Abstract
Aim
Despite prompt revascularization of acute myocardial infarction (AMI), substantial myocardial injury may occur, in part a consequence of ischaemia reperfusion injury (IRI). There has been considerable interest in therapies that may reduce IRI. In experimental models of AMI, sodium nitrite substantially reduces IRI. In this doubleblind randomized placebo controlled parallel-group trial, we investigated the effects of sodium nitrite administered immediately prior to reperfusion in patients with acute ST-elevation myocardial infarction (STEMI).
Methods and results
A total of 229 patients presenting with acute STEMI were randomized to receive either an i.v. infusion of 70 μmol sodium nitrite (n = 118) or matching placebo (n = 111) over 5 min immediately before primary percutaneous intervention (PPCI). Patients underwent cardiac magnetic resonance imaging (CMR) at 6–8 days and at 6 months and serial blood sampling was performed over 72 h for the measurement of plasma creatine kinase (CK) and Troponin I. Myocardial infarct size (extent of late gadolinium enhancement at 6–8 days by CMR-the primary endpoint) did not differ between nitrite and placebo groups after adjustment for area at risk, diabetes status, and centre (effect size −0.7% 95% CI: −2.2%, +0.7%; P = 0.34). There were no significant differences in any of the secondary endpoints, including plasma troponin I and CK area under the curve, left ventricular volumes (LV), and ejection fraction (EF) measured at 6–8 days and at 6 months and final infarct size (FIS) measured at 6 months.
Conclusions
Sodium nitrite administered intravenously immediately prior to reperfusion in patients with acute STEMI does not reduce infarct size.
Keywords: Acute myocardial infarction, Ischaemia-reperfusion injury, Cardioprotection, Nitrite
Introduction
Heart failure is a common long-term sequel of acute myocardial infarction. While prompt reperfusion in acute STEMI is a pre-requisite for limiting infarct size, the process of reperfusion itself causes injury (ischaemia reperfusion injury, IRI) due to opening of the mitochondrial permeability transition pore (MPTP) occurring ∼3 min after reperfusion.1
Direct ischaemic preconditioning (brief repetitive episodes of ischaemia and reperfusion prior to a major ischaemic insult) reduces IRI2 and can be replicated by a variety of pharmacological agents3 and by remote ischaemic preconditioning.4 In experimental models, several interventions administered during ischaemia or at reperfusion prior to opening of the MPTP (peri and postconditioning, respectively) have successfully reduced cardiac IRI.3 A small number of these have been successful in patients with acute STEMI,5–8 but several have not.9–11
Nitrite was in the past considered a relatively inert breakdown product of nitric oxide (NO), but the last decade has witnessed the emergence of evidence that nitrite is a bioactive substance with promising pharmacological properties in its own right. Plasma nitrite is derived from oxidation of endothelially derived NO12 and from the ingestion of dietary nitrate which is reduced following enterosalivary circulation to nitrite by oral commensal bacteria.13 In addition to non-enzymatic processes, favoured by hypoxic and acidic conditions akin to those prevailing in ischaemic myocardium, multiple enzymatic processes involving haem and molybdopterine-containing proteins are thought to be involved in its bioactivation (by reduction to NO)14 (for detail information, see Supplementary material online). Nitrite has been shown to have vasorelaxant and anti-platelet properties, that are enhanced by hypoxia.15,16 In a primate model, there was no evidence of tolerance to the vasorelaxant effects of sodium nitrite.17 Oral inorganic nitrate supplementation reduces the oxygen cost of submaximal exercise.18 In experimental models of AMI, sodium nitrite administered as a pre- or periconditioning agent has demonstrated very impressive cardioprotection.19–21,22 These effects are seen in the nanomolar concentration range in a murine model.22 An i.v. infusion of sodium nitrite administered over the final 5 min of a 120 min coronary occlusion in a canine model, which resulted in peak plasma levels of ∼5 µmol/L, markedly reduced IRI.20 In the Nitrites in Acute Myocardial Infarction (NIAMI) trial, we replicated the timing, duration and dose of this canine study20 in order to explore the efficacy of i.v. sodium nitrite immediately prior to primary percutaneous intervention (PPCI) in patients with STEMI. We administered the dose of this study on a per kg basis—we therefore gave 70 µmol nitrite over 5 min, assuming a mean body weight of 70 kg. We previously found that a 5 min infusion of 50 µmol of sodium nitrite in healthy individuals and patients with heart failure resulted in a peak concentration of ∼5 µmol/L.
Methods
Study design
NIAMI, funded by the UK Medical Research Council, was a multicentre, double-blind, randomized, placebo-controlled trial designed to study the effect of sodium nitrite administered intravenously over 5 min immediately prior to reperfusion by PPCI on infarct size in patients presenting with first acute STEMI. The detailed study protocol has been published.23 Regulatory approvals were obtained from Scotland A Research Ethics Committee and the Medicines and Healthcare products Regulatory Agency (EudraCT number 2010-023571-26). The study was registered (NCT01388504, ISRCTN57596739) and overseen by trial steering and data monitoring committees.23
Patients
We recruited patients from four centres (Aberdeen, St Georges Hospital London and Brighton in the UK and Queen Elizabeth Hospital, Adelaide, Australia).
Eligible patients were those presenting within 12 h of the onset of chest pain, with ECG features of first acute STEMI in whom the decision had been made to proceed with PPCI of the occluded culprit artery, and in whom TIMI flow was grade 0 or 1. Exclusion criteria included prior MI, CABG or previous PCI, cardiogenic shock, and contraindication to CMR.23 Owing to the remote risk of inducing methaemoglobinaemia in patients with G6PD deficiency, only patients of Northern European ancestry were recruited.
Consent
Full details of the consent process are reported elsewhere.23 Initial written consent was obtained where possible, with the majority of patients providing verbal agreement. Subsequently, fully informed written consent was sought.
Randomization
Patients were randomly allocated in a 1 : 1 ratio to active treatment (70 µmol sodium nitrite in 5 mL water) or matching placebo (5 mL 0.9% saline) using permuted blocks. The dose was based on an anticipated average patient weight of 70 kg, corresponding to 1 µmol/kg. Technical aspects of the PCI were left to clinician discretion and all the patients received dual anti-platelet therapy in accordance with current guidelines.
Scanning
Cardiac magnetic resonance was performed at 6–8 days and at 6 months using standardized sequences23 and analysed in a single-core laboratory. Cardiac magnetic resonance was performed at 6–8 days and at 6 months as published in the study protocol23 using internationally standardized sequences endorsed by the ESC24 and analysed in a single-core laboratory utilizing agreed thresholding and planimetry methods for area at risk (AAR) and IS.25,26
Blood sampling
Blood samples for the measurement of plasma troponin I and creatine kinase (CK) were collected prior to, and at 6, 12, 18, 24, 36, 48, 60 and 72 h after injection of the study medication and analysed in Aberdeen (ultra-Troponin I method on Siemens ADVIA Centaur automated immunoanalyser and Siemens ADVIA 2400 automated general chemistry analyser for total CK). In 17 patients, sampling for plasma nitrite was undertaken prior to infusion and 5 min after completion of the infusion of study medication and for blood methaemoglobin prior to 10 min and 2 h after commencing study medication.
Plasma nitrite was measured by high-pressure liquid ion chromatography with post-column derivatization using a dedicated analysis system (ENO-20 with Gilson 234 autoinjector, EPC-500 data processor and PowerChrome software; Eicom). Plasma was obtained by centrifugation of EDTA blood within minutes of collection, snap-frozen in liquid nitrogen and stored at −80°C. Frozen plasma samples were thawed in the presence of N-ethylmaleimide (10 mM final concentration) and deproteinized by methanol precipitation immediately prior to analysis.
Study endpoints
The primary endpoint was the difference in infarct size (expressed as a percentage of LV myocardial mass) between the active and placebo groups at 6–8 days post-infarct assessed by the extent of late gadolinium enhancement (LGE; by planimetry) on CMR.
Secondary endpoints were plasma total CK and troponin I area under the curve (AUC), infarct size (measured using CMR LGE hyperenhancement extent defined using a cut-off of 5-SD greater than the intensity in the remote myocardium)27 at 6–8 days with AAR as a covariate (measured by T2-weighted triple inversion recovery T2-W SPAIR or STIR with extent determined on the basis of a 2-SD cut-off); LVEF, LV end-diastolic volume (LVEDV) and LV end-systolic volume (LVESV) measured by CMR at 6–8 days and at 6 months after AMI; and ‘final’ infarct size (FIS) (CMR) assessed 6-month post-AMI.
Sample size
The sample size was estimated using simulation methods.23 Based on data from Botker et al.7 and from our CMR database of patients studied ∼1 week following acute STEMI, we assumed the mean infarct size in the placebo group would be 15–20%, and that a relevant treatment effect would reduce this by 4 absolute percentage points in the active group. We assumed that the mean AAR was 30% (SD 15) and the correlation between AAR and infarct size was 0.6. Simulations showed that 150 participants (75 in each group) were required to detect the anticipated effect size with 90% power at the 5% alpha level. In previous published studies of conditioning interventions in acute STEMI, data loss from randomized patients were high.7 Sample size was therefore inflated by ∼30–35% to account for loss of primary outcome data due to death, contraindication to or failure to proceed to CMR, or inadequate CMR quality. We planned to recruit between 200 and 210 participants.
Statistical analysis
Baseline and outcome data were described using appropriate summary measures. The primary outcome was analysed using analysis of covariance adjusting for AAR, diabetic status, and centre. Secondary outcomes were analysed using similar models. All analyses were per-protocol but the sensitivity of the primary outcome and blood measurement results to missing data were tested using multiple imputation and missing-not-at-random strategies.28,29 Multiple imputation models were run under the assumption primary outcome data being missing-at-random (conditional on measured covariates), firstly, a model including the covariates in the primary analysis strategy listed above. Secondly, a model that included additional baseline covariates: previous hypertension, previous angina, hyperlipidaemia, current smoking status, and sex. This was implemented using Stata's MI command using 10 imputations. The missing-not-at-random models were implemented using pattern mixture models which varied the difference delta between observed and missing data over a range of values of delta from −5% to +5% FIS. This was done in three ways: delta was equal in both arms; delta equal to zero in the placebo group only (i.e. no difference between missing and observed data in placebo group only); finally, delta equal to zero in the nitrite group only. All analyses were carried out using Stata 13 (StataCorp 2013. Stata Statistical Software: Release 13. College Station, TX, USA: StataCorp LP).
Results
Characteristics of the study population
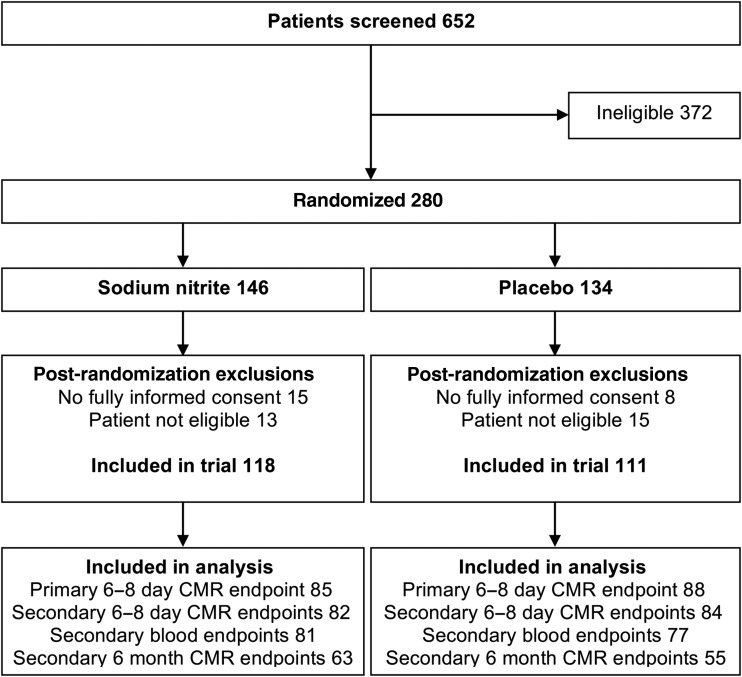
As shown in Figure 1 (CONSORT diagram), of 652 patients screened for eligibility, 372 were deemed at the time by the study team or (out of hours) by the treating cardiologist to be ineligible and 280 were randomized and received their allocated intervention. There were 51 of 280 (18%) post-randomization exclusions: in the nitrite group, there was 1 of 146 (<1%) and in the placebo group, there were 4 of 134 (3%) participants who did not survive the procedure or regain capacity and were therefore excluded; 14 of 146 (10%) in the nitrite group and 8 of 134 (6%) in the placebo group survived but did not consent to remain in the trial; and finally 13 of 146 (9%) and 11 of 134 (8%) thought at the time by the treating cardiologist to be eligible were subsequently found to be ineligible. We therefore included 229 patients who met the eligibility criteria and who gave fully informed consent to participate in the trial (118 received sodium nitrite and 111 placebo). Groups were well balanced for age, gender, TIMI grade, and pain-to-balloon time (Table 1). All the patients received the standard dual anti-platelet therapy pre-PCI, 50% received glycoprotein IIb3a inhibitors, and 89% received heparin pre/during PCI.
Figure 1.
CONSORT diagram.
Table 1.
Baseline characteristics
| Nitrite (n = 118) | Placebo (n = 111) | |
|---|---|---|
| Age (years) mean (SD) | 63 (12) | 64 (13) |
| Female | 22 (19) | 30 (26) |
| Weight (kg) median (IQR) | 82 (75, 91) | 77 (69, 89) |
| BMI mean (SD) | 28 (4) | 27 (4) |
| Previous conditions | ||
| Hypertension | 35 (30) | 35 (32) |
| hyperlipidaemia | 55 (47) | 52 (46) |
| diabetes | 14 (12) | 19 (17) |
| Current smoker | 53 (45) | 47 (42) |
| Infarct site (ECG) | ||
| Anterior | 46 (39) | 41 (37) |
| Other sites | 72 (61) | 70 (63) |
| Symptom to balloon time (min) | ||
| mean (SD) | 208 (119) | 238 (135) |
| median (25th, 75th) | 164 (127, 256) | 203 (133, 317) |
| TIMI grade pre-PCI | ||
| TIMI0 | 101 (91) | 105 (89) |
| TIMI1 | 9 (8) | 11 (9) |
| Missing | 1 (<1) | 2 (2) |
| Stenting of culprit lesion by PCI | 116 (98) | 110 (99) |
| TIMI grade post-PCI | ||
| TIMI0 | 2 (2) | 2 (2) |
| TIMI1 | 1 (<1) | – |
| TIMI2 | 1 (<1) | 1 (<1) |
| TIMI3 | 112 (95) | 107 (96) |
| Missing | 2 (2) | 1 (<1) |
| Other drug therapies pre/during PCI reported to have ‘conditioning’ effects | ||
| Nitrates | 99 (84) | 105 (95) |
| Morphine | 70 (59) | 66 (60) |
| Prior drug therapy | ||
| Beta-blocker | 12 (10) | 6 (5) |
| Calcium channel blockers | 24 (20) | 29 (26) |
| Statins | 3 (3) | 1 (<1) |
| Heparin | 103 (87) | 99 (89) |
| ACE inhibitors | – | 1 (0.9) |
| Nicorandil | – | – |
| Allopurinol | – | – |
Data are expressed as number (%) unless otherwise stated. TIMI, thrombolysis in myocardial infarction; BMI, body mass index kg/m2.
Primary outcome
Primary outcome data were obtained from 85 of 118 (72%) participants in the nitrite and 88 of 111 (79%) in the placebo group. The reasons for not obtaining primary outcome data were: participants declining CMR [26 (22%) and 18 (16%) in the nitrite and placebo groups, respectively] and unreadable scans (12 in total). The median infarct size was 22% in the nitrite and 20% in the placebo groups [difference −0.7% (95% CI: −2.2, +0.7; P = 0.30)] (Table 2). Multiple imputation sensitivity analyses gave similar results to the primary analysis, the treatment effect estimate from the comprehensive model was −0.8% (95% CI: −2.3, 0.8; P = 0.34). Results were robust to missing-not-at-random assumptions for all but implausible scenarios, for example, delta −5% in the nitrite group but zero in the placebo group.
Table 2.
Pre-specified primary and secondary outcome measures
| Measure | Nitrite | Placebo | Effect size (95% CI); P-value |
|---|---|---|---|
| Primary outcome | |||
| Infarct size at 6–8 days | n = 85 | n = 88 | |
| Mean (SD) | 22.9 (13.5) | 23.1 (13.2) | −0.7 (−2.2, 0.7); 0.30 |
| Median (25th, 75th) | 22 (12, 33) | 20 (13, 32) | |
| Area at risk | |||
| Mean (SD) | 33.1 (15.8) | 32.4 (14.1) | |
| Median (25th, 75th) | 31 (21, 44) | 32.5 (22.5, 42) | |
| Secondary outcomes | |||
| Troponin AUC | n = 81 | n = 87 | |
| Mean (SD) | 3734 (3091) | 3807 (3262) | −125 (−1139, 888); 0.81 |
| CK AUC | n = 81 | n = 87 | |
| Mean (SD) | 67 019 (42 446) | 59 574 (48 337) | 5766 (−8695, 20 288); 0.79 |
| Infarct size 6–8 days (5-SD) | n = 82 | n = 84 | |
| Mean (SD) | 14.5 (10.5) | 14.7 (11.2) | 0.1 (−2.5, 2.8); 0.92 |
| Median (25th, 75th) | 12 (6, 20) | 11.5 (7, 22) | |
| Area at risk (T2) | n = 39 | n = 46 | |
| Mean (SD) | 36.1 (24.8) | 38.1 (16.9) | |
| Median (25th, 75th) | 33 (19, 46) | 39 (26 ,51) | |
| Final infarct size at 6 months | n = 63 | n = 55 | −1.7 (−3.2, 5.5); 0.19 |
| Mean (SD) | 13.3 (8.7) | 15.0 (9.7) | |
| Median (25th, 75th) | 12 (7, 17) | 14 (8, 20) | |
| LVEDV (mL) at 6–8 days | n = 75 | n = 84 | −3.5 (−16.3, 9.2); 0.58 |
| Mean (SD) | 159 (41) | 162 (40) | |
| LVEDV (mL) at 6 months | n = 64 | n = 54 | |
| Mean (SD) | 159 (42) | 165 (37) | −5.0 (−19.8, 9.8); 0.50 |
| LVEDV (mL) (delta) | n = 63 | n = 51 | |
| Mean (SD) | −1 (29) | −3 (32) | 1.3 (−10.1, 12.6); 0.82 |
| LVESV (mL) at 6–8 days | n = 75 | n = 84 | |
| Mean (SD) | 85 (36) | 85 (32) | 0.5 (−10.4,11.3); 0.93 |
| LVESV (mL) at 6 months | n = 64 | n = 54 | |
| Mean (SD) | 75 (31) | 78 (28) | −2.7 (−13.7,8.3); 0.63 |
| LVDV mL (delta) | n = 63 | n = 63 | |
| Mean (SD) | 9 (25) | 6 (24) | 2.0 (−7.2, 11.2); 0.66 |
| LVEF % at 6–8 days | n = 75 | n = 84 | |
| Mean (SD) | 48 (11) | 50 (18) | −2.3 (−7.1, 2.4); 0.34 |
| LVEF % at 6 months | n = 64 | n = 54 | |
| Mean (SD) | 53 (9) | 53 (9) | −0.6 (−3.9, 2.7); 0.72 |
| LVEF % (delta) | n = 63 | n = 51 | |
| Mean (SD) | −5 (8) | −3 (22) | −1.7 (−7.6, 4.2); 0.57 |
Pre-specified primary and secondary outcome measures. LVEDV, LV, end-diastolic volume; LVESV, LV end-systolic volume; LVEF, LV ejection fraction; Delta, Change between first and second scan.
Secondary outcomes
Biomarker data for sufficient datapoints to create a 72-h AUC were available in 158 patients (81 nitrite, 77 placebo). Reasons for incomplete biomarker data included participants discharged before 72 h and patients declining to have (usually nocturnal) samples taken. Patients with more than two missing datapoints were excluded. The AUCs for both biomarkers were similar in the two treatment groups (see Table 2 and Figure 2). The effect size was −125 arbitrary units (95% CI: −1139, +888 units; P = 0.81) for Troponin I, and +5766 arbitrary units (95% CI: −8695, +20 288 units; P = 0.79) for CK. These results were consistent under all sensitivity analyses, except implausible scenarios.
Figure 2.
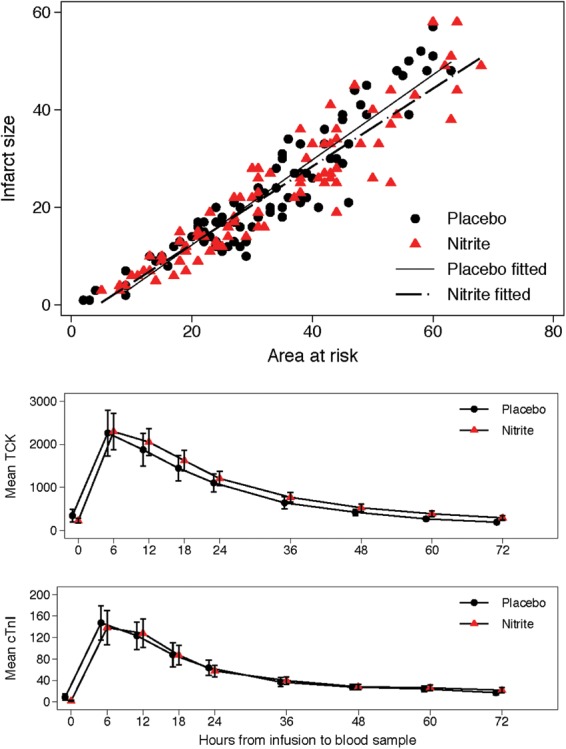
Relation between infarct size (late gadolinium enhancement planimetry on early scan) and area at risk (late gadolinium enhancement endocardial surface area (ESA) technique on early scan) in nitrite and placebo groups (upper panel). Serial measures of mean total creatine kinase and Troponin I immediately prior to and over 72 h following administration of nitrite and placebo. Nitrite did not significantly reduce the area under the curve for either biomarker (lower panel).
There was no significant difference in infarct size (5-SD technique) using AAR (T2) as a covariate between the nitrite and placebo groups (effect size +0.1% 95% CI −2.5, +2.8; P = 0.92), or in LVEDV, LVESV or LVEF at 6–8 days, at 6 months or in the changes in these variables between 6–8 days and 6 months (Table 2).
Final infarct size at 6 months
These data in 118 patients (63 in the nitrite group and 55 in the placebo group) did not differ significantly between the nitrite and placebo groups (median 12.0 vs. 14.0%). The effect size was −1.7 (95% CI: −3.2, +5.5; P = 0.19) (Table 2).
Table 3.
Pre-specified and post hoc subgroup analyses
| Effect size (95% CI); P-value | |
|---|---|
| Pre-specified subgroups | |
| Non-diabetics | −0.2 (−1.8, 1.3); 0.77 |
| Diabetics | −4.5 (−8.8, −0.2); 0.041 |
| Interaction | −4.3 (−8.9, 0.3); 0.067 |
| Post hoc subgroups | |
| No microvascular obstruction | −0.6 (−3.3, 2.1); 0.54 |
| Microvascular obstruction | −0.7 (−3.4, 1.9); 0.47 |
| Interaction | −0.1 (−3.9, 3.7); 0.94 |
| Anterior | −1.9 (−5.1, 1.4); 0.13 |
| Other site | 0.0 (−2.4, 2.4); 0.97 |
| Interaction | −1.9 (−5.9, 2.2); 0.24 |
| Late PCI | −1.7 (−3.8, 0.5); 0.045 |
| Early PCI | 2.9 (−1.3, 7.1); 0.070 |
| Interaction | 4.6 (−0.1, 9.3); 0.012 |
| AAR ≤40% | −1.2 (−5.3, 3.0); 0.46 |
| AAR >40% | −2.6 (−8.7, 3.5); 0.27 |
| Interaction | −1.4 (−8.8, 6.0); 0.62 |
All post hoc subgroup models corrected for AAR except the model exploring the 40% or less vs. more than 40% subgroup analysis which re-expressed AAR as a dichotomy and therefore did not include the AAR covariate. Late/early PCI, primary PCI performed after/before the median chest pain to PCI time (120 min); AAR ≤ or >40%, treatment effect in patients with area at risk ≤40% or >40% of LV mass using LGE ESA measurement on early scan.
Pre-specified and post hoc subgroup analysis
In diabetics, there was a treatment effect favouring nitrite, −4.5 (95% CI: −8.8, −0.2; P = 0.041) but not in non-diabetics [−0.2 (95% CI: −0.8, 1.3); P = 0.77]. The interaction was not significant (P = 0.067).
There was no interaction between treatment effect and infarct site (anterior vs. the remainder); in patients with chest pain to PCI times <120 min vs. the remainder; in those with or without microvascular obstruction; or those with an AAR of 40% or less vs. more than 40%.
Plasma nitrite measurements
These were performed immediately prior to commencing the study medication and 5 min after ceasing the study medication in 17 patients (11 nitrite, 6 placebo). Plasma nitrite [mean (SD), µmol/L] was similar at baseline [0.76 (0.14) vs. 0.73 (0.08)] but higher in the nitrite group 5 min after ceasing infusion [1.42 (0.96) vs. 0.18 (0.08); P = 0.008]. The fall in the placebo group was not due to haemolysis, consistent with nitrite uptake by ischaemic myocardial tissue or red blood cells.30
Blood methaemoglobin levels
Blood methaemoglobin levels were similar in the nitrite vs. placebo groups at baseline (0.52 ± 0.08 vs. 0.59 ± 0.04%), were higher at 10 min following study medication (0.66 ± 0.12 vs. 0.57 ± 0.05; P = 0.005) and similar in both groups at 2 h.
Safety data
Fifty serious adverse events (SAEs) were recorded in the safety dataset (280 participants where injection was started). There were 21 events in 19 of 146 participants (13%) in the nitrite group, 29 events in 25 of 134 participants in the (19%) placebo group. There was one death in the nitrite group and four in the placebo group: none was related to the study intervention. There were no suspected unexpected serious adverse reactions. Six SAEs were recorded as ‘possibly related’ to the study intervention but were listed in the protocol as expected events. Two were in the nitrite group and four in the placebo group.
Discussion
Ischaemia reperfusion injury is a potential target for therapeutic intervention in acute STEMI. In experimental models, a variety of pharmacological and non-pharmacological interventions are effective.3 Periconditioning with remote (limb) ischaemia,7 cyclosporine,5 and exenatide6 have been shown in phase 2 studies to reduce myocardial injury in patients presenting with STEMI. Initial studies suggested a benefit from graded opening of the occluded artery by repeated balloon inflation (direct postconditioning),8 but this was not confirmed by a larger study.11 Several other pre, peri, and postconditioning interventions have been ineffective in humans.9,10 Sodium nitrite has shown particular efficacy in reducing myocardial infarct size in experimental models when administered as either a pre or periconditioning agent.19–22 While several different mechanisms may contribute to cardioprotection (see Supplementary material online, Figure S1), in a murine model of nitrite-mediated cardiac periconditioning, reduction of nitrite to NO by deoxymyoglobin appeared to be the dominant mechanism of bioconversion.21 The NO so released S-nitrosylates complex 1 of the electron transport chain, reducing superoxide production during ischaemia and reperfusion,31 and thereby reducing opening of the MPTP, which initiates IRI.
In NIAMI, a 5 min i.v. infusion of sodium nitrite administered to patients with acute STEMI immediately prior to PPCI did not reduce infarct size. The findings are consistent for the primary endpoint and for each of the secondary endpoints, and the confidence intervals clearly exclude the a priori hypothesis.
We replicated the protocol employed in the Gonzalez canine study20 in which a 5 min infusion of sodium nitrite administered immediately prior to opening of the infarct-related artery substantially reduced infarct size. In our previous studies in healthy volunteers and in patients with heart failure given a dose of 50 µmol over 5 min (vs. 70 µmol over 5 min in NIAMI), peak plasma levels were ∼5 µmol/L (data not shown), identical to those achieved by the 5 min infusion in the Gonzalez study. The median time to reperfusion from the onset of infusion in NIAMI was 5 min, coinciding with this peak level; 5 min after ceasing the infusion (and reperfusion) circulating nitrite levels were still 1.4 µmol/L in the nitrite group (7.8-fold higher than in the placebo group). In a previous murine periconditioning study, even doses of sodium nitrite that only increased plasma levels from 0.7 to 0.9 µmol/L substantially reduced infarct size.22
The question arises whether a higher dose or a longer duration of infusion might have been effective as both of these strategies would have resulted in higher plasma nitrite concentrations. In the Gonzalez study, a 1 h infusion regime resulted in higher plasma levels than the 5 min regime, with significant haemodynamic changes (sometimes requiring infusion of saline to maintain blood pressure) and a statistically insignificant incremental reduction in infarct size,20 but we were concerned about safety in a first-in-disease-area study. The regime we employed was not associated with hypotension. We cannot exclude that a benefit might be observed with intracoronary administration of sodium nitrite. One previous study reported a beneficial effect of intracoronary administration of sodium nitrite as a preconditioning agent in a swine model.32 A single-centre trial in 80 patients with acute STEMI in which intra-coronary nitrite was administered at reperfusion has been completed, and the results are awaited.33
Another important question is whether particular subgroups may benefit from this intervention. In some previous studies, diabetic patients have been shown to be relatively resistant to conditioning agents.34 We therefore pre-specified an analysis according to diabetes status. On the contrary, in NIAMI there was a significant treatment benefit in the diabetes subgroup; however, the numbers were small and the interaction was not significant, therefore, this may well represent a type 2 error. It has been suggested that patients with larger infarcts and those reperfused relatively early may gain more benefit from conditioning strategies.9 In NIAMI, infarct size was relatively large (FIS 15% at 6 months in NIAMI vs. 7% in the placebo group at 1 month in the AARHUS remote periconditioning study).7 Post hoc subgroup analysis revealed no interaction for anterior vs. non anterior infarcts (anterior infarcts tend to be larger), those patients with larger vs. smaller areas at risk or those with shorter vs. longer chest pain to reperfusion times. Furthermore, it has been proposed that patients with totally occluded coronary arteries are more likely to benefit than those with some flow. Accordingly, our study included only patients with TIMI 0 or 1 flow, with the vast majority being 0.
Organic nitrates confer cardioprotection in experimental models35 Direct NO donors (and organic nitrates) appear to exhibit a biphasic dose response as conditioning agents, with lower doses reducing IRI while higher doses are ineffective.36 However, evidence from clinical studies demonstrating cardioprotection by organic nitrates is limited.37 Evidence from rodent models suggests that nitrite mediates the majority of its actions by conversion to NO.21 In experimental models a biphasic dose–response relationship is also observed for the cardioprotective effects of nitrite.22 It is therefore possible that the prior use of organic nitrates, which apart from NO, also generate nitrite and other NO-related metabolites during tissue biotransformation33,38 may have already induced a degree of cardioprotection, thereby limiting the potential for additional cardioprotection by nitrite. The deleterious effects of high doses of NO donors and nitrite are likely in part mediated via the generation of peroxynitrite, which may itself result in IRI.39 Furthermore, nitroglycerine causes increased electron leak from complex 1 and complex 11 of the electron transport chain that appears to be involved in the induction of second window preconditioning,40 but in contrast when nitrite is administered as a periconditioning agent on a background of organic nitrite therapy this could potentially favour peroxynitrite formation. Almost all of our patients had received organic nitrates acutely prior to reperfusion (>90% of whom had received glyceryl trinitrate), reflecting widespread clinical practice, none was receiving chronic organic nitrate therapy. This could be an important reason for the discrepancy between the preclinical data and these clinical data. Nevertheless, new therapies need to be effective when given to patients receiving standard therapy.
Study limitations
Potential limitations of this study are the exclusion of participant’s post-randomization and the loss of outcome data. There are a number of reasons why this occurred. The study was performed in an acute setting in which decisions regarding eligibility had to be made by the treating clinician very quickly. A small number were subsequently found by the study team to be ineligible. Some patients declined to give fully informed consent to remain in the study once they have had a chance to consider their initial decision. Others died or, having given fully informed consent, later declined to have, or were unable to complete, the CMR.
Following post-randomization exclusions, data loss for the primary endpoint was 24%, which was less than we anticipated in our sample size calculations. Compared with similar studies, data loss was modest. For example, in the AARHUS study the primary endpoint was the salvage index; 333 patients received the intervention; after post-randomization exclusions, 251 patients were deemed eligible for data analysis, and of this subgroup only 142 (57%) contributed data to this primary endpoint.7
The results of the main analysis were maintained in all sensitivity analyses except implausible scenarios (i.e. all missing data in the nitrite group had smaller infarct size but all missing in the placebo group had larger infarct size). Furthermore, there was significant loss of serial biomarker data over 72 h that would have compromised the AUC measurements principally due to patient refusal (usually for nocturnal samples) or early discharge before 72 h. These patients were not included in the biomarker analysis. Biomarker results were robust to sensitivity analysis.
Conclusions
This multi-centre, randomized, double-blind, placebo-controlled phase 2 trial showed that nitrite was ineffective when administered intravenously immediately prior to PPCI in patients presenting with first acute STEMI.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This study was funded by the UK Medical Research Council. Funding to pay the Open Access publication charges for this article was provided by the University of Aberdeen.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We would like to thank our patients and the staff of the cardiology and radiology/cardiac MRI departments at all sites. We are grateful for the support of the staff at the Centre for Healthcare Randomised Trials at the University of Aberdeen.
Appendix
The NIAMI investigators were: Aberdeen: Dr Nishat Siddiqi, Dr Christopher Neil, Mrs Margaret Bruce, Mr Graeme MacLennan, Dr Seonaidh Cotton, Dr Dana Dawson, Prof. Michael Frenneaux, Dr Satnam Singh, Dr Konstantin Schwarz, Mrs Baljit Jagpal, Dr Malcolm Metcalfe, Dr Andrew Stewart, Dr Andrew Hannah, Dr Noman Awsan, Dr Paul Broadhurst, Dr Duncan Hogg, Dr Deepak Garg, Mrs Elaine Slattery, Mrs Tracey Davidson, Mrs Alison McDonald, Dr Gladys McPherson.
St Georges Hospital London: Prof. Juan-Carlos Kaski, Dr Pitt O Lim, Research Sister Sue Brown, Dr Sofia A Papadopoulou, Dr Fatima Gonzalvez, Dr David Roy, Dr Sami Firoozi, Dr Richard Bogle, Dr Elved Roberts, Mr Jonathan Rhodes.
Royal Sussex County Hospital Brighton: Dr David Hildick-Smith, Dr Adam de Belder, Ms Nina Cooter, Ms Lorraine Bennett.
Queen Elizabeth Hospital Adelaide: Prof. John Horowitz, Dr Sharmalar Rajendran, Dr Rustem Dautov, Ms Marilyn Black, Ms Else Jansen.
Trial Steering Committee: Prof. Nicholas Boon, Prof. Allan Struthers, Dr William Toff.
Data Safety and Monitoring Committee: Prof. Henry Dargie, Prof. Chim Lang, Dr Peter Nightingale.
References
- 1.Halestrap AP, Clarke SJ, Javadov SA. Mitochondrial permeability transition pore opening during myocardial reperfusion--a target for cardioprotection. Cardiovasc Res. 2004;61:372–385. doi: 10.1016/S0008-6363(03)00533-9. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 3.Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 4.Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA, Vogel M, Sorensen K, Redington AN, MacAllister R. Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation. 2002;106:2881–2883. doi: 10.1161/01.cir.0000043806.51912.9b. [DOI] [PubMed] [Google Scholar]
- 5.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, Andre-Fouet X, Revel D, Kirkorian G, Monassier JP, Derumeaux G, Ovize M. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 6.Lonborg J, Vejlstrup N, Kelbaek H, Botker HE, Kim WY, Mathiasen AB, Jorgensen E, Helqvist S, Saunamaki K, Clemmensen P, Holmvang L, Thuesen L, Krusell LR, Jensen JS, Kober L, Treiman M, Holst JJ, Engstrom T. Exenatide reduces reperfusion injury in patients with ST-segment elevation myocardial infarction. Eur Heart J. 33:1491–1499. doi: 10.1093/eurheartj/ehr309. [DOI] [PubMed] [Google Scholar]
- 7.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 8.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I, Aupetit JF, Bonnefoy E, Finet G, Andre-Fouet X, Ovize M. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 9.Ludman AJ, Yellon DM, Hausenloy DJ. Cardiac preconditioning for ischaemia: lost in translation. Dis Model Mech. 3:35–38. doi: 10.1242/dmm.003855. [DOI] [PubMed] [Google Scholar]
- 10.Najjar SS, Rao SV, Melloni C, Raman SV, Povsic TJ, Melton L, Barsness GW, Prather K, Heitner JF, Kilaru R, Gruberg L, Hasselblad V, Greenbaum AB, Patel M, Kim RJ, Talan M, Ferrucci L, Longo DL, Lakatta EG, Harrington RA. Intravenous erythropoietin in patients with ST-segment elevation myocardial infarction: REVEAL: a randomized controlled trial. JAMA. 305:1863–1872. doi: 10.1001/jama.2011.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hahn JY, Song YB, Kim EK, Yu CW, Bae JW, Chung WY, Choi SH, Choi JH, Bae JH, An KJ, Park JS, Oh JH, Kim SW, Hwang JY, Ryu JK, Park HS, Lim DS, Gwon HC. Ischemic postconditioning during primary percutaneous coronary intervention: the POST Randomized Trial. Circulation. 2013;0:1889–1896. doi: 10.1161/CIRCULATIONAHA.113.001690. [DOI] [PubMed] [Google Scholar]
- 12.Shiva S, Wang X, Ringwood LA, Xu X, Yuditskaya S, Annavajjhala V, Miyajima H, Hogg N, Harris ZL, Gladwin MT. Ceruloplasmin is a NO oxidase and nitrite synthase that determines endocrine NO homeostasis. Nat Chem Biol. 2006;2:486–493. doi: 10.1038/nchembio813. [DOI] [PubMed] [Google Scholar]
- 13.Lundberg JO, Weitzberg E, Cole JA, Benjamin N. Nitrate, bacteria and human health. Nat Rev Microbiol. 2004;2:593–602. doi: 10.1038/nrmicro929. [DOI] [PubMed] [Google Scholar]
- 14.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR, Jr, Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL, Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat Chem Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maher AR, Milsom AB, Gunaruwan P, Abozguia K, Ahmed I, Weaver RA, Thomas P, Ashrafian H, Born GV, James PE, Frenneaux MP. Hypoxic modulation of exogenous nitrite-induced vasodilation in humans. Circulation. 2008;117:670–677. doi: 10.1161/CIRCULATIONAHA.107.719591. [DOI] [PubMed] [Google Scholar]
- 16.Srihirun S, Sriwantana T, Unchern S, Kittikool D, Noulsri E, Pattanapanyasat K, Fucharoen S, Piknova B, Schechter AN, Sibmooh N. Platelet inhibition by nitrite is dependent on erythrocytes and deoxygenation. PLoS One. 2012;7:e30380. doi: 10.1371/journal.pone.0030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO, III, Schechter AN, Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–1831. doi: 10.1161/CIRCULATIONAHA.107.712133. [DOI] [PubMed] [Google Scholar]
- 18.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J Appl Physiol. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 19.Dezfulian C, Shiva S, Alekseyenko A, Pendyal A, Beiser DG, Munasinghe JP, Anderson SA, Chesley CF, Vanden Hoek TL, Gladwin MT. Nitrite therapy after cardiac arrest reduces reactive oxygen species generation, improves cardiac and neurological function, and enhances survival via reversible inhibition of mitochondrial complex I. Circulation. 2009;120:897–905. doi: 10.1161/CIRCULATIONAHA.109.853267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez FM, Shiva S, Vincent PS, Ringwood LA, Hsu LY, Hon YY, Aletras AH, Cannon RO, 3rd, Gladwin MT, Arai AE. Nitrite anion provides potent cytoprotective and antiapoptotic effects as adjunctive therapy to reperfusion for acute myocardial infarction. Circulation. 2008;117:2986–2994. doi: 10.1161/CIRCULATIONAHA.107.748814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T. Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury. Proc Natl Acad Sci USA. 2008;105:10256–10261. doi: 10.1073/pnas.0801336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, Patel RP, Yet SF, Wang X, Kevil CG, Gladwin MT, Lefer DJ. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siddiqi N, Bruce M, Neil CJ, Jagpal B, Maclennon G, Cotton SC, Papadopoulo SA, Bunce N, Lim P, Schwarz K, Singh S, Hildick-Smith D, Horowitz JD, Madhani M, Boon N, Kaski JC, Dawson D, Frenneaux MP. Protocol: does sodium nitrite administration reduce ischaemia-reperfusion injury in patients presenting with acute ST segment elevation myocardial infarction? Nitrites in acute myocardial infarction (NIAMI) J Transl Med. 11:116. doi: 10.1186/1479-5876-11-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kramer CM, Barkhausen J, Flamm SD, Kim RJ, Nagel E Society for Cardiovascular Magnetic Resonance Board of Trustees Task Force on Standardized P. Standardized cardiovascular magnetic resonance (CMR) protocols 2013 update. J Cardiovasc Magn Reson. 2013;15:91. doi: 10.1186/1532-429X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flett AS, Hasleton J, Cook C, Hausenloy D, Quarta G, Ariti C, Muthurangu V, Moon JC. Evaluation of techniques for the quantification of myocardial scar of differing etiology using cardiac magnetic resonance. JACC Cardiovasc Imaging. 2011;4:150–156. doi: 10.1016/j.jcmg.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 26.O'Regan DP, Ahmed R, Neuwirth C, Tan Y, Durighel G, Hajnal JV, Nadra I, Corbett SJ, Cook SA. Cardiac MRI of myocardial salvage at the peri-infarct border zones after primary coronary intervention. Am J Physiol Heart Circ Physiol. 2009;297:H340–H346. doi: 10.1152/ajpheart.00011.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eitel I, Desch S, Fuernau G, Hildebrand L, Gutberlet M, Schuler G, Thiele H. Prognostic significance and determinants of myocardial salvage assessed by cardiovascular magnetic resonance in acute reperfused myocardial infarction. J Am Coll Cardiol. 55:2470–2479. doi: 10.1016/j.jacc.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 28.White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ. 2011;342:d40. doi: 10.1136/bmj.d40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White IR, Kalaitzaki E, Thompson SG. Allowing for missing outcome data and incomplete uptake of randomised interventions, with application to an Internet-based alcohol trial. Stat Med. 2011;30:3192–3207. doi: 10.1002/sim.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feelisch M, Fernandez BO, Bryan NS, Garcia-Saura MF, Bauer S, Whitlock DR, Ford PC, Janero DR, Rodriguez J, Ashrafian H. Tissue processing of nitrite in hypoxia: an intricate interplay of nitric oxide-generating and -scavenging systems. J Biol Chem. 2008;283:33927–33934. doi: 10.1074/jbc.M806654200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murillo D, Kamga C, Mo L, Shiva S. Nitrite as a mediator of ischemic preconditioning and cytoprotection. Nitric Oxide. 2011;25:70–80. doi: 10.1016/j.niox.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doganci S, Yildirim V, Bolcal C, Korkusuz P, Gumusel B, Demirkilic U, Aydin A. Sodium nitrite and cardioprotective effect in pig regional myocardial ischemia-reperfusion injury model. Adv Clin Exp Med. 2012;21:713–726. [PubMed] [Google Scholar]
- 33.Jones DA, Andiapen M, Van-Eijl TJ, Webb AJ, Antoniou S, Schilling RJ, Ahluwalia A, Mathur A. The safety and efficacy of intracoronary nitrite infusion during acute myocardial infarction (NITRITE-AMI): study protocol of a randomised controlled trial. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2013-002813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsang A, Hausenloy DJ, Mocanu MM, Carr RD, Yellon DM. Preconditioning the diabetic heart: the importance of Akt phosphorylation. Diabetes. 2005;54:2360–2364. doi: 10.2337/diabetes.54.8.2360. [DOI] [PubMed] [Google Scholar]
- 35.Yui H, Imaizumi U, Beppu H, Ito M, Furuya M, Arisaka H, Yoshida K. Comparative effects of verapamil, nicardipine, and nitroglycerin on myocardial ischemia/reperfusion injury. Anesthesiol Res Pract. 2011;2011:521084. doi: 10.1155/2011/521084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calvert JW, Lefer DJ. Myocardial protection by nitrite. Cardiovasc Res. 2009;83:195–203. doi: 10.1093/cvr/cvp079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bolli R, Becker L, Gross G, Mentzer R, Jr, Balshaw D, Lathrop DA. Myocardial protection at a crossroads: the need for translation into clinical therapy. Circ Res. 2004;95:125–134. doi: 10.1161/01.RES.0000137171.97172.d7. [DOI] [PubMed] [Google Scholar]
- 38.Janero DR, Bryan NS, Saijo F, Dhawan V, Schwalb DJ, Warren MC, Feelisch M. Differential nitros(yl)ation of blood and tissue constituents during glyceryl trinitrate biotransformation in vivo. Proc Natl Acad Sci USA. 2004;101:16958–16963. doi: 10.1073/pnas.0406075101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jung KH, Chu K, Ko SY, Lee ST, Sinn DI, Park DK, Kim JM, Song EC, Kim M, Roh JK. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 40.Gori T, Daiber A, Di Stolfo G, Sicuro S, Dragoni S, Lisi M, Munzel T, Forconi S, Parker JD. Nitroglycerine causes mitochondrial reactive oxygen species production: in vitro mechanistic insights. Can J Cardiol. 2007;23:990–992. doi: 10.1016/s0828-282x(07)70862-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.