This editorial refers to ‘Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischaemic myocardium: the Cardio133 Trial’†, by B.A. Nasseri et al., on page 1263
The heart failure epidemic, accelerated by global ageing, is a rampant contributor to worldwide morbidity and mortality, underscoring the escalating burden of non-communicable diseases. In the context of a growing prevalence in ischaemic heart disease, advances in the acute management of myocardial infarction with rapid percutaneous revascularization have reduced premature death but have resulted in a high incidence of chronic heart failure overloading healthcare systems.1 The malignant nature of the disease associated with poor outcomes reflects a shortcoming of current therapies, largely ineffective against infarction-triggered tissue destruction. Established therapies target afterload reduction and mineralocorticoid dysregulation to limit myocardial remodelling without, however, treating parenchymal loss that underlies disease. Loss of ventricular mass, compounded by maladaptive remodelling, leads to progressive organ deterioration necessitating recurrent hospitalizations and life-extending measures. Yet, left ventricular assist devices or heart transplantation are complex and costly procedures, available to a limited patient population. In the USA alone, ∼2500 heart transplants are performed annually. Due to donor paucity, >100 000 additional patients are left without hope for such a life-saving intervention. A pressing need thus exists for institution of innovative therapies that would reach beyond existing standards of care.
The emergence of the science-powered regenerative toolkit offers such a prospect, signalling a potential for radical transformation of future practices.2 Curative options are viewed as being paramount in addressing the evolving patho-demographic landscape. Applied to cardiovascular diseases, regenerative technologies—exemplified by stem cell-based protocols—aim at restoring normative structure and function. By seeking definitive solutions, regenerative approaches are predicted to offer a cost-effective outlook. To this end, translation of a growing body of knowledge underlying repair strategies necessitates deployment of robust regenerative platforms for delivery of quality medical and/or surgical algorithms. Indeed, roll-out of the regenerative model of care predicates a rigorous evidence-supported paradigm that will drive validated science into standardized and scalable clinical options.
Regenerative cardiology principles
The regenerative paradigm exploits new knowledge in disease pathogenesis and natural repair mechanisms. Conceived to halt or reverse disease progression, stem cell therapies are applied essentially as adjuvants to standard of care with the goal of furthering an otherwise limited self-renewal capacity of the human heart. That the adult heart is not a terminally differentiated organ, as traditionally assumed, was recently demonstrated in humans. As part of establishing a comprehensive human regenerative map, the cardiomyocyte turnover rate was estimated at ∼1%/year in young adults and 0.5%/year in the elderly, indicating that around half of the heart mass will be renewed over a person's life.3 In the setting of massive ischaemic injury, this renewal reserve becomes insufficient and thus unable to rescue a failing myocardium. Yet, introduction of exogenous or activation of endogenous progenitor cell pools within the permissive heart environment would offer unprecedented strategies to fortify reparative mechanisms.
Early after infarction, the goal is primarily cardioprotective, i.e. to salvage the jeopardized myocardium and prevent pathological remodelling. At later stages of florid left ventricular dysfunction, the goal becomes cardiorestorative, i.e. to reverse maladaptive remodelling and improve contractility.4 Beyond the original notion that transplanted cells would serve as building blocks to generate new muscle, novel evidence implicates that the repair process engenders a cross-talk between delivered cells and the host myocardium triggering reparative signalling and a regenerative response. In this regard, multiple processes have been traced, ranging from activation of resident cardiac progenitors and induction of cardiomyocyte division to modification of the tissue niche with an increase in neovascularization and reduction in scar burden. Amplification of the inherent regenerative activity of the heart is thus an attractive strategy for therapeutic cardiac repair deserving methodical exploration.
Clinical experience
Supported by pre-clinical studies, translation of regenerative paradigms has been tested in distinct clinical settings using various stem cell platforms.5 Early emphasis was placed on establishing quality control procedures through standard operating practices for the harvesting, isolation, and expansion of cell populations. Among multiple sources, the accessibility and ease of cell isolation and processing has catalysed reliance on the patient's own bone marrow as a renewable option. Bone marrow-derived mononuclear cells, unfractionated or enriched in progenitor subpopulations, have most frequently been used for treatment of acute myocardial infarction. Experience to date underscores a proven feasibility and safety profile, although study endpoints have not always been met and a sustained functional benefit remains uncertain. Case-controlled trials in patients with recent myocardial infarction in fact suggest only modest benefit with regard to recovery of left ventricular ejection fraction beyond standard reperfusion therapy. Variable outcome is further highlighted in the setting of chronic heart failure, where the experience is more limited.6 The results of the Cardio133 clinical study that uses a selected bone marrow progenitor subpopulation in combination with surgical revascularization are now reported.7
Conducted in 60 patients as a double-blinded, randomized, placebo-controlled protocol, the Cardio133 study was designed to determine the impact of intramyocardial transplantation of autologous CD133+ bone marrow-derived stem cells on left ventricular function in the setting of ischaemic heart failure and coronary artery bypass grafting.7 CD133 is a transmembrane cell surface receptor available for clinical-grade isolation of a stem cell population, shown to regenerate ischaemic myocardium in pre-clinical models. The Cardio133 study reports localized benefit, with improved myocardial perfusion in more left ventricular segments in the CD133 group than with placebo. Despite possible amelioration in regional perfusion and scar size, no significant effect was documented on global left ventricular function and clinical symptoms.7
It should be pointed out that in such combination regimens, the confounding impact of coronary artery bypass grafting renders difficult the distinction of a genuine effect ascribed to cell injection. Also, a dose of 5 million CD133+ cells was injected according to the Cardio133 study protocol. This may be insufficient, further compounded by a limited retention of cells upon delivery and the prevailing uncertainty of best timing for cell therapy. Importantly, variability in the regenerative potency of progenitor cell function and in the responsiveness of the recipient myocardium has been reported in heart failure, implicating patient age, severity of disease, cardiovascular risk factors, as well as individual genetic variance. The Cardio133 study investigators conclude that cell products with a greater and more predictable potential for cardiac regeneration may be better suited in heart failure therapy.7
Towards maximizing benefit of cell-based therapy
Trial results across studies undeniably lack uniformity, owing to the current lack of standardization of cell isolation and delivery protocols. Beyond intertrial unevenness, interpatient variability has been recognized, prompting the pursuit of optimization strategies in order to identify adequate cell sources and cell types, stratify and select patients most amenable to cell therapy, target ideal timing of intervention, and define favourable routes and modes of administration.8
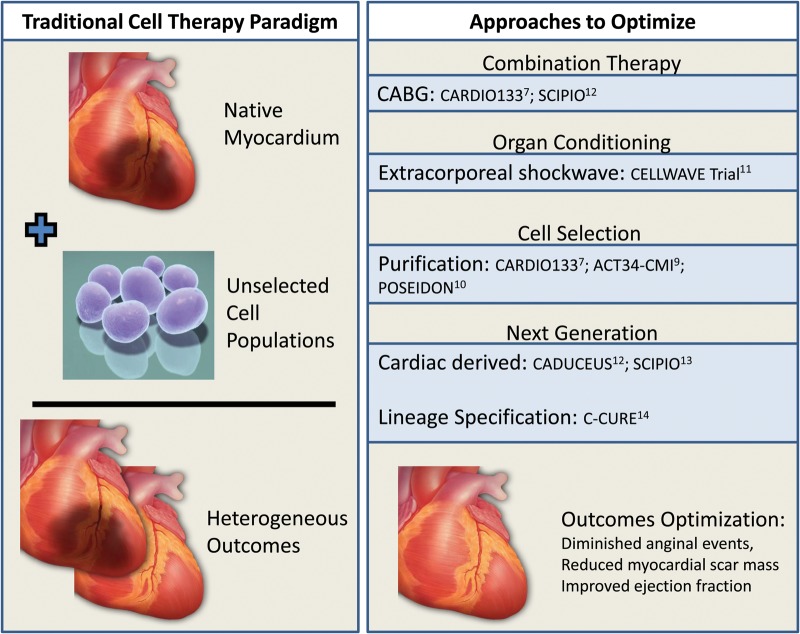
Among optimization modalities under consideration in clinical trials (Figure 1),9–14 the principle of conditioning the myocardial environment prior to cell delivery has been introduced. With this goal, the CELLWAVE trial was designed to assess the value of manipulating the target tissue to enhance the therapeutic benefit of stem cell therapy.11 Specifically to improve stem cell homing, the territory of interest was exposed to extracorporeal shockwave pre-treatment followed by cell infusion. This shockwave-facilitated strategy was associated with functional benefit when compared with cell therapy alone.11
Figure 1.
Evolution of stem cell paradigms. Early experience in the treatment of cardiovascular diseases employs stem cells in their native state. This approach has yielded variable outcomes. Increasingly, strategies that would optimize outcome are implemented in clinical trials. These include combination approaches that augment the efficacy of primary therapy; organ conditioning to prepare target tissue for subsequent cell delivery; cell selection to purify the desired cell population; and next-generation approaches including cardiac stem cells and lineage-specified progenitors.
In parallel with habituation schemes, distinct strategies have been proposed including the prospect of anatomically matching the regenerative cell source with the target organ.12,13 These approaches leverage the aptitude to derive resident stem cell populations by processing myocardial tissue excised during cardiac surgery or by endovascular biopsy. Resident cardiac stem cells have been evaluated in SCIPIO and CADECEUS trials.12,13 The CADECEUS study utilizes the cell cluster cardiosphere approach for derivation and propagation, while SCIPIO implements an antibody-based method to derive a C-kit+ population. Both studies reported reduced myocardial scar mass following cell treatment, indicative of therapeutic regeneration, with the SCIPIO trial also reporting improved left ventricular ejection fraction albeit in the setting of coronary artery bypass grafting.12,13
Alternatively, the rational design of next-generation cell biotherapeutics has been advanced. As a case in point, orienting non-resident stem cells towards cardiogenesis upgrades their regenerative potential while removing the need for patients to undergo myocardial harvest.14 To this end, cardiac development traits were induced in bone marrow-derived mesenchymal stem cells, establishing the first human scalable lineage-specified cardioreparative phenotype derived without heart tissue harvest. In the ensuing C-CURE clinical trial, patient-derived mesenchymal stem cells were converted into the cardiopoietic phenotype through priming with a cardiogenic growth factor-containing cocktail.14 The C-CURE trial demonstrated feasibility and safety of bone marrow-derived cardiopoietic stem cell therapy. This study also documented improvement in left ventricular ejection fraction compared with standard of care, with a decrease in left systolic end-systolic volumes and increase in 6 min walking distance.14 Next-generation stem cell technologies highlight continuous advances in regenerative science, progressively translated into phase III therapeutic protocols (e.g. CHART-1 trial ClinicalTrials.gov identifier NCT01768702) designed to validate benefit definitively in larger cohorts of patients with chronic heart failure.
In due course, the objectivity of comparative effectiveness outcome analysis15 with the aim to inform practice, improve care, and influence costs applied across regenerative platforms will serve as the foundation for future evidence-based standard of care.
Funding
Funding to pay the Open Access publication charges for this article was provided by Mayo Clinic.
Conflict of interest: none declared.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update. Circulation. 2014;129:399–410. doi: 10.1161/01.cir.0000442015.53336.12. [DOI] [PubMed] [Google Scholar]
- 2.Terzic A, Harper CM, Jr, Gores GJ, Pfenning MA. Regenerative medicine blueprint. Stem Cells Dev. 2013;22(Suppl 1):20–24. doi: 10.1089/scd.2013.0448. [DOI] [PubMed] [Google Scholar]
- 3.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartunek J, Vanderheyden M, Hill J, Terzic A. Cells as biologics for cardiac repair in ischaemic heart failure. Heart. 2010;96:792–800. doi: 10.1136/hrt.2007.139394. [DOI] [PubMed] [Google Scholar]
- 5.Telukuntla KS, Suncion VY, Schulman IH, Hare JM. The advancing field of cell-based therapy: insights and lessons from clinical trials. J Am Heart Assoc. 2013;2:e000338. doi: 10.1161/JAHA.113.000338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanganalmath SK, Bolli R. Cell therapy for heart failure: a comprehensive overview of experimental and clinical studies, current challenges, and future directions. Circ Res. 2013;113:810–834. doi: 10.1161/CIRCRESAHA.113.300219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasseri BA, Ebell W, Dandel M, Kukucka M, Gebker R, Doltra A, Knosalla C, Choi Y-H, Hetzer R, Stamm C. Autologous CD133+ bone marrow cells and bypass grafting for regeneration of ischemic myocardium: the Cardio133 trial. Eur Heart J. 2014;35:1263–1274. doi: 10.1093/eurheartj/ehu007. [DOI] [PubMed] [Google Scholar]
- 8.Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair—lessons from clinical trials. Nat Rev Cardiol. 2014 doi: 10.1038/nrcardio.2014.9. in press. [DOI] [PubMed] [Google Scholar]
- 9.Losordo DW, Henry TD, Davidson C, Sup Lee J, Costa MA, Bass T, Mendelsohn F, Fortuin FD, Pepine CJ, Traverse JH, Amrani D, Ewenstein BM, Riedel N, Story K, Barker K, Povsic TJ, Harrington RA, Schatz RA. ACT34-CMI Investigators. Intramyocardial, autologous CD34+ cell therapy for refractory angina. Circ Res. 2011;109:428–436. doi: 10.1161/CIRCRESAHA.111.245993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis-Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. doi: 10.1001/jama.2012.25321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assmus B, Walter DH, Seeger FH, Leistner DM, Steiner J, Ziegler I, Lutz A, Khaled W, Klotsche J, Tonn T, Dimmeler S, Zeiher AM. Effect of shock wave-facilitated intracoronary cell therapy on LVEF in patients with chronic heart failure: the CELLWAVE randomized clinical trial. JAMA. 2013;309:1622–1631. doi: 10.1001/jama.2013.3527. [DOI] [PubMed] [Google Scholar]
- 12.Malliaras K, Makkar RR, Smith RR, Cheng K, Wu E, Bonow RO, Marbán L, Mendizabal A, Cingolani E, Johnston PV, Gerstenblith G, Schuleri KH, Lardo AC, Marbán E. Intracoronary cardiosphere-derived cells after myocardial infarction: evidence of therapeutic regeneration in the final 1-year results of the CADUCEUS Trial (CArdiosphere-Derived aUtologous stem CElls to reverse ventricUlar dySfunction) J Am Coll Cardiol. 2014;63:110–122. doi: 10.1016/j.jacc.2013.08.724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet. 2011;378:1847–1857. doi: 10.1016/S0140-6736(11)61590-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo-Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. doi: 10.1016/j.jacc.2013.02.071. [DOI] [PubMed] [Google Scholar]
- 15.Terzic A, Nelson TJ. Regenerative medicine: advancing health care 2020. J Am Coll Cardiol. 2010;55:2254–2257. doi: 10.1016/j.jacc.2009.12.050. [DOI] [PubMed] [Google Scholar]