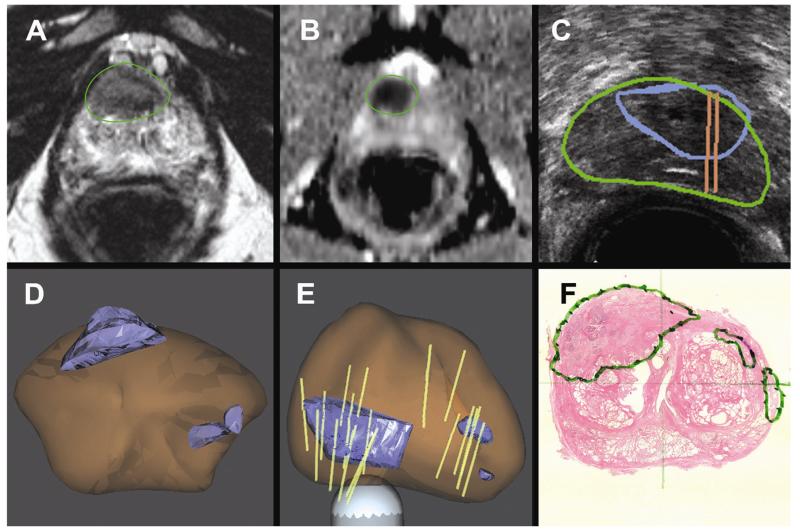
Fig. 3.
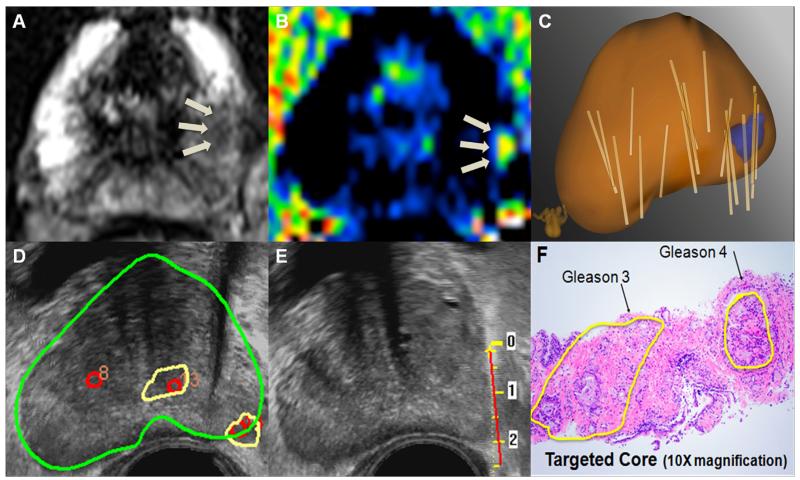
A 65-year-old man with PSA 8.5 on active surveillance after initial biopsy showed 1 mm of Gleason 3 + 3 cancer in 1 core. (A and B) Anterior lesion of highest suspicion identified on mpMRI. (C) Real-time US targeting of the corresponding lesion. (D and E) 3D models demonstrate the target (blue), prostate (brown), and biopsy cores (tan cylinders). Note that in panel E the 3D model is rotated, making the anterior tumor appear to be posterior. MR-US fusion confirmatory biopsy revealed Gleason 4 + 3 cancer. (F) Radical prostatectomy pathology confirmed a 2.3 cm Gleason 4 + 4 cancer centered in the right, anterior prostate. (Color version of figure is available online.)
Advantages of robotic tracking technology include the superior accuracy of robotic tracking and the ability of the robotic arm to immobilize the probe from the time of biopsy targeting to firing of the biopsy gun. The downsides include the relative bulkiness of the device and the more cumber-some procedure for biopsy when compared with freehand biopsies done with other fusion devices.
The UCLA group described the initial clinical use of this 3D biopsy tracking and targeting device in men on active surveillance and those with prior negative biopsy results[28,39]. Cancer was detected in 55% of subjects overall, including 94% of those with the highest level of suspicion on MRI [28]. In men with prior negative biopsy results and a suspicious MRI, cancer yield was 50%, with increasing detection of significant cancers and decreased detection of insignificant cancers using targeting instead of systematic biopsy [14]. The preferential detection of significant cancers is the most exciting aspect of targeted biopsy because it should both diminish the risk posed by cancers missed on conventional biopsy and help reduce overtreatment of low-risk disease.
1.4. Electromagnetic tracking
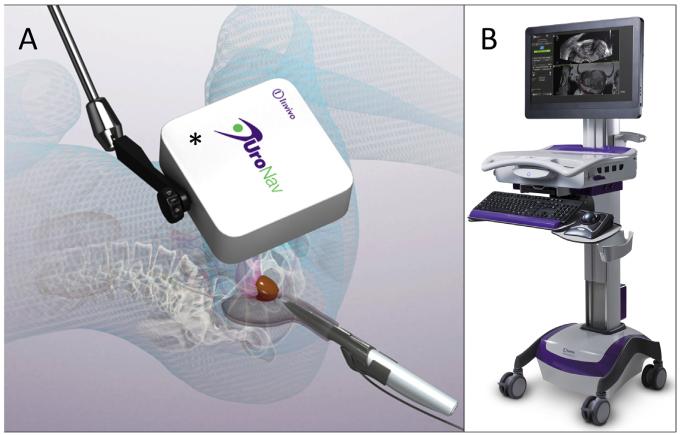
The UroNav device (Invivo, Gainesville, FL) tracks the location of the TRUS probe by attaching a sensor to the probe itself and following its location in space using a small electromagnetic field generator that is placed in close proximity to the patient (Fig. 4). The primary advantage of this technology is in allowing freehand use of the US probe during biopsy, which is more familiar to urologists. This results in a shorter learning curve and somewhat shorter procedure duration. The disadvantage is less accurate probe tracking when using electromagnetic instead of robotic tracking.
Fig. 4.
Photograph of the UroNav MR-US fusion device and generator. (A) An electromagnetic field generator (*) enables tracking of the TRUS probe thereby allowing targeted biopsy with the device (B). Images courtesy of Invivo Corporation. (Color version of figure is available online.)
The preclinical and early clinical development of this device was performed at the National Institutes of Health [40]. This group demonstrated cancer detection in 28% of men with low risk on MRI, 67% in those with moderate risk, and 89% in those with high suspicion [26]. In a separate study on men with prior negative biopsy results, cancer was detected in 37%, including 11% with high-risk Gleason score ≥8 cancer [41].
1.5. Image-based tracking with 3D US
The Urostation device (Koelis, LaTronche, France) uses real-time 3D TRUS to track the position of each biopsy core in a model of the prostate that is recreated with each fire of the biopsy gun rather than at a single time at the beginning of the biopsy session as is done with the Artemis and UroNav devices [42]. This and the simplicity of freehand use of the biopsy probe are the primary advantages of this device. The downsides include the fact that targets are displayed retrospectively, after each biopsy is performed and the potential for human error exists if the handheld probe is moved during image acquisition after each fire of the biopsy gun.
In a validation study of the European Society of Urogenital Radiology Scoring System using the Urostation device, 62 of 129 (48%) men with at least 1 prior negative biopsy result were found to have cancer. Among those with the highest level of suspicion on MRI, 83% had cancer on MR-US fusion biopsy [43].
1.6. Comparison of cognitive and fusion approaches to targeted biopsy
Although the benefit of targeted biopsy is clear, the extent to which a fusion device improves outcomes relative to cognitive fusion has yet to be established. To date, 2 publications have directly addressed this issue.
In a consecutive case series on 391 patients, Delongchamps et al. [44] compared cancer detection rates using visually targeted biopsy (n = 127), rigid registration with a fusion device (Esaote) (n = 131), and elastic registration with a fusion device (Urostation). Each subject underwent 10 to 12 core random biopsies and those with lesions on MRI also received targeted biopsy. Cancer detection for visually targeted biopsy was no better than random biopsy (P = 0.66). In contrast, both rigid and elastic device-based registration increased detection of high-grade cancer while reducing the number of cores and the detection of microfocal cancer.
Puech et al. [45] enrolled 95 men with a suspicious lesion seen on MRI in a prospective study. Each subject underwent a 12-core conventional biopsy and a 4-core targeted biopsy (2 using visual guidance and 2 using MR-US fusion software). Targeted biopsy detected clinically significant cancer in more men (67%) than conventional biopsy (52%) (P = 0.001). Cancer detection using cognitive fusion (47%) was not significantly different from that using fusion software (53%, P = 0.16).
Both cognitive and device-based fusion for targeted biopsy add value to conventional TRUS biopsy. Yet, the published studies comparing cognitive to device-based fusion provide conflicting results. We feel that in expert hands, cognitive fusion likely provides a benefit that is comparable to device-based fusion. However, for the general urologist looking to introduce targeted biopsy to his/her practice, fusion devices would decrease the learning curve, making the results quoted by experts more achievable. In addition, fusion devices provide the ability to track biopsy location to enable subsequent repeat biopsy in men on active surveillance.
1.7. Cancer risk assessment using targeted biopsy
The transition from systematic biopsy to image-targeted biopsy presents new issues for the clinical management of prostate cancer. Ideally, the additional confidence provided by improved biopsy techniques would result in more men opting for active surveillance. However, it is also possible that adoption of targeted biopsy could be used to justify more overtreatment. Currently, therapeutic decisions are heavily influenced by risk classification systems that are driven by biopsy results. These systems (D’Amico, NCCN, Epstein, etc.) were derived from conventional systematic biopsy. When tumors are more extensively sampled via targeted biopsy, the proportion of cores that are positive and the maximal CCL are greater than those with conventional biopsy [28,46]. As a result, targeted biopsy results in an increase in risk attribution relative to systematic biopsy.
Robertson et al. [47] demonstrated this important issue in a computer simulation study involving 107 reconstructed 3D models of whole-mount prostatectomy specimens. They determined that a 12-core TRUS biopsy correctly classified only 24% of prostates containing clinically significant cancer as high risk, as compared with 74% of cases using transperineal targeted biopsy with 4 cores. Furthermore, targeted biopsies reported significantly higher proportion of positive cores and greater maximum CCLs (P < 0.00001). They concluded that image-directed biopsy introduces a systematic increase in risk attribution when applying risk models from conventional TRUS biopsy.
At UCLA, 194 men on active surveillance underwent MR-US fusion targeted biopsy that includes both systematic and targeted sampling. Using systematic biopsy alone and applying the Epstein histologic criteria (Gleason 6, ≤2 cores cancer, and <50% of any core), 28% of men were reclassified on confirmatory biopsy as poor candidates for surveillance. Incorporating targeted biopsy raises the reclassified proportion to 41%. In some cases, this is because of identification of additional serious cancers, but in others (i.e., multiple cancerous cores from a single MRI target), it is a consequence of applying a classification system that fails to consider targeted biopsy.
Given the inflation in risk attribution, it is possible that targeted biopsy could be used to justify aggressive treatment of more men, thereby worsening the problem with overtreatment. To avoid this undesirable result, new criteria for risk stratification based on image-targeted biopsy must be developed and validated. For example, consider a man with low-volume Gleason score of 3 + 3 on conventional biopsy and low-volume Gleason score of 3 + 4 on targeted biopsy (Fig. 5). It is possible that targeted biopsy with device-based tracking of cancerous sites may be used to safely follow cancers that are currently believed to require treatment.
Fig. 5.
Illustrative case of a 66-year-old man with a PSA of 9 ng/mL. MRI demonstrates 2 image grade 3 targets. (A) T2-weighted MRI shows a left-sided lesion with moderately reduced signal. (B) Diffusion-weighted imaging confirms moderately restricted diffusion in the area of interest. (C) Fusion of US and MRI generates a 3D model. Individual biopsy cores (tan cylinders) are mapped on the model. (D) Targets are superimposed on real-time US to enable targeted biopsy. (E) Real-time tracking of biopsy cores ensures accurate biopsy of targeted lesions. (F) In this case, diagnostic Artemis fusion biopsy showed one core of Gleason 3 + 3 (1 mm) on systematic biopsy, thereby fulfilling Epstein criteria. However, targeted biopsy showed a focus of Gleason 3 + 4. Based on systematic biopsy alone, this patient would be considered an excellent candidate for active surveillance. Inclusion of targeted biopsy and application of current risk models would result in upstaging to intermediate risk and perhaps definitive treatment. Further study will be required to develop new risk assignment criteria based on targeted biopsy and to determine if men with small, Gleason 3 + 4 tumors on targeted biopsy are suitable for active surveillance. (Color version of figure is available online.)
1.8. Barriers to adoption
Several barriers exist that must be overcome for image-guided biopsy to be adopted as the new standard of care: additional evidence of benefit, proliferation of radiologic expertise in prostate MRI, and demonstration of cost-effectiveness.
Current clinical guidelines do not call for the use of MR-US fusion targeted prostate biopsy. The recent American Urological Association guideline on early detection of prostate cancer noted that prostate imaging, along with prostate-specific antigen derivatives and novel urinary markers, should be considered as a secondary test with potential utility for determining the need for a prostate biopsy, but with unproven benefit [48]. Additional data are needed to conclusively prove that targeted biopsy will increase benefit and reduce harm before incorporation into guidelines.
Accurate interpretation of prostate MRI requires substantial radiologic expertise. Such accuracy is mandatory for MR-US fusion targeted biopsy to be beneficial. Most publications describing the benefits of MRI arise from a small number of expert centers. For image-targeted biopsy to be widely adopted and maintain its benefit outside of centers of excellence, practicing radiologists need to be trained in prostate MRI interpretation.
Finally, the cost of prostate MRI and image-targeted biopsy is commonly cited as a deterrent to adoption. At face value, the new image-based approach seems more expensive than conventional TRUS biopsy. However, this does not take into account the repercussions of missed diagnoses on conventional biopsy or overtreatment because of uncertainty from systematic TRUS biopsy. A thorough cost-effectiveness analysis of MRI to aid localization of prostate abnormalities for biopsy was prepared for the National Health Service in the United Kingdom. It concludes that “under certain circumstances T2-MRI may be cost-effective compared with systematic TRUS” and calls for further studies to more conclusively analyze the cost-effectiveness of image-guided biopsy [49]. Additional research is required to determine if the savings associated with preferential diagnosis of clinically significant cancers is sufficient to offset the cost of MRI and fusion biopsy devices.
2. Conclusion
Multiparametric MRI enables greatly enhanced detection of localized prostate cancer when compared with US. Fusion of MRI with US to guide biopsy, whether done cognitively or using a device, allows the urologist to utilize the power of MRI in improving prostate cancer diagnosis and risk stratification in an office-based procedure. Targeted prostate cancer diagnosis enables increased detection of clinically significant prostate cancers while reducing detection of clinically insignificant cancers [14].
References
- [1].Turnbull LW, Buckley DL, Turnbull LS, Liney GP, Knowles AJ. Differentiation of prostatic carcinoma and benign prostatic hyperplasia: correlation between dynamic Gd-DTPA-enhanced MR imaging and histopathology. J Magn Reson Imaging. 1999;9:311–6. doi: 10.1002/(sici)1522-2586(199902)9:2<311::aid-jmri24>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- [2].Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-Medicare. J Urol. 2011;186:1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cancer Facts & Figures. Cancerorg n.d.
- [4].Gibbs P, Tozer DJ, Liney GP, Turnbull LW. Comparison of quantitativeT2 mapping and diffusion-weighted imaging in the normal and pathologic prostate. Magn Reson Med. 2001;46:1054–8. doi: 10.1002/mrm.1298. [DOI] [PubMed] [Google Scholar]
- [5].Vallancien G, Leo JP, Brisset JM. Transperineal prostatic biopsy guided by transrectal ultrasonography. Prog Clin Biol Res. 1987;243B:25–7. [PubMed] [Google Scholar]
- [6].Hodge KK, McNeal JE, Terris MK, Stamey TA. Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate. J Urol. 1989;142:71–4. doi: 10.1016/s0022-5347(17)38664-0. discussion74-5. [DOI] [PubMed] [Google Scholar]
- [7].Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, et al. Prostate Cancer: value of Multiparametric MR Imaging at 3 T for Detection—histopathologic correlation. Radiology. 2010;255:89–99. doi: 10.1148/radiol.09090475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Presti JCJ, O’Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003;169:125–9. doi: 10.1016/S0022-5347(05)64051-7. [DOI] [PubMed] [Google Scholar]
- [9].Gore JL, Shariat SF, Miles BJ, Kadmon D, Jiang N, Wheeler TM, et al. Optimal combinations of systematic sextant and laterally directed biopsies for the detection of prostate cancer. J Urol. 2001;165:1554–9. [PubMed] [Google Scholar]
- [10].Puech P, Potiron E, Lemaitre L, Leroy X, Haber G-P, Crouzet S, et al. Dynamic contrast-enhanced-magnetic resonance imaging evaluation of intraprostatic prostate cancer: correlation with radical prostatectomy specimens. Urology. 2009;74:1094–9. doi: 10.1016/j.urology.2009.04.102. [DOI] [PubMed] [Google Scholar]
- [11].Taira AV, Merrick GS, Galbreath RW, Andreini H, Taubenslag W, Curtis R, et al. Performance of transperineal template-guided mapping biopsy in detecting prostate cancer in the initial and repeat biopsy setting. Prostate Cancer Prostatic Dis. 2010;13:71–7. doi: 10.1038/pcan.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Fütterer JJ, Heijmink SW, Scheenen TW, Veltman J, Huisman HJ, Vos P, et al. Prostate cancer localization with dynamic contrast-enhanced MR imaging and proton MR spectroscopic imaging. Radiology. 2006;241:449–58. doi: 10.1148/radiol.2412051866. [DOI] [PubMed] [Google Scholar]
- [13].Rouse P, Shaw G, Ahmed HU, Freeman A, Allen C, Emberton M. Multi-parametric magnetic resonance imaging to rule-in and rule-out clinically important prostate cancer in men at risk: a cohort study. Urol Int. 2011;87:49–53. doi: 10.1159/000325880. [DOI] [PubMed] [Google Scholar]
- [14].Sonn GA, Chang E, Natarajan S, Margolis DJ, Macairan M, Lieu P, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2013 Mar 17; doi: 10.1016/j.eururo.2013.03.025. doi:pii: S0302-2838(13)00249-2. 10.1016/j.eururo.2013.03.025. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Park BK, Lee HM, Kim CK, Choi HY, Park JW. Lesion localization in patients with a previous negative transrectal ultrasound biopsy and persistently elevated prostate specific antigen level using diffusion-weighted imaging at three Tesla before rebiopsy. Invest Radiol. 2008;43:789–93. doi: 10.1097/RLI.0b013e318183725e. [DOI] [PubMed] [Google Scholar]
- [16].Simon J, Kuefer R, Bartsch GJ, Volkmer BG, Hautmann RE, Gottfried HW. Intensifying the saturation biopsy technique for detecting prostate cancer after previous negative biopsies: a step in the wrong direction. BJU Int. 2008;102:459–62. doi: 10.1111/j.1464-410X.2008.07560.x. [DOI] [PubMed] [Google Scholar]
- [17].Dickinson L, Ahmed HU, Allen C, Barentsz JO, Carey B, Fütterer JJ, et al. Scoring systems used for the interpretation and reporting of multiparametric MRI for prostate cancer detection, localization, and characterization: could standardization lead to improved utilization of imaging within the diagnostic pathway? J Magn Reson Imaging. 2013;37:48–58. doi: 10.1002/jmri.23689. [DOI] [PubMed] [Google Scholar]
- [18].Ukimura O, Hung AJ, Gill IS. Innovations in prostate biopsy strategies for active surveillance and focal therapy. Curr Opin Urol. 2011;21:115–20. doi: 10.1097/MOU.0b013e3283435118. [DOI] [PubMed] [Google Scholar]
- [19].Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–57. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Toi A, Neill MG, Lockwood GA, Sweet JM, Tammsalu LA, Fleshner NE. The continuing importance of transrectal ultrasound identification of prostatic lesions. J Urol. 2007;177:516–20. doi: 10.1016/j.juro.2006.09.061. [DOI] [PubMed] [Google Scholar]
- [21].Moore CM, Kasivisvanathan V, Eggener S, Emberton M, Fütterer JJ, Gill IS, et al. Standards of reporting for MRI-targeted biopsy studies (START) of the prostate: recommendations from an International Working Group. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.03.030. [DOI] [PubMed] [Google Scholar]
- [22].Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, et al. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59:962–77. doi: 10.1016/j.eururo.2011.02.034. [DOI] [PubMed] [Google Scholar]
- [23].Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-Van de Kaa CA, et al. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453–61. doi: 10.1148/radiol.11091409. [DOI] [PubMed] [Google Scholar]
- [24].Kasivisvanathan V, Dufour R, Moore CM, Ahmed HU, Abd-Alazeez M, Charman SC, et al. Transperineal magnetic resonance image targeted prostate biopsy versus transperineal template prostate biopsy in the detection of clinically significant prostate cancer. J Urol. 2013;189:860–6. doi: 10.1016/j.juro.2012.10.009. [DOI] [PubMed] [Google Scholar]
- [25].Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010;183:520–7. doi: 10.1016/j.juro.2009.10.022. [DOI] [PubMed] [Google Scholar]
- [26].Pinto PA, Chung PH, Rastinehad AR, Baccala AAJ, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011;186:1281–5. doi: 10.1016/j.juro.2011.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hricak H, Williams RD, Spring DB, Moon KL, Jr., Hedgcock MW, Watson RA, et al. Anatomy and pathology of the male pelvis by magnetic resonance imaging. AJR Am J Roentgenol. 1983;141:1101–10. doi: 10.2214/ajr.141.6.1101. [DOI] [PubMed] [Google Scholar]
- [28].Sonn GA, Natarajan S, Margolis DJA, Macairan M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013;189:86–91. doi: 10.1016/j.juro.2012.08.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hadaschik BA, Kuru TH, Tulea C, Rieker P, Popeneciu IV, Simpfendorfer T, et al. A novel stereotactic prostate biopsy system integrating pre-interventional magnetic resonance imaging and live ultrasound fusion. J Urol. 2011;186:2214–20. doi: 10.1016/j.juro.2011.07.102. [DOI] [PubMed] [Google Scholar]
- [30].Moore CM, Robertson NL, Arsanious N, Middleton T, Villers A, Klotz L, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013;63:125–40. doi: 10.1016/j.eururo.2012.06.004. [DOI] [PubMed] [Google Scholar]
- [31].Bezzi M, Kressel HY, Allen KS, Schiebler ML, Altman HG, Wein AJ, et al. Prostatic carcinoma: staging with MR imaging at 1.5 T. Radiology. 1988;169:339–46. doi: 10.1148/radiology.169.2.3174982. [DOI] [PubMed] [Google Scholar]
- [32].Perrotti M, Han KR, Epstein RE, Kennedy EC, Rabbani F, Badani K, et al. Prospective evaluation of endorectal magnetic resonance imaging to detect tumor foci in men with prior negative prostastic biopsy: a pilot study. J Urol. 1999;162:1314–7. [PubMed] [Google Scholar]
- [33].D’Amico AV, Tempany CM, Cormack R, Hata N, Jinzaki M, Tuncali K, et al. Transperineal magnetic resonance image guided prostate biopsy. J Urol. 2000;164:385–7. [PubMed] [Google Scholar]
- [34].van Herk M, Bruce A, Kroes AP, Shouman T, Touw A, Lebesque JV. Quantification of organ motion during conformal radiotherapy of the prostate by three dimensional image registration. Int J Radiat Oncol Biol Phys. 1995;33:1311–20. doi: 10.1016/0360-3016(95)00116-6. [DOI] [PubMed] [Google Scholar]
- [35].Deurloo KE, Steenbakkers RJ, Zijp LJ, de Bois JA, Nowak PJ, Rasch CR, et al. Quantification of shape variation of prostate and seminal vesicles during external beam radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:228–38. doi: 10.1016/j.ijrobp.2004.09.023. [DOI] [PubMed] [Google Scholar]
- [36].Ghilezan MJ, Jaffray DA, Siewerdsen JH, van Herk M, Shetty A, Sharpe MB, et al. Prostate gland motion assessed with cine-magnetic resonance imaging (cine-MRI) Int J Radiat Oncol Biol Phys. 2005;62:406–17. doi: 10.1016/j.ijrobp.2003.10.017. [DOI] [PubMed] [Google Scholar]
- [37].Baumann M, Mozer P, Daanen V, Troccaz J. Prostate biopsy tracking with deformation estimation. Med Image Anal. 2012;16:562–76. doi: 10.1016/j.media.2011.01.008. [DOI] [PubMed] [Google Scholar]
- [38].Guo Y, Werahera PN, Narayanan R. Image registration accuracy of a 3-dimensional transrectal ultrasound-guided prostate biopsy system. J Ultrasound Med. 2009;28:1561–8. doi: 10.7863/jum.2009.28.11.1561. [DOI] [PubMed] [Google Scholar]
- [39].Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, Lieu P, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011;29:334–42. doi: 10.1016/j.urolonc.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Xu S, Kruecker J, Turkbey B, Glossop N, Singh AK, Choyke P, et al. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13:255–64. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Vourganti S, Rastinehad A, Yerram NK, Nix J, Volkin D, Hoang A, et al. Multiparametric magnetic resonance imaging and ultrasound fusion biopsy detect prostate cancer in patients with prior negative transrectal ultrasound biopsies. J Urol. 2012 doi: 10.1016/j.juro.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Baumann M, Mozer P, Daanen V, Troccaz J. Lecture notes in computer science. Vol. 5761. Springer; Berlin, Heidelberg: 2009. Prostate biopsy assistance system with gland deformation estimation for enhanced precision; pp. 67–74. [DOI] [PubMed] [Google Scholar]
- [43].Portalez D, Mozer P, Cornud F, Renard-Penna R, Misrai V, Thoulouzan M, et al. Validation of the European Society of Urogenital Radiology scoring system for prostate cancer diagnosis on multiparametric magnetic resonance imaging in a cohort of repeat biopsy patients. Eur Urol. 2012;62:986–96. doi: 10.1016/j.eururo.2012.06.044. [DOI] [PubMed] [Google Scholar]
- [44].Delongchamps NB, Peyromaure M, Schull A, Beuvon F, Bouazza N, Flam T, et al. Prebiopsy magnetic resonance imaging and prostate cancer detection: comparison of random and targeted biopsies. J Urol. 2013;189:493–9. doi: 10.1016/j.juro.2012.08.195. [DOI] [PubMed] [Google Scholar]
- [45].Puech P, Rouvière O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal US-MR fusion guidance versus systematic biopsy—Prospective multicenter study. Radiology. 2013;268:461–9. doi: 10.1148/radiol.13121501. [DOI] [PubMed] [Google Scholar]
- [46].Haffner J, Lemaitre L, Puech P, Haber G-P, Leroy X, Jones JS, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011;108:E171–8. doi: 10.1111/j.1464-410X.2011.10112.x. [DOI] [PubMed] [Google Scholar]
- [47].Robertson NL, Hu Y, Ahmed HU, Freeman A, Barratt D, Emberton M. Prostate cancer risk inflation as a consequence of image-targeted biopsy of the prostate: a computer simulation study. Eur Urol. 2013 Jan 3; doi: 10.1016/j.eururo.2012.12.057. pii: S0302-2838(12)01587-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Carter HB, Albertsen PC, Barry MJ, Etzioni R, Freedland SJ, Greene KL, et al. Early detection of prostate cancer: AUA guideline. J Urol. 2013;190:419–26. doi: 10.1016/j.juro.2013.04.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Mowatt G, Scotland G, Boachie C. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation. Health Technol. 2013;17:vii–xix. 1–281. doi: 10.3310/hta17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013;23:43–50. doi: 10.1097/MOU.0b013e32835ad3ee. [DOI] [PMC free article] [PubMed] [Google Scholar]