Abstract
Studies have identified the risk factors like folic acid deficiency during gestational period, family history for orofacial clefts, drugs like antiepileptic, vitamin A. But, the data regarding the folic acid status in children with cleft lip/palate is hardly evaluated in depth. Here, an assessment of folic acid and DNA damage were carried out in children with orofacial anomalies. Folic acid level and DNA damage were evaluated by folic acid assay (direct chemiluminescent technology) and single cell gel electrophoresis or comet assay method respectively. The mean value of plasma folic acid by direct chemiluminescent technology was 6.5±3.6 nmol/L and the normal value in children ranges from 11.3 to 47.6 nmol/L. The amount of damaged DNA, measured as the tail length of the comet in cases, was 19.4±8.9 μm and the mean percentage of DNA in tail was 16.5±3.7. Folic acid deficiency could be the reason for DNA damage.
Key words: cleft lip, cleft palate, comet assay, orofacial clefts, MTHFR gene
Introduction
Orofacial anomalies are the most common craniofacial birth defects that arise as a result of failure of facial embryonic processes to fuse. It is important for its significant lifelong morbidity and complex etiology.1 The average prevalence of orofacial clefts is 1/700 live births.2 Apart from the cosmetic appearance children often suffer from comorbidities like feeding difficulties, ear infections, hearing loss, speech and language delay.3 Despite surgical interventions, associated comorbidities and social problems make their lives challenging. Several studies have identified the risk factors like folic acid deficiency during gestational period, family history for orofacial clefts, drugs like antiepileptic, vitamin A. But, the data regarding the folic acid status in children with cleft lip/palate is hardly evaluated in depth. Here, an assessment of folic acid was carried out in children with cleft lip and palate. The present study would add to the existing knowledge of folic acid deficiency behind most of the genetic damages and this knowledge can be eventually applied for prevention, treatment and prognosis in individuals having orofacial clefts.
Case Report
The research plan was approved by the Human Ethics Committee of Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER, India). Clinically diagnosed cases of isolated cleft lip, isolated cleft palate and cleft lip with cleft palate were included and those associated with other major congenital anomalies were excluded from the study. Ten children aged between 0-10 years with various types of orofacial clefts who attended Pediatrics Outpatient Department of JIPMER were the cases for the current study.
Folic acid was measured by direct chemiluminescent technology. A plasma sample of 150 was dispensed with 50 μL of DTT/releasing agent into a cuvette and incubated for 4.7 min at 37°C. Then 100 μL of folate binding protein and 200 μL solid phase was dispensed and incubated for 6.3 min at 37°C. Dispense 100 μL of lite reagent and incubated for 3 min at 37°C. Separate, aspirate and wash the cuvettes with wash 1. Dispense 300 μL each of acid reagent (R1) and base reagent (R2) to initiate the chemiluminescent reaction.
By venipuncture, 1 mL of blood was collected under strict aseptic conditions and subjected to centrifuging at 1500 rpm. After 30 min of centrifugation, the buffy coat containing lymphocytes was removed carefully using a micropipette. The slides were pre coated with 1% NMA (normal melting point agarose) and allowed to dry for 10 min. When the first layer had solidified a second layer containing 20 μL of separated lymphocytes mixed with 60 μL of warm 0.5% LMA (low melting point agarose) was layered using coverslip and gelled at 4°C for 15 min. After 15 min of solidification at 4°C the third layer was made with 75 μL of LMA and refrigerated for 15 min to prevent the loss of sample.
After the third layer of gel was set, the slides were immersed in lysis solution for 1 h at 4°C. With this treatment the cell membrane and nuclear membrane were lysed and the majority of proteins were removed to expose the nucleoids. The slides were then placed in a horizontal gel electrophoresis tank. The slides were left in the high pH (>13) buffer for 30 min to allow unwinding of DNA and expression of alkali labile sites and another 30 min at 300 mA, 0.74V/cm for the movement of DNA fragments if any, towards anode through the gel. After electrophoresis the slides were washed gently with neutralization buffer. Staining was done with silver nitrate solution. The stained slides were visualized using a 20x objective on a bright field light microscope and captured using CCD camera and they were scored using comet score software.
In this study six children were males, six were females and four patients had cleft lip, seven cleft palate and one cleft lip with palate (Figure 1). The mean value of plasma folic acid is found to be 6.5±3.6 nmol/L (Table 1) and the normal value of folic acid in children varies from 11.3 to 47.6 nmol/L.4,5 In the current study the DNA damage was assessed by the single cell gel electrophoresis or comet assay which is considered as a simple and sensitive method to assess DNA damage in a single cell. The comet assay is based on the principle that damaged DNA moves towards anode forming a comet shaped image and the undamaged DNA remains within the cell. The amount of damaged DNA is measured as the tail length of the comet (Figure 2). The mean tail length in cases is 19.4±8.9 μm. Mean percentage of DNA in tail is 16.5±3.7 (Table 1).
Figure 1.
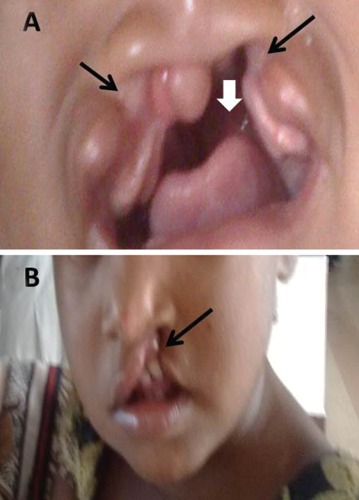
Photographs showing bilateral (A) and unilateral (B) cleft lip with palate. White arrow showing cleft palate and black arrow showing cleft lip.
Table 1.
Parameters of DNA damage and folic acid level among the case.
| No. of cases | Tail length (μm) | % DNA in tail | Level of folic acid (nmol/L) |
|---|---|---|---|
| 1 | 28.8 | 14.6 | 8.1 |
| 2 | 7.2 | 14.5 | 5.4 |
| 3 | 17.0 | 15.0 | 6.7 |
| 4 | 26.4 | 18.5 | 4.6 |
| 5 | 30.4 | 20.9 | 4.7 |
| 6 | 9.7 | 10.9 | 3.8 |
| 7 | 16.2 | 23.6 | 7.7 |
| 8 | 33.5 | 17.5 | 13.9 |
| 9 | 18.5 | 15.5 | 10.2 |
| 10 | 14.9 | 19.1 | 6.2 |
| 11 | 7.6 | 11.1 | 7.5 |
| 12 | 22.6 | 17.6 | 4.6 |
| Mean | 19.4±8.9 | 16.5±3.7 | 6.5±3.6 |
Figure 2.
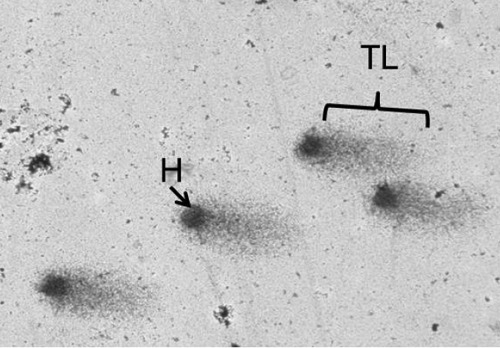
Showing picture of comet. Dark globular shaped area indicates head (H) and tail length (TL).
Discussion
Folate is required for the synthesis of DNA and RNA, the building blocks of cells.6 Folic acid deficiency causes DNA damage in the form of single/double strand DNA breaks, alkali labile sites (apurinic/apyrimidinic sites), DNA cross links, base/base pair damages. Most of the damaged DNA is cleared/repaired from circulation. If a stable mutation has occurred in the stem cells which may accumulate over months or years and may end up being responsible for various health conditions in childhood or later life, in present or future generations.
Cleft lips/palate are the most common craniofacial birth defects and the second most common among all congenital anomalies. The various types of clefts are isolated cleft lip, isolated cleft palate and cleft lip with cleft palate (Figure 1). In 40% to 60% of persons with birth defects the cause is unknown. Genetic factors such as chromosomal abnormalities and mutant genes account for approximately 15%: environmental factors produce approximately 10%; combination of genetic and environmental influences (multifactorial inheritance) produces 20% to 25%.7 On extensive literature search, there was no literature showed the status of folic acid in orofacial clefts. The present study had been undertaken to assess the level of folic acid in cleft/palate.
Despite the detection folic acid deficiency and damaged DNA in orofacial clefts in this study, the cause for the same remains unknown due to the complex interplay between genetic and environmental factors. Genetic factors like mutation and its consequences could be hypothesized to be the causes of folic acid deficiency in orofacial clefts.
A study by Shaw et al. has shown mutation in MTHFR gene can cause orofacial clefting. Mutation in MTHR gene causes diminished level of serum folic acid and increased serum homocysteine level in the children with clefts.8 Apart from mutation and its consequences, children with orofacial clefts often suffer from comorbidities like feeding difficulties, ear infections, hearing loss, speech and language delay and dental problems. In this study all patients were belong to low economic status. The above said factors could be the reasons for folic acid deficiency in orofacial clefts.
Bille et al. revealed increased occurrence of breast cancer among females with cleft lip and/or cleft palate, primary brain cancer among females with cleft palate and primary lung cancer among males with both cleft lip and cleft palate.9 So, this folic acid status should be taken into consideration. Further extension of the study by folic acid supplementation and comparison of folic acid before and after supplementation, collection of adequate exposure data (beginning from pregnancy and during childhood) and a thorough look on clinical features will further enhance our knowledge about folic acid deficiency in children with orofacial clefts. It also gives an opportunity to advice on lifestyle changes for the betterment of health in people with cleft lip/palate.
Conclusions
Folic acid deficiency and DNA damage are there in children with isolated cleft lip, isolated cleft palate and cleft lip with palate. Identification and modulation of causative factors can reduce folic acid deficiency in clefts, which further can reduce the morbidity and prevent long-term complications of orofacial clefts.
Acknowledgments
The authors would like to thank Prof. Bishnu Bhat, Department of Pediatrics, for analyzing the data and scientific discussion during manuscript writing.
Funding Statement
Funding: the work was supported by an Institutional Research Grant (JIPMER, India).
References
- 1.Letra A, Menezes R, Govil M, et al. Follow-up association studies of chromosome region 9q and nonsyndromic cleft lip/palate. Am J Med Genet A 2010;152A:1701-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brito LA, Meira JGC, Kobayashi GS, Passos-Bueno MR. Genetics and management of the patient with orofacial cleft. Plast Surg Int 2012;2012:782821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jugessur A, Farlie PG, Kilpatrick N. The genetics of isolated orofacial clefts: from genotypes to subphenotypes. Oral Dis 2009;15:437-53 [DOI] [PubMed] [Google Scholar]
- 4.Ghadban R, Almourani R, Staros EB. Folate (folic acid). Dec 11,2013. Available from: http://emedicine.medscape.com/article/2085523-overviewAccessed: Jan 18, 2014 [Google Scholar]
- 5.Fischbach FT, Dunning MB. Ovid Technologies I. A manual of laboratory and diagnostic tests. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2008. pp 141-143 [Google Scholar]
- 6.Brito A, Hertrampf E, Olivares M, et al. [Folate, vitamin B12 and human health]. Rev Médica Chile 2012;140:1464-75 [Article in Spanish] [DOI] [PubMed] [Google Scholar]
- 7.Sadler TW, Langman J. Langman’s medical embryology. Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012 [Google Scholar]
- 8.Shaw GM, Rozen R, Finnell RH, et al. Infant C677T mutation in MTHFR, maternal periconceptional vitamin use, and cleft lip. Am J Med Genet 1998;80:196-8 [DOI] [PubMed] [Google Scholar]
- 9.Bille C, Winther JF, Bautz A, et al. Cancer risk in persons with oral cleft-a population-based study of 8,093 cases. Am J Epidemiol 2005;161:1047-55 [DOI] [PMC free article] [PubMed] [Google Scholar]


