Abstract
Aim
To investigate the link between serum vaspin levels and physical activity and/or physical fitness in Japanese.
Methods
A total of 156 subjects (81 men and 75 women) was enrolled in this cross-sectional study. Serum vaspin levels, physical activity by uniaxial accelerometers, peak oxygen uptake, and metabolic risk parameters were evaluated. We also assessed anthropometric and body composition parameters.
Results
Serum vaspin levels were over the level of 10 ng/mL in 15 subjects (9.6 %: Vaspin High group). In Vaspin Low group (<5 ng/mL: 74 men and 67 women), serum vaspin levels were 0.12 ± 0.18 ng/mL in men and 0.39 ± 0.70 ng/mL in women. Peak oxygen uptake was significantly and positively correlated with serum vaspin levels even after adjusting for age, physical activity evaluated by Σ[metabolic equivalents × h per week (METs⋅h/w)], BMI, and other confounding factors in men. In turn, physical activity was significantly and positively correlated with serum vaspin levels even after adjusting for confounding factors in women.
Conclusion
Serum vaspin levels were closely associated with physical fitness in men and physical activity in women independent of body composition in this Japanese cohort.
Keywords: Vaspin, Japanese, Physical activity, Peak oxygen uptake, Body composition
Introduction
Visceral adipose tissue-derived serine proteinase inhibitor (vaspin) was identified as an adipokine, which is predominantly secreted from visceral adipose tissue in a rat model of type 2 diabetes (Otsuka Long-Evans Tokushima Fatty rats: OLETF rats) [1]. The mRNA expression of vaspin is associated with the degree of obesity and insulin resistance in OLETF rats. The injection of recombinant human vaspin into obese (diet-induced) mice ameliorates insulin resistance, and it may be a compensatory factor in the status of obesity [2]. In obese adults [3–5] and children [6, 7], serum vaspin levels increase with the degree of obesity and decrease with weight reduction by lifestyle modification or surgery. In addition, low vaspin levels were found to be in chronic hemodialysis patients [8] and also in patients who recently experienced ischemic events [9]. Recently, Teshigawara et al. [10] measured serum vaspin levels by a wide-range RIA system, and they found that approximately 7 % of the population demonstrated more than approximately a 10-fold higher concentration of serum vaspin levels compared with the rest of the population. They discovered that a minor allele of rs77060950 in the SERPINA12 gene was significantly associated with higher serum levels of vaspin in Japanese.
It is well known that regular physical activity reduces resting blood pressure, fasting blood glucose, triglycerides, abdominal fat accumulation, and insulin responses to an oral glucose challenge test, and increases high density (HDL) cholesterol [11–14]. Sawada et al. [15] reported that low cardiorespiratory fitness was closely associated with cancer mortality in Japanese men. Sandvik et al. [16] also showed that physical fitness was a graded, independent, long-term predictor of mortality from cardiovascular causes in healthy, middle-aged men. Taken together, physical activity and/or physical fitness may modify serum vaspin levels.
However, the link between serum vaspin levels and physical activity and/or physical fitness independent of body composition in a Japanese population is not fully discussed. In addition, whether increases in physical activity and/or physical fitness are beneficial for serum vaspin levels, and what effects this has on serum vaspin levels, remains to be investigated in a longitudinal study. Therefore, in this pilot cross-sectional study, we evaluated the relationship between serum vaspin levels and physical activity and/or physical fitness in apparently healthy Japanese patients.
Methods
Subjects
We enrolled 156 subjects (81 men and 75 women) who met the following criteria: (1) wanted to volunteer in this cross-sectional, investigative study at Okayama Southern Institute of Health, Okayama Health Foundation, Okayama, Japan; (2) had received anthropometric, physical activity, peak oxygen uptake, blood pressure (BP) measurements, and blood examinations including serum vaspin levels; (3) received no medications for diabetes, hypertension, and/or dyslipidemia; and (4) provided written informed consent (Table 1).
Table 1.
Clinical characteristics of Vaspin Low group subjects
| Men | Women | |
|---|---|---|
| Number of subjects | 74 | 67 |
| Age (year) | 45.4 ± 17.6 | 43.2 ± 18.0 |
| Height (cm) | 170.9 ± 6.0 | 158.2 ± 6.6 |
| Body weight (kg) | 66.6 ± 9.7 | 52.5 ± 7.3 |
| Body mass index (kg/m2) | 22.8 ± 3.0 | 21.0 ± 3.0 |
| Abdominal circumference (cm) | 80.8 ± 8.0 | 76.2 ± 9.5 |
| Body fat percentage (%) | 19.4 ± 6.0 | 27.4 ± 6.1 |
| Peak oxygen uptake (mL/kg/min) | 37.2 ± 9.3 | 30.5 ± 7.9 |
| Physical activity (METs⋅h/w) | 13.2 ± 8.5 | 13.6 ± 10.5 |
| Systolic blood pressure (mmHg) | 133.2 ± 15.7 | 122.8 ± 16.8 |
| Diastolic blood pressure (mmHg) | 82.9 ± 12.0 | 75.0 ± 11.9 |
| Blood profile | ||
| Vaspin (ng/mL) | 0.12 ± 0.18 | 0.39 ± 0.70 |
| Triglyceride (mg/dL) | 98.4 ± 59.2 | 72.5 ± 48.4 |
| HDL cholesterol (mg/dL) | 55.4 ± 13.9 | 67.4 ± 14.1 |
| Blood glucose (mg/dL) | 93.1 ± 9.6 | 88.4 ± 9.0 |
| Number of subjects with smoking habits (%) | 33 (44.6 %) | 4 (6.0 %) |
Mean ± SD
METs⋅h/w: Σ [metabolic equivalents × h per week (METs⋅h/w)]
Ethical approval for the study was obtained from the Ethical Committee of Okayama Health Foundation, Okayama, Japan.
Blood sampling and assays
After the subjects fasted and rested overnight for 10–12 h, blood samples were collected in order to determine the serum levels of vaspin, high density lipoprotein (HDL) cholesterol, triglycerides (L Type Wako Triglyceride H, Wako Chemical, Osaka, Japan), and blood glucose. Serum vaspin levels were measured using a commercially available enzyme-linked immunosorbent assay (Adipogen, Seoul, South Korea). Coefficients of variance for intra- and inter-assays were 1.3–3.8 % and 3–9 %, respectively. When serum vaspin levels were under the measurement limit (<0.016 ng/mL), 0 ng/mL was applied and used for analysis. Blood glucose was measured by the glucose-oxidant method. Samples were frozen and stored (−80 °C) until analysis in the same assay.
Anthropometric and body composition measurements
Anthropometric and body compositions were evaluated based on the following parameters: height, body weight, abdominal circumference, and body composition. The abdominal circumference was measured at the umbilicus in standing subjects after a normal exhalation [17]. Body mass index (BMI) was calculated by weight/[height]2 (kg/m2). The body fat percentage was measured by DEXA (QDR4500, Hologic Inc., Waltham, MA, USA), which is accepted as an accurate standard [18]. The DEXA measurement consisted of a whole body scan using an array beam [19]. The subjects removed all metal objects, and were positioned in the supine position with their hands placed on either side of the body and their legs held 10 cm apart according to the specifications of the manufacturer. All scans were analyzed according to the manufacturer’s instructions [20].
Physical activity
Physical activity was measured by the Kenz Lifecorder (LC; SUZUKEN Co Ltd, Nagoya, Japan), a recent addition to the growing number of uniaxial accelerometer options; it offers comparable instrument outputs, with several potentially attractive features for researchers and practitioners. The LC displays reasonable estimates of physical activity intensity and energy expenditures under controlled conditions on a treadmill [21], over 24 h of typical daily activities undertaken in a respiratory chamber [21], and in a free-living environment using double-labeled water as the criterion method [22]. Furthermore, when compared with many other accelerometers, the LC can potentially simplify the data interpretation process by reducing the time spent and the need for advanced technical expertise or software programs [23]. The subjects were taught how to use the instrument, and were told to wear it on their belt or waist band at the right midline of the thigh from the moment they got up until they went to bed except while bathing or swimming, for seven consecutive days [24]. The activity monitor was firmly attached to their clothes at the waist by a clip.
Exercise testing
After blood sampling, peak oxygen uptake was measured using a maximal graded exercise test with bicycle ergometers (Excalibur V2.0, Lode BV, Groningen, Netherlands). The initial work load was 30–60 w, and the work rate was increased thereafter by 15 w/min until the subject could not maintain the required pedaling frequency (60 rpm) [25]. During the latter stages of the test, each subject was verbally encouraged by the test operators to give their maximal effort. In addition, an ECG was monitored continuously while recording the heart rate (HR). The expired gas was collected, and the rates of oxygen consumption (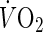 ) and carbon dioxide production (
) and carbon dioxide production (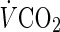 ) were measured breath-by-breath using a cardiopulmonary gas exchange system (Oxycon Alpha, Mijnhrdt B.V., The Netherlands). The achievement of peak oxygen uptake was accepted if the following two conditions were met: the subject’s maximal HR was >95 % of the age-predicted maximal HR (220 − age), and the
) were measured breath-by-breath using a cardiopulmonary gas exchange system (Oxycon Alpha, Mijnhrdt B.V., The Netherlands). The achievement of peak oxygen uptake was accepted if the following two conditions were met: the subject’s maximal HR was >95 % of the age-predicted maximal HR (220 − age), and the 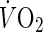 curve showed a leveling-off.
curve showed a leveling-off.
Blood pressure (BP) measurements at rest
Resting systolic and diastolic BP (SBP and DBP) were measured indirectly using a mercury sphygmomanometer placed on the right arm of the seated participant after at least 15 min of rest.
Cigarette smoking
The data on cigarette smoking were obtained through structured interviews conducted by public health nurses trained for this study. The subjects were asked if they currently smoked cigarettes. When the answer was “yes”, they were classified as current smokers. In the case of a “no” answer, they were classified as non-smokers. We could not classify those who used to smoke but had since stopped smoking.
Statistical analysis
All data are expressed as mean ± SD values. Pearson’s correlation coefficients were calculated and used to test the significance of the linear relationship between continuous parameters, where p < 0.05 was considered statistically significant. A multiple logistic was also performed to test the relationship between serum vaspin levels and physical activity, and between serum vaspin levels and peak oxygen uptake. The variance inflation factor (VIF) was used to assess multicollinearity.
Results
As with previous reports in Japanese [8, 10], serum vaspin levels were stratified into two subgroups. The measurement of serum vaspin levels revealed that the majority displayed vaspin levels less than 5 ng/mL (Vaspin Low group). A minor fraction (n = 15: 7 men and 8 women, 9.6 %) displayed much higher levels ranging from 23.12 to 149.37 ng/mL (Vaspin High group >10 ng/mL) as previously reported [10]. Subjects in Vaspin High group were reported to be genetically defined [10]. Therefore, we mainly analyzed in the Vaspin Low group.
The measurements of parameters in the Vaspin Low group were summarized in Table 1. Serum vaspin levels were 0.12 ± 0.18 ng/mL in men and 0.39 ± 0.70 ng/mL in women. Physical activity evaluated by the LC was 13.2 ± 8.5 METs⋅h/w in men and 13.6 ± 10.5 METs⋅h/w in women. Peak oxygen uptake was 37.2 ± 9.3 mL/kg/min in men and 30.5 ± 7.9 mL/kg/min in women (Table 1).
The simple correlation analysis between serum vaspin levels and clinical parameters was evaluated (Table 2). Serum vaspin levels were weakly and positively correlated with triglyceride in men (r = 0.254, p = 0.0292) and peak oxygen uptake in women (r = 0.261, p = 0.0331) (Table 2) in the Vaspin Low group. However, significant relationships between serum vaspin levels and other parameters were not noted in the Vaspin Low group or in total subjects.
Table 2.
Simple correlation analysis between serum vaspin levels and clinical parameters
| Men | Women | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Low group | Total | Low group | |||||
| r | p | r | p | r | p | r | p | |
| Age (year) | 0.099 | 0.3801 | −0.104 | 0.3763 | −0.017 | 0.8820 | −0.180 | 0.1452 |
| Height (cm) | −0.053 | 0.6353 | 0.162 | 0.1670 | 0.049 | 0.6751 | 0.202 | 0.1018 |
| Body weight (kg) | −0.064 | 0.5675 | 0.096 | 0.4149 | 0.046 | 0.6944 | 0.018 | 0.8872 |
| Body mass index (kg/m2) | −0.050 | 0.6560 | 0.008 | 0.9461 | 0.018 | 0.8810 | −0.096 | 0.4419 |
| Abdominal circumference (cm) | 0.056 | 0.6169 | −0.012 | 0.9167 | −0.030 | 0.7991 | −0.157 | 0.2053 |
| Body fat percentage (%) | 0.074 | 0.5098 | −0.055 | 0.6396 | −0.036 | 0.7570 | −0.196 | 0.1123 |
| Peak oxygen uptake (mL/kg/min) | −0.141 | 0.2080 | 0.093 | 0.4314 | 0.002 | 0.9833 | 0.261 | 0.0331 |
| Physical activity (METs⋅h/w) | −0.141 | 0.2085 | −0.187 | 0.1112 | 0.055 | 0.6395 | 0.222 | 0.0715 |
| Systolic blood pressure (mmHg) | −0.081 | 0.4697 | 0.192 | 0.1013 | −0.067 | 0.5658 | −0.067 | 0.5658 |
| Diastolic blood pressure (mmHg) | 0.011 | 0.9230 | 0.010 | 0.9336 | −0.059 | 0.6180 | −0.059 | 0.6180 |
| Blood profile | ||||||||
| Triglyceride (mg/dL) | 0.054 | 0.6333 | 0.254 | 0.0292 | 0.107 | 0.3629 | −0.081 | 0.5129 |
| HDL cholesterol (mg/dL) | 0.026 | 0.8159 | −0.057 | 0.6291 | −0.145 | 0.2157 | 0.029 | 0.8163 |
| Blood glucose (mg/dL) | −0.133 | 0.2362 | −0.079 | 0.5021 | −0.138 | 0.2380 | −0.117 | 0.3459 |
METs⋅h/w: Σ [metabolic equivalents × h per week (METs⋅h/w)]
Bold values are statistically significant (p < 0.05)
Table 3 showed the results of multiple logistic regression analysis of serum vaspin levels (under median, over median) according to quartiles of physical activity. The odds ratio of serum vaspin levels according to quartiles of physical activity was 0.318 in men and 4.000 in women (not adjusted), In women, the odds ratio of serum vaspin levels according to quartiles of physical activity was 13.190 (1.441–120.696) at a significant level even after adjusting for age, peak oxygen uptake, BMI, abdominal circumference, body fat percentage, and cigarette smoking designation (Table 3). VIF values for all variables indicated the absence of multicollinearity among the selected variables. However, significant associations between serum vaspin levels and physical activity were not observed in men.
Table 3.
Odds ratio of serum vaspin levels according to quartiles of physical activity in Vaspin Low group
| Quartiles of physical activity (METs⋅h/w) | ||||
|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | |
| Men | ||||
| Number of subjects | 18 | 19 | 19 | 18 |
| Mean ± SD | 3.9 ± 1.4 | 9.6 ± 2.0 | 14.6 ± 1.5 | 24.8 ± 7.0 |
| Model 1 | 1 | 0.364 (0.095–1.386) | 0.556 (0.147–2.103) | 0.318 (0.081–1.244) |
| Model 2 | 1 | 0.358 (0.093–1.375) | 0.570 (0.148–2.193) | 0.332 (0.082–1.346) |
| Model 3 | 1 | 0.309 (0.076–1.255) | 0.463 (0.114–1.869) | 0.236 (0.054–1.035) |
| Model 4 | 1 | 0.627 (0.130–3.027) | 0.648 (0.134–3.124) | 0.525 (0.098–2.819) |
| Women | ||||
| Number of subjects | 17 | 17 | 17 | 16 |
| Mean ± SD | 4.3 ± 1.6 | 8.8 ± 1.4 | 13.6 ± 2.1 | 28.7 ± 10.6 |
| Model 1 | 1 | 3.429 (0.827–14.211) | 2.700 (0.657–11.089) | 4.000 (0.935–17.115) |
| Model 2 | 1 | 3.325 (0.794–13.927) | 2.662 (0.646–10.967) | 3.712 (0.818–16.844) |
| Model 3 | 1 | 3.216 (0.755–13.695) | 2.599 (0.625–10.805) | 3.367 (0.652–13.382) |
| Model 4 | 1 | 4.301 (0.889–20.794) | 4.488 (0.877–22.979) | 13.190 (1.441–120.969) |
METs⋅h/w: Σ [metabolic equivalents × h per week (METs⋅h/w)]
Data were analyzed by multiple logistic regression analysis
Quartiles of physical activity (METs⋅h/w)
Bold value indicates that the 95 % confidence interval does not cross 1
Model 1, not adjusted; Model 2, adjusted for age; Model 3, adjusted for age and peak oxygen uptake; Model 4, adjusted for age, peak oxygen uptake, BMI, abdominal circumference, cigarette smoking habit, and body fat percentage
The odds ratio of serum vaspin levels (under median, over median) according to quartiles of peak oxygen uptake was 3.143 in men and 3.056 in women (not adjusted) (Table 4). In men, the odds ratio of serum vaspin levels according to quartiles of peak oxygen uptake was 39.956 (2.477–644.577) at a significant level even after adjusting for age, physical activity, BMI, abdominal circumference, body fat percentage, and cigarette smoking habit (Table 4). VIF values for all variables indicatied the absence of multicollinearity among the selected variables. However, significant associations between serum vaspin levels and peak oxygen uptake in women were not noted.
Table 4.
Odds ratio of serum vaspin levels according to quartiles of peak oxygen uptake in Vaspin Low group
| Quartiles of peak oxygen uptake (ml/kg/min) | ||||
|---|---|---|---|---|
| Q 1 | Q 2 | Q 3 | Q 4 | |
| Men | ||||
| Number of subjects | 18 | 19 | 19 | 18 |
| Mean ± SD | 26.6 ± 4.0 | 32.7 ± 1.1 | 39.5 ± 4.0 | 50.0 ± 3.6 |
| Model 1 | 1 | 1.143 (0.307–4.254) | 1.746 (0.472–6.454) | 3.143 (0.804–12.286) |
| Model 2 | 1 | 1.238 (0.326–4.694) | 2.219 (0.541–9.107) | 5.249 (0.924–29.816) |
| Model 3 | 1 | 1.518 (0.376–6.127) | 3.182 (0.697–14.533) | 8.354 (1.255–55.622) |
| Model 4 | 1 | 2.242 (0.452–11.127) | 7.652 (1.195–48.988) | 39.956 (2.477–644.577) |
| Women | ||||
| Number of subjects | 17 | 17 | 17 | 16 |
| Mean ± SD | 21.9 ± 2.2 | 27.1 ± 1.1 | 32.0 ± 2.0 | 41.8 ± 5.2 |
| Model 1 | 1 | 1.630 (0.411–6.460) | 2.619 (0.655–10.480) | 3.056 (0.739–12.632) |
| Model 2 | 1 | 1.762 (0.405–7.657) | 2.957 (0.596–14.659) | 3.600 (0.606–21.369) |
| Model 3 | 1 | 1.682 (0.386–7.334) | 2.834 (0.568–14.145) | 2.184 (0.293–16.267) |
| Model 4 | 1 | 1.338 (0.253–7.083) | 1.780 (0.277–11.444) | 1.626 (0.170–15.565) |
Data were analyzed by multiple logistic regression analysis
Bold values indicate that the 95 % confidence interval does not cross 1
Model 1, not adjusted; Model 2, adjusted for age; Model 3, adjusted for age and physical activity; Model 4, adjusted for age, physical activity, BMI, abdominal circumference, cigarette smoking habit, and body fat percentage
Discussion
In this study, we accurately evaluated the relationship between serum vaspin levels and physical activity using a uniaxial accelerometer and/or physical fitness in apparently healthy Japanese patients for the first time. Physical activity in women and physical fitness in men were closely associated with serum vaspin levels, even after adjusting for confounding factors such as body composition, in Vaspin Low group.
Youn et al. [26] reported that physical training for 4 weeks resulted in significantly increased vaspin levels in normal glucose-tolerant individuals, impaired glucose tolerance and type 2 diabetes groups, and changes in serum vaspin levels were significantly correlated with changes in peak oxygen uptake [26]. In turn, Cho et al. [27] reported that the obese group with low cardiorespiratory fitness levels showed significantly higher vaspin concentrations than the obese group with moderately high cardiorespiratory fitness levels; and BMI, abdominal circumference, peak oxygen uptake, and fasting insulin explained approximately 18 % of the individual variations in serum vaspin concentration in young Korean men (age 23.8 ± 2.5 years). Kadoglou et al. [28] also showed that there were no significant differences of serum vaspin levels between type 2 diabetes mellitus patients and non-healthy subjects, with and without active lifestyles.
In this study, subjects in the Vaspin High group were genetically defined [10]; we excluded those subjects and analyzed subjects in the Vaspin Low group. By accurately evaluating physical activity and physical fitness, we revealed the positive association between serum vaspin levels and physical fitness in men, and physical activity in women even after adjusting for age, BMI, and other confounding factors in Japanese not taking any medications. Many factors such as age, BMI, type 2 diabetes, and race may affect serum vaspin levels. However, the administration of recombinant vaspin improved glucose tolerance and insulin sensitivity in obese mice [1] and altered the expression of relevant insulin resistance genes including leptin, glucose transporter-4, adiponectin, resistin and TNF-α [1]. In rats, vaspin injection into the arcuate nucleus of the hypothalamus significantly decreased feeding compared to vehicle, where reduction of nueropeptide Y and increase of proopiomelanocortin mRNA levels mediate feeding inhibition [29]. Taken together, increasing physical activity and/or physical fitness may increase serum vaspin levels resulting in a protective effect, preventing obesity and insulin resistance in some Japanese. In addition, serum vaspin levels in this study were lower than those in a previously reported study. Vaspin is a compensatory factor in the status of obesity and insulin resistance [2]; apparently healthy subjects not taking any medications and younger subjects (20s: 54 subjects) without insulin resistance may affect our results. The findings of this pilot study may suggest the effect of physical activity and/or physical fitness on serum vaspin levels is independent of body composition in subjects with lower insulin resistance.
Potential limitations still remain in this study. First, our study was a cross-sectional, but not longitudinal study. The effect of long-term physical activity and/or physical fitness could not be evaluated clearly. Second, 156 subjects in our study voluntarily underwent measurements: they were therefore more likely to be health-conscious, and selection bias may exist. Third, we could not explain the gender difference and a clear mechanism for the relationship between serum vaspin levels and physical activity and/or physical fitness. The difference of reference value of physical fitness and physical activity between men and women may induce the gender difference in this study. In fact, in the Vaspin Low group, serum vaspin levels in women were higher than those in men, and peak oxygen uptake in men was higher than that in women. However, it seems reasonable to suggest that promoting physical activity in women and physical fitness in men might result in increasing serum vaspin levels in some Japanese men and women in clinical practice. To confirm these findings, further prospective studies in the Japanese population with larger sample sizes are required.
Acknowledgments
This research was supported in part by Research Grants from the Ministry of Health, Labour, and Welfare of Japan.
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Hida K, Wada J, Eguchi J, Zhan H, Baba M, Seida A, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA. 2005;102:10610–10615. doi: 10.1073/pnas.0504703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wada J. Vaspin: a novel serpin with insulin-sensitizing effects. Expert Opin Investig Drugs. 2008;17:327–333. doi: 10.1517/13543784.17.3.327. [DOI] [PubMed] [Google Scholar]
- 3.Chang HM, Park HS, Park CY, Song YS, Jang YJ. Association between serum vaspin concentrations and visceral adipose tissue in Korean subjects. Metabolism. 2010;59:1276–1281. doi: 10.1016/j.metabol.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Chang HM, Lee HJ, Park HS, Kang JH, Kim KS, Song YS, et al. Effects of weight reduction on serum vaspin concentrations in obese subjects: modification by insulin resistance. Obesity (Silver Spring) 2010;18:2105–2110. doi: 10.1038/oby.2010.60. [DOI] [PubMed] [Google Scholar]
- 5.Handisurya A, Riedl M, Vila G, Maier C, Clodi M, Prikoszvich T, et al. Serum vaspin concentrations in relation to insulin sensitivity following RYGB-induced weight loss. Obes Surg. 2010;20:198–203. doi: 10.1007/s11695-009-9882-y. [DOI] [PubMed] [Google Scholar]
- 6.Suleymanoglu S, Tascilar E, Pirgon O, Tapan S, Meral C, Abaci A. Vaspin and its correlation with insulin sensitivity induced in obese children. Diabetes Res Clin Pract. 2009;84:325–328. doi: 10.1016/j.diabres.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Lee MK, Jakal Y, Im JA, Kim E, Lee SH, Park JH, et al. Reduced serum vaspin concentration in obese children following short-term intensive lifestyle modification. Clin Chim Acta. 2010;411:381–385. doi: 10.1016/j.cca.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Inoue J, Wada J, Teshigawara S, Hida K, Nakatsuka A, Takatori Y, et al. The serum vaspin levels are reduced in Japanese chronic hemodialysis patients. BMC Nephrol. 2012;13:163. doi: 10.1186/1471-2369-13-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aust G, Richter O, Rohm S, Kerner C, Hauss J, Kloting N, et al. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis. 2009;204:262–266. doi: 10.1016/j.atherosclerosis.2008.08.028. [DOI] [PubMed] [Google Scholar]
- 10.Teshigawara S, Wada J, Hida K, Nakatsuka A, Eguchi J, Murakami K, et al. Serum vaspin concentrations are closely related to insulin resistance, and rs77060950 at SERPINA12 genetically defines distinct group with higher serum levels in Japanese population. J Clin Endocrinol Metab. 2012;97:E1202–E1207. doi: 10.1210/jc.2011-3297. [DOI] [PubMed] [Google Scholar]
- 11.Miyatake N, Takahashi K, Wada J, Nishikawa H, Morishita A, Suzuki H, et al. Daily exercise lowers blood pressure and reduces visceral fat in overweight Japanese men. Diabetes Res Clin Pract. 2003;62:149–157. doi: 10.1016/S0168-8227(03)00176-1. [DOI] [PubMed] [Google Scholar]
- 12.Oshida Y, Yamanouchi K, Hayamizu S, Sato Y. Long-term mild jogging increases insulin action despite no influence on body mass index or VO2 max. J Appl Physiol. 1989;66:2206–2210. doi: 10.1063/1.344319. [DOI] [PubMed] [Google Scholar]
- 13.Yamanouchi K, Shinozaki T, Chikada K, Nishikawa T, Ito K, Shimizu S, et al. Daily walking combined with diet therapy is a useful means for obese NIDDM patients not only to reduce body weight but also to improve insulin sensitivity. Diabetes Care. 1985;18:775–778. doi: 10.2337/diacare.18.6.775. [DOI] [PubMed] [Google Scholar]
- 14.Miyatake N, Nishikawa H, Morishita A, Kunitomi M, Wada J, Suzuki H, et al. Daily walking reduces visceral adipose tissue areas and improves insulin resistance in Japanese obese subjects. Diabetes Res Clin Pract. 2002;58:101–107. doi: 10.1016/S0168-8227(02)00129-8. [DOI] [PubMed] [Google Scholar]
- 15.Sawada SS, Muto T, Tanaka H, Lee IM, Paffenbager RS, Jr, Shindo M, et al. Cardiorespiratory fitness and cancer mortality in Japanese men: a prospective study. Med Sci Sports Exerc. 2003;35:1546–1550. doi: 10.1249/01.MSS.0000084525.06473.8E. [DOI] [PubMed] [Google Scholar]
- 16.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med. 1993;328:533–537. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 17.Definition and the diagnostic standard for metabolic syndrome—Committee to Evaluate Diagnostic Standards for Metabolic Syndrome. Nihon Naika Gakkai Zasshi. 2005;94:794–809 (in Japanese). [PubMed]
- 18.Wang J, Heymsfield SB, Aulet M, Thornton JC, Pierson RN., Jr Body fat from body density: underwater weighing vs. dual-photon absorptiometry. Am J Physiol. 1989;256:E829–E834. doi: 10.1152/ajpendo.1989.256.6.E829. [DOI] [PubMed] [Google Scholar]
- 19.Gustafsson L, Jacobson B, Kusoffsky L. X-ray spectrophotometry for bone-mineral determinations. Med Biol Eng. 1974;12:113–119. doi: 10.1007/BF02629842. [DOI] [PubMed] [Google Scholar]
- 20.Herd RJ, Blake GM, Parker JC, Ryan PJ, Fogelman I. Total body studies in normal British women using dual energy X-ray absorptiometry. Br J Radiol. 1993;66:303–308. doi: 10.1259/0007-1285-66-784-303. [DOI] [PubMed] [Google Scholar]
- 21.Kumahara H, Schutz Y, Ayabe M, Yoshioka M, Yoshitake Y, Shindo M, et al. The use of uniaxial accelerometry for the assessment of physical-activity-related energy expenditure: a validation study against whole-body indirect calorimetry. Br J Nutr. 2004;91:235–423. doi: 10.1079/BJN20031033. [DOI] [PubMed] [Google Scholar]
- 22.Yamada Y, Yokoyama K, Noriyasu R, Osaki T, Adachi T, Itoi A, et al. Light-intensity activities are important for estimating physical activity energy expenditure using uniaxial and triaxial accelerometers. Eur J Appl Physiol. 2009;105:141–152. doi: 10.1007/s00421-008-0883-7. [DOI] [PubMed] [Google Scholar]
- 23.McClain JJ, Sisson SB, Washington TL, Craig CL, Tudor-Locke C. Comparison of Kenz Lifecorder EX and ActiGraph accelerometers in 10-yr-old children. Med Sci Sports Exerc. 2007;39:630–638. doi: 10.1249/mss.0b013e3180313056. [DOI] [PubMed] [Google Scholar]
- 24.Clemes SA, Griffiths PL. How many days of pedometer monitoring predict monthly ambulatory activity in adults? Med Sci Sports Exerc. 2008;40:1589–1595. doi: 10.1249/MSS.0b013e318177eb96. [DOI] [PubMed] [Google Scholar]
- 25.Miyachi M, Tanaka H, Yamamoto K, Yoshioka A, Takahashi K, Onodera S. Effects of one-legged endurance training on femoral arterial and venous size in healthy humans. J Appl Physiol. 2001;90:2439–2444. doi: 10.1152/jappl.2001.90.6.2439. [DOI] [PubMed] [Google Scholar]
- 26.Youn BS, Kloting N, Kratzsch J, Lee N, Park JW, Song ES, et al. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377. doi: 10.2337/db07-1045. [DOI] [PubMed] [Google Scholar]
- 27.Cho JK, Han TK, Kans HS. Combined effects of body mass index and cardio/respiratory fitness on serum vaspin concentrations in Korean young men. Eur J Appl Physiol. 2010;108:347–353. doi: 10.1007/s00421-009-1238-8. [DOI] [PubMed] [Google Scholar]
- 28.Kadoglou NP, Vrabas IS, Kapelouzou A, Angelopoulou N. The association of physical activity with novel adipokines in patients with type 2 diabetes. Eur J Intern Med. 2012;23:137–142. doi: 10.1016/j.ejim.2011.10.020. [DOI] [PubMed] [Google Scholar]
- 29.Brunetti L, DiNisio C, Recinella L, Chiavaroli A, Leone S, Ferrante C, et al. Effects of vaspin, chemerin and omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptide. 2011;32:1866–1871. doi: 10.1016/j.peptides.2011.08.003. [DOI] [PubMed] [Google Scholar]


