Abstract
Purpose
Cost-effectiveness analysis of voriconazole compared with fluconazole, both with galactomannan monitoring, for prevention of invasive fungal infections (IFI) in patients receiving allogeneic hematopoietic cell transplantation (HCT)
Methods
A decision analytic economic model was developed to estimate the drug costs associated with planned prophylaxis, supplemental prophylaxis, and empirical therapy and costs associated with treatment of suspected or documented IFI. We estimated incremental cost-effectiveness ratios (ICERs) at 6 months, 12 months, and lifetime. A bootstrap analysis was conducted to estimate the uncertainty of the clinical trial results.
Results
At 12 months, mean total fungal-infection-related costs were not significantly different for voriconazole prophylaxis than for fluconazole prophylaxis for a subpopulation with acute myeloid leukemia (AML) but were significantly higher for voriconazole prophylaxis for the study population and in a subpopulation of those with other underlying diseases. The cost per IFI avoided ($66,919) and the cost per life-year gained (LYG) ($5,453) were also lower for the AML subgroup than for the complete trial population ($812,990/IFI avoided; and dominated for cost/LYG). ICERs were more favorable for voriconazole at 6 months and when the generic price was used for oral voriconazole. Bootstrap analysis results for the cost per LYG showed that the probability of voriconazole being cost-effective is 33% for the complete study population and 85% for the AML subpopulation, for a maximum willingness to pay for 1 year of life gained of $50,000.
Conclusion
Voriconazole prophylaxis was significantly more costly than fluconazole for the complete study population but may be cost-effective for those undergoing an allogeneic HCT for AML. Use of generic oral voriconazole will have a favorable impact on the costs and cost-effectiveness of prophylaxis with voriconazole compared with fluconazole for all undergoing allogeneic HCT.
Introduction
Patients undergoing allogeneic hematopoietic cell transplantation (HCT) are at high risk of invasive fungal infections (IFIs), especially those caused by Candida species and Aspergillus species.1 Given the high mortality rates associated with these infections and the high costs of their treatment, preventive strategies are now part of standard care.2 Placebo-controlled trials in those undergoing either autologous or allogeneic HCT have shown that fluconazole decreases Candida infection and IFI-related deaths after HCT (reviewed in McCoy et al., 20091). Only one study, which primarily enrolled people with allogeneic HCT, showed a reduction in overall mortality associated with fluconazole prophylaxis.3,4
Because fluconazole has no activity against Aspergillus species or other molds, randomized controlled trials of prophylaxis with itraconazole, posaconazole, and voriconazole, drugs that are active against Aspergillus species, have been completed in patients undergoing allogeneic HCT (reviewed in McCoy et al., 20091). In particular, the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) 0101 clinical trial was a randomized, double-blind, multicenter study of fluconazole versus voriconazole, with regular serum galactomannan monitoring for IFI, for the prevention of IFI in allogeneic HCT recipients with follow-up for all participants up to 12 months.5 The results of the BMT CTN 0101 clinical trial indicated that voriconazole was not superior to fluconazole when considering the primary endpoint of fungal-free survival (alive and free from presumptive, probable, or proven IFI) at 6 months; prophylaxis with either voriconazole or fluconazole gave similar results. However, there were trends to fewer Aspergillus species infections and less frequent empirical antifungal therapy administered in patients receiving prophylaxis with voriconazole compared with those receiving prophylaxis with fluconazole.
The economic evaluation reported here used data from the BMT CTN 0101 clinical trial to estimate the cost-effectiveness of prophylaxis with voriconazole compared with fluconazole in patients undergoing allogeneic HCT. Previous analyses have been performed to determine whether clinical benefits that have been shown using prophylaxis regimens that are active against both Aspergillus and Candida species might result in offsetting cost savings and favorable cost-effectiveness ratios.6,7 The clinical benefits that have been shown include reduced need for empirical therapy and reduced cases of IFI.5,8,9,10 None of the published economic evaluations have focused on those undergoing allogeneic HCT. In addition, all the recent economic evaluations of voriconazole, posaconazole, or micafungin have been based on clinical endpoints assessed either at the end of planned prophylaxis or soon afterward. The BMT CTN 0101 clinical trial followed patients for 12 months after allogeneic HCT, including at least 6 months after the end of planned prophylaxis (either 100 or 180 days).5 In addition, data were collected on all other prophylaxis regimens used during the clinical trial period, as well as data on empirical therapy use for those suspected of having an IFI allowing for a more comprehensive assessment of the outcomes.
Methods
The study used a decision analytic approach to estimate the costs and cost-effectiveness of voriconazole compared with fluconazole, both with galactomannan monitoring, for prevention of IFI in patients receiving allogeneic HCT. Estimates were generated for all trial participants, as well as for two different population subgroups within the trial population: those with acute myeloid leukemia (AML) and those with all other underlying diseases (i.e., acute lymphoblastic leukemia, chronic myelogenous leukemia, myelodysplastic syndrome, non-Hodgkin lymphoma). The subgroup analyses were performed for two reasons: (1) there was an imbalance in the number of AML patients in the two prophylaxis groups in the full study population and (2) there was a greater risk of IFI and death and in a post-hoc analysis a higher FFS in the voriconazole cohort in the AML patients. As in the full study population, the patients in the AML fluconazole and voriconazole cohorts were evenly balanced with respect to bone marrow versus peripheral blood graft source, transplant type, and incidence of graft versus host disease. In addition, the patients in the AML prophylaxis cohorts received similar immunosuppressant therapy to all subjects during the trial. A bootstrap analysis was performed to represent the uncertainty in the clinical trial results. Sensitivity of the results to changes in oral voriconazole costs and IFI treatment costs were also performed.
Model Structure
A decision analytic approach was taken for the analysis (see Figure 1). Three time periods were considered in the analysis: 6 months, 12 months, and lifetime. During the trial time period, a patient could be assigned to planned prophylaxis with either voriconazole or fluconazole. Within the 6-month or 12-month time period, each patient could be either diagnosed with a possible, presumptive, probable, or proven IFI or not and could either die or not. Cause of death could be related to either the IFI or to the underlying disease. The no-IFI branch of the decision tree included those with no IFI and those with a possible IFI (meets the clinical criterion for a lower respiratory tract infection but lacks mycological confirmation), some of whom were given a short course of empirical therapy but who never progressed to presumptive, probable, or proven IFI as defined in the Wingard et al. study.5 The IFI branch of the decision tree includes patients with one or more presumptive, probable, or proven IFI. Because there were no statistical differences between the adverse events associated with different types of prophylaxis observed in the clinical trial, adverse events were considered to occur equally in all branches of the decision tree and were not costed separately. Table 1 presents a listing of the clinical outcomes of the BMT CTN 0101 study that were used to derive the probabilities for the decision tree model and for the measurement of cost inputs for the model.
Figure 1.
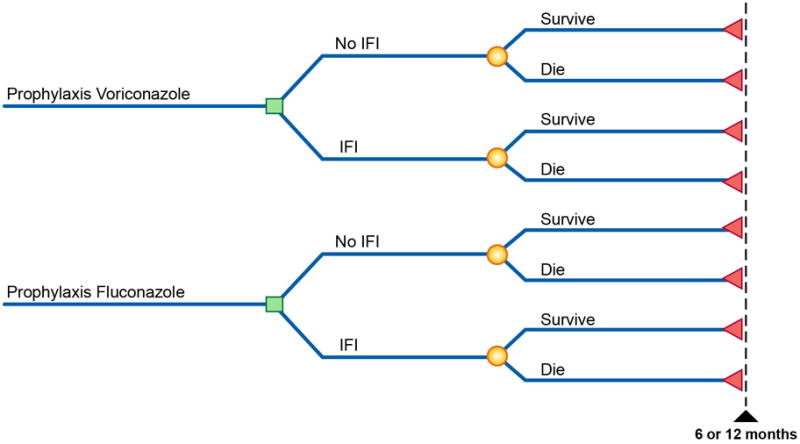
Table 1. Clinical Outcomes at 6 and 12 Months.
| Input Parameter | Voriconazole (n=304) |
Fluconazole (n=295) |
|---|---|---|
| 6 Months | ||
| Total presumptive, probable, or proven IFIsa | 22 | 33 |
| Aspergillus—probable or proven | 9 | 17 |
| Candida—probable or proven | 3 | 3 |
| Other/multiple—probable or proven | 2 | 4 |
| Presumptive | 8 | 9 |
| Survival rate at 6 months | 81.2% | 80% |
| Percentage taking empirical therapy | 24.4 | 30.2 |
| Percentage taking supplemental prophylaxis | 31.9 | 19.0 |
| 12 Months | ||
| Total presumptive, probable, or proven IFIsa | 38 | 41 |
| Aspergillus—probable or proven | 17 | 21 |
| Candida—probable or proven | 6 | 3 |
| Other/multiple—probable or proven | 7 | 7 |
| Presumptive | 8 | 10 |
| Survival rate at 12 months | 67.8% | 70.3% |
| Percentage taking empirical therapy | 24.7 | 30.5 |
| Percentage taking supplemental prophylaxis | 32.6 | 19.7 |
Analysis Populations
The study population was the same as the study population used in Wingard et al.5 and included patients ≥ 2 years of age who were receiving a related or unrelated hematopoietic graft for acute myelogenous leukemia in first or second complete remission or early relapse, acute lymphoblastic, biphenotypic or undifferentiated leukemia in first or second complete remission, chronic myelogenous leukemia in the chronic or accelerated phase, myelodysplastic syndrome, or receiving related donor transplant for chemosensitive nonHodgkin or Hodgkin lymphoma. Two subpopulations were evaluated according to their underlying disease: acute myeloid leukemia (AML) and “other diagnosis.”
Efficacy Variables
The primary measure of effectiveness in the 6- and 12-month cost-effectiveness analysis used in this analysis (and one of the key outcome variables in the primary publication of the BMT CTM 0101 study) was the incidence of presumptive, probable, or proven IFIs from transplant to 6 and 12 months, respectively. The incidence of IFIs at 6 and 12 months was determined using competing risk models in which death was a competing risk. Deaths or infections that happened after the 12-month threshold (i.e., 12 months or more elapsed from the day treatment started) were not considered. Time to first infection was considered for three patients with two IFIs.
For the lifetime cost-effectiveness analysis, the primary benefit outcome of interest was average expected life-years for the study population or for either of the two subpopulations for each prophylaxis group. Life-years were estimated for each patient in the trial for both survivors and non-survivors of the 1-year prophylaxis period. Life-years for the non-survivors were estimated using the time to death during the trial. Life expectancy for each 1-year survivor was estimated as the product of (1) the remaining life expectancy for these patients determined using the general population annual mortality rates from the 2004 United States Life Tables11 by their sex, nationality, ethnicity, and age at the time of entry into the clinical trial and (2) the annual relative risk of mortality post HCT based on their underlying disease.12 Relative risk data from Wingard and colleagues12 did not include relative risk of mortality compared with general population mortality rates between 1 and 2 years and after 15 years post HCT. For the analysis, it was assumed that the relative risk of mortality between 1 and 2 years post HCT was equal to the relative risk from 2 to 3 years post HCT for each underlying disease. The relative risk after year 15 was assumed to remain constant and was calculated by taking the average of the relative risks of mortality from years 10 through 15 for each underlying disease. Data on chronic myelogenous leukemia were not presented by Wingard and colleagues12; as a proxy, the relative risks of mortality for the severe aplastic anemia disease group were used. Life-years were discounted at a rate of 3% annually.
Drug and IFI Treatment Costs
The economic evaluation of the BMT CTN 0101 trial data was conducted from the perspective of a health care provider and included direct health care costs only. The resources or events used for costing included planned prophylaxis; supplemental prophylaxis therapy; empirical antifungal therapy; and treatment of presumptive, probable, and proven IFIs. Resources used or events (i.e., supplemental prophylaxis or empirical therapy and IFIs) were classified as occurring during the 6-month period if the resource use or event started less than 6 months from the planned prophylaxis start date. Similarly, a resource use or event was classified as occurring during the 12-month period if the resource use or event started within 12 months of the planned prophylaxis start date. Fungal-infection-related resources used or events that started after 12 months were not collected in the clinical trial and therefore were not included in the cost-effectiveness analyses. The IFIs were classified into three groups: Aspergillus, Candida, and others.5 For the patients with two or more IFIs, all infections were considered for costing purposes.
All costs are presented in 2011 United States (US) dollars and are shown in Table 2.
Table 2. Unit Costs for Treatment of Fungal Infections and Antifungal Drugs.
| Cost Variable | Cost in 2011 US$ |
|---|---|
| Treatment of presumptive, probable, and proven IFIs | |
| Invasive Aspergillusa | $79,358b |
| Invasive Candidaa | $29,419b |
| Other IFIa | $15,001b |
| Alternative value for treatment costsc | $48,011d |
| Planned prophylaxise | |
| Voriconazole (oral) | |
| Aged <12 years branded (generic) | $67.31 ($36.72)/dayf |
| Aged ≥12 years branded (generic) | $134.63 ($73.44)/dayf |
| Fluconazole (oral) | |
| Aged < 12 years | $7.11/dayf |
| Aged ≥12 years | $16.11/dayf |
| Empirical antifungal therapyh | |
| Amphotericin B—lipid formulation (IV) | $761.79/dayf |
| Voriconazole (IV) | $149.54/dayf |
| Itraconazole (IV) | $7.71/dayf |
| Caspofungin (IV) | $302.01/dayf |
| Fluconazole (IV) | $26.65/dayf |
| Micafungin (IV) | $93.50/dayf |
| Supplemental Prophylaxisg | |
| Amphotericin B—lipid formulation (IV) | $756.19/dayf |
| Voriconazole (oral) branded (generic) | $128.59 ($70.14)/dayf |
| Itraconazole (oral) | $20.89/dayf |
| Caspofungin (IV) | $307.24/dayf |
| Fluconazole (oral) | $15.59/dayf |
| Micafungin (IV) | $93.50/dayf |
IFI = invasive fungal infection; IV = intravenous.
Costs include incremental hospitalization costs for patients with IFI compared with those without IFI with the same underlying diagnosis.
Data source: Collins et al., 2008.13
A single average cost was assumed for all IFI types for the alternative IFI treatment cost scenario based on the value used in the O'Sullivan and colleagues14 cost-effectiveness analysis.
Data source: O'Sullivan et al., 2009.14
Dosing for planned prophylaxis was taken from the BMT CTN 0101 clinical trial protocol: 400 mg/day for those aged ≥ 12 years; 200 mg/day for those aged < 12 years and weighing ≥ 20 kg; 100 mg/day for those aged < 12 years and weighing < 20 kg.
Data source: Red Book online, 2011.15
Empirical therapy was assumed to use an IV formulation and supplemental prophylaxis to use an oral formulation where possible. Dosing was based on the BMT CNT 0101 clinical trial protocol for voriconazole and fluconazole and on prescribing information for the other antifungal drugs used during the clinical trial and weighted based on the age distribution.
The costs of proven or probable Aspergillus, Candida, and other IFIs were taken from the Wilson and colleagues16 and Collins and colleagues13 studies and inflated to 2011 dollars using the medical price component of the Consumer Price Index. The Wilson and colleagues16 study used the Maryland Hospital Discharge Data Set and a case-control estimation method to estimate the incremental costs attributable to different types of IFI. Collins and colleagues13 used the Wilson costs inflated to 2006 US dollars in their cost-effectiveness analysis. In the current study, the cost of presumptive IFIs was estimated as a weighted average of the cost of Aspergillus, Candida, and other IFIs, with weights derived from the relative frequency of the different types of probable or proven infections for each treatment arm.
The cost of the planned prophylaxis regimen for each patient was estimated as the duration of the regimen (in days) times the cost of voriconazole or fluconazole per day according to age group (i.e., < 12 years, and ≥ 12 years). The cost of supplemental prophylaxis or empirical therapy was estimated as the duration of therapy (in days) times the cost of therapy per day based on age group. The duration of supplemental prophylaxis or empirical therapy was determined as the minimum of the end date of the therapy, date of fungal infection, or 12‐month date minus start date of therapy plus 1. In two cases, end date of fluconazole as supplemental prophylaxis therapy was missing. In these cases, the duration was imputed as the average duration of fluconazole used as supplemental prophylaxis therapy.
The dosing and unit costs assumed for each antifungal drug used by each trial participant were derived as follows. For planned prophylaxis, the dosing was assumed to be that designated in the protocol, with fixed dosing for those over 12 years of age and weight-based dosing for those 12 years of age and under. US average weight was used for those under 5 years and those over 5 years.17 The cost per milligram for the base-case analysis was estimated from the branded price for oral voriconazole and an average generic price for fluconazole using the Wholesale Acquisition Cost from Red Book Online.15 In a sensitivity analysis, the generic price for oral voriconazole was estimated using Red Book Online.15 Costs per milligram were then converted into daily costs using the weighted average dose for the two treatment groups. Similar methods were used to estimate the daily costs for empirical therapy and supplemental prophylaxis from the trial data indicating which drugs were used for each patient receiving supplemental prophylaxis or empirical drug treatment. The trial data did not include dosing or formulation used for each drug. We assumed that empirical therapy would always be delivered using an intravenous (IV) formulation, whereas supplemental prophylaxis would be given orally unless the drug was not available in that formulation. We also assumed for both empirical therapy and supplemental prophylaxis that dosing would be based on the prescribing information or, for voriconazole and fluconazole, the same dose as was used for planned prophylaxis. In an alternative analysis, the generic price for oral voriconazole, from Red Book Online,15 was used for all patients who took oral therapy.
Statistical Analyses
The primary goal of the economic evaluation was to assess whether prophylaxis with voriconazole was cost-effective compared with prophylaxis with fluconazole based on the decision tree diagram shown in Figure 1. Incremental cost-effectiveness ratios (ICERs), defined as the difference in average costs for the group randomized to voriconazole compared with the average costs for the group randomized to fluconazole divided by the difference in average outcomes for the two groups, were estimated. Outcomes used for the ICER estimates included IFI avoided and life-year gained.
Mathematically, the ICER pertaining to this study is given by the following equation:
| (1) |
where ΔCVF is the difference in the average cost for the two prophylaxis groups (CV − CF) and ΔEVF is the difference in average effectiveness (EV − EF) between voriconazole (denoted V) and fluconazole (denoted F). Voriconazole would be seen as cost-effective (good value for money) if ICERVF < RC, where RC is the maximum willingness to pay for an additional unit of effectiveness.
Due to uncertainty determining RC and in the estimation of ICERVF, a probabilistic sensitivity analysis was conducted and presented in the form of cost-effectiveness acceptability curves (CEAC)18 (Figure 2). The CEAC allows identification of the probability—i.e., proportion of (ΔCVF, ΔEVF) pairs—for which voriconazole is cost-effective given the data, for any value of RC. This method of presentation of the uncertainty of the cost-effectiveness results is especially valuable when the differences between alternative treatments in costs or efficacy are small, as is the case with the efficacy differences from this study.
Figure 2.
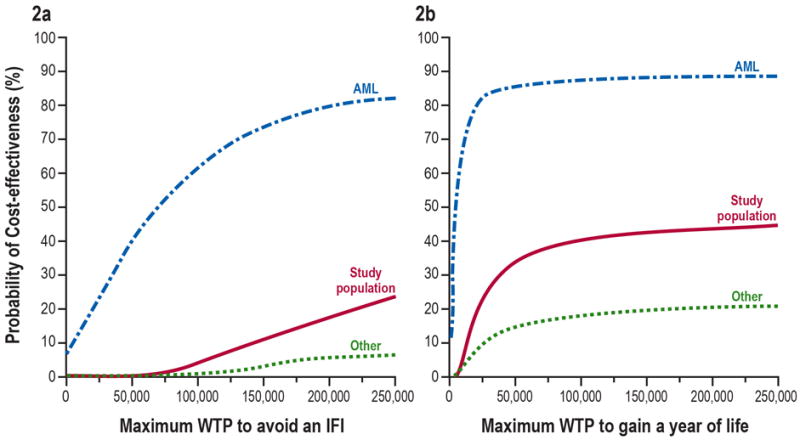
The primary economic analysis generated results for the study population and the two subpopulations in the form of CEACs using, NBCVF the net benefit associated with voriconazole treatment, expressed in units of money. The NBCVF is derived from equation (1) as follows:
| (2) |
Equation (2) states that voriconazole is cost-effective if net benefits are positive. A bootstrap method, with 1,000 replications, was used to produce the CEACs in each population and for each efficacy measure. In each bootstrap replication, we selected (with replacement) two random samples i of size niV and niF of cost and effect outcomes pairs, one from clinical trial enrollees in the voriconazole group and one from clinical trial enrollees in the fluconazole group, where niV and niF are the original sample sizes of each treatment group in the population of interest. We used the bootstrap resample for each treatment arm to calculate the mean costs and 95% confidence intervals (CiV and CiF) (i.e., prophylaxis, empirical treatment, and IFI costs) and efficacy values (EiV and EiF (i.e., incidence of IFI and overall survival). The bootstrap 95% CIs were used instead of the parametric confidence intervals due to skewness of cost distributions. The means for each treatment arm were then used to calculate (1) the bootstrapped ICERiVF and (2) the bootstrapped net benefit statistic, NBjiCVF, for a range of values for RCj (maximum willingness to pay for an additional unit of effectiveness): j = $50………$250,000 by $50 increments. Finally, for each value of RCj, we calculated the proportion of times that NBjiCVF > 0. These proportions were plotted against their corresponding values of RCj to produce the cost-effectiveness acceptability curve.
Support for this study was provided by grant #U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute.
Results
Table 3 presents the mean fungal-infection-related costs and the two measures of benefit, IFI avoided in the 12-month period after the allogeneic HCT and life-years gained, for each treatment group for the total trial population and the AML subgroup. Table 3 also presents the differences in mean fungal-infection-related costs and benefits between voriconazole and fluconazole. Mean total fungal-infection-related costs for the voriconazole prophylaxis arm were significantly higher than for the fluconazole arm at 12 months for the complete study population (i.e., the 95% CI did not contain zero) but were not significantly different for the AML subpopulation. At 12 months, costs other than those for planned prophylaxis were similar between the two treatment groups for the study population ($11,775 for voriconazole versus $11,727 for fluconazole) but were lower for voriconazole ($12,777) than fluconazole ($16,368) in the AML subpopulation.
Table 3. Cost-effectiveness of Voriconazole Versus Fluconazole in Patients With Allogeneic Hematopoietic Cell Transplants, 12-Month Outcomes.
| Total Study Population | AML Subpopulation | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Outcome | Voriconazole | Fluconazole | Difference | Voriconazole | Fluconazole | Difference |
| Clinical outcomes | ||||||
| IFI events | 0.127 | 0.137 | -0.011 | 0.145 | 0.228 | -0.083 |
| Life-years | 8.219 | 8.269 | -0.05 | 7.911 | 6.891 | 1.020 |
| Costsa | ||||||
| Planned prophylaxis | $9,774 | $1,104 | $8,670b | $10,142 | $990 | $9,152b |
| Other prophylaxis | $3,162 | $1,165 | $1,997 | $2,809 | $897 | $1,911 |
| Empirical therapy | $1,744 | $2,284 | -$540 | $1,805 | $2,400 | -$595 |
| IFI treatment | $6,870 | $8,278 | -$1,408 | $8,164 | $13,070 | -$4,906 |
| Total | $21,549 | $12,831 | $8,718b | $22,919 | $17,358 | $5,562 |
| Incremental cost-effectiveness ratios | ||||||
| Incremental cost per IFI avoided | $812,990 | $66,919 | ||||
| Incremental cost per life-year gained | Dominatedc | $5,453 | ||||
AML = acute myeloid leukemia; IFI = invasive fungal infection.
Costs in 2011 US$.
95% confidence limits estimated using the bootstrap analysis do not include zero.
Dominated means that treatment with voriconazole was both more expensive and resulted in fewer estimated life years than treatment with fluconazole.
Table 4 presents the cost-effectiveness estimates for cost per IFI avoided and cost per life-year gained for the base case 6- and 12-month data and several alternative scenarios. In the 12-month base case, the cost per IFI avoided is lower for the AML subgroup ($66,919) than for the complete study population ($812,990). Similarly, the cost per life-year gained is favorable for the AML subpopulation but unfavorable for the complete study population. The ICERs for the 12-month time period comparing oral voriconazole at the generic price with voriconazole at the branded price were more favorable for generic voriconazole (e.g., $9,683 versus $66,919 per IFI avoided and $789 versus $5,453 per life-year gained for the AML subpopulation). In Table 4, the cost-effectiveness ratios (per IFI avoided) for the 6-month base case are more favorable for voriconazole than those for 12-month data (e.g., $34,576 versus $66,919 per IFI avoided for the AML subpopulation), because of the greater reduction in IFIs with voriconazole seen at 6 months than at 12 months.
Table 4. Univariate Sensitivity Analysis for Cost-effectiveness of Voriconazole Versus Fluconazole in Patients With Allogeneic Hematopoietic Cell Transplantsa.
| Cost per IFI Avoided | Cost per Life-year Gained | |||||
|---|---|---|---|---|---|---|
|
|
||||||
| Scenario | Total Study | AML | Other Diagnosisb | Total Study | AML | Other Diagnosisb |
| 12 Months | ||||||
| Base case | $812,990 | $66,919 | Dominatedc | Dominatedc | $5,453 | Dominatedc |
| Generic oral voriconazole | $384,099 | $9,683 | Dominatedc | Dominatedc | $789 | Dominatedc |
| Alternative IFI treatment costs | $881,219 | $71,210 | Dominatedc | Dominatedc | $5,802 | Dominatedc |
| 6 Months | ||||||
| Base case | $196,622 | $34,576 | $2,872,064 | n/a | n/a | n/a |
| Generic oral voriconazole | $78,629 | Dominantd | $1,498,005 | n/a | n/a | n/a |
| Alternative IFI treatment costs | $210,202 | $44,503 | $2,922,074 | n/a | n/a | n/a |
AML = acute myeloid leukemia; IFI = invasive fungal infection.
Costs in 2011 US$.
“Other diagnosis” underlying diseases subpopulation.
Dominated means that treatment with voriconazole was both more expensive and resulted in either fewer fungal infections avoided or fewer estimated life years than treatment with fluconazole.
Dominant means that treatment with voriconazole was both less expensive and resulted in more fungal infections avoided.
The uncertainty associated with these estimated ICERs is presented using CEACs in Figure 2 using the 12-month trial outcomes for the three populations examined (study population, AML subpopulation, and “other diagnosis” underlying diseases subpopulation). For every threshold value, the probability of voriconazole being cost-effective compared with fluconazole is much higher in the AML subpopulation than in the complete study population and in the “other diagnosis” subpopulation. For example, the probability of voriconazole being cost-effective (per additional IFI) compared with fluconazole at a maximum willingness to pay value of $50,000 for each additional IFI avoided is 41% in the AML subpopulation, compared with 0.4% in the complete study population and 0.1% in the “other diagnosis” subpopulation. Similarly, the probability of voriconazole being cost-effective (per life-year gained) compared with fluconazole at a maximum willingness to pay value of $50,000 for an extra year of life is 85% in the AML subpopulation, compared with 33% in the complete study population and 15% in the “other diagnosis” subpopulation.
Discussion
In this analysis, the fungal-infection-related costs and the cost-effectiveness of voriconazole compared with fluconazole have been estimated using data from the BMT CTN 0101 clinical trial in patients undergoing allogeneic HCT and published costs for antifungal drugs and for the treatment of IFIs. The analysis showed that at 12 months, mean total fungal-infection-related costs for the voriconazole prophylaxis arm were significantly higher than for the fluconazole prophylaxis arm for the complete study population and for the “other diagnosis” underlying diseases subpopulation, but were not significantly different for the AML subpopulation. Because of these differences, the cost per IFI avoided and the cost per life-year gained were also more favorable for the AML subgroup than for the complete trial population or the “other diagnosis” subpopulation. The cost per IFI avoided was more favorable at 6-months than at 12-months because of the smaller difference in IFIs after 12 months. The cost-effectiveness ratios were all more favorable for voriconazole when the generic price for oral voriconazole was substituted for the branded price. The results of the bootstrap analyses, which reflect the uncertainty in the clinical trial results, showed that the probability of voriconazole being cost-effective is greater than 41% per additional IFI or greater than 85% per life-year gained at thresholds of $50,000 for the AML subpopulation.
The improved cost-effectiveness of voriconazole in the AML subpopulation over that of the total population may be related to the greater risk for developing IFIs in patients with AML. The Cox proportional hazards model conducted in the BMT CTN 0101 study found that the AML subpopulation had significantly greater risk for death and IFI, as well as greater efficacy of voriconazole. As our cost analysis reported in this paper demonstrated that voriconazole may be more cost-effective for the AML population than for the general population, one could consider that fluconazole may be more appropriate in the non-AML HCT population with ALL, chronic myelogenous leukemia, myelodysplastic syndrome, and non-Hodgkin lymphoma, where risk of IFI and IFI-related mortality is lower.
In order to allow for health plans or hospitals to use the results of this analysis in situations where the distribution of patients among the underlying diseases or the costs of antifungal drugs or treatment of IFI differ from those assumed in the analysis, an Excel model is available at the BMT CTN website (www.bmtctn.net). For example, wholesale acquisition costs were assumed for the drugs used for empirical therapy. If health plans or hospitals are able to obtain discounted prices for these drugs, the offsetting cost savings with voriconazole because of less need for empirical therapy will be reduced. The impact of such price discounts can be estimated using the Excel model.
Several economic evaluations of antifungal prophylaxis in patients with hematological malignancies have been performed for posaconazole or micafungin. Six economic evaluations of posaconazole were performed using data from a clinical trial of prophylaxis up to 84 days in patients receiving intense chemotherapy for hematological malignancies with clinical endpoints determined at 100 days compared with itraconazole or fluconazole.13,19,20,21,22,23 The results of these studies all showed that posaconazole was cost saving and was associated with longer life expectancy than standard therapy with either itraconazole or fluconazole. A cost-effectiveness analysis comparing voriconazole with posaconazole prophylaxis, in those undergoing induction with chemotherapy for AML using observational data of a switch from use of voriconazole to posaconazole in a single hospital, showed cost saving and greater therapeutic success with posaconazole.24 An economic evaluation for posaconazole compared with fluconazole or itraconazole using data from a clinical trial of 112 days of prophylaxis in patients with graft-versus-host disease following allogeneic HCT for AML or myelodysplastic syndrome showed an increased cost and life expectancy with posaconazole resulting in an estimated cost per life-year gained of $15,300.25 Finally, two cost-effectiveness analyses of micafungin prophylaxis were performed using data from a clinical trial of patients undergoing HCT, 50% of whom received autologous transplants. In the trial, prophylaxis was continued up to 42 days post transplant and outcomes were assessed at 4 weeks after stopping prophylaxis. In both studies, micafungin prophylaxis was associated with lower total costs and fewer IFIs than fluconazole prophylaxis.26,27
When the time horizon for the analysis presented in this article was shortened to 6 months, which is similar to the time horizons used in most previously published cost-effectiveness analyses for antifungal prophylaxis with posaconazole, micafungin, or voriconazole in patients with hematological malignancies,13,19,20,21,22,23,24,26,27 the cost-effectiveness ratios were more favorable for voriconazole because the observed reduction in IFIs was greater between zero and 6 months than between zero and 12 months. In particular, for the AML subpopulation, the cost per IFI avoided was $34,576 at 6 months compared with $66,919 at 12 months. Whether the same attenuation of the benefit of antifungal prophylaxis would have been observed in the other clinical trials had they had a longer follow-up time period is unknown. However, if such attenuation had occurred, there would have been a similar unfavorable impact on the cost-effectiveness ratios based on those clinical trials.
No clinical trial or observational data are currently available to assess the cost-effectiveness of voriconazole compared with posaconazole in an allogeneic HCT population similar to that included in the BMT CTN 0101 clinical trial. No head-to-head studies of the two drugs for prophylaxis in this population have been completed. A prospective observational study of 106 patients undergoing allogeneic HCT has been completed. This study estimated a rate of breakthrough IFIs at 6 months for those given posaconazole prophylaxis (7.5%) that was similar to the rate observed in the BMT CTN 0101 clinical trial for voriconazole (7.3%).28 However, in the posaconazole observational study, only 7% of the study population had AML as the underlying disease, whereas in the BMT CTN 0101 clinical trial, 44% of the voriconazole prophylaxis arm had AML as the underlying disease. Thus, the available data are not sufficient to determine the comparative effectiveness and cost-effectiveness of prophylaxis with voriconazole and posaconazole in those undergoing allogeneic HCT.
The decision analytic model used to derive the cost-effectiveness estimates had several limitations. The population included in the model base-case analyses was assumed to be similar to that included in the BMT CTN 0101 clinical trial. Secondly, the costs of adverse events associated with either voriconazole or fluconazole prophylaxis were not estimated because there were no significant differences in these adverse events noted during the clinical trial.5 Costs for treating IFIs were taken from estimates for all transplant patients rather than limited to those with HCT since data for the latter were not available. An assumption was also made for the lifetime analysis of cost per life-year gained that no additional costs related to IFIs following the allogeneic HCT were incurred after the 12-month trial time period. It is also possible that the increased costs with voriconazole compared with fluconazole may be overestimated since we did not incorporate costs of adjunctive tests performed as part of a pre-emptive treatment strategy. For example, the voriconazole costs would be overestimated if galactomannan testing is routinely used for those receiving fluconazole prophylaxis but not for those receiving voriconazole prophylaxis. Since galactomannan testing was performed routinely in both prophylaxis arms in the BMT CTN 0101 clinical trial, the impact on costs and outcomes of differential use of galactomannan testing between the prophylaxis groups could not be assessed. In addition, there may be utility in galactomannan screening in practice in those taking voriconazole because, although the risk of aspergillosis is lower when taking voriconazole, it does not disappear. This may be due in part to the known inter-patient variability of blood levels of voriconazole.
Finally, the data for estimating expected life-years for the 12-month survivors by age and underlying disease were incomplete; thus, assumptions were made based on available data. In particular, the relative risk of mortality compared with the general population mortality in the second year after transplant was assumed to be the same as that in the third year after transplant. However, since this possible underestimate of mortality in the second year is applied to both prophylaxis groups and since the difference in survival rates at 12 months was small, the impact of this assumption on the difference in life expectancy between the two groups should be small.
Conclusion
The results of this study indicate that voriconazole prophylaxis may be a cost-effective choice at a cost per life-year gained threshold of $50,000 compared with fluconazole prophylaxis in those undergoing an allogeneic HCT for AML. For other underlying diseases, the relative efficacy of voriconazole compared with fluconazole on the incidence of IFIs was very close to one and, therefore, the cost-effectiveness of voriconazole compared with fluconazole is likely to be unfavorable at branded prices. The availability of generic oral voriconazole will have a favorable impact on the cost-effectiveness of voriconazole compared with fluconazole.
Supplementary Material
Contributor Information
Josephine Mauskopf, RTI Health Solutions.
Costel Chirila, RTI Health Solutions.
Jon Graham, RTI Health Solutions.
Iris Gersten, The EMMES Corporation.
Richard T. Maziarz, Knight Cancer Institute, Oregon Health and Science University.
Lindsey Baden, Brigham and Women's Hospital/Dana-Farber Cancer Institute.
Javier Bolaños-Meade, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.
Janice M. Y. Brown, Stanford University School of Medicine.
Thomas Walsh, Weill Cornell Medical Center of Cornell University.
Mary M. Horowitz, Medical College of Wisconsin.
Joanne Kurtzberg, Duke University.
Kieren A. Marr, Johns Hopkins University School of Medicine.
John R. Wingard, University of Florida College of Medicine.
Helen Leather, University of Florida College of Medicine.
References
- 1.McCoy D, Depestel DD, Carver PL. Primary antifungal prophylaxis in adult hematopoietic stem cell transplant recipients: current therapeutic concepts. Pharmacotherapy. 2009;29(11):1306–25. doi: 10.1592/phco.29.11.1306. [DOI] [PubMed] [Google Scholar]
- 2.Freifeld AG, Bow EJ, Sepkowitz KA, et al. Clinical practice guidelines for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2011;52(4):427–431. e56–93. doi: 10.1093/cid/ciq147. [DOI] [PubMed] [Google Scholar]
- 3.Marr KA, Seidel K, Slavin MA, et al. Prolonged fluconazole prophylaxis is associated with persistent protection against candidiasis-related death in allogeneic marrow transplant recipients: long-term follow-up of a randomized placebo-controlled trial. Blood. 2000;96(6):2055–61. [PubMed] [Google Scholar]
- 4.Slavin MA, Osborne B, Adams R, et al. Efficacy and safety of fluconazole prophylaxis for fungal infections after marrow transplantation—a prospective, randomized, double-blind study. J Infect Dis. 1995;171(6):1545–52. doi: 10.1093/infdis/171.6.1545. [DOI] [PubMed] [Google Scholar]
- 5.Wingard JR, Carter SL, Walsh TJ, et al. Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116(24):5111–8. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pechlivanoglou P, De Vries R, Daenen SM, et al. Cost benefit and cost effectiveness of antifungal prophylaxis in immunocompromised patients treated for haematological malignancies: reviewing the available evidence. Pharmacoeconomics. 2011;29(9):737–51. doi: 10.2165/11588370-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Lyseng-Williamson KA. Posaconazole: a pharmacoeconomic review of its use in the prophylaxis of invasive fungal disease in immunocompromised hosts. Pharmacoeconomics. 2011;29(3):251–68. doi: 10.2165/11206800-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Ullmann AJ, Lipton JH, Vesole DH, et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N Engl J Med. 2007;356(4):335–47. doi: 10.1056/NEJMoa061098. Erratum in: N Engl J Med. 2007; 357(4):428. [DOI] [PubMed] [Google Scholar]
- 9.Cornely OA, Maertens J, Winston DJ, et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N Engl J Med. 2007;356(4):348–59. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 10.van Burik JA, Ratanatharathorn V, Stepan DE, et al. National Institute of Allergy and Infectious Diseases Mycoses Study Group. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39(10):1407–16. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 11.Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56(9):1–39. [PubMed] [Google Scholar]
- 12.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–9. doi: 10.1200/JCO.2010.33.7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins CD, Ellis JJ, Kaul DR. Comparative cost-effectiveness of posaconazole versus fluconazole or itraconazole prophylaxis in patients with prolonged neutropenia. Am J Health Syst Pharm. 2008;65(23):2237–43. doi: 10.2146/ajhp070588. [DOI] [PubMed] [Google Scholar]
- 14.O'Sullivan AK, Pandya A, Papadopoulos G, et al. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health. 2009;12(5):666–73. doi: 10.1111/j.1524-4733.2008.00486.x. [DOI] [PubMed] [Google Scholar]
- 15.Red Book Online, accessed through Micromedex 2.0. Thomson Reuters; 2011. [Google Scholar]
- 16.Wilson LS, Reyes CM, Stolpman M, et al. The direct cost and incidence of systemic fungal infections. Value Health. 2002;5(1):26–34. doi: 10.1046/j.1524-4733.2002.51108.x. [DOI] [PubMed] [Google Scholar]
- 17.McDowell MA, Fryar CD, Ogden CL, et al. National Health Statistics Reports. Number 10. Hyattsville, MD: National Center for Health Statistics; 2008. [accessed 2011 Dec 21]. Anthropometric reference data for children and adults: United States, 2003–2006. http://www.cdc.gov/nchs/data/nhsr/nhsr010.pdf. [Google Scholar]
- 18.Briggs A, Fenn P. Confidence intervals or surfaces? Uncertainty on the cost-effectiveness plane. Health Econ. 1998;7(8):723–40. doi: 10.1002/(sici)1099-1050(199812)7:8<723::aid-hec392>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 19.Greiner RA, Meier Y, Papadopoulos G, et al. Cost-effectiveness of posaconazole compared with standard azole therapy for prevention of invasive fungal infections in patients at high risk in Switzerland. Oncology. 2010;78(3-4):172–80. doi: 10.1159/000313696. [DOI] [PubMed] [Google Scholar]
- 20.O'Sullivan AK, Pandya A, Papadopoulos G, et al. Cost-effectiveness of posaconazole versus fluconazole or itraconazole in the prevention of invasive fungal infections among neutropenic patients in the United States. Value Health. 2009;12(5):666–73. doi: 10.1111/j.1524-4733.2008.00486.x. [DOI] [PubMed] [Google Scholar]
- 21.Dranitsaris G, Khoury H. Posaconazole versus fluconazole or itraconazole for prevention of invasive fungal infections in patients undergoing intensive cytotoxic therapy for acute myeloid leukemia or myelodysplasia: a cost effectiveness analysis. Support Care Cancer. 2011;19(11):1807–13. doi: 10.1007/s00520-010-1022-7. [DOI] [PubMed] [Google Scholar]
- 22.Stam WB, O'Sullivan AK, Rijnders B, et al. Economic evaluation of posaconazole vs. standard azole prophylaxis in high risk neutropenic patients in the Netherlands. Eur J Haematol. 2008;81(6):467–74. doi: 10.1111/j.1600-0609.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 23.Rely K, Alexandre PK, Escudero GS. Cost effectiveness of posaconazole versus fluconazole/itraconazole in the prophylactic treatment of invasive fungal infections in Mexico. Value Health. 2011;14(5 Suppl 1):S39–42. doi: 10.1016/j.jval.2011.05.032. Spanish. [DOI] [PubMed] [Google Scholar]
- 24.Al-Badriyeh D, Slavin M, Liew D, et al. Pharmacoeconomic evaluation of voriconazole versus posaconazole for antifungal prophylaxis in acute myeloid leukaemia. J Antimicrob Chemother. 2010;65(5):1052–61. doi: 10.1093/jac/dkq076. [DOI] [PubMed] [Google Scholar]
- 25.O'Sullivan AK, Weinstein MC, Pandya A, et al. Cost-effectiveness of posaconazole versus fluconazole for prevention of invasive fungal infections in U.S. patients with graft-versus-host disease. Am J Health Syst Pharm. 2012;69(2):149–56. doi: 10.2146/ajhp110149. [DOI] [PubMed] [Google Scholar]
- 26.Schonfeld W, Wang Cheng J, Tong KB, et al. Cost-effectiveness analysis of antifungal prophylaxis in patients undergoing hematopoietic stem cell transplantation. Clin Ther. 2008;30(5):964–73. doi: 10.1016/j.clinthera.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 27.Sohn HS, Lee TJ, Kim J, et al. Cost-effectiveness analysis of micafungin versus fluconazole for prophylaxis of invasive fungal infections in patients undergoing hematopoietic stem cell transplantation in Korea. Clin Ther. 2009;31(5):1105–15. doi: 10.1016/j.clinthera.2009.05.011. discussion 1066-8. [DOI] [PubMed] [Google Scholar]
- 28.Winston DJ, Bartoni K, Territo MC, et al. Efficacy, safety, and breakthrough infections associated with standard long-term posaconazole antifungal prophylaxis in allogeneic stem cell transplantation recipients. Biol Blood Marrow Transplant. 2011 Apr;17(4):507–15. doi: 10.1016/j.bbmt.2010.04.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


