Abstract
Neural circuits that translate sensory information into motor commands are organized in a feedforward manner converting sensory information into motor output. The superior colliculus (SC) follows this pattern as it plays a role in converting visual information from the retina and visual cortex into motor commands for rapid eye movements (saccades). Feedback from movement to sensory regions is hypothesized to play critical roles in attention, visual image stability, and saccadic suppression, but in contrast to feedforward pathways, motor feedback to sensory regions has received much less attention. The present study used voltage imaging and patch-clamp recording in slices of rat SC to test the hypothesis of an excitatory synaptic pathway from the motor layers of the SC back to the sensory superficial layers. Voltage imaging revealed an extensive depolarization of the superficial layers evoked by electrical stimulation of the motor layers. A pharmacologically isolated excitatory synaptic potential in the superficial layers depended on stimulus strength in the motor layers in a manner consistent with orthodromic excitation. Patch-clamp recording from neurons in the sensory layers revealed excitatory synaptic potentials in response to glutamate application in the motor layers. The location, size, and morphology of responsive neurons indicated they were likely to be narrow-field vertical cells. This excitatory projection from motor to sensory layers adds an important element to the circuitry of the SC and reveals a novel feedback pathway that could play a role in enhancing sensory responses to attended targets as well as visual image stabilization.
Keywords: superior colliculus, voltage imaging
Introduction
The superior colliculus (SC) is a midbrain structure critically involved in rapid eye movement (saccade) generation, target selection, and spatial attention. Saccades are produced by the coordinated activity of at least three feedforward pathways. One pathway from frontal and parietal cortices targets the intermediate/deep (motor) layers of the SC (Fries, 1984; Segraves and Goldberg, 1987; Hall and Lee, 1997; Sommer and Wurtz, 2000). A second pathway from the same cortical areas targets the motor layers of the SC through the basal ganglia (Stanton et al., 1988; Hikosaka et al., 2000). The third pathway from the superficial sensory layers of the SC targets the deeper motor layers of the SC (Behan and Appell, 1992; Lee et al., 1997; Isa et al., 1998; Ozen et al., 2000, 2004). This pathway may underlie ultra-short latency, express saccades, which are linked to behavioral tasks requiring disengagement of attention (Boch et al., 1984; Fischer and Weber, 1993; Paré and Munoz, 1996). In contrast to feedforward pathways, feedback pathways from motor control neurons to sensory neurons are less well understood, although they are thought to play critical roles in spatial attention, visual image stability, and visual suppression. For example, feedback from frontal eye fields modulates the responsiveness of sensory neurons in extrastriate cortex area V4 (Moore and Armstrong, 2003; Moore and Fallah, 2004), consistent with signaling a location for spatial attention. In monkeys, movement information from SC motor layers influences sensory neurons in frontal cortex through the medial dorsal thalamus (Sommer and Wurtz, 2002). This corollary discharge is thought to signal upcoming movements to distinguish self-induced from world-induced motion. The ability to make this distinction is critical for maintaining a stable visual scene. An inhibitory feedback pathway in rodent SC projects from motor to sensory layers (Lee et al., 2007; Phongphanphanee et al., 2011), and may underlie saccadic suppression.
Excitatory feedback from motor to sensory layers of the SC was proposed over 40 years ago to account for the enhancement of the response of visual neurons in SC to a visual stimulus when it was used by a monkey to target a saccade (Goldberg and Wurtz, 1972; Wurtz and Mohler, 1976a). This result led to the proposal of a corollary discharge that propagated upward to the superficial layers to enhance the discharge of sensory neurons. Enhancement has since been found in many brain regions (Wurtz and Mohler, 1976a; Hikosaka and Wurtz, 1983; Moran and Desimone, 1985; Boch, 1986; Colby et al., 1996; Lamme et al., 2000; Li and Basso, 2008), but a direct excitatory pathway from SC motor layers to sensory layers has yet to be described. Here, we used voltage imaging and patch-clamp recording in slices of the rodent SC to obtain the first evidence that the motor layer in the SC sends direct excitatory inputs to superficial layer sensory neurons. This feedback targets narrow-field vertical neurons in the sensory layers. Their projections to the lateral geniculate nucleus (Diamond et al., 1991; Karten et al., 1997) may provide SC motor layers with access to visual cortical streams and interact with sensory signals in striate and extrastriate visual cortex.
Materials and Methods
Slice preparation.
Slices of SC were prepared from Sprague Dawley rats of either sex. The rat is a species that is widely used in the study of SC circuitry (Isa and Hall, 2009; Phongphanphanee et al., 2011). Since the SC is highly conserved (Vanegas, 1984), our examination of circuitry should have relevance to more visual animals. All procedures were approved by the animal care and use committee of the University of Wisconsin–Madison. Animals were rendered unconscious with vaporized isoflurane or with CO2 and decapitated. As described previously (Vokoun et al., 2010), brains were removed and immersed in ice-cold cutting solution containing the following (in mm): 124 NaCl, 3.2 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1 CaCl2, 6 MgSO4, and 10 glucose, bubbled with 95% O2/5% CO2. Slices (400 μm) were cut in a Leica 1200S tissue slicer and maintained in cutting solution for 30 min at room temperature (21−24°C). Slices were then transferred to artificial CSF (aCSF; cutting solution with 2.5 mm CaCl2 and 1.2 mm MgCl2) for 30–60 min before initiating patch-clamp experiments or staining with voltage-sensitive dye. All physiological experiments were conducted while superfusing with 95% O2/5% CO2 bubbled aCSF at room temperature. Patch-clamp recording was performed with slices from 2- to 4-week-old animals and voltage imaging was performed with slices from 3- to 6-week-old animals.
Voltage imaging.
For voltage imaging experiments slices were stained for 45 min with 0.05 mg/ml RH482 (synthesized in this laboratory) at room temperature. Imaging was performed with instrumentation described previously (Wu and Cohen, 1993; Chang and Jackson, 2006; Vokoun et al., 2010). A 464 channel fiber-optic photodiode camera with hexagonal geometry collected light and amplified signals to 5 V/nA of photocurrent. Signals were digitized at a frame rate of 5 kHz using a DAP5200 data acquisition board (Microstar Laboratories) and read into a PC. Data acquisition and stimulation were all controlled by a PC running a computer program developed in this laboratory (Chang and Jackson, 2006). Slices were illuminated with a 100 W tungsten-halogen bulb driven by a Kepco ATE 36–30 power supply. Light passed through a 700 ± 25 nm bandpass filter and was collected by a 10× Olympus objective (NA 0.4). The center-to-center distance between the fields of adjacent photodiodes was 67 μm. Four successive trials of data were acquired at 10 s intervals and averaged. Slices were stimulated with 0.2 ms, 20–100 μA pulses using aCSF-filled micropipettes (tip 5–10 μm), with current supplied by a WPI A385 stimulus isolator gated by computer using the imaging software. An electronically controlled shutter limited light exposure to times of data acquisition.
Voltage imaging traces from individual photodiodes were exported and analyzed on an iMac desktop computer (Apple). The computer program Volumetry v.7 (Dr. G.W. Hennig) was used to construct maximal projection images, sequential plots, and spatiotemporal maps. Maximal projections were used to establish the spatial extent of the responses (Figs. 1C, 2B). Spatiotemporal maps were used in the analysis of the positions of peak responses along the dorsoventral axis (Fig. 1C).
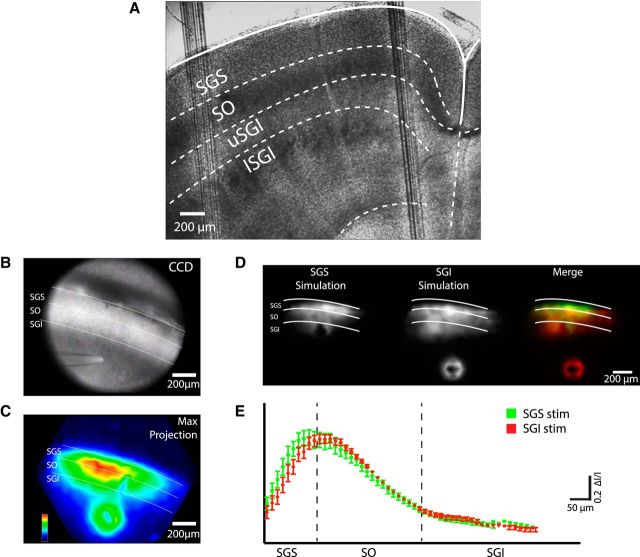
Figure 1.
Upward spread of electrical excitation in a slice of the rat SC. A, Low-magnification view (5×) of a coronal brain slice containing the SC, with demarcations of collicular laminae labeled with white lines. Laminar boundaries are indicated by dashed white curves. B, CCD image of a slice with stimulation electrode in the SGI. C, Map of maximum optical signals from a voltage-sensitive absorbance dye, with peak response amplitude normalized to the maximum in this field of view and encoded as color according to the temperature scale in the lower left corner. Stimulation (100 μA, 0.2 ms) was applied to a site in the SGI through a glass micropipette that is visible in B. The map (C) shows response spread from the SGI to the SO and SGS. White lines highlight the boundaries between lamella in this section and throughout the figure. D, Maps of maximum optical signal (encoded as brightness) for stimulation in SGS (left) and SGI (center). Encoding responses to SGS stimulation with a green scale and responses to SGI stimulation with a red scale, the superimposed images highlight different spatial patterns of responses to stimulation at these two sites, with regions of overlap revealed as yellow (right). E, Plot of maximum response to SGI and SGS stimulation versus distance along a dorsoventral axis (average from seven slices). The response to SGS stimulation peaks in the SGS (green) and the response to SGI stimulation peaks in the SO (red). These peaks appear at significantly different locations (p = 0.024; see text).
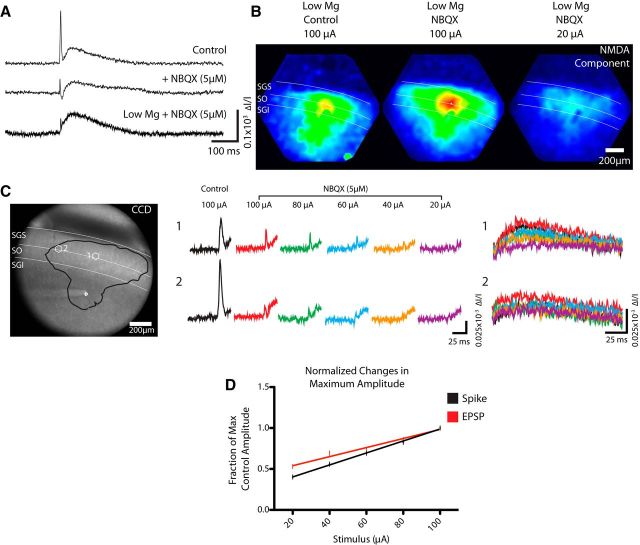
Figure 2.
A, Optical traces from a location in the SGS show a control response with a rapid initial spike and slow afterdepolarization (top trace). NBQX (5 μm) reduced both components (middle trace). Reducing Mg2+ to 100 μm enhanced the activation of NMDA receptors, revealing an NMDA receptor-mediated EPSP in the same time window in which the afterdepolarization appeared under control conditions (lower trace). B, Map of the maximum signal in the 10–200 ms time window, with amplitude encoded as color according the scale in Figure 1B. The first map shows the afterdepolarization evoked by 100 μA stimulation in the SGI; the second shows the NMDA receptor-mediated EPSP in NBQX and low Mg2+, again with 100 μA stimulus current. The third map shows the NMDA receptor-mediated EPSP evoked by 20 μA. The three maps were all normalized to the maximum in the middle map. C, A CCD image shows the site of stimulation and sites from which two traces are displayed to the right. The region with responses within 50% of the maximum is outlined in black. Two hexagonal locations, labeled 1 and 2, were selected to show the two response components for different stimulus currents. Middle traces show initial spikes evoked by different current intensities indicated above. Right traces show NMDA receptor-mediated EPSPs (in the 10–200 ms poststimulus time window) evoked by the same current intensities (indicated by colors used for initial-spike traces). D, Plots of initial-spike and NMDA receptor-mediated EPSP amplitudes recorded in NBQX/low Mg2+ versus stimulus current. Each is normalized to the value of the event evoked by 100 μA in control solution. Reducing the stimulus current reduced the spike by ∼3-fold and the EPSP by <2-fold. The fitted lines have significantly different slopes and intercepts (see text).
The AMPA-type glutamate receptor antagonist 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX) was obtained from Sigma-Aldrich.
Patch-clamp recording.
Slices were viewed in a Zeiss Axioskop microscope with IR-DIC optics and a 63× objective. Patch electrodes fabricated from borosilicate glass filled with 130 mm K-gluconate, 10 mm HEPES, 7 mm KCl, 1 mm EGTA, 2 mm Na2ATP, and 2 mm MgATP, pH 7.2, had resistances of 3–5 MΩ. After establishing whole-cell recordings, voltage or current was measured with an Axopatch 200B amplifier (Molecular Devices) connected through a Digidata 1440A interface to a PC running pClamp 9.0. Iontophoresis of glutamate was performed using high-resistance electrodes (28–32 MΩ) filled with 1 m l-glutamate. l-glutamate was ejected by 1 s pulses of −500 nA using a WPI A385 stimulus isolator.
Analysis of patch-clamp data was performed using Clampfit. The frequency of spontaneous synaptic events was determined by a template-matching search within 9 s stretches of voltage using the computer program Volumetry. Quantification of spike adaptation was performed by analyzing recordings of neurons driven to spike by current injection for 1 s. The interspike intervals were determined by detecting spikes via a threshold search.
For the analysis of spontaneous EPSCs voltage-clamp recordings at −70 mV from responsive and nonresponsive neurons were filtered at 1 kHz off-line. Events larger than 7 pA were detected using the computer program Synaptosoft, which was used to determine amplitude, rise time, and e-fold decay time. Averaged values from responsive and nonresponsive neurons were computed and plotted as bar graphs. For event averages the event detection function of Clampfit was used to select events by template matching. Events were averaged for each cell and then averaged over cells (Fig. 6B).
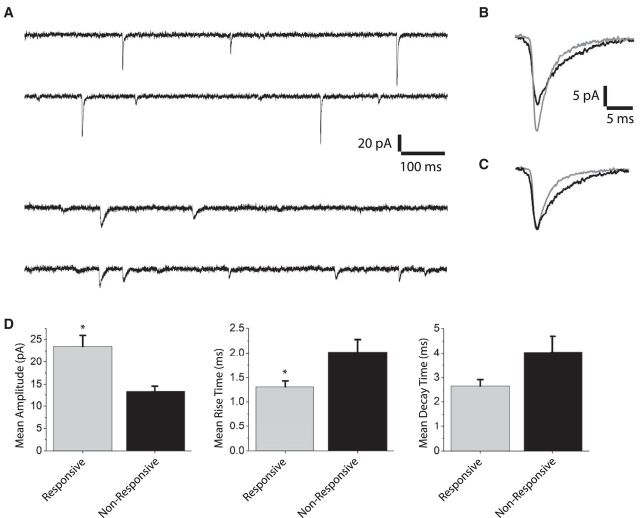
Figure 6.
Spontaneous synaptic currents from responsive and unresponsive SO neurons. A, Voltage-clamp traces from a responsive neuron (top two traces) and an unresponsive neuron (bottom two traces) held at −70 mV. A total of 103 events from 6 responsive and 125 events from 7 nonresponsive neurons were detected by template matching analysis and averaged for each cell. B, Averaged plots for all responsive (red) and unresponsive cells (black) were superimposed, and (C) normalized to their maximum amplitude. The amplitude, rise time, and e-fold decay time were determined with the mini analysis program Synaptosoft, and average values are plotted in D. Responsive neurons have a significantly higher mean amplitude (p = 0.002) and significantly shorter mean rise time (p = 0.034). The difference in mean decay time was not significant (p = 0.09) when based on cell number, but when we used the number of EPSCs the difference in decay times was significant.
Morphology.
The fluorescent tracer Lucifer yellow was added to the patch pipette solution (2–5 mg/ml) to obtain morphology of neurons after recording. Slices were fixed with 4% paraformaldehyde and mounted on gelatin-coated slides, dehydrated in ethanol, and cleared in winter green oil. Images of labeled neurons were acquired with an Ultima two-photon microscope (Prairie Technologies). Maximal projections of two-photon image stacks were constructed in Volumetry. Neuronal outlines were created in Photoshop CS6 (Adobe Systems) using layers masking and freehand highlighting of the neuronal soma and dendrites.
Statistical analysis.
Statistical analysis was performed in Prism 5 (GraphPad Software). Statistical significance was assessed with an unpaired student's t test using p < 0.05 as the criterion for significance.
Results
Imaging population activity in the SC
The SC is a laminated structure (Fig. 1A). In coronal section, the stratum griseum superficiale (SGS) lies immediately below the zonal layer on the dorsal surface of the SC. The SGS is sometimes divided into two sublayers, an upper and lower. Below the SGS is the stratum opticum (SO), which contains neurons but is made up largely of fibers arising from the retina and visual cortex. Together the SGS and SO are referred to as the superficial or sensory layers, a terminology we will use here. Below the SO lies the stratum griseum intermediale (SGI), which is subdivided into either two or three layers. Figure 1A shows the upper and lower layers as uSGI and lSGI, respectively. These layers contain the neurons that project to eye movement centers in the brainstem and are thus considered motor. We will refer to these layers as intermediate and deep, or as motor layers (May, 2006).
To map out the spread of population activity within and between these layers, we performed voltage imaging experiments in rat SC slices stained with a voltage-sensitive dye, to track electrical activity as a change in light intensity. A CCD image with twice the magnification of Figure 1A shows an SC slice used in an experiment with a stimulating electrode positioned in the SGI (Fig. 1B). Electrical stimulation at this site elicited responses through much of the region in view, as illustrated by a map of peak response amplitude with intensity encoded as color (Fig. 1C). Responses spread dorsally into the superficial, sensory layers and then laterally throughout the extent of the superficial layers. Electrical activity initiated around the stimulating electrode positioned in the SGI, but the SO and SGS responded with much larger amplitude signals that then spread laterally over a far more extensive area than in the SGI. The spread through the SO and SGS was very striking, and much greater than that seen with direct stimulation of the SGS itself (Vokoun et al., 2010). Furthermore, the spatial profile of responses along the dorsoventral axis differed between responses evoked by stimulation in the SGI and SGS. Maximal responses to SGS stimulation appeared within the SGS, whereas responses to SGI stimulation peaked at a more ventral location, in the SO and lower SGS. Figure 1D shows response maps from the same slice, with stimulation in the SGS (left) or SGI (center). Using red for responses to SGI stimulation and green for responses to SGS stimulation, these maps, when combined, revealed a yellow band of overlap flanked by a dorsally located green band with a greater response to SGS stimulation and a ventrally located red band with a greater response to SGI stimulation (Fig. 1D, right). Plotting response versus position along the dorsoventral axis showed an offset in the peak response to SGI versus SGS stimulation (Fig. 1E). Responses to SGS stimulation peaked at 97 ± 15 μm, whereas responses to SGI stimulation peaked at 146 ± 16 μm (N = 7, p = 0.024) from the dorsal surface. Thus, stimulation of the SGI activates a population of neurons within the lower superficial layers that are more ventral than the population of neurons activated by direct SGS stimulation.
The spread of electrical activity from the SGI to the SO and SGS illustrated in Figure 1 has three possible explanations. (1) The stimulus could activate cell bodies in the SGI that project orthodromically to the superficial layers. (2) Axons originating from neurons in the superficial layers could be activated antidromically when we stimulate the SGI. Such antidromic activation could include collaterals of descending axons that form excitatory synapses in the superficial layer. (3) The stimulus could activate axons of passage originating outside the SC and targeting superficial layers. To distinguish the first possibility from the second two, we modified our experimental protocol to accentuate the differences between orthodromic and antidromic responses. Optical responses within the SC exhibit two temporal components: a rapid initial spike (1–5 ms) followed by a slower afterdepolarization (10–200 ms; Fig. 2A, top trace; Vokoun et al., 2010). To resolve spikes and synaptic responses more clearly, we applied the AMPA-type glutamate receptor antagonist NBQX, which reduced but did not eliminate both the initial spike and the afterdepolarization (Fig. 2A, middle trace). We then reduced Mg2+ to unblock and thus enhance synaptic responses mediated by NMDA-type glutamate receptors (Fig. 2A, bottom trace). Under these conditions, electrical stimulation in the SGI still evoked responses in the superficial layers (Fig. 2B), but the initial spike reflected action potentials with no synaptic contributions from AMPA receptors. Now the slower, long-duration component largely reflected an NMDA receptor-mediated EPSP (Fig. 2A, bottom trace).
This separation of the two response components in NBQX and low Mg2+ created a condition in which we could resolve antidromic and orthodromic responses by varying the stimulus strength. It is well established that the sensitivity of a cell to extracellular stimulation is inversely proportional to its size, and that larger stimulus currents are generally needed to activate smaller structures (Tehovnik et al., 2006). Our constant current stimulation sets up an electrical field, E, that varies with distance from the stimulus electrode, and induces a potential across the membrane of a neuron of diameter D that is proportional to DE (Hibino et al., 1991). (Orientation and time-dependent contributions are irrelevant and have been neglected.) A typical neuronal soma with a 10 μm diameter will therefore be subjected to a >10-fold larger voltage gradient compared with a typical submicron axon. Furthermore, the axon hillock emanating from a neuron soma generally has a higher density of Na+ channels making it more excitable. For these two reasons, as we reduce the stimulus current, responses will become smaller but they will also be enriched in orthodromic responses due to the much greater reduction in the activation of fine distal axons relative to large neuronal cell bodies and dendrites, and more excitable axon hillocks.
Titrating down the stimulus current reduced the initial spike more than the late NMDA receptor-mediated synaptic potential, as can be seen in both selected traces (Fig. 2C), and in a plot of amplitude (normalized to the maximum at 100 μA) versus stimulus current (Fig. 2D). The plots of these two components had significantly different slopes (p = 0.024) and intercepts (p = 0.0012), indicating that the initial spike and late EPSP differ in their sensitivity to stimulus current. The weaker decline in EPSP amplitude with stimulus current supports the interpretation that this component has a greater contribution from the activation of larger nerve cell bodies than fine axons, whereas the initial spike reflects predominantly antidromic activation arising from the depolarization of axons. Reducing the stimulus current filtered out the antidromic spike but preserved the NMDA receptor-mediated EPSP. These results thus indicate that the first possible explanation put forward above, an ascending excitatory orthodromic projection, makes a significant contribution to the responses seen in the superficial layers following SGI stimulation.
Mapping responses with glutamate application
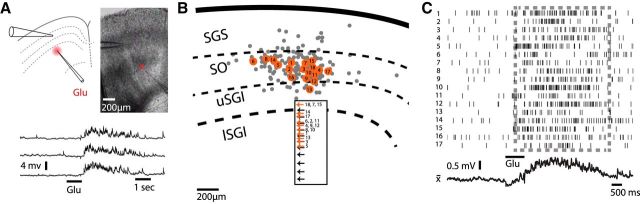
To map synaptic pathways at the cellular level we performed whole-cell patch-clamp recordings in SC slices, and used l-glutamate iontophoresis to activate neuronal cell bodies and dendrites at selected locations. Iontophoresis of l-glutamate within 100 μm of the cell body of a neuron under recording directly depolarized the neuron and elicited an intense train of action potentials (Fig. 3A). Placing the iontophoresis electrode beyond 100 μm from the cell body of a neuron in the SGI failed to induce action potentials in any neurons tested (data not shown). Applying l-glutamate in the superficial layers elicited subthreshold EPSPs in 5 of 12 neurons in SGI (Fig. 3B,C). Deep neurons responsive to glutamate tended to lie directly ventral to the site of application (Fig. 3C). This observation confirmed the presence of a descending excitatory synaptic pathway projecting from the sensory to motor layers (Behan and Appell, 1992; Lee et al., 1997; Saito and Isa, 2003, 2005 Helms et al., 2004). In most cases only a single location was tested with the iontophoretic electrode but with unresponsive neurons moving the stimulus did not produce a response, indicating that the qualitative nature of the response was not sensitive to position. Nevertheless, the fraction of intermediate/deep neurons receiving excitatory synaptic inputs from superficial layers should be taken as a lower bound reflecting both uncertainties of positioning the iontophoresis electrode and severing of axons during slice preparation.
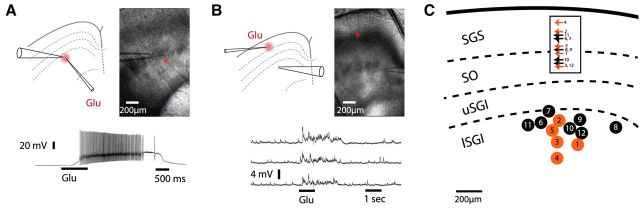
Figure 3.
Iontophoresis of l-glutamate evoked action potentials and synaptic potentials in patch-clamped SC neurons. A, A patch-clamped neuron (current-clamp mode) near the site of l-glutamate application responded directly with a depolarization and action potentials to l-glutamate application < 100 μm from the recording. In A and B the narrow iontophoretic pipette labeled Glu indicates the site of l-glutamate application and the wider patch pipette indicates the location of the neuron under recording. B, An SGI neuron responded with synaptic potentials to l-glutamate application in the SGS (in 5 of 12 neurons). Three voltage traces show three consecutive trials, with the neuron under recording responding with a burst of subthreshold EPSPs. C, A diagram depicting the locations of all 12 neurons from which recordings were made. Numbered black circles denote glutamate-unresponsive neurons and orange circles denote glutamate-responsive neurons. The sites of application are indicated in the SGS corresponding to each numbered neuron. The iontophoretic pipette contained 1 m l-glutamate and delivered 1 s, 500 nA pulses in each trial (bar below each trace).
Applying glutamate in the SGI (mainly lSGI) elicited EPSPs in superficial layer neurons (Fig. 4A), but the fraction of responsive neurons was small (10%; 17 of 168 neurons). Neurons were generally held near the Cl− Nernst potential (−71 mV) to avoid contamination from inhibitory synaptic inputs. Inhibitory potentials of opposite sign could be seen when the neurons were held at less negative potentials of approximately −50 mV. Plotting the location of the responsive and unresponsive neurons shows that responses were confined largely to neurons whose cell bodies resided in the SO (Fig. 4B). No neurons with cell bodies in the SGS ever responded with EPSPs to l-glutamate application in the SGI (n = 16). Following l-glutamate application, the 17 responsive neurons in the SO presented a high rate of EPSPs for ∼2 s (Fig. 4A,C), with a time course similar to that of the action potentials observed in neurons depolarized directly by glutamate (Fig. 3A). This suggests that the synaptic potentials observed in distant neurons in the SO were activated by presynaptic action potentials initiated in the SGI. These single-neuron experiments provide direct evidence for an upward excitatory projection from the intermediate/deep layers to the superficial layers of the SC. The finding that only 10% of the SO neurons were responsive to SGI stimulation suggests that this pathway is sparser than either the descending excitatory (Fig. 3) or the ascending inhibitory pathways within the SC (Lee et al., 1997; Saito and Isa, 2003, 2005; Helms et al., 2004; Lee et al., 2007). The confinement of responsive neurons to the SO is consistent with the location of the peak of the optical response to SGI stimulation (Fig. 1D,E). The extension of depolarization into the SGS then reflected voltage spread from neuronal cell bodies in the SO into their dendrites in the SGS. Figure 4C shows the timing of the peaks of the EPSPs from each of the 17 responsive neurons in the SO, with the mean current trace from these neurons combined in the plot below. This illustrates the general temporal pattern in the responses of SO neurons to the activation of the ascending synaptic pathway by l-glutamate iontophoresis.
Figure 4.
Iontophoresis of l-glutamate in the SGI evokes synaptic potentials in SO neurons. A, Application of l-glutamate (as in Fig. 3) to the SGI elicited synaptic potentials in a patch-clamped SO neuron (in 17 of 168 neurons tested). Three voltage traces show subthreshold EPSPs resulting from the activation of the ascending excitatory pathway. B, A diagram as in Figure 3C depicts the locations of all 168 neurons from which recordings were made. Gray circles denote unresponsive neurons and orange circles with numbers indicate responsive neurons. The sites of application are indicated in the SGI corresponding to each numbered neuron. C, The time of occurrence of each synaptic potential in all 17 responsive neurons is represented by a vertical line with each neuron numbered according to its location in B. The dashed grey rectangle highlights the time of high activity. The trace below the raster plot presents the average voltage response from all responsive neurons.
Properties of SO neurons receiving synaptic inputs from the SGI
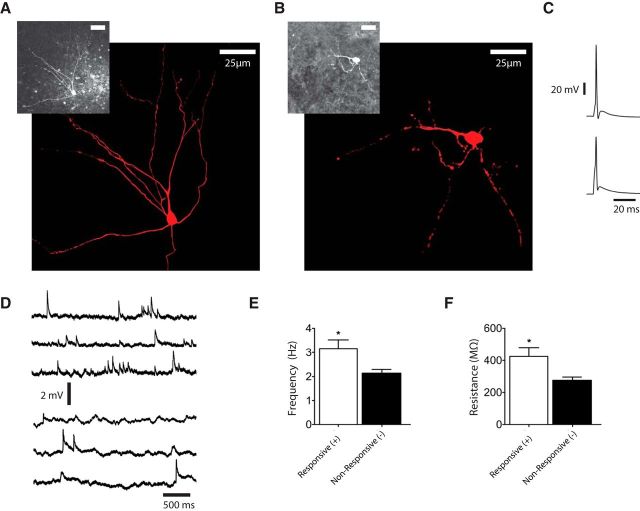
Because the fraction of SO neurons receiving excitatory inputs from the SGI was small, we compared the responsive and unresponsive neurons in more detail to identify distinguishing cellular properties and explore the possibility that they belong to distinct classes of neurons. Neurons were filled with a fluorescent dye to assess their morphology. Of those neurons responding with EPSPs to the application of l-glutamate in the SGI, we were able to visualize five and they all had smaller dendritic arbors with very little horizontal spread (Fig. 5B). Of 14 visualized unresponsive cells, 10 had larger dendritic arbors with extensive horizontal spread (Fig. 5A). Injection of current pulses evoked action potentials, and in both responsive and unresponsive neurons the action potentials were followed by afterdepolarizations (Fig. 5C), qualitatively resembling the population responses observed with voltage-sensitive dye (Fig. 2A; Vokoun et al., 2010). In the absence of stimulation both types of neurons generated spontaneous EPSPs (Fig. 5D). Responsive neurons in the SO could be distinguished from unresponsive neurons by their significantly higher spontaneous EPSP frequency (Fig. 5E; p = 0.019). Furthermore, responsive neurons were observed to have a higher resistance than unresponsive neurons (Fig. 5F; p = 0.023), supporting the morphological observation that responsive neurons are smaller.
Figure 5.
Properties of SO neurons responsive and unresponsive to l-glutamate application in the SGI. Morphological reconstructions of an unresponsive (A) and a responsive (B) neuron. Neurons were filled with Lucifer yellow during recording and imaged with a two-photon microscope. Z-stack projections (insets) were traced in Photoshop (in red). C, Action potentials elicited by brief current pulses in the neuron shown in A (top trace), and B (bottom trace). D, Current-clamp records reveal spontaneous EPSPs in a responsive neuron (top three traces) and an unresponsive neuron (bottom three traces). E, The average EPSP frequency in the 151 unresponsive neurons was lower than the average from the 17 responsive neurons (p = 0.020). F, Whole-cell resistance was measured under voltage clamp and responsive neurons had a higher resistance than unresponsive neurons (p = 0.023).
The different frequency of EPSPs between responsive and unresponsive neurons prompted a more detailed analysis of spontaneous events. Switching to voltage clamp, we recorded spontaneous EPSCs. The traces revealed different appearances between the two cell types (Fig. 6A), and averages of all the events revealed a difference in amplitude (Fig. 6B). Normalizing these two averages revealed that synaptic events in responsive cells have faster kinetics (Fig. 6C). Further quantification revealed that the spontaneous EPSCs of responsive cells had larger amplitudes, shorter rise times, and shorter decay times (Fig. 6D). The differences in amplitude and rise time were statistically significant, and the p value for decay times was 0.09. This statistical analysis used a more conservative approach based on the number of neurons. However, a statistical analysis based on the number of events made the differences highly significant for all quantities. Since we do not know how much of the variance reflects differences between neurons and how much reflects variations in EPSCs recorded from a single neuron, the significance of the difference in decay times remains in question but the overall comparison clearly indicates that the spontaneous EPSCs differ in both amplitude and kinetics between responsive and unresponsive cells. This indicates differences in receptor properties and density between SO neurons depending on whether or not they receive excitatory inputs from the SGI.
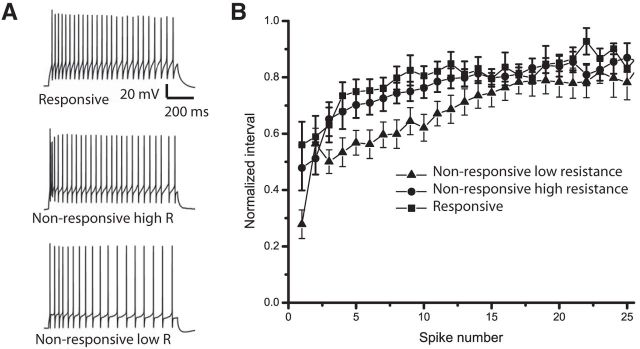
Brief pulses of current elicited single action potentials in SO neurons (Fig. 5C), and sustained current injection elicited trains of action potentials (Fig. 7A). The interval between successive action potentials progressively increased to varying degrees as current was maintained, indicating spike adaptation of SO neurons. This spike adaptation was quite variable, as noted previously for SGI neurons (Saito and Isa, 1999), but it appeared that there was less spike adaptation in SO neurons receiving excitatory inputs from the SGI. To quantify this behavior we determined the interspike intervals throughout a 1 s train and normalized to the maximum interspike interval within that train. Neurons were held within 5 mV of −60 mV and different stimulus currents were tested. The degree of spike adaptation was similar for different currents so we selected a current that evoked spikes over the entire 1 s pulse. When all responsive neurons were compared with all unresponsive neurons it was unclear whether they were different, but we were concerned that the unresponsive group may include responsive neurons with efferent axons severed during slicing or where the iontophoretic electrode missed the efferent neurons. We therefore ranked unresponsive SO neurons by resistance, and compared spike adaptation ratio in the top 20 (high resistance) unresponsive neurons from the bottom 20 (low-resistance) unresponsive neurons. This division was based on the finding noted above that responsive SO neurons had a higher resistance than unresponsive SO neurons (Fig. 5F). Plotting normalized interspike interval versus interval number within the train showed a clear difference in the degree and time course of spike adaptation between responsive neurons and low-resistance unresponsive neurons (Fig. 7B). In contrast, the high-resistance unresponsive neurons were similar to the responsive neurons, and clearly different from the low-resistance unresponsive neurons. The intervals increased 1.67-fold in responsive neurons and 1.89-fold increase in high-resistance, unresponsive neurons, compared with a 3.0-fold increase in the low-resistance, unresponsive neurons. This indicates nearly twofold greater spike adaptation in the low-resistance, unresponsive neurons. This distinction was evident regardless of whether the spike adaptation ratios were measured using the first spike interval of a train, or the averages of the first three or first five spike intervals of a train. In each case, the responsive neurons were indistinguishable from the high-resistance unresponsive neurons (p > 0.25), but were significantly different from the low-resistance unresponsive neurons (p < 0.03). Thus, neurons receiving excitatory inputs from the SGI had somewhat more than half as much spike adaptation compared with low-resistance unresponsive neurons.
Figure 7.
Spike adaptation in SO neurons. A, Neurons were depolarized with 1 s current pulses to elicit trains of action potentials. The top trace was from a responsive neuron. The middle trace was from an unresponsive neuron with a high resistance. The bottom trace was from an unresponsive neuron with a low resistance. B, The interspike intervals were normalized to the longest interval in trains such as those displayed in A and averaged across 10 responsive neurons and the 20 unresponsive neurons with the highest resistance and the 20 unresponsive neurons with the lowest resistance (for analysis of spike adaptation see text).
The distinct spike adaptation properties, spontaneous EPSPs and EPSCs, and resistance values of responsive neurons suggest they may belong to a particular neuronal class. We therefore attempted to identify the neurons receiving upward excitatory inputs. The extensive lateral spread of depolarization in the SGS seen in voltage imaging (Fig. 1A–C; Vokoun et al., 2010) includes both antidromic and orthodromic responses. If the orthodromic component contributes to this lateral spread then the neurons would have to be excitatory, and either have extensive intralaminar projections or activate other neighboring excitatory cells with extensive projections. Three classes of neurons within the superficial SC have been identified as excitatory: wide-field vertical cells, narrow-field vertical cells, and marginal cells (Langer and Lund, 1974; Albano et al., 1979; Mooney et al., 1988; Hall and Lee, 1993, 1997; Lee et al., 2001; Chomsung et al., 2008; Isa and Hall, 2009). Marginal cells are located within the stratum zonale, at sites more dorsal than our voltage imaging and patch-clamp results suggest. Narrow-field vertical cells and wide-field vertical cells, on the other hand, are both found in the lower SGS and SO (Langer and Lund, 1974; Albano et al., 1979; Mooney et al., 1985; Chomsung et al., 2008). The smaller size, inferred from their high resistance (Fig. 5F), and the smaller dendritic arbor (Fig. 5A,B) of responsive neurons, favor narrow-field vertical cells as the most likely targets of the upward excitatory pathway. Although this identification must be regarded as tentative, the vertically projecting dendritic arbors of these neurons extending into the SGS would account for the spread of the depolarization into the superficial layers (Fig. 1A–C). We thus propose that a population of glutaminergic neurons located in the SGI form excitatory synapses with neurons located in the SO, and these neurons, in turn, depolarize adjacent neurons in the SGS. Since the upward inhibitory pathway from the SGI to the SGS targets wide-field vertical cells predominantly (Lee et al., 2007), the two opposing ascending signals from the SGI are able to influence different neuronal populations in their feedback control of the sensory layers. Although other neuronal cell types in the SO may also receive excitatory inputs from the deep layers, any additional parallel stream of information would have to be even sparser than that targeting the narrow-field vertical cells. Further investigation of the physiological properties of superficial neurons receiving excitatory synaptic inputs from the SGI will be an important goal for future studies.
Discussion
The present study provides evidence for a novel, excitatory feedback pathway from the motor layers of the deep SC to the sensory layers in the superficial SC. Using voltage imaging in rodent slices we found that electrical stimulation of the intermediate/deep layers of the SC elicited robust responses in superficial layers. Using pharmacological methods combined with titration of electrical current intensity, we showed that the activity in the superficial layers evoked by stimulation in the motor layer included a substantial orthodromic contribution. We then showed that application of l-glutamate to the intermediate/deep layers, a procedure that precludes antidromic responses and activation of fibers of passage, elicited EPSPs in neurons of the superficial SC. This result provides compelling evidence for excitatory synaptic feedback from motor to sensory layers of the SC. The present study offers the first clear evidence for a motor layer-to-sensory layer excitatory projection in the mammalian SC.
Our physiological and morphological analyses showed that this pathway targets neurons in the superficial layers of the SC, providing a route for motor information to access sensory neurons of the SC and possibly visual cortical pathways. Indeed, a previous study noted that EPSPs could be elicited in SGS neurons by glutamate application in ventral locations of the SC (Lee et al., 2007). However, the glutamate in that study was applied in the SO or uSGI and the aim of that work was to identify an upward inhibitory pathway so the significance of the EPSPs was not discussed. The most parsimonious interpretation of our cellular and network level experiments is that collaterals of gaze center excitatory neurons of the SGI project upward to the SO. However, it is also possible that these neurons are different from the neurons projecting to gaze centers. Experiments using antidromic activation of the predorsal bundle are required to address this issue.
The small size, location, and morphology of the neurons receiving this ascending excitatory projection make it likely that they are narrow-field vertical cells. Iontophoretic mapping indicated an approximately columnar arrangement above the projecting neurons. However, responses to SGI stimulation spread laterally within the SGS and SO (Figs. 1C, 2C) well beyond the vertical column indicated by iontophoresis (Fig. 3B). Lateral spread in these experiments may include antidromic responses as well as polysynaptic spread of the orthodromic response mediated by putative narrow-field cells. Responses to SGI stimulation initially propagate upward and then laterally (Vokoun et al., 2010), and the pathway elucidated here may mediate the first step of this sequence.
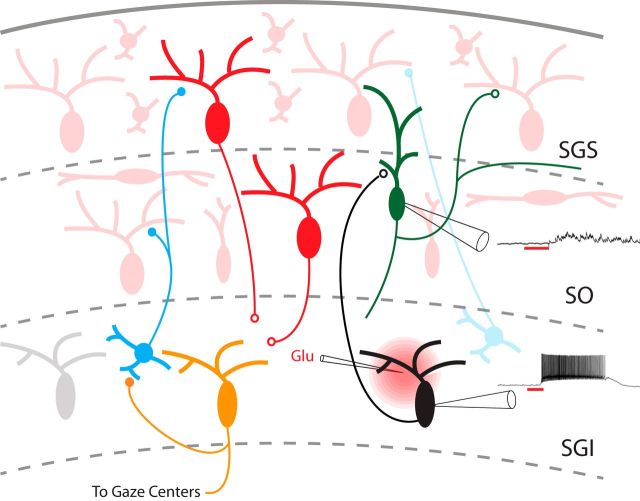
This interpretation would extend the role of SGI projections in both exciting and inhibiting sensory layers. Figure 8 incorporates this new element of synaptic circuitry into the established circuitry of the SC, which includes a descending projection illustrated with red neurons and an ascending inhibitory projection illustrated with blue neurons (Behan and Appell, 1992; Lee et al., 1997, 2007; Ozen et al., 2000; Isa and Hall, 2009). The neuron projecting upward in the SGI is represented in black, with ascending collaterals forming synapses on narrow-field vertical cells in the SO (green). Another SGI neuron projecting to gaze centers and to upward projecting inhibitory neurons (Phongphanphanee et al., 2011) is represented in orange. Whether the upward pathway arises from gaze center axon collaterals is an interesting possibility that cannot be addressed with the data at hand.
Figure 8.
Interlaminar synaptic circuitry in the rat SC. Starting with the circuit arising from a study of an ascending inhibitory pathway (Phongphanphanee et al., 2011), we added an ascending excitatory pathway. Axon collaterals from a premotor neuron (black) in the SGI form synapses on a narrow-field vertical cell (green). The circuit also illustrates the descending excitatory pathway (red to black) and the ascending inhibitory pathway (blue to red). Examples of responses to l-glutamate application in the SGI are shown for an SGI neuron (lower trace) and a responsive SO neuron (upper trace).
If the neurons targeted in this upward pathway are indeed narrow-field vertical cells, their projection to the lateral geniculate nucleus (Albano et al., 1979; Diamond et al., 1991) would provide eye movement control circuits with direct access to sensory neurons in the superficial layers and disynaptic access to visual cortical processing streams. This pathway also appears to be distinct from the recently identified ascending inhibitory pathway, which targets wide-field vertical cells. Since wide-field cells project to the pulvinar nucleus of the thalamus (Lee et al., 2007), the pathway described here can influence different sensory circuits from the inhibitory pathway.
The finding of excitatory feedback from motor to sensory SC is potentially important for the circuitry underlying at least two behavioral phenomena. Making an eye movement to a visual stimulus enhances the discharge of neurons encoding the sensory aspects of the target compared with when the visual stimulus is ignored. This phenomenon, called enhancement, was discovered over 40 years ago and was proposed to underlie spatial attention (Goldberg and Wurtz, 1972; Wurtz and Mohler, 1976b). The investigators proposed that motor signals provide feedback signals to the superficial layers of the SC to enhance sensory neuronal responses. Enhancement is found in many brain regions, including prefrontal cortex (Boch, 1986), V4 (Moran and Desimone, 1985), the lateral intraparietal area (Colby et al., 1996), the substantia nigra pars reticulata (Hikosaka and Wurtz, 1983), and even V1 (Wurtz and Mohler, 1976a; Lamme et al., 2000). Indeed, contrast sensitivity of neurons in the superficial SC increases when monkeys simply prepare to move their eyes, before actually moving them (Li and Basso, 2008). Sensory enhancement is also found in rodents performing tasks requiring attention (Sakata et al., 2002; Fritz et al., 2007) and in owls in response to activation of eye movement pathways (Winkowski and Knudsen, 2007, 2008). The work in birds indicates that the isthmus nucleus (parabigeminal nucleus in mammals) has reciprocal connections with the optic tectum (the avian homolog of the SC) forming a winner-takes-all circuit with neurons of the superficial layers. (Wang et al., 2004, 2006; Marín et al., 2007, 2012). The avian isthmus-tectal circuit shows attentional modulations similar to those found in the sensory cortex and SC of monkeys (Li and Basso, 2005, 2008; Asadollahi et al., 2010). However, the circuit in birds targets wide-field vertical neurons in the SO that project to the nucleus rotundus (the caudal pulvinar in mammals; Karten et al., 1997; Major et al., 2000). The novel pathway we report here thus provides a parallel route through which information about impending eye movements can reach higher areas of visual cortex through the lateral geniculate nucleus. This pathway in mammals is a strong candidate to underlie the enhancement of sensory neuronal responses.
Although this novel excitatory pathway can play a role in enhancement of sensory responses seen in superficial SC, other parallel pathways for attention are likely to exist. For example, recent work in monkeys reveals that inactivation of the intermediate SC inhibits monkeys' performance in a motion-change detection task when the motion information appears in the response field of the inactivated region of the SC (Zénon and Krauzlis, 2012). Despite the attention deficit, simultaneous recordings of motion-selective neurons in the medial temporal area showed that the enhancement of neuronal responses was unaltered. This indicates that the enhancement of medial temporal area sensory responses is not what underlies the ability to perform this task. The extent to which this result is task specific is unclear. Perhaps other attention tasks not requiring the detection of a change in information would reveal altered sensory enhancement. Electrophysiological investigations of these kinds of behavioral tasks may resolve different forms of attention mediated by different neuronal substrates.
A second behavioral phenomenon to which excitatory feedback from the motor to sensory layer of the SC may contribute is visual image stabilization. Although we move our eyes many times a second activating different visual neurons with each movement, we perceive a visual scene that is stable. It is thought that this stability arises from a shifting or remapping of receptive fields of sensory neurons in the lateral intraparietal region of the cerebral cortex concomitant with each eye movement. Monkeys trained on a task requiring remapping that received complete transection of forebrain commissures show intact remapping of receptive fields in the parietal cortex, indicating that the eye movement signal driving the remapping arises from a subcortical source (Dunn and Colby, 2010; Dunn et al., 2010). The upward excitatory pathway within the SC that targets neurons with access to the visual cortex through the lateral geniculate nucleus could provide that signal. Thus, subcortical feedback arising from the SC may also play a critical role in visual image stabilization.
Footnotes
This work was supported by National Institutes of Health grants EY019963, NS072905, and NS078301, and a Parkinson's disease Summer Student Fellowship (C.R.V.). We thank Dr. Grant Hennig for adapting his Volumetry software to facilitate data analysis in the present study. We thank Dr. Helen Scharfman for helpful advice on tissue clearing and imaging morphology.
The authors declare no competing financial interests.
References
- Albano JE, Norton TT, Hall WC. Laminar origin of projections from the superficial layers of the superior colliculus in the tree shrew, Tupaia glis. Brain Res. 1979;173:1–11. doi: 10.1016/0006-8993(79)91090-4. [DOI] [PubMed] [Google Scholar]
- Asadollahi A, Endler F, Nelken I, Wagner H. Neural correlates of binaural masking level difference in the inferior colliculus of the barn owl (Tyto alba) Eur J Neurosci. 2010;32:606–618. doi: 10.1111/j.1460-9568.2010.07313.x. [DOI] [PubMed] [Google Scholar]
- Behan M, Appell PP. Intrinsic circuitry in the cat superior colliculus: projections from the superficial layers. J Comp Neurol. 1992;315:230–243. doi: 10.1002/cne.903150209. [DOI] [PubMed] [Google Scholar]
- Boch R. Behavioral modulation of neuronal activity in monkey striate cortex: excitation in the absence of active central fixation. Exp Brain Res. 1986;64:610–614. doi: 10.1007/BF00340501. [DOI] [PubMed] [Google Scholar]
- Boch R, Fischer B, Ramsperger E. Express-saccades of the monkey: reaction times versus intensity, size, duration and eccentricity of their targets. Exp Brain Res. 1984;55:223–231. doi: 10.1007/BF00237273. [DOI] [PubMed] [Google Scholar]
- Chang PY, Jackson MB. Heterogeneous spatial patterns of long-term potentiation in rat hippocampal slices. J Physiol. 2006;576:427–443. doi: 10.1113/jphysiol.2006.112128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomsung RD, Petry HM, Bickford ME. Ultrastructural examination of diffuse and specific tectopulvinar projections in the tree shrew. J Comp Neurol. 2008;510:24–46. doi: 10.1002/cne.21763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colby CL, Duhamel JR, Goldberg ME. Visual, presaccadic, and cognitive activation of single neurons in monkey lateral intraparietal area. J Neurophysiol. 1996;76:2841–2852. doi: 10.1152/jn.1996.76.5.2841. [DOI] [PubMed] [Google Scholar]
- Diamond IT, Conley M, Fitzpatrick D, Raczkowski D. Evidence for separate pathways within the tecto-geniculate projection in the tree shrew. Proc Natl Acad Sci U S A. 1991;88:1315–1319. doi: 10.1073/pnas.88.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CA, Colby CL. Representation of the ipsilateral visual field by neurons in the macaque lateral intraparietal cortex depends on the forebrain commissures. J Neurophysiol. 2010;104:2624–2633. doi: 10.1152/jn.00752.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn CA, Hall NJ, Colby CL. Spatial updating in monkey superior colliculus in the absence of the forebrain commissures: dissociation between superficial and intermediate layers. J Neurophysiol. 2010;104:1267–1285. doi: 10.1152/jn.00675.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B, Weber H. Express saccades and visual attention. Behav Brain Sci. 1993;16:553–567. doi: 10.1017/S0140525X00031575. [DOI] [Google Scholar]
- Fries W. Cortical projections to the superior colliculus in the macaque monkey: a retrograde study using horseradish peroxidase. J Comp Neurol. 1984;230:55–76. doi: 10.1002/cne.902300106. [DOI] [PubMed] [Google Scholar]
- Fritz JB, Elhilali M, David SV, Shamma SA. Does attention play a role in dynamic receptive field adaptation to changing acoustic salience in A1? Hear Res. 2007;229:186–203. doi: 10.1016/j.heares.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972;35:560–574. doi: 10.1152/jn.1972.35.4.560. [DOI] [PubMed] [Google Scholar]
- Hall WC, Lee P. Interlaminar connections of the superior colliculus in the tree shrew. I. The superficial gray layer. J Comp Neurol. 1993;332:213–223. doi: 10.1002/cne.903320206. [DOI] [PubMed] [Google Scholar]
- Hall WC, Lee P. Interlaminar connections of the superior colliculus in the tree shrew. III: the optic layer. Vis Neurosci. 1997;14:647–661. doi: 10.1017/S095252380001261X. [DOI] [PubMed] [Google Scholar]
- Helms MC, Ozen G, Hall WC. Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. J Neurophysiol. 2004;91:1706–1715. doi: 10.1152/jn.00705.2003. [DOI] [PubMed] [Google Scholar]
- Hibino M, Shigemori M, Itoh H, Nagayama K, Kinosita K., Jr Membrane conductance of an electroporated cell analyzed by submicrosecond imaging of transmembrane potential. Biophys J. 1991;59:209–220. doi: 10.1016/S0006-3495(91)82212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. I. Relation of visual and auditory responses to saccades. J Neurophysiol. 1983;49:1230–1253. doi: 10.1152/jn.1983.49.5.1230. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Takikawa Y, Kawagoe R. Role of the basal ganglia in the control of purposive saccadic eye movements. Physiol Rev. 2000;80:953–978. doi: 10.1152/physrev.2000.80.3.953. [DOI] [PubMed] [Google Scholar]
- Isa T, Hall WC. Exploring the superior colliculus in vitro. J Neurophysiol. 2009;102:2581–2593. doi: 10.1152/jn.00498.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isa T, Endo T, Saito Y. The visuo-motor pathway in the local circuit of the rat superior colliculus. J Neurosci. 1998;18:8496–8504. doi: 10.1523/JNEUROSCI.18-20-08496.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ, Cox K, Mpodozis J. Two distinct populations of tectal neurons have unique connections within the retinotectorotundal pathway of the pigeon (Columba livia) J Comp Neurol. 1997;387:449–465. doi: 10.1002/(SICI)1096-9861(19971027)387:3<449::AID-CNE10>3.0.CO%3B2-G. [DOI] [PubMed] [Google Scholar]
- Lamme VA, Supèr H, Landman R, Roelfsema PR, Spekreijse H. The role of primary visual cortex (V1) in visual awareness. Vision Res. 2000;40:1507–1521. doi: 10.1016/S0042-6989(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Langer TP, Lund RD. The upper layers of the superior colliculus of the rat: a Golgi study. J Comp Neurol. 1974;158:418–435. doi: 10.1002/cne.901580404. [DOI] [PubMed] [Google Scholar]
- Lee PH, Helms MC, Augustine GJ, Hall WC. Role of intrinsic synaptic circuitry in collicular sensorimotor integration. Proc Natl Acad Sci U S A. 1997;94:13299–13304. doi: 10.1073/pnas.94.24.13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Schmidt M, Hall WC. Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J Neurosci. 2001;21:8145–8153. doi: 10.1523/JNEUROSCI.21-20-08145.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Sooksawate T, Yanagawa Y, Isa K, Isa T, Hall WC. Identity of a pathway for saccadic suppression. Proc Natl Acad Sci U S A. 2007;104:6824–6827. doi: 10.1073/pnas.0701934104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci. 2005;25:11357–11373. doi: 10.1523/JNEUROSCI.3825-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Basso MA. Preparing to move increases the sensitivity of superior colliculus neurons. J Neurosci. 2008;28:4561–4577. doi: 10.1523/JNEUROSCI.5683-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major DE, Luksch H, Karten HJ. Bottlebrush dendritic endings and large dendritic fields: motion-detecting neurons in the mammalian tectum. J Comp Neurol. 2000;423:243–260. doi: 10.1002/1096-9861(20000724)423:2<243::AID-CNE5>3.0.CO%3B2-5. [DOI] [PubMed] [Google Scholar]
- Marín GJ, Duran E, Morales C, González-Cabrera C, Sentis E, Mpodozis J, Letelier JC. Attentional capture? Synchronized feedback signals from the isthmi boost retinal signals to higher visual areas. J Neurosci. 2012;32:1110–1122. doi: 10.1523/JNEUROSCI.4151-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín G, Salas C, Sentis E, Rojas X, Letelier JC, Mpodozis J. A cholinergic gating mechanism controlled by competitive interactions in the optic tectum of the pigeon. J Neurosci. 2007;27:8112–8121. doi: 10.1523/JNEUROSCI.1420-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321–378. doi: 10.1016/S0079-6123(05)51011-2. [DOI] [PubMed] [Google Scholar]
- Mooney RD, Klein BG, Rhoades RW. Correlations between the structural and functional characteristics of neurons in the superficial laminae and the hamster's superior colliculus. J Neurosci. 1985;5:2989–3009. doi: 10.1523/JNEUROSCI.05-11-02989.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney RD, Nikoletseas MM, Hess PR, Allen Z, Lewin AC, Rhoades RW. The projection from the superficial to the deep layers of the superior colliculus: an intracellular horseradish peroxidase injection study in the hamster. J Neurosci. 1988;8:1384–1399. doi: 10.1523/JNEUROSCI.08-04-01384.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T, Armstrong KM. Selective gating of visual signals by microstimulation of frontal cortex. Nature. 2003;421:370–373. doi: 10.1038/nature01341. [DOI] [PubMed] [Google Scholar]
- Moore T, Fallah M. Microstimulation of the frontal eye field and its effects on covert spatial attention. J Neurophysiol. 2004;91:152–162. doi: 10.1152/jn.00741.2002. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Ozen G, Augustine GJ, Hall WC. Contribution of superficial layer neurons to premotor bursts in the superior colliculus. J Neurophysiol. 2000;84:460–471. doi: 10.1152/jn.2000.84.1.460. [DOI] [PubMed] [Google Scholar]
- Özen G, Helms MC, Hall WC. The intracollicular neuronal network. In: Hall WC, Moschovakis MA, editors. The superior colliculus: new approaches for studying sensorimotor integration. Boca Raton, FL: CRC; 2004. pp. 147–158. [Google Scholar]
- Paré M, Munoz DP. Saccadic reaction time in the monkey: advanced preparation of oculomotor programs is primarily responsible for express saccade occurrence. J Neurophysiol. 1996;76:3666–3681. doi: 10.1152/jn.1996.76.6.3666. [DOI] [PubMed] [Google Scholar]
- Phongphanphanee P, Mizuno F, Lee PH, Yanagawa Y, Isa T, Hall WC. A circuit model for saccadic suppression in the superior colliculus. J Neurosci. 2011;31:1949–1954. doi: 10.1523/JNEUROSCI.2305-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Isa T. Electrophysiological and morphological properties of neurons in the rat superior colliculus. I. Neurons in the intermediate layer. J Neurophysiol. 1999;82:754–767. doi: 10.1152/jn.1999.82.2.754. [DOI] [PubMed] [Google Scholar]
- Saito Y, Isa T. Local excitatory network and NMDA receptor activation generate a synchronous and bursting command from the superior colliculus. J Neurosci. 2003;23:5854–5864. doi: 10.1523/JNEUROSCI.23-13-05854.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Isa T. Organization of interlaminar interactions in the rat superior colliculus. J Neurophysiol. 2005;93:2898–2907. doi: 10.1152/jn.01051.2004. [DOI] [PubMed] [Google Scholar]
- Sakata S, Kitsukawa T, Kaneko T, Yamamori T, Sakurai Y. Task-dependent and cell-type-specific Fos enhancement in rat sensory cortices during audio-visual discrimination. Eur J Neurosci. 2002;15:735–743. doi: 10.1046/j.1460-9568.2002.01905.x. [DOI] [PubMed] [Google Scholar]
- Segraves MA, Goldberg ME. Functional properties of corticotectal neurons in the monkey's frontal eye field. J Neurophysiol. 1987;58:1387–1419. doi: 10.1152/jn.1987.58.6.1387. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol. 2000;83:1979–2001. doi: 10.1152/jn.2000.83.4.1979. [DOI] [PubMed] [Google Scholar]
- Sommer MA, Wurtz RH. A pathway in primate brain for internal monitoring of movements. Science. 2002;296:1480–1482. doi: 10.1126/science.1069590. [DOI] [PubMed] [Google Scholar]
- Stanton GB, Goldberg ME, Bruce CJ. Frontal eye field efferents in the macaque monkey: I. Subcortical pathways and topography of striatal and thalamic terminal fields. J Comp Neurol. 1988;271:473–492. doi: 10.1002/cne.902710402. [DOI] [PubMed] [Google Scholar]
- Tehovnik EJ, Tolias AS, Sultan F, Slocum WM, Logothetis NK. Direct and indirect activation of cortical neurons by electrical microstimulation. J Neurophysiol. 2006;96:512–521. doi: 10.1152/jn.00126.2006. [DOI] [PubMed] [Google Scholar]
- Vanegas H, editor. Comparative neurology of the optic tectum. New York: Plenum; 1984. [Google Scholar]
- Vokoun CR, Jackson MB, Basso MA. Intralaminar and interlaminar activity within the rodent superior colliculus visualized with voltage imaging. J Neurosci. 2010;30:10667–10682. doi: 10.1523/JNEUROSCI.1387-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Major DE, Karten HJ. Morphology and connections of nucleus isthmi pars magnocellularis in chicks (Gallus gallus) J Comp Neurol. 2004;469:275–297. doi: 10.1002/cne.11007. [DOI] [PubMed] [Google Scholar]
- Wang Y, Luksch H, Brecha NC, Karten HJ. Columnar projections from the cholinergic nucleus isthmi to the optic tectum in chicks (Gallus gallus): a possible substrate for synchronizing tectal channels. J Comp Neurol. 2006;494:7–35. doi: 10.1002/cne.20821. [DOI] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Top-down control of multimodal sensitivity in the barn owl optic tectum. J Neurosci. 2007;27:13279–13291. doi: 10.1523/JNEUROSCI.3937-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkowski DE, Knudsen EI. Distinct mechanisms for top-down control of neural gain and sensitivity in the owl optic tectum. Neuron. 2008;60:698–708. doi: 10.1016/j.neuron.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Cohen LB. Fluorescent and luminescent probes for biological activity. London: Academic; 1993. [Google Scholar]
- Wurtz RH, Mohler CW. Enhancement of visual responses in monkey striate cortex and frontal eye fields. J Neurophysiol. 1976a;39:766–772. doi: 10.1152/jn.1976.39.4.766. [DOI] [PubMed] [Google Scholar]
- Wurtz RH, Mohler CW. Organization of monkey superior colliculus: enhanced visual response of superficial layer cells. J Neurophysiol. 1976b;39:745–765. doi: 10.1152/jn.1976.39.4.745. [DOI] [PubMed] [Google Scholar]
- Zénon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489:434–437. doi: 10.1038/nature11497. [DOI] [PMC free article] [PubMed] [Google Scholar]