SUMMARY
RNase III represents a family of dsRNA-specific endoribonucleases required for RNA maturation and gene regulation. The mechanism of action has been well characterized for the bacterial enzyme, but is not clear for eukaryotic RNase IIIs. Here, we describe the structure of Saccharomyces cerevisiae RNase III (Rnt1p) post-cleavage complex and explain the basis of its affinity for RNA stems capped with an NGNN tetraloop. The structure shows specific interactions between a new structural motif located at the end of Rnt1p dsRNA-binding domain (dsRBD) and the guanine nucleotide in the second position of the loop. Strikingly, structural and biochemical analyses indicate that the dsRBD and N-terminal domain function as two rulers measuring the distance between the tetraloop and the cleavage site. This unusual mechanism of substrate selectivity represents an example of the evolution of substrate selectivity and provides a framework for understanding the mechanism of action of eukaryotic RNase IIIs.
INTRODUCTION
Members of the RNase III family are involved in RNA interference (RNAi) (Hutvagner and Zamore, 2002) and RNA maturation (Court, 1993; Lamontagne et al., 2001; Nicholson, 1999). RNase IIIs are found in all species except archaebacteria (Lykke-Andersen and Garrett, 1997). The family members are identified by their homology to the Escherichia coli RNase III (Robertson et al., 1968) and can be divided into four classes (Lamontagne et al., 2001; Nicholson, 2003) based on protein features (Figure 1A). Typically, class I enzymes possess a single RNase III domain (RIIID) followed by a dsRNA-binding domain (dsRBD); class II is defined by the presence of an N-terminal domain (NTD), a RIIID, and a dsRBD; class III enzymes contain N-terminal P-rich and RS-rich domains followed by two RIIIDs and a dsRBD (Lee et al., 2003); and class IV possesses N-terminal helicase, DUF283, and PAZ domains followed by two RIIIDs and a dsRBD (Zhang et al., 2002). It is the N-terminal extension (NTE) beyond the RIIID that distinguishes eukaryotic RNase IIIs (classes II–IV) from bacterial enzymes (class I).
Figure 1. New Mode of Substrate Recognition by RNase III.
(A) Domain structures are illustrated for Homo sapiens Dicer (HsDicer, UniProtKB Q9UPY3), Giardia intestinalis Dicer (GiDicer, UniProtKB A8BQJ3), Homo sapiens Drosha (HsDrosha, UniProtKB Q9NRR4), Saccharomyces cerevisiae Rnt1p (ScRnt1p, UniProtKB Q02555), Kluyveromyces polysporus Dcr1 (KpDcr1, UniProtKB A7TR32), Aquifex aeolicus RNase III (AaRNase III, UniProtKB O67082), and Bacillus subtilis Mini-III (BsMini-III, UniProtKB O31418). The scale on top indicates the polypeptide lengths. The red box in RIIID represents the RNase III signature sequence.
(B) The Rnt1p:RNA complex contains two NTD dimers (blue/red), one RIIID dimer (cyan/orange), two dsRBDs (pale cyan/light orange), and two 34-nt RNAs (grey/dark grey) in addition to Mg2+ ions and solvent molecules (not shown).
(C) Distinct modes of dsRNA recognition by ScRnt1p (left) and AaRNase III (right) are illustrated. The RIIIDs are shown as ribbon diagrams outlined with transparent molecular surfaces, dsRBDs as solid molecular surfaces, and RNAs as tube-and-stick models. The span of the two dsRBDs along dsRNA is indicated with a double-headed arrow.
See also Figure S1.
Dicer produces small interfering (si) and micro (mi) RNAs involved in mediating RNAi. The precursor of siRNA is long dsRNA, whereas that of miRNA is pre-miRNA, a stem-loop structure produced by Drosha from an RNA primary transcript known as pri-RNA. Bacterial RNase III can function as either a processing enzyme (Court, 1993) or a binding protein (Oppenheim et al., 1993). As the only RNase III protein in Saccharomyces cerevisiae (Sc), Rnt1p is involved in the production of small nuclear RNA (snRNA) (Abou Elela and Ares, 1998), pre-rRNA (Abou Elela et al., 1996), and small nucleolar RNA (snoRNA) (Chanfreau et al., 1998). It is also involved in the degradation of several mRNAs (Danin-Kreiselman et al., 2003; Meaux et al., 2011). Deletion of RNT1 perturbs the cell cycle and growth, increases the telomere length, inhibits ribosome production, impairs cell wall stress response, and induces temperature sensitivity (Catala et al., 2008). While Rnt1p normally cleaves local stem loop structure, it was shown that the enzyme could direct cleavage in trans by binding to small guide RNA in analogy to the mechanism of RNAi, which is absent in S. cerevisiae (Lamontagne and Abou Elela, 2007).
The structure and mechanism of bacterial RNase III have been well characterized (Court et al., in press; Gan et al., 2008; Gan et al., 2006; Nicholson, 2011), whereas those of the eukaryotic enzymes remain unclear. The structure and mechanism of bacterial RNase III cannot explain the function of features unique for eukaryotic enzymes like the NTE and the two additional side chains found in the cleavage site (Du et al., 2008; Weinberg et al., 2011). Furthermore, several eukaryotic enzymes developed affinity for substrates with a specific structure and sequence, which cannot be explained by the basic mechanism of dsRNA recognition found in bacterial enzyme. For example, Rnt1p does not cleave dsRNA under physiological conditions but specifically recognizes short RNA hairpins with conserved NGNN tetraloops (G2-loop, Lebars et al., 2001). Substrate recognition by Rnt1p is mediated by dsRBD, while the cleavage is performed at a fixed distance from the tetraloop by RIIID (Ghazal and Elela, 2006; Lamontagne and Elela, 2001; Lamontagne and Elela, 2004; Lamontagne et al., 2003; Lamontagne et al., 2000). However, it is not clear how Rnt1p selects these substrates and how the enzyme positions its cleavage site at a fixed distance from the tetraloop. Until now, the exact contribution of the tetraloop, especially the conserved guanine nucleotide, to substrate binding and cleavage is still being debated (Ghazal and Elela, 2006; Hartman et al., 2013; Lavoie and Abou Elela, 2008; Wang et al., 2011; Wu et al., 2004).
Here, we report the crystal structure of Rnt1p in complex with a G2-loop and explain how the catalytic complex of eukaryotic RNase III is assembled and explain the basis of Rnt1p affinity for short RNAs with specific structure and sequence. Surprisingly, a new structural motif near the C-terminus of dsRBD specifically recognizes the conserved guanine in the second position of the tetraloop. Intriguingly, the NTD acts as a second ruler that measures together with the dsRBD the distance between the tetraloop and the cleavage site.
RESULTS
New Binding Mode of dsRNA by RNase III
Recombinant Rnt1p was incubated with a 34-nucleotide (nt) RNA stem-loop (Figure S1A). The resulting mixture yielded crystals of an Rnt1p:RNA complex. However, the SDS-PAGE analysis of the crystal content indicated that the protein was degraded into two fragments (Figure S1B), a 14-kDa fragment containing the NTD (residues 42–151) and a 32-kDa fragment containing the RIIID and dsRBD (ΔNTD, residues 197–457). The asymmetric unit contained an intertwined dimer of NTD, a ΔNTD, and a 34-nt RNA (Figure S1C). It has been shown that the dimerization of RIIID is required for dsRNA processing by bacterial RNase III in that side chains from both RIIID subunits are involved in the cleavage of each RNA strand and the cleavage of both strands creates the characteristic 2-nt 3′ overhang in the product (Gan et al., 2006). Like bacterial RNase III, Rnt1p also functions as a homodimer (Lamontagne et al., 2000). Thus, the biological assembly of Rnt1p was obtained by expanding the asymmetric unit via a crystallographic 2-fold axis. It revealed a ΔNTD dimer in complex with an RNA pseudoduplex, formed by two 34-nt RNAs joined by their 2-nt 3′ overhangs, and two NTD dimers (Figure 1B). Similar to the Aquifex aeolicus RNase III (AaRNase III):RNA complex (Gan et al., 2006), the ScΔNTD:RNA complex also contained a pseudoduplex RNA formed by two product RNAs (Figure 1C). Unlike bacterial RNase III, however, Rnt1p has the NTD (Figure 1A). The NTD, separated from the ΔNTD due to protein degradation, formed an intertwined dimer recognizing both ends of the ΔNTD:RNA complex (Figure 1B). The extra NTD is due to crystallization condition but its position does not affect the biochemically predicted positioning of RIIID relative to the cleavage site (vide infra).
The RIIID and dsRBD in both AaRNase III and Rnt1p are connected by a 7-residue flexible linker (Figure S2). The structure of this linker is well defined in the Rnt1p:RNA (this work) and AaRNase III:RNA (Gan et al., 2006) complexes. Comparison between the ScΔNTD:RNA and AaRNase III:RNA structures shows that while the RIIID dimer of the two enzymes adopt the same position relative to the RNA, the span of the two dsRBDs differs by 66 Å (Figure 1C). This new conformation of the enzyme significantly increases the buried surface between protein and RNA, which measures 5520 Å2 in the case of AaRNse III and 8372 Å2 in the case of ScΔNTD. Thus, Rnt1p adopts a novel RNA-binding mode that permits the recognition of the defining substrate elements without disrupting the classical alignment of dsRNA with the two cleavage sites in the RNase III catalytic complex.
Structure of Eukaryotic RNase III Post-Cleavage Complex
Unlike bacterial RNase III, the cleavage site of eukaryotic RNase III enzymes contains six conserved side chains (Figure 2A). The Rnt1p:RNA structure shows that the enzyme processes dsRNA by a single RNA cleavage event on each RNA strand to generate products with the 2-nt 3′ overhang (Figure 2B, left). In the post-cleavage complex, each cleavage site is composed of the 3′ - and 5′-termini of a cleaved RNA strand, two Mg2+ ions, four water molecules, and six catalytic side chains (Figure 2B, right). The distance between the 3′-hydroxyl oxygen and the 5′-phosphate phosphorus is 3.1 Å, which corresponds to the van der Waals distance between these two atoms. This arrangement of the RNA within the cleavage site indicates that the Rnt1p:RNA structure represents a catalytic stage immediately after the hydrolysis of the scissile bond. Statistics for the quality of the structure is listed in Table 1.
Figure 2. Cleavage Site Assembly of Eukaryotic RNase III.
(A) Structure-based sequence alignment of ScRnt1p (this work), KpDcr1 (PDB entry 3RV0), GiDicer (PDB entry 2FFL), HsDicer (PDB entry 2EB1), and AaRNase III, (PDB entry 2NUG). The RIIIID signature sequence is indicated and the conserved amino acid residues in the cleavage site highlighted (in red). The boxed residues, N5 and K6, are unique for eukaryotic enzymes.
(B) The dsRNA-processing center of Rnt1p has two cleavage sites (left). Stereoview on the right shows the cleavage site assembly. Electron density (annealed omit Fo-Fc, contoured at 4.5 σ) is shown as grey nets for the Mg2+ ions (black spheres) and coordinating water molecules (red spheres). Residues are illustrated as sticks in atomic colors [N in blue, C in green (protein) or orange (RNA), O in red, P in orange, and Mg in black]. Mg2+ coordination is indicated by solid lines, hydrogen bonds by dashed lines, and the distance between the 3′ hydroxyl oxygen of Cyt34 and the phosphorous of Cyt1 (3.1 Å) by a double-headed arrow.
(C–F) Stereoviews show the superposition of the Rnt1p cleavage site assembly (in green) on that of AaRNase III (C), KpDcr1 (D), GiDicer (E), or HsDicer (F). The AaRNase III, KpDcr1, GiDicer, and HsDicer structures were adopted from the PDB as specified in (A) and shown in magenta.
See also Figure S2.
Table 1.
Data Collection and Structure Refinement Statistics
| Data Collection | |
|---|---|
| Wavelength (Å) | 1.0 |
| Space group | C2221 |
| Cell dimensions | |
| a, b, c (Å) | 158.0, 183.8, 61.3 |
| α, β, γ (°) | 90, 90, 90 |
| Resolution (Å) | 40-2.50 (2.65-2.50)a |
| Number of unique reflections | 31,010 (4,870) |
| Rmerge (%)b | 6.6 (78.4) |
| I/σ | 14.45 (1.82) |
| Completeness (%) | 98.7 (97.5) |
| Redundancy | 4.7 (4.3) |
| Structure Refinement | |
| Resolution | 40-2.5 (2.63-2.50) |
| Rwork (%)c | 21.6 (41.0) |
| Rfree (%)d | 23.9 (40.8) |
| Number of Atoms | |
| Protein | 4,081 |
| RNA | 574 |
| Water | 98 |
| B factors | |
| Protein | 81.5 |
| RNA | 58.9 |
| Water | 62.2 |
| Root-mean-square deviation | |
| Bond lengths (Å) | 0.003 |
| Bond angles (°) | 0.777 |
| Ramachandran plot | |
| Most favored region (%) | 92.1 |
| Additional allowed region (%) | 6.5 |
| Generously allowed region (%) | 0.7 |
| Disallowed region (%) | 0.7 |
Values in parentheses are for the highest resolution shell.
Rmerge = Σ|(I − <I>)|/σ(I), where I is the observed intensity.
Rwork = Σhkl | |Fo| − |Fc| | / Σhkl |Fo|, calculated from working dataset.
Rfree is calculated from 3% of data randomly chosen and not included in refinement.
All six catalytic residues conserved in eukaryotic RNase IIIs (N1, D2, D3, E4, N5, and K6, Figure 2A) were well defined. The side chain of N5 was found to interact with two water molecules and one oxygen of the 5′ phosphate, while the side-chain ε-amino group of K6 interacted with one oxygen of the 5′ phosphate and another from the carboxylic group of D3 (Figure 2B). The interaction of these two side chains with the leaving phosphate group via specific hydrogen bonds indicate that they play important roles in stabilizing the transition state.
Superposition of the Rnt1p:RNA and the AaRNase III:RNA (Gan et al., 2008) structures showed identical positioning of the E1, D2, D3, and E4 side chains (Figure 2C). Comparison of the Rnt1p:RNA with three RNA-free eukaryotic RNase IIIs (MacRae et al., 2006; Takeshita et al., 2007; Weinberg et al., 2011) revealed substantial conformational changes of the D2, N5, and K6 side chains upon the formation of cleavage site assembly (Figures 2D, 2E, and 2F), demonstrating that the cleavage site assembly of eukaryotic RNase III is formed and configured for cleavage only when all the components are present.
New RNA-Binding Motif Unique to Rnt1p
Bacterial RNase III recognizes dsRNA with four RNA-binding motifs (RBMs), RBMs 1 and 2 in the dsRBD and RBMs 3 and 4 in the RIIID (Figure 3A, left). RBMs 1 and 3 form multiple hydrogen bonds with the O2′ hydroxyl groups, while RBMs 2 and 4 each projects a loop into the minor groove. These four RBMs are conserved in Rnt1p (Figure 3A, right). Surprisingly, the Rnt1p:RNA structure revealed another RBM that is specialized in the recognition of the conserved guanine in the G2-loop, which we named RBM0 (Figure 3B). The RBM0 is formed by two additional α-helices (α3, α4) near the C terminus of dsRBD (Figure 3C). The α3 helix was also seen previously in the structures of truncated dsRBD of Rnt1p in complex with RNA (Wang et al., 2011; Wu et al., 2004), but α4 was not observed before. These two helices transform the conserved αβββα fold of dsRBD (Ramos et al., 2000) into a new αβββααα fold. Thus, Rnt1p recognizes its substrate using five RBMs (0, 1, and 2 in dsRBD; 3 and 4 in RIIID), corresponding to residues 445–455, 370–381, 392–399, 265–269, and 292–312, respectively (Figure S2).
Figure 3. New RNA-Binding Motif, RBM0, Identifies the NGNN Tetraloop.
(A) The arrangement of the RBMs of AaRNase III (PDB entry 2EZ6) is shown on the left and that of ScRnt1p (this work) on the right. Only one subunit of the protein dimer is shown. The RIIID is shown in cyan and dsRBD in pale cyan. The RBMs are in blue and the linker between the two domains in red. Stem-loop RNA is shown as a molecular surface in grey or light grey.
(B) RBM0 identifies the AGUC tetraloop. The dsRBD, outlined with a transparent molecular surface, is shown as a ribbon diagram with the RBM0 highlighted in blue. The RNA backbone is shown as a ribbon diagram with the four nucleotides in the tetraloop as sticks colored by atom (N in blue, O in red, C in grey, and P in orange).
(C) On the left, dsRBD proteins used in structural analysis (in cyan/blue, this work; in yellow, PDB entry 1T4L; in pink, PDB entry 2LBS) are illustrated with RBMs indicated with dashed boxes. On the right, the three dsRBD structures are superimposed.
(D) Interaction map for the binding of RBMs 0 and 1 to the AGUC tetraloop via interaction(s) with the base, ribose, and/or backbone of the RNA. Residues of RBMs 0 and 1 are colored in blue and cyan, respectively.
(E) RBM0 is required for substrate cleavage. The Long- or Short-G2 substrate was incubated alone (no enz), with recombinant Rnt1p (Rnt1p), or with enzymes lacking the RBM0 (ΔRBM0). The reactions were carried out in multiple turnover (substrate excess) and physiological salt (150 mM KCl) conditions. S and P indicate the position of intact substrate and cleavage product, respectively. The asterisk indicates a secondary cleavage product. The sizes of the different RNA fragments are indicated on the left. The substrates are illustrated on top.
(F) RBM0 is required for substrate binding. Increasing amounts of Long- or Short-G2 were injected into surface-bound, full-length or ΔRBM0 enzyme. Shown is the binding curve from surface plasmon resonance. The ratio of the resonance unit change (RU) over the theoretical maximal RU (Rmax) obtained for each binding assay is presented in the form of a graph.
Sequence-Specific Tetraloop Recognition by dsRBD
The Rnt1p:RNA structure showed that the dsRBD recognizes the AGUC tetraloop with RBMs 0 and 1 (Figure 3B). As summarized in Figure 3D, a total of 10 hydrogen bonds are formed between the tetraloop and residues in RBM0 (R445, I448, R450, S453, and V454) and RBM1 (Q373, Y375, S376, G379, and A381), demonstrating that the tetraloop is required for recognition. Four of the 10 hydrogen bonds are base-specific, including one between Ade15 and RBM1 residue Q373 (Figure S3A) and three between Gua16 and RBM0 residues R445, I448, and S453 (Figure S3B). In contrast, interactions of Uri17 with RBM1 (Figure S3C) and of Cyt18 with RBM0 (Figure S3D) are not base-specific. As illustrated in Figure S3E, Gua16 is the most conserved, Ade15 is less conserved, and Uri17 and Cyt18 are not conserved. It has been shown previously that Gua16 is essential for both binding and cleavage of G2 substrates, whereas mutations of the other three nucleotides reduce, but do not block, cleavage (Lamontagne et al., 2003; Lamontagne et al., 2004). Thus, the requirement of an individual nucleotide in the tetraloop for cleavage appears to be dictated by its degree of base-specific interactions.
To evaluate the biochemical significance of RBM0, we prepared a truncated Rnt1p that lacks residues 446–471 (ΔRBM0). It was purified (Figures S1D, S1E) and tested for RNA binding and cleavage using G2 substrates with either long or short RNA duplex (Long- or Short-G2, Figure S1A) under multiple turn-over (RNA excess) and physiological salt (150 mM KCl) conditions. As shown in Figure 3E, Rnt1p was able to cleave both substrates efficiently while ΔRBM0 did not, indicating that RBM0 is required for Rnt1p function. To distinguish between defects in catalysis and RNA binding, we examined the impact of deleting the RBM0 on RNA binding in the absence of Mg2+ using surface plasmon resonance. As shown in Figure 3F, full-length Rnt1p efficiently bound to both G2 substrates whereas the ΔRBM0 did not bind the Short-G2 and bound very poorly to the Long-G2. The weak binding detected with the Long-G2 is consistent with specific role of RBM0 in recognizing the tetraloop and suggests that the other four RBMs may recognize the RNA duplex albeit inefficiently. We conclude that the RBM0 of Rnt1p is required for substrate binding and cleavage.
Clamp-Shaped Pocket Tailored for the Selection of Unpaired Guanosines
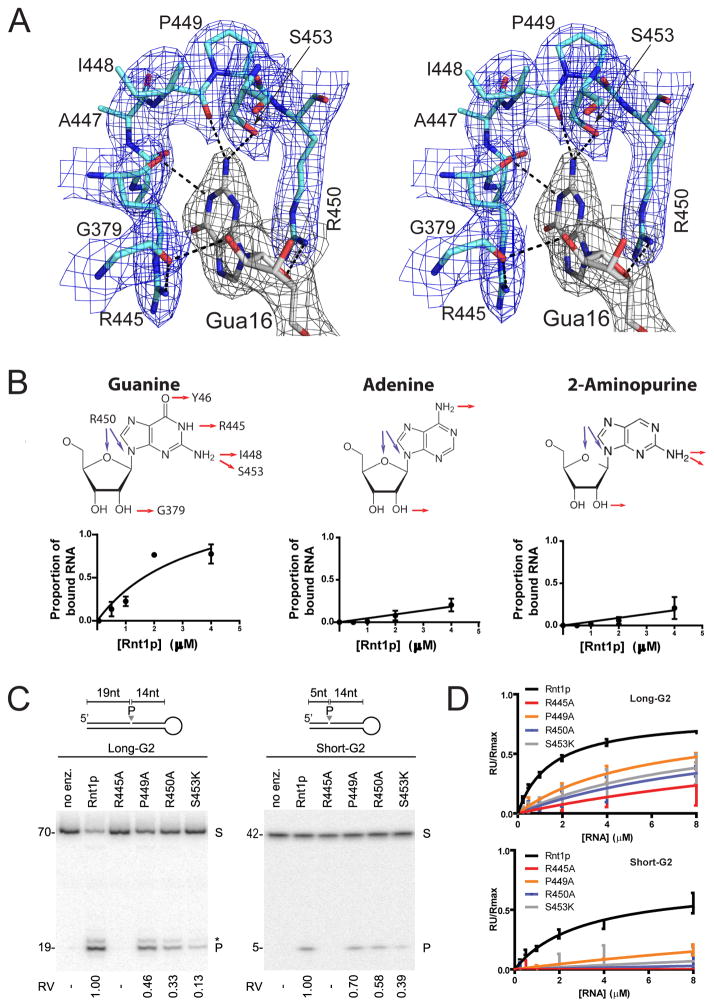
Inspection of the structure near the tetraloop revealed that the conserved guanosine (Gua16) is snuggly positioned into a clamp-shaped pocket formed by one RBM1 residue (G379) and seven RBM0 residues (R445, A446, A447, I448, P449, R450, and S453), which we call G-clamp (Figure 4A). The G-clamp structure is tailored to the shape and chemical moieties of the protruding guanosine and as such it is responsible for the sequence requirement of Rnt1p substrates. Moreover, side-by-side comparison of the Rnt1p:RNAAGUC-capped structure (this work) with the dsRBD:RNAAGAA-capped structure (Wu et al., 2004) shows distinct locations of the guanine base in the tetraloop (Figures S4A, S4B). Therefore, the G-clamp not only recognizes the guanosine in the second position of the loop but also reshapes the loop to better fit the enzyme structure.
Figure 4. G-clamp Is Tailored for the Recognition of Gua16.
(A) Stereoview showing that the conserved Gua16 base is buried in the G-clamp formed by eight residues from RMBs 0 and 1. Residues are illustrated as sticks [N in blue, O in red, P in orange, and C in pale cyan (amino acids) or grey (nucleotide)] outlined with electron density map (blue/grey nets, 2Fo-Fc, contoured at 1.2 σ). Dashed lines indicate hydrogen bonds.
(B) Guanine-specificity of the G-clamp. Rnt1p substrates carrying adenine or 2-aminopurine in the second position of the NGNN loop were tested for binding to Rnt1p using electrophoretic mobility gel shift assay (EMSA). The top panels show the structure of the three bases; hydrogen bonds formed between each base and the G-clamp are indicated by red and blue arrows. The bottom panels show the binding curves of the three substrates derived from gel shift assays.
(C) Contribution of G-clamp residues to RNA cleavage. Four G-clamp residues directly interacting with Gua16 of the tetraloop were individually mutated and the impact on enzyme activities was tested using Long- or Short-G2 as described in Figure 3E. Relative velocities (RV), the cleavage rates obtained with the mutants relative to that obtained by the wild type, are indicated at bottom.
(D) Impact of G-clamp mutations on substrate binding. The binding kinetics of different mutations to Long- or Short-G2 were assessed using surface plasmon resonance and the ratio of the resonance unit (RU) over the theoretical maximal RU (Rmax) obtained for each binding assay is presented in the form of a graph.
See also Figure S4 and Tables S1 and S2.
To evaluate the effect of the G-clamp structure on substrate selection, we substituted the Gua16 base with either adenine or 2-aminopurine, which eliminates its specific interaction with R445 (Figure 4B). The impact on protein binding was then tested using the electrophoretic mobility shift assay (EMSA). Rnt1p bound efficiently the unmodified RNA but not the RNAs with modified bases in that position. This suggests that other interactions, like those observed with Y46 or those with I448 and S453, are not sufficient for efficient binding to the RNA. To more clearly evaluate the relative contribution of G-clamp residues that directly interact with Gua16 to the enzyme function, we replaced residues R445, P449, R450, and S453 individually with alanine or lysine and tested the impact on RNA binding and cleavage (Figures 4C and 4D). The substitution of R445 blocked the cleavage and binding of both G2 substrates. In contrast, the substitution of P449, R450, or S453 did not block but reduced the binding and cleavage of the two substrates. As expected, the binding to Short-G2, which limits interactions between the enzyme and the RNA beyond the cleavage site, was more affected by the G-clamp mutations than the binding to Long-G2 (Figure 4D). However, the cleavage of Short-G2 was not more affected than the cleavage of Long-G2 by these mutations (Figure 4C), suggesting that enzyme-RNA interactions beyond the cleavage site do not directly contribute to cleavage efficiency. The R445 requirement for cleavage can be explained by its side chain stacking with the Gua16 base, the formation of a hydrogen bond with the Gua16 base, and the formation of a hydrogen bond with the G379 carbonyl that forms a hydrogen bond with the Gua16 O2′-OH (Figure 4A). We conclude that residue R445 is required for substrate binding and cleavage, while the intact structure of the G-clamp is necessary for optimal affinity and activity.
Residue Q373 forms a single base-specific hydrogen bond with Ade15, the first nucleotide in the tetraloop (Figure S3A), and the Q373A mutation had similar effects as the P449A, R450A, or S453K mutation that did not block but reduced the cleavage of the two G2 substrates (Figures S3F, 4C).
Rnt1p Interacts with the RNA Stem Upstream of the Cleavage Sites
The conserved RBMs of Rnt1p (RMBs 1–4, Figure S2) interact with the first five base pairs, the 9th base pair, and the scissile bond downstream of the tetraloop, demonstrating that the structural basis for recognizing dsRNA is conserved between bacterial and yeast enzymes. The Rnt1p:RNA structure shows that RBM1 interacts with base pairs 1–4, the α2 helix of the dsRBD interacts with base pair 5 (Figure 5A), RBMs 2 and 4 interact with base pair 9 (Figure 5B), and RBM3 recognizes the scissile bond (not shown). Therefore, using RBMs 0–2 and the α2 helix the Rnt1p dsRBD interacts with the tetraloop (Figure 3D) and the RNA stem (Figures 5A, 5B) by forming a total of 18 hydrogen bonds to the RNA, confirming that the role of dsRBD in substrate recognition is conserved in Rnt1p. Seventeen out of the 18 are directed at the tetraloop and its neighboring five base pairs (Figures 3D, 5C), which explains why Rnt1p can bind to a short RNA hairpin with a 5-base pair stem (Lamontagne et al., 2003), a substrate much shorter than what bacterial enzyme can (Gan et al., 2006).
Figure 5. dsRBD Interacts with the Stem down to the 9th Base Pair below the Tetraloop.
(A) Stereoview showing the interactions between RBM1 and the first four base pairs below the tetraloop and that between the α2 K421 and the 5th base pair. Residues are shown as sticks in atomic colors [N in blue, C in green (protein) or grey (RNA), O in red, and P in orange]. Dashed lines indicate hydrogen bonds. A “zoomed out” view indicating the position of the RBMs within the Rnt1p:RNA structure is shown on the right.
(B) Stereoview showing the interactions between the 9th base pair below the tetraloop and residues K392 from RBM2 and K311 from RBM4.
(C) Interaction map between the Rnt1p dsRBD and the first five base pairs below the tetraloop.
(D and E) Disruption of RBM2 interaction with the 9th base pair below the tetraloop alters RNA binding and cleavage. Residue K392 was mutated and the impact of the mutation was tested on substrate cleavage and binding as described in Figures 4C and 4D.
See also Tables S1 and S2.
To determine the significance of the interaction between RBM2 and the 9th nucleotide, which is located midway between the upper stem-loop and cleavage site, we mutated the interacting amino acid K392 and tested the effect on RNA binding and cleavage. Comparison of the cleavage kinetic parameters of the wild type and the K392A mutant indicated that the mutation did not affect the binding of Long-G2 but reduced the amount of cleaved RNA by ~35% (Figures 5D, 5E). Consistently, analysis of the kinetic parameters of the Long-G2 cleavage indicated slower catalysis and decreased catalytic efficiency with little effect on the KM (Table S1). Similarly, the K392A mutation impaired the cleavage of the Short-G2 substrates. However, unlike Long-G2, binding to Short-G2 was significantly inhibited by the K392A mutation (Table S1). This result is consistent with previous studies suggesting that the 9th base pair is important for Rnt1p binding (Lamontagne et al., 2003). We conclude that the interaction of Rnt1p with the middle of the RNA stem is required for optimal binding and cleavage and is particularly critical for forming stable complexes with short RNA substrates.
Interaction between the NTD and RNA Increases Precision of Cleavage Site Selection
Deletion of the Rnt1p NTD impairs cleavage at high salt conditions, which was suggested to contribute to the stability of the protein-RNA complex (Lamontagne et al., 2000; Lavoie and Abou Elela, 2008). However, the mechanism by which the NTD contributes to substrate selection and catalysis remained unclear. As shown in Figure 6A, the NTD has an all-helical fold that forms an intertwined dimer, as was shown for Kluyveromyces polysporus Dcr1 (KpDcr1, Weinberg et al., 2011). Surprisingly, however, the NTD dimer was found in contact with the AGUC tetraloop via four hydrogen bonds between four amino acid residues (Y46 and H54 from one subunit, K58 and H54 from the other) and three nucleotide residues (Gua14, Ade15, and Gua16). Residue Y46 forms a base-specific hydrogen bond with Gua16. In addition, one of the two H54 side chain stacks with the Ade15 base. This set of NTD-tetraloop interactions has also been observed in two other crystal forms (data not shown), indicating that this arrangement is highly preferred. As Ade15 and Gua16 are conserved in G2-loops (Figure S3E), this finding suggests that the NTD directly contributes to substrate selection. To test this possibility, we examined the impact of deleting the NTD on cleavage when compared to the wild type and the RBM0 deletion. Cleavage reactions were performed first under low salt conditions (10 mM KCl) with excess protein to allow the detection of any residual activity. As shown in Figures 6B and 6C, wild-type enzyme cleaved the two G2 substrates almost to completion, whereas both the ΔNTD and ΔRBM0 showed reduced cleavage. Furthermore, the deletion of NTD resulted in the appearance of additional cleavage products, corresponding to the cleavage at non-canonical sites, 5–8 nucleotides below the tetraloop. Interestingly, the Short-G2 showed reduced binding with both the wild type and ΔNTD relative to the Long-G2 (Table S1). Consistent results were obtained when the cleavage reactions were carried out under either the low salt but multiple turnover conditions or the crystallization conditions (data not shown). We conclude that the Rnt1p NTD increases the precision of the cleavage site selectivity by directly interacting with Gua16 and the 5′ end region of the G2-loop.
Figure 6. NTD Dimer Interacts with RNA to Increase Binding Affinity and the Precision of Cleavage Site Selection.
(A) The NTD dimer of Rnt1p interacts with three nucleotides within or near the AGUC tetraloop. NTD1 (blue) and NTD2 (red) are shown as ribbons. RNA backbone is shown in orange. Contacting residues are shown as sticks. Dashed lines indicate hydrogen bonds.
(B, C) Deletion of the NTD impairs cleavage site selection of G2 substrates. The substrates were labeled at their 5′ (B) or 3′ (C) ends. Wild-type enzyme (Rnt1p), enzyme lacking the RBM0 (ΔRBM0), or that lacking the NTD (ΔNTD) were incubated with either Long- or Short-G2 and the cleavage products visualized under single turnover (enzyme excess) and low salt (10 mM KCl) conditions. Alternative cleavage sites observed in the absence of the NTD are indicated by “#”. The cleavage rates are indicated as relative velocities (RV) at bottom.
(D) The double-ruler architecture ensures the cleavage accuracy. The protein is illustrated as a molecular surface and color-coded as in (A). The RNA is shown as a cartoon in grey for the tetraloop and in blue and red for the stem. Gua16 is highlighted as a ball-and-stick illustration.
See also Figure S5 and Tables S1 and S2.
The structural and functional data suggest that two rulers are dedicated for the mechanism of Rnt1p action: Ruler 1 is the RIIID1-dsRBD1 fragment; Ruler 2 is the RIIID2:NTD1/NTD2 complex (Figure 6D). Upon the Rnt1p:RNA complex formation, both rulers are sufficiently “stiff” owing to the protein-protein (RIIID1–RIIID2, RIIID1-dsRBD1, RIIID2-NTD1/NTD2, and dsRBD1-NTD1/NTD2) and protein-RNA interactions. Both rulers interact with the NGNN tetraloop; Ruler 1 recognizes the Gua16 base and Ruler 2 secures the Gua16 recognition. Hence, the two RNA strands of the substrate are cleaved accurately 14 and 16 nucleotides, respectively, downstream from the tetraloop.
DISCUSSION
The majority of RNase III natural substrates are short RNA molecules with specific features located at a fixed distance from the scissile bonds, such as the 3′ overhang and internal or terminal loops (Nicholson, 2003). In this study, we reveal a mechanism by which a eukaryotic RNase III recognizes a terminal tetraloop sequence in harmony with the formation of the catalytic complex. Recognition of the tetraloop is achieved by an extended dsRBD with two additional α-helices, α3 and α4. This αβββααα fold of the dsRBD creates a unique guanosine-specific binding motif that we call the G-clamp. Surprisingly, the Rnt1p:RNA structure also revealed specific contacts between the NTD dimer and the 5′ end region of the tetraloop. Deletion of the NTD led to cleavage at alternative site(s), suggesting that this motif increases the precision of the cleavage site selectivity. Together, the work presented here demonstrates a new mechanism for RNase III substrate selectivity whereby the dsRBD and the NTD dimer jointly bind the tetraloop and position the RIIID dimer along the stem at a fixed distance from the tetraloop.
Eukaryotic RNase IIIs feature an NTE of variable lengths and domain structures (Figure 1A). Yeast Rnt1p is an excellent model system for studying such NTEs. It contains a single NTD, but the linker between the NTD and RIIID (residues 155–190) is 46-ressidue long (Figure S2). Secondary structure and disorder prediction suggests that this linker is disordered and thus flexible. On one hand, the degradation of Rnt1p into the NTD and ΔNTD fragments is, at least in part, due to the length and flexibility of this linker. On the other hand, it is this long and flexible linker that could allow the NTD dimer to reach and interact with the dsRBD and the AGUC tetraloop. It is not clear, however, which NTD within the NTD dimer is connected to which RIIID in the RIIID dimer. We believe that the intact Rnt1p protein could form the same complex because this complex is in excellent agreement with previous studies, which indicated that the NTD of Rnt1p could interact with itself and the dsRBD (Lamontagne et al., 2000) while contacting the 5′ side of the tetraloop (Lavoie and Abou Elela, 2008). Whether the NTD and dsRBD from the same Rntp1 are involved in the recognition of Gua16 in the AGNN tetraloop remains to be seen.
The Rnt1p:RNA structure explains the capacity of Rnt1p to play a general role in RNA processing and regulation in yeast despite its high specificity to a given RNA structure. With just one exception, the natural substrates of Rnt1p contain the guanine nucleotide in the second position of the tetraloop (Jules Gagnon, Mathieu Lavoie, and Sherif Abou Elela, unpublished data). Therefore, the structural basis for the processing of all but one natural substrates of Rnt1p has been provided by the new structure. The one substrate RNA that is not capped with the G2-loop (snR48) contains an adenine nucleotide in the second position (the AAGU tetraloop), for which the binding mode by the enzyme remains to be revealed.
The Presence of NTD Increases the Accuracy and Efficiency of Yeast RNase III
Eukaryotic RNase IIIs each contains an NTE that includes the helicase, DUF283 and PAZ domains in Dicer, the P-rich and RS-rich domains in Drosha, or the NTD in KpDcr1 and Rnt1p (Figure 1A). These domains could specifically interact with RNA and influence cleavage in different ways. It has been suggested that Dicer uses the PAZ domain to recognize the end of dsRNA substrate (Zhang et al., 2004) and uses the helicase domain to recognize the loop/bulge structure of short hairpin RNAs for accurate processing (Gu et al., 2012). The Rnt1p:RNA structure demonstrates that the NTD is a well-defined structural domain that exhibits an all-helical fold and forms an intertwined dimer (Figure 1B), which is similar to that of the KpDcr1 (Weinberg et al., 2011). However, unlike the KpDcr1 NTD dimer, which stacks on the back of its RIIID dimer, the Rnt1p NTD extends away (Figure S5A). On one hand, the length of the linker connecting the NTD to RIIID in KpDcr1 is too short to permit the extended NTD-RIIID arrangement as observed in Rnt1p (Figure S2 and S5A). On the other hand, the Rnt1p NTD dimer may not adopt a KpDcr1-like position because the electrostatic potential between the corresponding surfaces of the NTD and the RIID is not compatible (Figure S5C). The distinct orientations of Rnt1p and KpDcr1 domains reflect the difference between the enzymes’ modes of substrate selection. In the case of Rnt1p, the enzyme extends to recognize the terminal tetraloop with both the NTD and the dsRBD, whereas in the case of KpDcr1, neither the NTD nor the dsRBD are positioned to measure the length from the end of a dsRNA molecule to the cleavage sites (Weinberg et al., 2011).
The Rnt1p:RNA structure indicates a direct role of the NTD dimer in substrate selection. This mode of substrate recognition, in which the NTD assists in selecting the cleavage site at a given distance, is not possible in bacterial RNase III because it lacks an NTE. During evolution, however, eukaryotic RNase IIIs have acquired an NTE to enhance the basic dsRNA substrate specificity conferred by the dsRBD.
How Does Eukaryotic RNase III Process dsRNA?
Despite the wealth of information on the function of eukaryotic RNase III enzymes, the biochemistry of these enzymes remains obscure (Li et al., 2010; MacRae and Doudna, 2007). The Rnt1p:RNA structure represents the catalytic state immediately after cleavage, and as such, provides a unique opportunity to look at how the eukaryotic RIIID dimer cleaves dsRNA. The dimerization of RIIID creates the catalytic valley in which the two cleavage sites form the processing center. Each strand of the bound RNA aligns at a cleavage site and the two sites are spaced such that the cleavage of the two stands creates the 2-nt 3′ overhangs on the product ends (Figure 2). Each cleavage site requires six conserved amino acids side chains, among which the general organization of E1, D2, D3, and E4 is preserved from bacteria to human, whereas the involvement of N5 and K6 is unique for eukaryotes. In the Rnt1p:RNA structure, the N5 and K6 side chains interact with the 5′ phosphate group of the cleaved scissile bond, showing how they are directly involved in the formation of the cleavage site assembly of eukaryotic RNase IIIs. We hypothesize that these two residues participate in the stabilization of the transition state during catalysis.
The Rnt1p dsRBD Exhibits a New Mode of Sequence-Specific Selection of dsRNA
The classical mode of dsRNA recognition involves interactions between the dsRBD and two minor grooves of dsRNA in order to discriminate between RNA and DNA duplexes. These interactions do not contribute directly to cleavage site selection but instead increase substrate affinity. As such, E. coli RNase III can accurately cleave double stranded RNA after the deletion of its dsRBD (Sun et al., 2001); Giardia intestinalis Dicer (GiDicer, MacRae et al., 2006) and Bacillus subtilis Mini-III (BsMini-III, Redko et al., 2008) do not even have a dsRBD (Figure 1A). In contrast, the dsRBD of Rnt1p was shown to be required for the recognition of dsRNA capped with NGNN tetraloop (Nagel and Ares, 2000). However, the basis of this substrate selectivity remained unclear, because previous structures using truncated dsRBD did not show that the Gua16 in the tetraloop is specifically recognized by the new structural motif we termed the G-clamp. The G-clamp structure is formed by seven RBM0 and one RBM1 residues, resulting in perfect shape complementarity and base-specific hydrogen bonds (Figure 4). The discovery of this G-specific motif illustrates how the dsRBD may acquire affinity to RNA with specific motif and opens the door for designing other clamp structures capable of targeting RNAs harboring different tetraloop sequences and broaden the substrate specificity of RNase III.
The 7-residue flexible linker between the RIIID and dsRBD of AaRNase III (Figure S2) allows substantial conformational changes between the two domains, suggesting that RNase III first binds its substrate using one dsRBD, and by free rotation of the RNA-bound dsRBD with respect to the RIIID dimer subsequently brings the RNA in alignment with the catalytic valley for cleavage (Gan et al., 2005). Similarly, the dsRBD of Rnt1p may also perform the initial recognition of the substrate using RBM0, leading to further binding to the stem through interactions with RBMs 1–4. Indeed, in the absence of Mg2+, Rnt1p formed a stable complex with RNA in a dsRBD-dependent manner (Lavoie and Abou Elela, 2008).
The Double-Ruler Mechanism for Substrate Selection
Soon after the discovery of bacterial RNase III, it was noted that the cleavage products of long dsRNAs by this enzyme are of similar size, giving rise to the idea that the enzyme acts as a molecular ruler measuring the number of nucleotides from one end to its cleavage site (Court, 1993; Nicholson, 1999). However, it was Rnt1p that provided the first concrete example of cleavage at a fixed distance from a specific RNA structure, namely the NGNN tetraloop (Chanfreau et al., 2000). This pattern of cleavage is induced by a combination of structural and base-specific interactions with the stem-loop as summarized in Figure 7A. The Rnt1p:RNA structure shows that the initial binding and positioning box (IBPB), which consists of the conserved NGNN tetraloop, is recognized by RBMs 0 and 1 as well as the NTD dimer, the binding stability box (BSB) is recognized by RBM1 and the α2 helix of dsRBD, and the cleavage efficiency box (CEB) is recognized by RBM3. In addition to these established RNA epitopes (Lamontagne et al., 2003), the structure also identifies the middle box (MB) as a site for the interaction of RBMs 2 and 4 with the 9th base pair below the tetraloop. We conclude that the MB is required for stabilizing the protein:RNA complex, especially when the substrate is too short to allow all the RBMs of both subunits to contact the RNA.
Figure 7. Double-Ruler Mechanism for Substrate Selection by Rnt1p.
(A) Schematic representation of Rnt1p interactions with G2 substrates. Nucleotides are shown as rectangles, while RBMs and NTDs as ellipsoids. C1 and C2 indicate the two canonical cleavage sites. The four boxes of the RNA substrate are outlined with dashed lines. Sites of hydrogen bonds formed between proteins and RNA backbone are shown as shaded rectangles and labeled with residue numbers only, whereas nucleotides forming base-specific hydrogen bonds are labeled by both one-letter code and residue number. Lowercase letters c, f, and s indicate, respectively, positions of chemical interference (Ghazal and Elela, 2006), hydrogen bonds requirement for cleavage (Lavoie and Abou Elela, 2008), and sites where hydrogen bonds were observed with the ribose 2′-OH in the dsRBD:RNA structure (PDB entry 1T4L).
(B) Schematic representation of the ΔNTD interactions with the G2 substrates. C1 and C2 indicate the two canonical cleavage sites by Rnt1p. C* signifies additional cleavage sites produced by the ΔNTD.
Together, the structural and functional information suggests a new mechanism of substrate recognition, the double-ruler mechanism, which uses the NTD dimer as a second ruler to ensure the precision of cleavage (Figure 6D). The additional cleavage sites on the G2 substrates observed in the in vitro cleavage reaction catalyzed by ΔNTD (Figures 6B and 6C) are summarized in Figure 7B. This unusual double-ruler mechanism for substrate selection represents an example of molecular ruler evolution and provides a framework for understanding the mechanism of eukaryotic RNase III action.
EXPERIMENTAL PROCEDURES
Crystal Structure Determination
The Rnt1p:RNA complex was made by incubation prior to crystallization. Initial screening for crystallization conditions was carried out with a Hydra II Plus One robot system (Matrix Technologies Corporation). X-ray diffraction data were collected at the SER-CAT 22-ID beamline of the Advanced Photon Source and processed using program XDS (Kabsch, 2010). The structure was solved by molecular replacement (MR) and difference Fourier synthesis. X-ray data and structure refinement statistics are listed in Table 1. The MR search was very difficult due to protein degradation. For details, see Supplemental Experimental Procedures.
In vitro Cleavage Assays
In vitro cleavage reactions were conducted as described (Lavoie and Abou Elela, 2008). For a brief description, see Supplemental Experimental Procedures.
Electrophoretic Mobility Shift Assays (EMSA)
EMSA assays were performed as described (Lamontagne et al., 2001) with less than 10 fmols of radiolabeled RNA and Rnt1p concentrations ranging from 500 to 4000 nM. Kd values were calculated using a single binding site non-linear regression model. The experiment was repeated three times.
Surface Plasmon Resonance
RNA binding over Rnt1p was monitored using the T200 Biacore system (GE healthcare life sciences, Baie d’Urfe Quebec, Canada) in 30 mM HEPES (pH 7.5), 150 mM KCl, and 10 nM EDTA. Different versions of His-tagged Rnt1p (Lamontagne et al., 2001) were immobilized on Nickel-NTA chip via the His-tag prior to RNA injection. The surface was washed with 350 mM EDTA and 0.1% SDS between each sample. Steady state levels of binding were calculated relative to the amount of protein bound to the chip for each RNA concentration to generate RU/Rmax values. The experiment was repeated three times. Kd values were calculated using a single binding site non-linar regression model and presented in Table S1.
Supplementary Material
Highlights.
The structure of yeast RNase III (Rnt1p) post-cleavage complex is determined
The substrate-binding mode of Rnt1p is distinct from that of bacterial RNase III
A new RNA-binding motif of Rnt1p functions as a guanine-specific clamp
Rnt1p uses an unusual double-ruler mechanism for substrate selection
Acknowledgments
We thank Catherine Desrosiers for help with protein purification, Jules Gagnon for tetraloop sequence conservation analysis, and Donald Court, George Mackie, and Alexander Wlodawer for discussion. X-ray diffraction data were collected at the SER-CAT 22-ID beamline of the Advanced Photon Source, Argonne National Laboratory. This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (X.J.) and a grant from the Canadian Institute of Health Research (S.A.E.).
Footnotes
ACCESSION NUMBER
Atomic coordinates and structure factors have been deposited in the Protein Data Bank (PDB) under entry code 4OOG.
Supplemental Experimental Procedures, Supplemental References, five Supplemental Figures, and two Supplemental tables can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abou Elela S, Ares M., Jr Depletion of yeast RNase III blocks correct U2 3′ end formation and results in polyadenylated but functional U2 snRNA. EMBO J. 1998;17:3738–3746. doi: 10.1093/emboj/17.13.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abou Elela S, Igel H, Ares M., Jr RNase III cleaves eukaryotic preribosomal RNA at a U3 snoRNP-dependent site. Cell. 1996;85:115–124. doi: 10.1016/s0092-8674(00)81087-9. [DOI] [PubMed] [Google Scholar]
- Catala M, Tremblay M, Samson E, Conconi A, Abou Elela S. Deletion of Rnt1p alters the proportion of open versus closed rRNA gene repeats in yeast. Mol Cell Biol. 2008;28:619–629. doi: 10.1128/MCB.01805-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanfreau G, Buckle M, Jacquier A. Recognition of a conserved class of RNA tetraloops by Saccharomyces cerevisiae RNase III. Proc. Natl. Acad. Sci. USA. 2000;97:3142–3147. doi: 10.1073/pnas.070043997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanfreau G, Legrain P, Jacquier A. Yeast RNase III as a key processing enzyme in small nucleolar RNAs metabolism. J Mol Biol. 1998;284:975–988. doi: 10.1006/jmbi.1998.2237. [DOI] [PubMed] [Google Scholar]
- Court DL. RNA processing and degradation by RNase III. In: Belasco JG, Brawerman G, editors. Control of Messenger RNA Stability. New York: Academic Press; 1993. pp. 71–116. [Google Scholar]
- Court DL, Gan J, Liang Y-H, Shaw GX, Tropea JE, Costantino N, Waugh DS, Ji X. RNase III: Genetics and Function; Structure and Mechanism. Annu Rev Genet. doi: 10.1146/annurev-genet-110711-155618. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danin-Kreiselman M, Lee CY, Chanfreau G. RNAse III-mediated degradation of unspliced pre-mRNAs and lariat introns. Mol Cell. 2003;11:1279–1289. doi: 10.1016/s1097-2765(03)00137-0. [DOI] [PubMed] [Google Scholar]
- Du Z, Lee JK, Tjhen R, Stroud RM, James TL. Structural and biochemical insights into the dicing mechanism of mouse Dicer: A conserved lysine is critical for dsRNA cleavage. Proc Natl Acad Sci USA. 2008;105:2391–2396. doi: 10.1073/pnas.0711506105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan J, Shaw G, Tropea JE, Waugh DS, Court DL, Ji X. A stepwise model for double-stranded RNA processing by ribonuclease III. Mol Microbiol. 2008;67:143–154. doi: 10.1111/j.1365-2958.2007.06032.x. [DOI] [PubMed] [Google Scholar]
- Gan J, Tropea JE, Austin BP, Court DL, Waugh DS, Ji X. Intermediate states of ribonuclease III in complex with double-stranded RNA. Structure (Camb) 2005;13:1435–1442. doi: 10.1016/j.str.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Gan J, Tropea JE, Austin BP, Court DL, Waugh DS, Ji X. Structural insight into the mechanism of double-stranded RNA processing by ribonuclease III. Cell. 2006;124:355–366. doi: 10.1016/j.cell.2005.11.034. [DOI] [PubMed] [Google Scholar]
- Ghazal G, Elela SA. Characterization of the reactivity determinants of a novel hairpin substrate of yeast RNase III. J Mol Biol. 2006;363:332–344. doi: 10.1016/j.jmb.2006.08.028. [DOI] [PubMed] [Google Scholar]
- Gu S, Jin L, Zhang Y, Huang Y, Zhang F, Valdmanis PN, Kay MA. The loop position of shRNAs and pre-miRNAs is critical for the accuracy of dicer processing in vivo. Cell. 2012;151:900–911. doi: 10.1016/j.cell.2012.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman E, Wang Z, Zhang Q, Roy K, Chanfreau G, Feigon J. Intrinsic dynamics of an extended hydrophobic core in the S.cerevisiae RNase III dsRBD contributes to recognition of specific RNA binding sites. J Mol Biol. 2013;425:546–562. doi: 10.1016/j.jmb.2012.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, Zamore PD. RNAi: nature abhors a double-strand. Current opinion in genetics & development. 2002;12:225–232. doi: 10.1016/s0959-437x(02)00290-3. [DOI] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Crystallogr D. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne B, Abou Elela S. Short RNA guides cleavage by eukaryotic RNase III. PLoS ONE. 2007;2:e472. doi: 10.1371/journal.pone.0000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamontagne B, Elela SA. Purification and characterization of Saccharomyces cerevisiae Rnt1p nuclease. Methods Enzymol. 2001;342:159–167. doi: 10.1016/s0076-6879(01)42543-2. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Elela SA. Evaluation of the RNA determinants for bacterial and yeast RNase III binding and cleavage. J Biol Chem. 2004;279:2231–2241. doi: 10.1074/jbc.M309324200. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Ghazal G, Lebars I, Yoshizawa S, Fourmy D, Elela SA. Sequence dependence of substrate recognition and cleavage by yeast RNase III. J Mol Biol. 2003;327:985–1000. doi: 10.1016/s0022-2836(03)00231-6. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Hannoush RN, Damha MJ, Abou Elela S. Molecular requirements for duplex recognition and cleavage by eukaryotic RNase III: discovery of an RNA-dependent DNA cleavage activity of yeast Rnt1p. J Mol Biol. 2004;338:401–418. doi: 10.1016/j.jmb.2004.02.059. [DOI] [PubMed] [Google Scholar]
- Lamontagne B, Larose S, Boulanger J, Elela SA. The RNase III family: a conserved structure and expanding functions in eukaryotic dsRNA metabolism. Curr Issues Mol Biol. 2001;3:71–78. [PubMed] [Google Scholar]
- Lamontagne B, Tremblay A, Abou Elela S. The N-terminal domain that distinguishes yeast from bacterial RNase III contains a dimerization signal required for efficient double-stranded RNA cleavage. Mol Cell Biol. 2000;20:1104–1115. doi: 10.1128/mcb.20.4.1104-1115.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie M, Abou Elela S. Yeast ribonuclease III uses a network of multiple hydrogen bonds for RNA binding and cleavage. Biochemistry. 2008;47:8514–8526. doi: 10.1021/bi800238u. [DOI] [PubMed] [Google Scholar]
- Lebars I, Lamontagne B, Yoshizawa S, Aboul-Elela S, Fourmy D. Solution structure of conserved AGNN tetraloops: insights into Rnt1p RNA processing. EMBO J. 2001;20:7250–7258. doi: 10.1093/emboj/20.24.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Li WM, Barnes T, Lee CH. Endoribonucleases - enzymes gaining spotlight in mRNA metabolism. The FEBS journal. 2010;277:627–641. doi: 10.1111/j.1742-4658.2009.07488.x. [DOI] [PubMed] [Google Scholar]
- Lykke-Andersen J, Garrett RA. RNA-protein interactions of an archaeal homotetrameric splicing endoribonuclease with an exceptional evolutionary history. EMBO J. 1997;16:6290–6300. doi: 10.1093/emboj/16.20.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae IJ, Doudna JA. Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr Opin Struct Biol. 2007;17:138–145. doi: 10.1016/j.sbi.2006.12.002. [DOI] [PubMed] [Google Scholar]
- MacRae IJ, Zhou K, Li F, Repic A, Brooks AN, Cande WZ, Adams PD, Doudna JA. Structural basis for double-stranded RNA processing by Dicer. Science. 2006;311:195–198. doi: 10.1126/science.1121638. [DOI] [PubMed] [Google Scholar]
- Meaux S, Lavoie M, Gagnon J, Abou Elela S, van Hoof A. Reporter mRNAs cleaved by Rnt1p are exported and degraded in the cytoplasm. Nucleic Acids Res. 2011;39:9357–9367. doi: 10.1093/nar/gkr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel R, Ares M., Jr Substrate recognition by a eukaryotic RNase III: the double-stranded RNA-binding domain of Rnt1p selectively binds RNA containing a 5′-AGNN- 3′ tetraloop. RNA. 2000;6:1142–1156. doi: 10.1017/s1355838200000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson AW. Function, mechanism and regulation of bacterial ribonucleases. FEMS microbiology reviews. 1999;23:371–390. doi: 10.1111/j.1574-6976.1999.tb00405.x. [DOI] [PubMed] [Google Scholar]
- Nicholson AW. The ribonuclease superfamily: forms and functions in RNA maturation, decay, and gene silencing. In: Hannon GJ, editor. RNAi: A Guide to Gene Silencing. Cold Spring Harbor: New York: Cold Spring Harbor Laboratory Press; 2003. pp. 149–174. [Google Scholar]
- Nicholson AW. Ribonuclease III and the role of double-stranded RNA processing in bacterial systems. In: Nicholson AW, editor. Ribonucleases. Berline-Heidelberg; Spinger: 2011. pp. 269–297. [Google Scholar]
- Oppenheim AB, Kornitzer D, Altuvia S, Court DL. Posttranscriptional control of the lysogenic pathway in bacteriophage lambda. Prog Nucleic Acid Res Mol Biol. 1993;46:37–49. doi: 10.1016/s0079-6603(08)61017-x. [DOI] [PubMed] [Google Scholar]
- Ramos A, Grunert S, Adams J, Micklem DR, Proctor MR, Freund S, Bycroft M, St Johnston D, Varani G. RNA recognition by a Staufen double-stranded RNA-binding domain. EMBO J. 2000;19:997–1009. doi: 10.1093/emboj/19.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redko Y, Bechhofer DH, Condon C. Mini-III, an unusual member of the RNase III family of enzymes, catalyses 23S ribosomal RNA maturation in B. subtilis. Mol Microbiol. 2008;68:1096–1106. doi: 10.1111/j.1365-2958.2008.06207.x. [DOI] [PubMed] [Google Scholar]
- Robertson HD, Webster RE, Zinder ND. Purification and properties of ribonuclease III from Escherichia coli. J Biol Chem. 1968;243:82–91. [PubMed] [Google Scholar]
- Sun W, Jun E, Nicholson AW. Intrinsic double-stranded-RNA processing activity of Escherichia coli ribonuclease III lacking the dsRNA-binding domain. Biochemistry. 2001;40:14976–14984. doi: 10.1021/bi011570u. [DOI] [PubMed] [Google Scholar]
- Takeshita D, Zenno S, Lee WC, Nagata K, Saigo K, Tanokura M. Homodimeric structure and double-stranded RNA cleavage activity of the C-terminal RNase III domain of human Dicer. J Mol Biol. 2007;374:106–120. doi: 10.1016/j.jmb.2007.08.069. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hartman E, Roy K, Chanfreau G, Feigon J. Structure of a yeast RNase III dsRBD complex with a noncanonical RNA substrate provides new insights into binding specificity of dsRBDs. Structure. 2011;19:999–1010. doi: 10.1016/j.str.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg DE, Nakanishi K, Patel DJ, Bartel DP. The inside-out mechanism of Dicers from budding yeasts. Cell. 2011;146:262–276. doi: 10.1016/j.cell.2011.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Henras A, Chanfreau G, Feigon J. Structural basis for recognition of the AGNN tetraloop RNA fold by the double-stranded RNA-binding domain of Rnt1p RNase III. Proc Natl Acad Sci USA. 2004;101:8307–8312. doi: 10.1073/pnas.0402627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Brondani V, Billy E, Filipowicz W. Human Dicer preferentially cleaves dsRNAs at their termini without a requirement for ATP. EMBO J. 2002;21:5875–5885. doi: 10.1093/emboj/cdf582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Kolb FA, Jaskiewicz L, Westhof E, Filipowicz W. Single processing center models for human Dicer and bacterial RNase III. Cell. 2004;118:57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.