Abstract
Allergic rhinitis (AR) is characterized by Th2 polarized immune response. Soluble HLA (sHLA) molecules play an immunomodulatory activity. Two different studies evidenced that both patients with seasonal AR (SAR) and patients with perennial AR (PAR) had higher sHLA-G levels than normal controls. The aim of this study was to compare sHLA-G serum levels in SAR and PAR patients, also considering allergen-specific IgE. One hundred sixty-eight AR patients were enrolled, 94 with SAR and 74 with PAR. A group of 116 healthy subjects was considered as control. sHLA-G and allergen-specific IgE serum levels were determined by immunoenzymatic method. SAR patients had significantly higher levels of sHLA-G than PAR patients (p = 0.0194). sHLA-G was moderately related to allergen-specific IgE both in SAR (r = 0.497) and in PAR patients (r = 0.584). The present study provides evidence that sHLA-G serum levels depend on the type of allergy and are related to allergen-specific IgE serum levels. These findings may suggest that sHLA-G could be a biomarker of allergic reaction.
Keywords: Allergic rhinitis, IgE, perennial allergens, pollens, sHLA-G
Allergic rhinitis (AR) is sustained by mucosal inflammation, which is orchestrated by T helper (Th) 2 cells.1 Th2 cells play an important role in the pathogenesis of AR, as IL-4 promotes isotype switching to IgE synthesis.2 Moreover, regulatory T (Treg) cells play an important role in controlling the Th2-biased response.3 Indeed, a defect in Treg cells has been evidenced in allergic patients.4 Therefore, allergic diseases are presently considered as immunoregulatory disorders.
In this regard, interest in HLA-G antigens has been shown by the possibility that they may be involved in inflammatory diseases.5 HLA-G is a human, nonclassic major histocompatibility complex molecule expressed in human-privileged sites and plays an essential role in the development of maternal tolerance to genetically different fetal tissues.6 Soluble HLA-G (sHLA-G) derives from the secretion of soluble isoforms as well as from the shedding of proteolytically cleaved surface isoforms. Several HLA-G–mediated mechanisms have been described, including an induction of Th2-like cytokine profile, through stimulation of IL-3, IL-4, and IL-10 secretion.7 Numerous studies provided evidence that sHLA-G serum levels are increased in different pathological conditions, including immune-mediated disorders, transplantation, and malignancies.8
Recently, it has been evidenced that patients with AR due to pollen allergy had significantly higher sHLA-G levels than healthy controls.9 Furthermore, it was shown that patients with AR due to perennial allergens had more elevated sHLA-G levels than healthy controls.10
Therefore, the aim of the present study was to evaluate whether there was a difference between patients with seasonal AR (SAR) and patients with perennial AR (PAR), also considering the possible relationship with allergen-specific IgE.
METHODS
Subjects
Two hundred eighty-four subjects, 116 healthy subjects (46 men; mean age, 44.3 years) and 168 patients (80 men; mean age, 38.7 years), with AR were included in the study. AR was diagnosed according to validated criteria proposed by Allergic Rhinitis and Its Impact on Asthma ARIA guidelines.11
Based on a consistent relationship between sensitization type and clinical history, patients were subdivided in two groups: SAR patients (94 patients) and PAR patients (74 patients). Patients either with acute upper respiratory infections; undergoing specific immunotherapy; or using nasal or oral corticosteroids, antileukotrienes, and antihistamines within the previous 4 weeks were excluded.
Blood samples were collected from patients to determine sHLA-G and allergen-specific IgE serum levels.
The study was conducted with the approval of the local Ethics Committee and after obtaining written informed consent by all participants.
sHLA Molecules Immunoassay
sHLA-G molecules were determined by a commercially available immunoenzymatic assay (EXBIO, Praha, Czech Republic) and was performed according to the Manufacturer's Instructions. Results were reported as concentration of sHLA-G in nanograms per milliliter in samples (where 100 U/mL corresponds to 40–50 ng/mL according to the 2004 Soluble HLA-G Workshop, Essen, Germany). The coefficient of variation for intraassay variation was 7.6% and for interassay variation was 5.8%.
Allergen-Specific IgE Measurement
Serum levels of specific IgE (sIgE) were detected by the Immunofluorescent Assay procedure (ImmunoCAP; Thermo Fisher Scientific, Milan, Italy) in peripheral blood samples from patients. The allergen of interest, covalently coupled to ImmunoCAP, reacted with the sIgE in the patient sample. After washing away nonspecific IgE, enzyme-labeled antibodies against IgE were added to form a complex. After incubation, unbound enzyme–anti-IgE was washed away and the bound complex was then incubated with a developing agent. After stopping the reaction, the fluorescence of the eluate was measured. Quantitative sIgE concentrations were expressed in kilounits per liter according to the traceable calibration of the 2nd World Health Organization International Reference Preparation for human IgE. sIgE levels were considered positive if they were >0.35 kU/L.
Statistical Analysis
Descriptive statistics were first performed and quantitative parameters were reported as median, minimum, and maximum values with first and third quartiles (interquartile range). The nonparametric Wilcoxon's test was used to compare samples. The nonparametric Kruskal-Wallis rank test was performed to evaluate the analysis of variance between groups of patients.
Relationships were analyzed by Spearman test. All tests were two sided and a value of p < 0.05 was considered statistically significant. The package “S-Plus” (MathSoft Corp., Cambridge, MA) was used for all of the analyses.
RESULTS
Figure 1 shows that sHLA-G levels significantly changed (Kruskal-Wallis test; p < 0.0001) among the groups of subjects. The median (range, 25th–75th percentile) of serum sHLA-G levels was 46.36 ng/mL (25th–75th percentile, 29.75–78.86 ng/mL) in SAR patients, 37.27 ng/mL (25th–75th percentile, 10.05–66.22 ng/mL) in PAR patients, and 6.75 ng/mL (25th–75th percentile, 4.14–9.69 ng/mL) in normal subjects. The post hoc analysis reveals that SAR patients have significantly higher levels than PAR patients (p = 0.0194); healthy subjects have lower levels than both SAR and PAR patients (p < 0.0001 for both).
Figure 1.
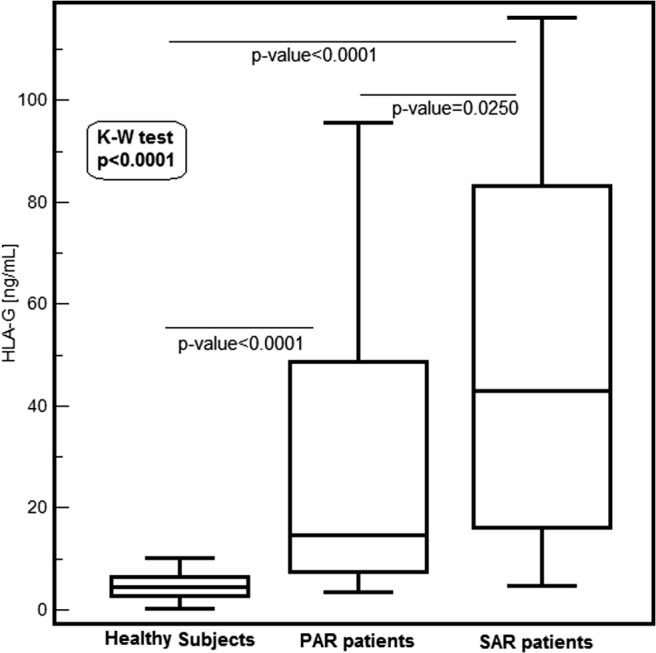
Serum soluble HLA-G (sHLA-G; ng/mL) distribution in healthy subjects and allergic patients, evaluated separately in patients with seasonal allergic rhinitis (SAR) and with perennial AR (PAR). Values are represented as medians (horizontal lines), quartiles (25th and 75th percentiles, boxes), and ranges (vertical lines) and p values between groups.
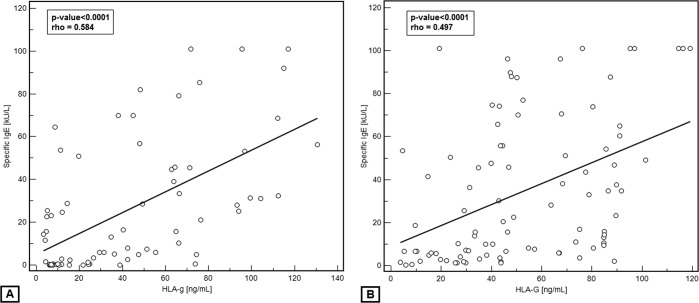
There was a significant and moderate relationship between HLA-G levels and allergen-specific IgE levels both in SAR patients (r = 0.497 and p < 0.0001) and in PAR patients (r = 0.584 and p < 0.0001), as shown in Fig. 2.
Figure 2.
Relationship between serum soluble HLA-G (sHLA-G) and serum allergen-specific IgE in patients with (A) perennial allergic rhinitis (PAR) and (B) with seasonal AR (SAR).
DISCUSSION
AR is characterized by a Th2-polarized inflammation. Th2-derived cytokines, such as IL-4 and IL-13, are the main pathogenic factors able to induce, maintain, and amplify the allergic inflammation.12 In addition, a defect of Treg cells characterizes allergic disorders.4 In this regard, sHLA-G molecules might play an important role in the mechanisms of immune tolerance toward allergens because, like their surface membrane bound counterparts, they have immunosuppressive properties. In fact, sHLA-G molecules inhibit T-cell proliferation induced by allogeneic dendritic cells13 and, in analogy to sHLA-A, -B, and -C molecules, induce apoptosis of T and NK CD8+ cells and inhibit cytotoxic T-cell activity through CD8 ligation by Fas/sFasL interaction.14 The immunoregulatory characteristics of HLA-G have prompted a number of studies aimed at evaluating the expression of sHLA-G in sera from patients affected by a variety of disorders of the immune system as reported by an extensive review.8
Two separate studies evidenced that both SAR and PAR patients had higher sHLA-G serum levels than normal controls.9,10 The present study therefore aimed at comparing sHLA-G in SAR and PAR patients, also considering allergen-specific IgE.
Results obtained show that SAR patients have higher sHLA-G levels than PAR patients. In addition, a significant and moderate relationship exists between sHLA-G and allergen-specific IgE in both AR groups.
These findings are consistent with previous studies indicating that pollen allergy is characterized by more intense clinical and inflammatory phenomena than AR because of perennial allergens.15,16 In addition, the moderate relationship between sHLA-G and IgE underlines the role exerted by sHLA-G in allergic inflammation.
Therefore, sHLA-G might be involved in maintaining the Th2-polarized immune response in allergic patients and in tolerogenic defective mechanisms.
In conclusion, the present study provides the evidence that sHLA-G depends on the type of allergy and allergen-specific IgE levels. These findings might reinforce the concept that sHLA-G could be a biomarker of allergic reaction.
ACKNOWLEDGMENTS
The authors thank Angelo Corsico (Respiratory Disease Unit, Fondazione IRCCS Policlinico S. Matteo, Pavia) for clinical data, Giorgia Testa and Cristina Torre (Allergy Lab, Fondazione IRCCS Policlinico S. Matteo) for outstanding technical support, and Vania Giunta (Allergy Lab, Fondazione IRCCS Policlinico S. Matteo) for data analysis.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Romagnani S. Biology of human TH1 and TH2 cells. J Clin Immunol 15:121–129, 1995 [DOI] [PubMed] [Google Scholar]
- 2. Durham SR, Ying S, Varney VA, et al. Cytokine messenger RNA expression for IL-3, IL-4, IL-5, and granulocyte/macrophage-colony-stimulating factor in the nasal mucosa after local allergen provocation: Relationship to tissue eosinophilia. J Immunol 148:2390–2394, 1992 [PubMed] [Google Scholar]
- 3. Imada M, Simons EF, Jay FT, Hayglass KT. Allergen-stimulated interleukin-4 and interferon-gamma production in primary culture: Responses of subjects with allergic rhinitis and normal controls. Immunology 85:373–380, 1995 [PMC free article] [PubMed] [Google Scholar]
- 4. Umetsu DT, DeKruyff RH. The regulation of allergy and asthma. Immunol Rev 212:238–255, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Carosella ED, Moreau P, Aractingi S, Rouass-Freiss N. HLA-G: A shield against inflammatory aggression. Trends Immunol 10:553–555, 2001 [DOI] [PubMed] [Google Scholar]
- 6. Carosella ED, Horuzsko A. HLA-G and cancer. Semin Cancer Biol 17:411–412, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Kanai T, Fujii T, Kozuma S, et al. Soluble HLA-G influences the release of cytokines from allogeneic peripheral blood mononuclear cells in culture. Mol Hum Reprod 7:195–200, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Pistoia V, Morandi F, Wang X, Ferrone S. Solubile HLA-G: Are they clinically relevant? Semin Cancer Biol 17:469–479, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciprandi G, Colombo BM, Contini P, et al. Soluble HLA-G and HLA-A,-B,-C serum levels in patients with allergic rhinitis. Allergy 63:1335–1338, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Ciprandi G, Contini P, Murdaca G, et al. Soluble HLA-G molecule in patients with perennial allergic rhinitis. Int Arch Allergy Immunol 150:278–281, 2009 [DOI] [PubMed] [Google Scholar]
- 11. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 108(suppl):S147–S334, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Bachert C, van Kempen M, Van Cauwenberge P. Regulation of proinflammatory cytokines in seasonal allergic rhinitis. Int Arch Allergy Immunol 118:375–379, 1999 [DOI] [PubMed] [Google Scholar]
- 13. Le Friec G, Laupéze B, Fardel O, et al. Soluble HLA-G inhibits human dendritic cell-triggered allogeneic T-cell proliferation without altering dendritic differentiation and maturation processes. Hum Immunol 64:752–761, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Contini P, Ghio M, Poggi A, et al. Soluble human HLA-A, -B, -C, and -G molecules induce apoptosis in T and NK CD8+ cells and inhibit cytotoxic T cell activity through CD8 ligation. Eur J Immunol 33:125–134, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Gelardi M, Maselli Del Giudice A, Candreva T, et al. Nasal resistance and allergic inflammation depend on allergen type. Int Arch Allergy Immunol 141:384–389, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Ciprandi G, Cirillo I. Monosensitization and polysensitization in allergic rhinitis. Eur J Intern Med 22:e75–e79, 2011 [DOI] [PubMed] [Google Scholar]