Abstract
Nasal polyposis (NP) has a great impact on quality of life (QOL) and its management involves a combination of medical therapy and surgery. To the authors' knowledge, no publication has extensively examined NP after optimal medical treatment based on subjective evaluations. The aim of this prospective study was designed to evaluate the QOL in NP patients after (1) a short course of oral steroids, (2) initial 3-month course of macrolide, and (3) long-term treatment with intranasal steroids. A total of 55 patients with grades I and II NP were consecutively treated with oral prednisolone at 25 mg in a single dose for 2 weeks, macrolide at 250 mg daily for the first 3 months, and long-term intranasal steroids. Patients were followed up and evaluated at baseline and 3, 6, and 12 months for QOL measure. At baseline, patients with grade I and grade II NP showed significantly worse QOL scores on all Rhinosinusitis Disability Index domains, particularly for physical function (4.59 ± 1.41) and were significantly higher in social function (3.16 ± 1.17). At 3, 6, and 12 months of treatment, patients showed a significant improvement in all impaired QOL domains compared with baseline after optimal medical therapy (p < 0.05). These results suggest that the optimal medical treatment to improve QOL incorporates medical polypectomy with a short course of oral steroids in addition to macrolide and this can be maintained by long-term intranasal steroid therapy.
Keywords: Aspirin sensitivity, asthma, grade I and I nasal polyps, loss sense of smell, macrolide, medical polypectomy, nasal obstruction, nasal polyposis, quality of life, topical intranasal steroids
Nasal polyposis (NP) is characterized by a recurrent sinonasal mucosal inflammatory process with the presence of two or more symptoms that include obstruction, congestion or discharge, facial pain or pressure and reduced sense of smell for >12 weeks. The etiology of NP is unknown but it is detected and monitored with pathological endoscopic findings and/or computed tomography scan changes within the osteomeatal complex and/or sinuses.1 The prevalence of NP in the general population is considered to be ∼4% and can be associated with different systemic and respiratory diseases such as cystic fibrosis, rhinitis, and asthma with or without aspirin sensitivity.2 Approximately 4.6% of patients with NP have acetylsalicylic acid triad: bronchial asthma, chronic rhinosinusitis, and aspirin insensitivity.3 Patients with NP seem to have a more pronounced disease burden than those without polyps.4 There is a relative lack of documentation in prospective, randomized, controlled trials regarding improvement of quality of life (QOL) after medical treatment of NP, which could suggest that surgical treatment of NP has a better effect on QOL than medical treatment.5 QOL improvement after NP treatment is correlated with nasal symptom improvement. One of the most frequently used validated questionnaires to assess QOL is the Rhinosinusitis Disability Index (RSDI).6 RSDI uses three subscales (emotional, physical, and functional) to combine measurements of general health status and disease-specific QOL. Nasal endoscopy has a definite role in detecting nasal polyps.7 NP is primarily a disease to be managed medically and optimal medical therapy is needed before and after surgery. A short course of the systemic steroid prednisolone at 25 mg daily for 14 days, serving as “medical polypectomy,” is equally effective as simple polypectomy with a snare. Intranasal corticosteroids as basic long-term therapy clearly reduce the size of nasal polyps with significant effects on blockage symptom scores and objective measures of nasal patency but with reduced effect on the loss of the sense of smell.8 Macrolide antibiotics have high anti-inflammatory activities and decrease the virulence of colonizing bacteria, which leads to shrinkage of nasal polyps by suppressing cytokine production of inflammatory cells in the paranasal sinus epithelium.9 The main aim of this study was (1) to evaluate the QOL outcomes in patients with grade I and grade II NP after optimal medical therapy, (2) to evaluate the frequency of various diseases in NP patients, and (3) to correlate between the nasal symptoms score and QOL after medical therapy.
MATERIALS AND METHODS
Study Population
A total of 55 patients with NP grades I and II (Lildholdt classification) were included in this prospective study from April 2011 to February 2013 with an age range of 18–65 years with a mean age of 51.47 years (SD, ±14.35). Female patients outnumbered male patients (54.5 and 45.5%, respectively). All patients were examined and treated for the entire duration of the study at the Outpatient Otorhinolaryngology Clinic. Approval for this study was obtained from the Ethics Committee and a signed informed consent was obtained from all patients.
Inclusion and Exclusion Criteria
The inclusion criteria included patient age of 18–65 years, nasal symptoms for >12 weeks, and NP grades I and II, and the exclusion criteria were NP grade III (high indication for surgical treatment), surgically treated patient with NP, allergy to macrolide antibiotics, patients with absolute contraindications for systemic steroids, and patients with severe structural abnormalities caused by deviated nasal septum.
Study Design
After a 4-week washout period of both oral and intranasal steroids (week 0), 55 patients received systemic steroid (oral prednisone) at 25 mg in a single daily dose for 2 weeks, followed by maintenance using a long-term intranasal steroid (e.g., mometasone furoate aqueous nasal spray at 200 μg/day). Macrolide antibiotic at 250 mg daily was given as single dose for the first 3 months. An initial baseline evaluation before treatment was performed followed by three follow-up evaluations at the 3, 6, and 12 months of treatment (Fig. 1). Nasal symptoms and QOL were scored by the RSDI questionnaire and all of the recruited patients underwent skin-prick test.
Figure 1.
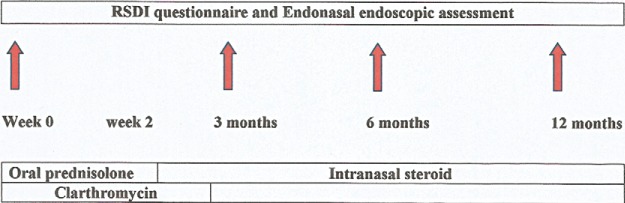
Study algorithm. Patients with nasal polyposis (NP) treated with a short course of oral prednisolone followed by long-term intranasal steroid. Clarithromycin was given for the initial 3 months.
QOL and RSDI Assessment
All patients with grades I and II NP fulfilling the inclusion criteria were assessed subjectively before and after medical treatment by filling in the RSDI questionnaire comprised of 28 questions divided into three domains (physical, mental, and social). Scale scores range from 0 to 100% and lower scores indicate better QOL. Each domain has 10 levels of answers based on tables that permit only one answer. These scores were taken and compared at the different follow-up study interval (i.e., baseline and 3, 6, and 12 months).
Nasal Symptoms Score
Nasal obstruction, loss of smell, rhinorrhea, and sneezing were recorded and severity of these symptoms was assessed and scored on subsequent follow-up as 0, excellent; 1 and 2, very good; 3 and 4, good; 5 and 6 fair; 7 and 8, poor; and 9 and 10, very poor.
Statistical Analysis
Data analysis was performed with the statistical package IBM SPSS Version 19.0 (IBM Company, United Kingdom) using mean ± SD. A value of p < 0.05 was considered statistically significant. All data were assessed for normal distribution. A paired t-test was used to compare the score of mean over time. QOL (physical, mental, and social) and treatment were compared to baseline scores by two-tailed paired Student's t-test and differences between groups were assessed using the t-test. A chi-square test was used to look for association (numbers and percentages) among the group. ANOVA was used to compare 55 patients' percentages of QOL and nasal symptoms scores for different time visit.
RESULTS
A total number of 55 NP patients were prospectively recruited and enrolled into the study with baseline comparison of disease–severity scores (i.e., QOL scores) and a 1-year follow-up. All patients received medical cointerventions that include oral prednisolone, clarithromycin, and continuous intranasal steroid application and allergy therapy. Follow-up data were available for 55 (91%) of the 60 patients at the end of this study.
Frequency of Various Diseases in Nasal Polyps
Before treatment, 20 patients with NP were diagnosed as hypertensive (36.4%). Allergic rhinitis and asthmatic patients were seen in 17 (30.9%) and 19 (34.5%) of NP patients, respectively. Eczematous disease was reported in 5 (9.1%) patients and aspirin intolerance in only 3 (5.5%) patients. Positive skin-prick test was seen in 17 (30.9%) and 38 (69.1%) patients had negative skin-prick test. Among 55 patients with NP only, 39 (70.9%) were under medication (Table 1).
Table 1.
Number and percentages of disease condition by duration visit (n = 55)
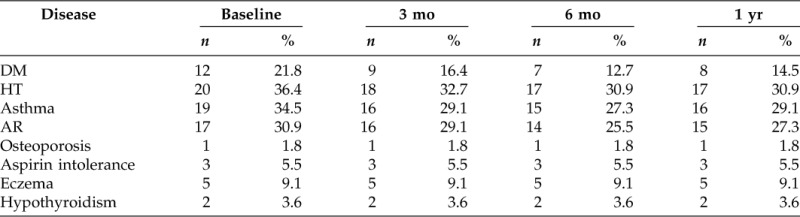
DM = diabetes mellitus; HT = hypertension; AR = allergic rhinitis.
QOL (RSDI) Assessment
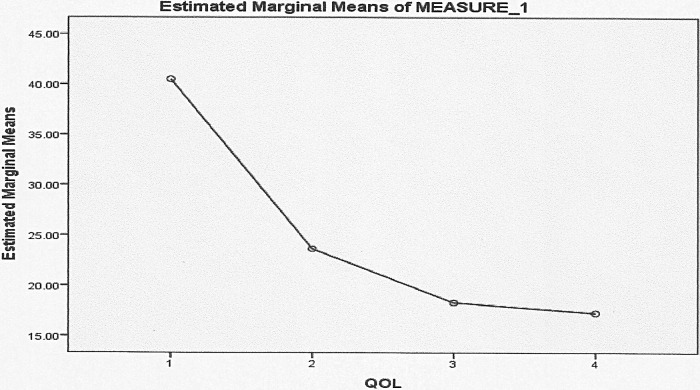
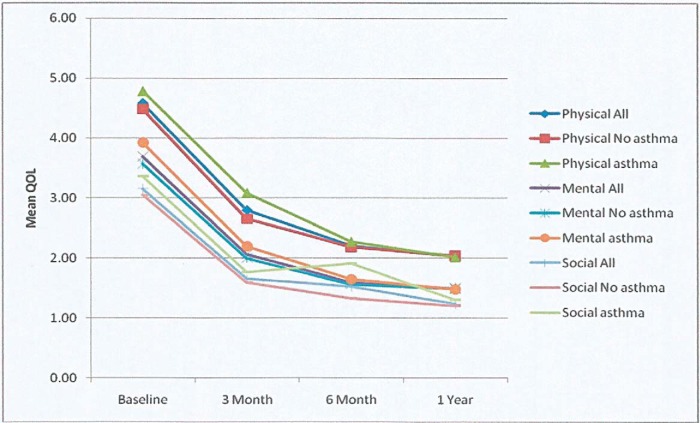
ANOVA was used to compare 55 patients' percentages of QOL for different duration visits. Pairwise comparisons showed the QOL baseline (40.52 ± 12.07) was significant at 3 month (23.66 ± 9.85), 6 month (18.32 ± 8.42), and 1 year (17.28 ± 8.79) follow-up (Table 2). In comparison of percentages of QOL by time visit, patients with NP had significantly worse QOL scores before treatment. At 3, 6, and 12 months, patients with NP showed a similar significant improvement in QOL. No significant differences were observed on QOL between 6 and 12 months (Fig. 2). In all domains of RSDI, patients with NP showed significantly worse physical function (4.59 ± 1.41) QOL scores than social function (3.16 ± 1.17). All of the RSDI domains are statistically correlated to NP patients with asthma (Table 3). At baseline, asthmatic patients with NP had worse scores of QOL (p < 0.05) than nonasthmatic patients with NP in physical, mental, and social functioning. Before treatment asthmatic patients showed lower physical, mental, and social functioning than nonasthmatic patients. At 6 and 12 months, similar QOL improvements were observed for physical and mental functions in asthmatic and nonasthmatic patients except for social function, where the former had worse QOL score than the latter (Fig. 3).
Table 2.
Comparison of mean percentages QOL by duration visit
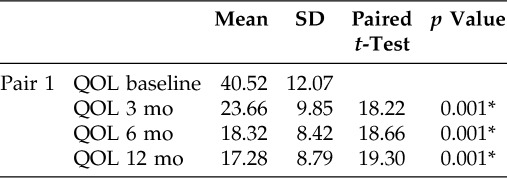
*Significant value of p < 0.05.
QOL = quality of life.
Figure 2.
General linear model one-way repeated measure to show quality of life (QOL) in patients with nasal polyposis (NP) for different duration visits. All patients had significantly worsened scores before treatment at baseline.
Table 3.
Summary of comparison of mean of physical, mental, and social components in asthmatic and nonasthmatic patients with NP
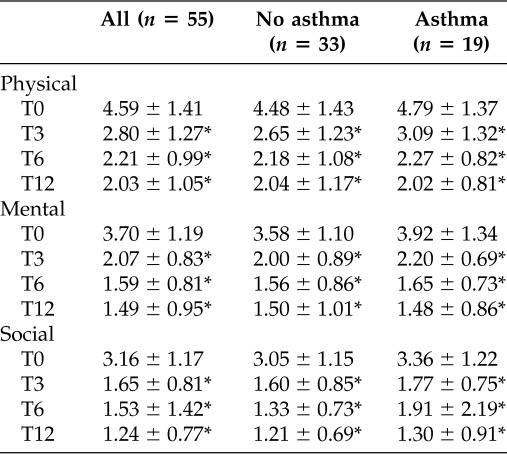
*Paired t-test with baseline, p < 0.001.
T0, baseline; T1, 3 mo; T2, 6 mo; T3, 1 yr.
NP = nasal polyposis.
Figure 3.
Quality of life (QOL) improvement in patients with nasal polyposis (NP). Rhinosinusitis Disability Index (RSDI) domains (physical, mental, and social functioning) in asthmatic and nonasthmatic NP patients. Paired t-test with baseline,*p < 0.05, at 3, 6, and 12 months of medical treatment compared with baseline.
Nasal Symptoms Scores
Patients scored loss of smell and nasal obstruction as the major complaints, while other nasal symptoms were much less frequent and discomforting. At 3, 6, and 12 months all nasal symptoms significantly improved compared with baseline. However, loss of sense of smell worsened at 12 months compared with 6 months. At baseline, asthmatic patients with nasal polyps had higher scores of nasal obstruction and loss of sense of smell than nonasthmatic patients. At 3 and 6 months, asthmatic and nonasthmatic patients scored similar improvement in nasal obstruction and loss of sense of smell except at 12 months, where loss of sense of smell worsened in nonasthmatic patients as compared to asthmatic patients (Fig. 4).
Figure 4.
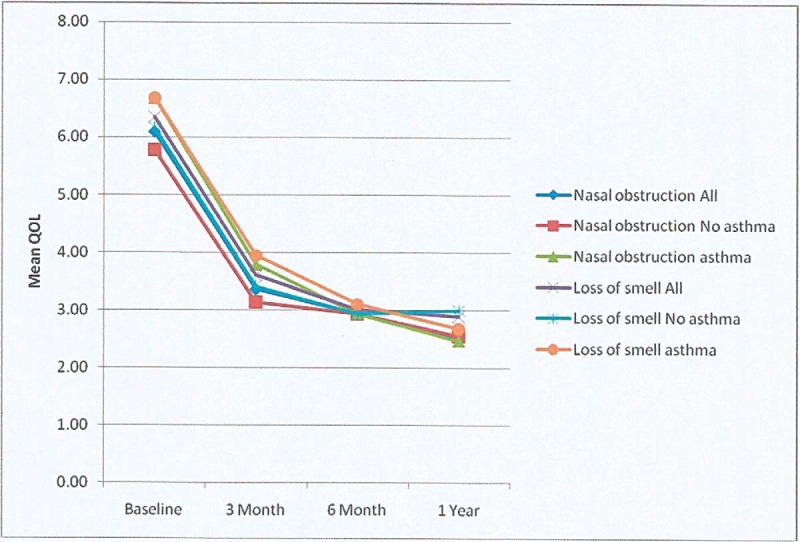
Comparison of mean quality of life (QOL) concerning loss of sense of smell and nasal obstruction in asthmatic and nonasthmatic patients with nasal polyposis (NP) by duration visits.
DISCUSSION
The evaluation of NP is presently impeded by a lack of valid specific instruments to measure QOL and insufficient data to assess the effect of gender, duration of therapy, or comorbidity with asthma or aspirin sensitivity on QOL. This study has been developed to highlight the effects of treatment of NP on QOL. All patients with a polyposis score 1 or 2 on the Lildholdt classification were treated with the same regime.10 Our study includes 55 subjects from baseline to 1 year and were all examined and followed up by the same otolaryngologist. The characteristics of the population were similar to the literature with reference to age (mean, 51 years), sex ratio (1.19), prevalence of associated asthma (34.5%), and sensitivity to aspirin (5.5%).11–13 The majority of the literature reviewed dealt with a single steroid, used either orally or as topical spray. Our study highlighted that (i) patients with NP have a significantly worse QOL in all RSDI domains (physical functioning was lower than the mental functioning), (ii) steroid treatment led to similar improvement in all QOL domains in NP with or without asthma (except for social functioning where asthmatic patients had worse QOL scores than nonasthmatic patients), and (iii) both steroid and clarithromycin treatments maintained improvement in nasal symptoms. At baseline, atopic patients with NP had worse scores of QOL (p < 0.05) than nonasthmatic patients with NP in physical, mental, and social functioning. At 6 and 12 months of medical therapy, similar QOL improvement was seen for both physical and mental functioning in atopic and nonatopic patients except for social functioning where the former had worse QOL score than the latter. The study also investigated the impact of NP indicating that nasal polyps impair QOL in all Short Form 36 domains, viz., that intranasal steroids improved the symptoms and the QOL in patients with NP (i.e., general health, vitality, social functioning, and mental health domains).14 Intranasal steroids are more effective than placebo in polyp size reduction.15 Our study reveals that QOL in patients with NP is impaired where the physical health is more impaired than the mental health and a short course of oral steroids caused a significant improvement in all domains of RSDI and long-term treatment with clarithromycin and intranasal steroids maintained this improvement. The two most disabling symptoms in baseline nasal polyp patients' were reduced loss of the sense of smell and nasal obstruction. Nasal symptoms clearly improved after oral steroid treatment and intranasal steroids maintained this effect except sense of the smell, which was a drawback at 12 months in nonasthmatic patients more than asthmatic patients. It has been shown that there is a clear improvement in nasal symptoms after 2 weeks commencement of oral steroids, especially for nasal obstruction and reduced sense of smell.16 Intranasal steroids (fluticasone or beclomethasone) have gained acceptance as an alternative treatment to surgery for NP and are effective in reducing nasal symptoms when compared with placebo.17 Combined oral and intranasal steroids seem to be effective to treat NP by improving the sense of smell, nasal obstruction, and other nasal symptoms.18 Nasal obstruction was a major complaint in NP patients and the long-lasting correction of olfactory dysfunction can be achieved through the combination of nasalization and a low dose of nasal steroids.19 Three major studies were dedicated to systemic steroid efficacy, which had a genuine improvement in all symptoms, especially on anosmia, which correlates well with the polyp volume reduction.8,16,20 Short-term systemic steroid administration thus appears to have the same efficacy as polypectomy, but the improvement proves to be of short duration.20 Over a dozen placebo-controlled studies were published but the therapeutic effect was mostly studied for periods restricted to a few weeks.20,15 Topical therapy has a definite beneficial effect on the clinical disorders and on the polyp size but shows little activity on the sense of smell dysfunction (Fig. 4).
A study of 20 patients with CRS and nasal polyps treated for a minimum of 3 months with clarithromycin at 400 mg/day revealed that in the group whose polyps were reduced in size, the IL-8 levels decreased fivefold. The IL-8 levels were also significantly higher before macrolide treatment than in the group whose polyps showed no change.21 In another uncontrolled trial, 40 patients were treated with roxithromycin at 150 mg for at least 8 weeks, where the lower-grade polyps shrunk in about one-half of the patients. The investigators found no correlation between treatment effect and the extent of eosinophilia in the tissue.22
A reported study revealed a greater impairment of QOL when NP was associated with asthma and physical and mental functioning were significantly lower in asthmatic patients than in a nonasthmatic patients.14 Asthma had an adverse impact on vitality and general health compared with rhinosinusitis alone but no significant differences in postoperative Short Form 36 scores from patients with rhinosinusitis alone.23 This study revealed that asthmatic patients had a higher nasal symptom score and worse QOL for physical, mental, and social functioning than nonasthmatic patients. However, asthmatic and nonasthmatic patients had similar nasal symptoms and polyp size and scored similar QOL domains after oral steroids, and these effects were maintained by long-term intranasal steroid therapy. The sense of smell worsened more at 12 months in nonasthmatic patients than in asthmatic patients with NP. In this study, a short course of oral steroids resulted in significant reduction of polyp size in asthmatic patients, which was maintained on long-term intranasal steroids. Few studies have been conducted to assess the impact of aspirin sensitivity on QOL in patients with nasal polyps. It has indicated that aspirin sensitivity has no further negative impact on QOL and no significant differences in nasal symptoms, polyp size, and QOL between aspirin-tolerant and aspirin-sensitive asthmatic patients before and after a short course of oral steroids or on long-term intranasal steroid treatment.24 In our study, there was no significant difference in polyp size score between right and left nasal cavities in aspirin-tolerant patients before and after a short course of oral prednisolone and long-term intranasal steroid. On the contrary, there was a significant difference noted in polyp size between right and left nasal cavities in aspirin-intolerant patients. In conclusion, the strength of this study includes sample size, length of follow-up, the prospective nature of data collection, and rigorous methodology used to qualify QOL. The main limitation is that it was a subjective evaluation of QOL by RSDI questionnaire leading to bias. A very small sample of patients with aspirin intolerance resulted in biasness when compared with 52 patients with aspirin tolerance.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Fokkens WJ, Lund VJ, Mullol J; Eurpoea Position Paper on Rhinosinusitis and Nasal Polyps Group. Latest review European position paper on rhinosinusitis and nasal polyps. Rhinology Suppl, 1–136, 2012 [PubMed] [Google Scholar]
- 2. Hedman J, Kaprio J, Poussa T, Neiminen MM. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol 28:717–722, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Kim JW, Hong SL, Kim YK. Histological and immunological features of non-eosinophilic nasal polyps. Otolaryngol Head Neck Surg 137:925–930, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Soler ZM, Smith TL. Quality of life outcomes after functional endoscopic sinus surgery. Otolaryngol Clin North Am 43:605–612, x, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ragab SM, Lund VJ, Scadding G. Impact of chronic rhinosinusitis therapy on quality of life: A prospective randomized controlled trial. Rhinology 48:305–311, 2010 [DOI] [PubMed] [Google Scholar]
- 6. Benninger MS, Senior BA. The development of the Rhinosinusitis Disability Index. Arch Otolaryngol Head Neck Surg 123:1175–1179, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Shahizon AM, Suraya A, Rozmnan Z, et al. Correlation of computed tomography and nasal endoscopic findings in chronic rhinosinusitis. Med J Malaysia 63:211–215, 2008 [PubMed] [Google Scholar]
- 8. Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroids powder for nasal polyp. A double-blind, placebo-controlled study of budesonide. Clin Otolaryngol 20:26–3028, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Katsuta S, Osafune H, Takita R. Therapeutic effect of roxithromycin on chronic sinusitis with nasal–polyps clinical, computed tomography, and electron microscopy analysis (in Japanese). Nihon Jibiinkoka Gakkai Kaiho 105:1189–1197, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Vendelo Johansen L, Illum P, Kristensen S, et al. The effect of budesonide (Rhinocort) in the treatment of small and medium-sized nasal polyps. Clin Otolaryngol 18:524–527, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Brown BL, Harner SG, Van Dellen RG. Nasal polypectomy in patients with asthma and sensitivity to aspirin. Arch Otolaryngol 105:413–416, 1979 [DOI] [PubMed] [Google Scholar]
- 12. Drake-Lee AB, Lowe D, Swanston A, Grace A. Clinical profile and recurrence of nasal polyps. J Laryngol Otol 98:783–793, 1984 [DOI] [PubMed] [Google Scholar]
- 13. Larsen K, Tos M. The estimated incidence of symptomatic nasal polyps. Acta Otolaryngol 122:179–182, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Radenne F, Lamblin C, Vandezande LM, et al. Quality of life in nasal polyposis. J Allergy Clin Immunol 104:79–84, 1999 [DOI] [PubMed] [Google Scholar]
- 15. Blomqvist EH, Lundblad L, Anggård A, et al. A randomized controlled study evaluating medical treatment versus surgical treatment in addition to medical treatment of nasal polyposis. J Allergy Clin Immunol 107:224–228, 2001 [DOI] [PubMed] [Google Scholar]
- 16. van Camp P, Clement PA. Results of oral steroid treatment in nasal polyposis. Rhinology 32:5–9, 1994 [PubMed] [Google Scholar]
- 17. Holmberg K, Juliusson S, Balder B, et al. Fluticasone propionate aqueous nasal spray in the treatment of nasal polyposis. Ann Allergy Asthma Immunol 78:270–276, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Norès JM, Avan P, Bonfils P. Medical management of nasal polyposis: A study in a series of 152 consecutive patients. Rhinology 41:97–102, 2003 [PubMed] [Google Scholar]
- 19. Jankowski R, Bodino C. Evolution of symptoms associated to nasal polyposis following oral steroid treatment and nasalization of the ethmoid–radical ethmoidectomy is functional surgery for NPS. Rhinology 41:211–219, 2003 [PubMed] [Google Scholar]
- 20. Lildholdt T, Mygind N. Effect of corticosteroids on nasal polyps: Evidence from controlled trials. In Nasal Polyposis: An Inflammatory Disease and Its Treatment. Mygind N, Lildholdt T. (Eds). Copenhagen, Denmark: Munksgaard, 160–169, 1997 [Google Scholar]
- 21. Yamada T, Fujieda S, Mori S, et al. Macrolide treatment decreased the size of nasal polyps and IL-8 levels in nasal lavage. Am J Rhinol 14:143–148, 2000 [DOI] [PubMed] [Google Scholar]
- 22. Nonaka M, Pawankar R, Tomiyama S, Yagi T. A macrolide antibiotic, roxithromycin, inhibits the growth of nasal polyp fibroblasts. Am J Rhinol 13:267–272, 1999 [DOI] [PubMed] [Google Scholar]
- 23. Winstead W, Barnett S. Impact of endoscopic sinus surgery on global health perception: An outcomes study. Otolaryngol Head Neck Surg 119:486–491, 1998 [DOI] [PubMed] [Google Scholar]
- 24. Alobid I, Benítez P, Bernal-Sprekelsen M. Nasal polyposis and its impact on quality of life. Comparison between the effects of medical and surgical treatments. Allergy 60:452–458, 2005 [DOI] [PubMed] [Google Scholar]