Abstract
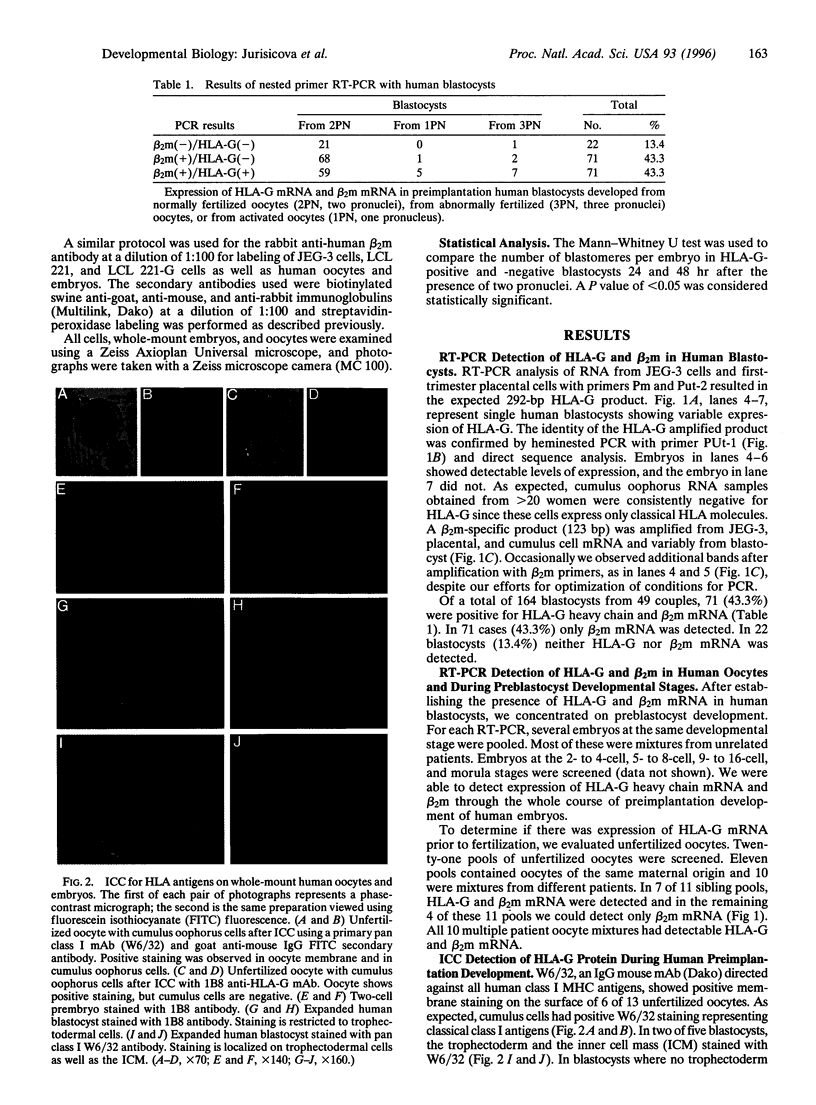
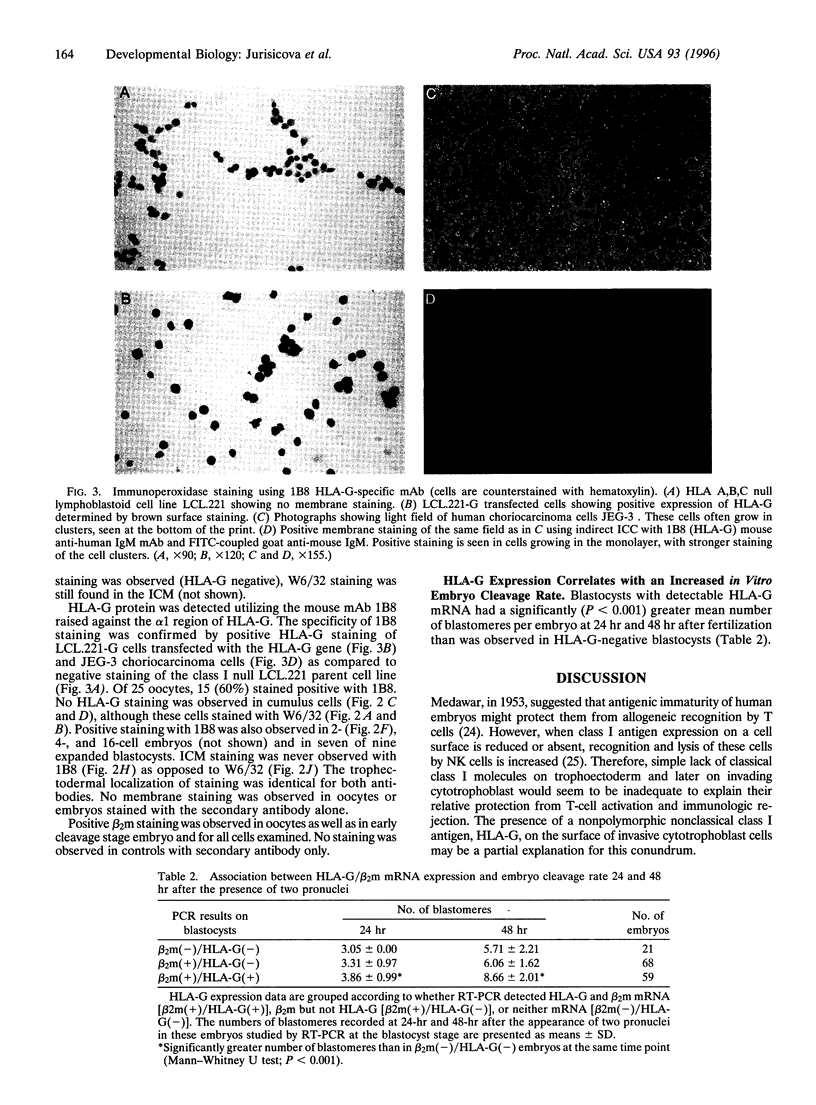
HLA-G is a nonclassical class I major histocompatibility complex molecule with a restricted pattern of expression that includes the placental extravillus cytotrophoblast cells in direct contact with maternal tissues. Circumstantial evidence suggests that HLA-G may play a role in protection of the semiallogeneic human fetus. We examined whether HLA-G is expressed during the critical period of preimplantation human development and whether expression of this molecule could be correlated with the cleavage rate of embryos. Using reverse transcription PCR on surplus human embryos and unfertilized oocytes from patients undergoing in vitro fertilization we detected HLA-G heavy chain mRNA in 40% of 148 of blastocysts tested. The presence of HLA-G mRNA was also detected in unfertilized oocytes and in early embryos, but not in control cumulus oophorus cells. beta 2-Microglobulin mRNA was also found in those embryos expressing HLA-G. In concordance with our mRNA data, a similar proportion of embryos stained positive for HLA-G utilizing a specific monoclonal antibody. Interestingly, expression of HLA-G mRNA was associated with an increased cleavage rate, as compared to embryos lacking HLA-G transcript. Thus, HLA-G could be a functional homologue of the mouse Qa-2 antigen, which has been implicated in differences in the rate of preimplantation embryo development. To our knowledge, the presence of HLA-G mRNA and protein in human preimplantation embryos and oocytes has not been reported previously. The correlation of HLA-G mRNA expression with cleavage rate suggests that this molecule may play an important role in human pre-embryo development.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton V. N., Hawes S. M., Taylor C. T., Parsons J. H. Development of spare human preimplantation embryos in vitro: an analysis of the correlations among gross morphology, cleavage rates, and development to the blastocyst. J In Vitro Fert Embryo Transf. 1989 Feb;6(1):30–35. doi: 10.1007/BF01134578. [DOI] [PubMed] [Google Scholar]
- Bose R., Mahadevan M. M. Embryo associated immunosuppressor factor(s) secreted by preembryo and serum estradiol levels are predictive of pregnancy outcome: effect of gonadotropin releasing hormone agonist (GnRHa) treatment of patients undergoing in vitro fertilization and embryo transfer (IVF-ET). Immunol Invest. 1992 Feb;21(1):25–38. doi: 10.3109/08820139209069360. [DOI] [PubMed] [Google Scholar]
- Brady G., Iscove N. N. Construction of cDNA libraries from single cells. Methods Enzymol. 1993;225:611–623. doi: 10.1016/0076-6879(93)25039-5. [DOI] [PubMed] [Google Scholar]
- Brownell M. S., Warner C. M. Ped gene expression by embryos cultured in vitro. Biol Reprod. 1988 Nov;39(4):806–811. doi: 10.1095/biolreprod39.4.806. [DOI] [PubMed] [Google Scholar]
- Clark D. A., Lee S., Fishell S., Mahadevan M., Goodall H., Ah Moye M., Schechter O., Stedronska-Clark J., Daya S., Underwood J. Immunosuppressive activity in human in vitro fertilization (IVF) culture supernatants and prediction of the outcome of embryo transfer: a multicenter trial. J In Vitro Fert Embryo Transf. 1989 Feb;6(1):51–58. doi: 10.1007/BF01134582. [DOI] [PubMed] [Google Scholar]
- Daya S., Clark D. A. Production of immunosuppressor factor(s) by preimplantation human embryos. Am J Reprod Immunol Microbiol. 1986 Jul;11(3):98–101. doi: 10.1111/j.1600-0897.1986.tb00039.x. [DOI] [PubMed] [Google Scholar]
- Desoye G., Dohr G. A., Motter W., Winter R., Urdl W., Pusch H., Uchanska-Ziegler B., Ziegler A. Lack of HLA class I and class II antigens on human preimplantation embryos. J Immunol. 1988 Jun 15;140(12):4157–4159. [PubMed] [Google Scholar]
- Dohr G. A., Motter W., Leitinger S., Desoye G., Urdl W., Winter R., Wilders-Truschnig M. M., Uchanska-Ziegler B., Ziegler A. Lack of expression of HLA [corrected] class I and class II molecules on the human oocyte. J Immunol. 1987 Jun 1;138(11):3766–3770. [PubMed] [Google Scholar]
- Ellis S. HLA G: at the interface. Am J Reprod Immunol. 1990 Jul;23(3):84–86. doi: 10.1111/j.1600-0897.1990.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Geraghty D. E., Koller B. H., Orr H. T. A human major histocompatibility complex class I gene that encodes a protein with a shortened cytoplasmic segment. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9145–9149. doi: 10.1073/pnas.84.24.9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbard S. B., Gollnick S. O., Warner C. M. A highly sensitive method for the detection of cell surface antigens on preimplantation mouse embryos. J Immunol Methods. 1984 Mar 30;68(1-2):137–146. doi: 10.1016/0022-1759(84)90144-3. [DOI] [PubMed] [Google Scholar]
- Goldbard S. B., Warner C. M. Genes affect the timing of early mouse embryo development. Biol Reprod. 1982 Sep;27(2):419–424. doi: 10.1095/biolreprod27.2.419. [DOI] [PubMed] [Google Scholar]
- Gonen Y., Jacobson W., Casper R. F. Gonadotropin suppression with oral contraceptives before in vitro fertilization. Fertil Steril. 1990 Feb;53(2):282–287. doi: 10.1016/s0015-0282(16)53282-8. [DOI] [PubMed] [Google Scholar]
- Ishitani A., Geraghty D. E. Alternative splicing of HLA-G transcripts yields proteins with primary structures resembling both class I and class II antigens. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3947–3951. doi: 10.1073/pnas.89.9.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koller B. H., Marrack P., Kappler J. W., Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990 Jun 8;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- Kovats S., Main E. K., Librach C., Stubblebine M., Fisher S. J., DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990 Apr 13;248(4952):220–223. doi: 10.1126/science.2326636. [DOI] [PubMed] [Google Scholar]
- Manaseki S., Searle R. F. Natural killer (NK) cell activity of first trimester human decidua. Cell Immunol. 1989 Jun;121(1):166–173. doi: 10.1016/0008-8749(89)90014-2. [DOI] [PubMed] [Google Scholar]
- McMaster M. T., Librach C. L., Zhou Y., Lim K. H., Janatpour M. J., DeMars R., Kovats S., Damsky C., Fisher S. J. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995 Apr 15;154(8):3771–3778. [PubMed] [Google Scholar]
- Meziou W., Chardon P., Fléchon J. E., Kalil J., Vaiman M. Expression of beta 2-microglobulin on preimplantation pig embryos. J Reprod Immunol. 1983 Mar;5(2):73–80. doi: 10.1016/0165-0378(83)90002-5. [DOI] [PubMed] [Google Scholar]
- Morales P., Corell A., Martínez-Laso J., Martín-Villa J. M., Varela P., Paz-Artal E., Allende L. M., Arnaiz-Villena A. Three new HLA-G alleles and their linkage disequilibria with HLA-A. Immunogenetics. 1993;38(5):323–331. doi: 10.1007/BF00210473. [DOI] [PubMed] [Google Scholar]
- Pérarnau B., Siegrist C. A., Gillet A., Vincent C., Kimura S., Lemonnier F. A. Beta 2-microglobulin restriction of antigen presentation. Nature. 1990 Aug 23;346(6286):751–754. doi: 10.1038/346751a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Taylor C. T., Melling G. C., Kingsland C. R., Johnson P. M. Expression of the CD46 antigen, and absence of class I MHC antigen, on the human oocyte and preimplantation blastocyst. Immunology. 1992 Jan;75(1):202–205. [PMC free article] [PubMed] [Google Scholar]
- Sauer M. V., Bustillo M., Rodi I. A., Gorrill M. J., Buster J. E. In-vivo blastocyst production and ovum yield among fertile women. Hum Reprod. 1987 Nov;2(8):701–703. doi: 10.1093/oxfordjournals.humrep.a136617. [DOI] [PubMed] [Google Scholar]
- Sawicki J. A., Magnuson T., Epstein C. J. Evidence for expression of the paternal genome in the two-cell mouse embryo. Nature. 1981 Dec 3;294(5840):450–451. doi: 10.1038/294450a0. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Demonstration by class I gene transfer that reduced susceptibility of human cells to natural killer cell-mediated lysis is inversely correlated with HLA class I antigen expression. Eur J Immunol. 1989 Mar;19(3):447–451. doi: 10.1002/eji.1830190306. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., DeMars R. Production of human cells expressing individual transferred HLA-A,-B,-C genes using an HLA-A,-B,-C null human cell line. J Immunol. 1989 May 1;142(9):3320–3328. [PubMed] [Google Scholar]
- Shukla H., Swaroop A., Srivastava R., Weissman S. M. The mRNA of a human class I gene HLA G/HLA 6.0 exhibits a restricted pattern of expression. Nucleic Acids Res. 1990 Apr 25;18(8):2189–2189. doi: 10.1093/nar/18.8.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinks M. T., Sellens M. H., Dealtry G. B., Fernandez N. Preimplantation mouse embryos express Mhc class I genes before the first cleavage division. Immunogenetics. 1993;38(1):35–40. doi: 10.1007/BF00216388. [DOI] [PubMed] [Google Scholar]
- Tian Z., Xu Y., Warner C. M. Removal of Qa-2 antigen alters the Ped gene phenotype of preimplantation mouse embryos. Biol Reprod. 1992 Aug;47(2):271–276. doi: 10.1095/biolreprod47.2.271. [DOI] [PubMed] [Google Scholar]
- Warner C. M., Gollnick S. O. Expression of H-2K major histocompatibility antigens on preimplantation mouse embryos. Biol Reprod. 1993 May;48(5):1082–1087. doi: 10.1095/biolreprod48.5.1082. [DOI] [PubMed] [Google Scholar]
- Warner C. M., Gollnick S. O., Flaherty L., Goldbard S. B. Analysis of Qa-2 antigen expression by preimplantation mouse embryos: possible relationship to the preimplantation-embryo-development (Ped) gene product. Biol Reprod. 1987 Apr;36(3):611–616. doi: 10.1095/biolreprod36.3.611. [DOI] [PubMed] [Google Scholar]
- Warner C. M., Gollnick S. O., Goldbard S. B. Linkage of the preimplantation-embryo-development (Ped) gene to the mouse major histocompatibility complex (MHC). Biol Reprod. 1987 Apr;36(3):606–610. doi: 10.1095/biolreprod36.3.606. [DOI] [PubMed] [Google Scholar]
- Xu Y., Jin P., Mellor A. L., Warner C. M. Identification of the Ped gene at the molecular level: the Q9 MHC class I transgene converts the Ped slow to the Ped fast phenotype. Biol Reprod. 1994 Oct;51(4):695–699. doi: 10.1095/biolreprod51.4.695. [DOI] [PubMed] [Google Scholar]
- Xu Y., Jin P., Warner C. M. Modulation of preimplantation embryonic development by antisense oligonucleotides to major histocompatibility complex genes. Biol Reprod. 1993 May;48(5):1042–1046. doi: 10.1095/biolreprod48.5.1042. [DOI] [PubMed] [Google Scholar]
- Yelavarthi K. K., Fishback J. L., Hunt J. S. Analysis of HLA-G mRNA in human placental and extraplacental membrane cells by in situ hybridization. J Immunol. 1991 Apr 15;146(8):2847–2854. [PubMed] [Google Scholar]
- Zijlstra M., Bix M., Simister N. E., Loring J. M., Raulet D. H., Jaenisch R. Beta 2-microglobulin deficient mice lack CD4-8+ cytolytic T cells. Nature. 1990 Apr 19;344(6268):742–746. doi: 10.1038/344742a0. [DOI] [PubMed] [Google Scholar]






