Abstract
A 55-year old man was treated with sunitinib 50 mg/day for 4 weeks on and 2 weeks off, as a first-line therapy for metastatic renal cell carcinoma. During the fourth week of the first cycle, he was admitted to the Emergency Department with abdominal pain and vomiting. Acute acalculous cholecystitis was diagnosed. Sunitnib-associated cholecystitis is a rare adverse event previously reported in few cases. The mechanism behind this complication is not fully understood, although vascular endothelial dysfunction may play a role. The use of this drug is expanding in clinical oncology, and physicians should be aware of this life-threating adverse event.
Key words: acalculous cholecystitis, sunitinib malate, angiogenic agents, receptor tyrosine kinase
Introduction
Sunitinib is an oral multitarget tyrosine kinase inhibitor (TKI), with targets including vascular endothelial growth factor receptor (VEGFR) and platelet-derived growth factor receptor (PDGFR). It is recommended for the treatment of advanced renal cell carcinoma (RCC) based on a phase III trial that randomized patients with metastatic RCC to receive either sunitinib or interferon-α and observed a longer progression-free survival and higher response rates in patients treated with sunitinib.1 This agent has showed a wide spectrum of adverse events such as diarrhea, fatigue, nausea and stomatitis. However, sunitinib-related acute cholecystitis has been previously reported in only three case reports. Here, we report this serious event in a patient with RCC.
Case Report
A Caucasian 55-year old man presented with abdominal pain, hematuria and weight loss. His medical history included smoking. Computed tomography (CT) was performed and revealed multiple pulmonary nodules and hypervascular expansive mass in the right kidney of 8.2 cm (Figure 1). The patient was referred to our hospital and underwent right nephrectomy. The postoperative period was uneventful. The pathologic examination showed clear-cell renal carcinoma. Pulmonary nodules were biopsied and confirmed metastasis of clear-cell renal carcinoma.
Figure 1.
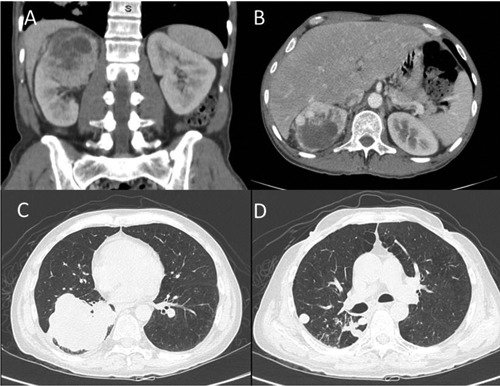
Initial presentation: hypervascular expansive mass in the right kidney (A and B) and multiple lung metastases (C and D).
Forty-two days after nephrectomy, sunitinib was started at a dose of 50 mg per day for 4 weeks on followed by 2 weeks off. In the fourth week of treatment, the patient presented with nausea, vomiting and post-prandial pain in the right upper quadrant. On physical examination, the patient was tachycardic, febrile, with painful abdominal palpation and Murphy’s sign. Laboratory tests showed leukocyte count of 16,540/mm3, neutrophil 11,040/mm3, amylase 98 U/L, lipase 42 U/L, C-reactive protein 98 mg/L (reference value <10 mg/L), bilirubin 0.58 mg/dL, aspartate aminotransferase 42 U/L, alanine aminotransferase 31 U/L. We performed a CT scan of the abdomen that revealed dilatation of the gallbladder lumen and pericholecystic fluid collection without biliary calculus (Figure 2). Ultrasonography confirmed these findings. Acute cholecystitis was diagnosed. Treatment with antibiotics (ceftriaxone and metronidazole) was started and sunitinib therapy was discontinued immediately. Due to severe sepsis at presentation, we decided to perform cholecystectomy. Pathological exam of the gallbladder confirmed cholecystitis and absence of calculosis (Figure 3). In the immediate postoperative period, the patient demonstrated improvement in the abdominal pain and resolution of the symptoms. He was discharged after 12 days. Sunitinib was reintroduced after 3 weeks in the same dose and was maintained for 6 months until disease-progression without new serious adverse events.
Figure 2.
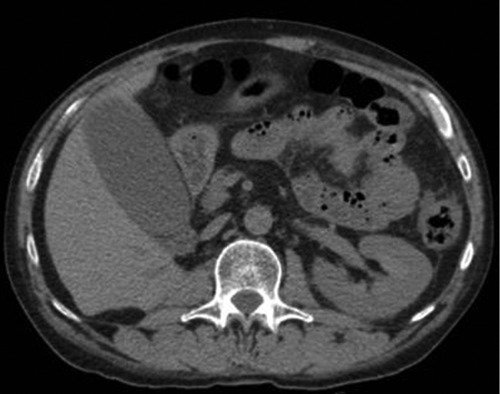
Computed tomographic scan revealed dilatation of the gallbladder lumen and pericholecystic fluid collection.
Figure 3.
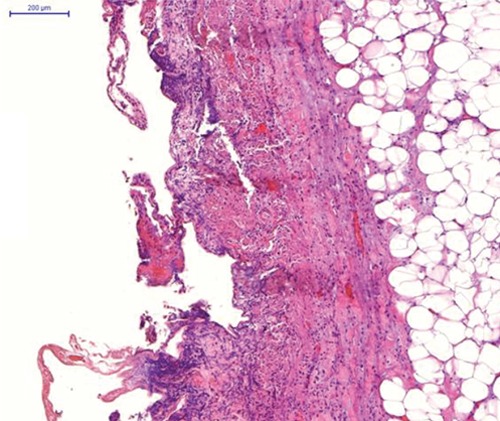
Cholecystitis: predominantly mucosal-based inflammatory infiltrate (hematoxylin and eosin x20).
Discussion
Sunitinib-related acute cholecystitis has been previously reported in three patients: one with gastrointestinal stromal tumors, one with clear-cell renal carcinoma, and one with cromophobe RCC.2-4 All three presented with acalculous cholecystitis, which is an acute necroinflammatory disease of the gallbladder associated with high morbidity and mortality. Known risk factors for its development are sepsis, parenteral nutrition, opiates and conditions leading to immunosuppression.5
Sunitinib has TKI activity against VEFGR and PDGFR and carries a high risk for adverse vascular events such as hypertension, proteinuria, thromboembolism, decline in left ventricle eject fraction and myocardial infarction.1,6,7
The etiology of gallbladder toxicity is uncertain, although such toxicity has also been reported for others TKIs.8-10 There is clear evidence that VEGF plays a role in modulating cholangiocyte proliferation in response to cholestasis and that cholangiocytes express VEGFR.11 Inhibition of VEGFR in biliary tract cells can represent an imbalance of stress adaption, causing biliary diseases. Another possible mechanism is disturbance of the homeostasis between the endothelium and platelets as a result of TKI-induced endothelial cell damages, allowing micothrombi formation and ischemia.12 In addition, the antiangiogenic activity of sunitinib in the gallbladder epithelium and muscularis layers may contribute to necroinflammatory alterations observed in cholecystitis. Considering the drug’s biliary excretion pattern, these events could be exacerbated by drug accumulation in the gallbladder lumen.13
Rosen et al. performed a phase I trial including patients with solid tumors treated with motesanib, a TKI against VEGFR, PDGFR and Kit and observed that the treatment was associated with increased gallbladder volume, decreased ejection fraction and biliary sludge. In this trial, three patients had cholecystitis that resolved after drug discontinuation.10
Our patient did not have major risk factors for developing acalculous cholecystitis except for a relative immunosuppressed state secondary to cancer. We evaluated whether or not acute cholecystitis in our patient was caused by sunitinib with the use of the Naranjo scale, which assesses the probability of a drug-related adverse event.14 Its score for our patient was five, indicating a probable association of acute cholecystitis with sunitinib (Table 1).
Table 1.
Adverse drug reaction probability scale (Naranjo scale) in the present case.
| Yes | No | Do not know | Score | |
|---|---|---|---|---|
| 1. Are there previous conclusive reports on this reaction? | 0 | |||
| 2. Did the adverse event appear after the suspected drug was given? | 2 | |||
| 3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was given? | 0 | |||
| 4. Did the adverse reaction appear when the drug was re-administered? | 0 | |||
| 5. Are there alternative causes that could have caused the reaction? | 2 | |||
| 6. Did the reaction reappear when a placebo was given? | 0 | |||
| 7. Was the drug detected in any body fluid in toxic concentrations? | 0 | |||
| 8. Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? | 0 | |||
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | 0 | |||
| 10. Was the adverse event confirmed by any objective evidence? | 1 | |||
| Scoring | 5 |
Sunitinib is associated with other adverse events such as nausea, vomiting, asthenia and abdominal pain, symptoms that mimic gallbladder disorders. It is possible that a portion of patients presenting these adverse reactions
may be suffering from a slight degree of gallbladder toxicity. Consequently, this event may be underreported in trials and in clinical practice.
Due to few cases reported, there is no standard management. In our case, the patient presented severe sepsis and cholecystectomy was therefore required in addition to drug discontinuation. Other reports showed that the discontinuation of sunitinib was sufficient to recover.3,4
Conclusions
We report a case of sunitinib-associated acalculous acute cholecystitis. The use of sunitinib is expanding in other solid tumors and physicians should therefore be aware of this life-threatening adverse event.
References
- 1.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115-24 [DOI] [PubMed] [Google Scholar]
- 2.Nakano K, Suzuki K, Morita T. Life-threatening acute acalculous cholecystitis in a patient with renal cell carcinoma treated by sunitinib: a case report. J Med Case Rep 2012;6:69-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gomez-Abuin G, Karam AA, Mezzadri NA, Bas CA. Acalculous cholecystitis in a patient with metastatic renal cell carcinoma treated with sunitinib. Clin Genitourin Cancer 2009;7:62-3 [DOI] [PubMed] [Google Scholar]
- 4.de Lima Lopes G, Jr, Rocha Lima CM. Emphysematous cholecystitis in a patient with gastrointestinal stromal tumor treated with sunitinib. Pharmacotherapy 2007;27:775-7 [DOI] [PubMed] [Google Scholar]
- 5.Ryu JK, Ryu KH, Kim KH. Clinical features of acute acalculous cholecystitis. J Clin Gastroenterol 2003;36:166-9 [DOI] [PubMed] [Google Scholar]
- 6.Daher IN, Yeh ET. Vascular complications of selected cancer therapies. Nat Clin Pract Cardiovasc Med 2008;5:797-805 [DOI] [PubMed] [Google Scholar]
- 7.Aparicio-Gallego G, Afonso-Afonso FJ, Leon-Mateos L, et al. Molecular basis of hypertension side effects induced by sunitinib. Anticancer Drugs 2011;22:1-8 [DOI] [PubMed] [Google Scholar]
- 8.Sanda M, Tamai H, Deguchi H, et al. Acalculous cholecystitis in a patient with hepatocellular carcinoma on sorafenib. ISRN Gastroenterol 2011;2011:201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aihara Y, Yoshiji H, Yamazaki M, et al. A case of severe acalculous cholecystitis associated with sorafenib treatment for advanced hepatocellular carcinoma. World J Gastrointest Oncol 2012;4:115-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosen LS, Lipton L, Price TJ, et al. The effect of different dosing regimens of motesanib on the gallbladder: a randomized phase 1b study in patients with advanced solid tumors. BMC Cancer 2013;13:242-52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaudio E, Barbaro B, Alvaro D, et al. Vascular endothelial growth factor stimulates rat cholangiocyte proliferation via an autocrine mechanism. Gastroenterology 2006;130:1270-82 [DOI] [PubMed] [Google Scholar]
- 12.Elice F, Rodeghiero F, Falanga A, Rickles FR. Thrombosis associated with angiogenesis inhibitors. Best Pract Res Clin Haematol 2009;22:115-28 [DOI] [PubMed] [Google Scholar]
- 13.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol 2006;24:25-35 [DOI] [PubMed] [Google Scholar]
- 14.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45 [DOI] [PubMed] [Google Scholar]


