Abstract
Cancer chemoprevention by phytochemicals may be one of the most feasible approaches for cancer control. Phytochemicals obtained from vegetables, fruits, spices, teas, herbs and medicinal plants, such as terpenoids and other phenolic compounds, have been proven to suppress experimental carcinogenesis in various organs in pre-clinical models. Recent studies have indicated that mechanisms underlying chemopreventive potential may be a combination of antioxidant, anti-inflammatory, immune-enhancing, and hormone modulation effects, with modification of drug metabolizing enzymes, influence on cell cycle and cell differentiation, induction of apoptosis, suppression of proliferation and angiogenesis playing roles in the initiation and secondary modification stages of neoplastic development. Specific features of prostate cancer, such as high prevalence and long latency period provides ample opportunities for chemopreventive agents to work at various stages of disease progression. Finally, suitable populations with appropriate risk factors, including the presence of pre-malignant lesions and genetic predispositions, need to be well characterized for future chemopreventive interventions. Here we review naturally occurring dietary terpenoids as useful agents for prostate cancer chemoprevention with reference to their classes and sources.
Keywords: Terpenoids, cancer chemoprevention, biomarkers, prostate cancer
INTRODUCTION
It is estimated that 218,890 new cases of prostate cancer will be diagnosed in the United States in the year 2007. This makes prostate cancer the number one cancer diagnosis for men for the second year in a row, accounting for 29% of all new cancers. Besides, diagnosis of new cases, approximately, 27,050 men will die of prostate cancer this year which is down about one percent from 2006, but still contributes to 9% of total cancer-related deaths in American men (1). This continued decrease in deaths can hopefully be attributed in part to higher long term success in prostate cancer treatments and early screening for diagnosis. Prostate cancer is an important target for chemoprevention because of its long latency and high prevalence (2, 3). Surgery, radiotherapy, and brachytherapy are potentially curative treatments for localized prostate carcinoma, when watchful waiting is not an option. Hormonal therapies also known as ‘androgen deprivation therapy’ reduces the testosterone levels which drive prostate cancer cells to multiply are usually effective initially, but given enough time, hormone refractory disease eventually emerges (4, 5). This is a common and deadly form of the disease, lacks curative options and is the main cause of prostate cancer-related mortality. The only treatment with a demonstrated survival benefit is docetaxel, which lengthens the life of patients with a mean of less than 3 months, despite often grueling side effects (6). Limited options for the management of prostate cancer and its increasing incidence necessitate search for novel preventive approaches for this disease. One such approach is through chemoprevention, a means of cancer management by which the occurrence of the disease can be entirely prevented, slowed, or reversed by the administration of one or more naturally occurring and/ or synthetic compounds, as an alternative to treatment of cancer cases after clinical symptoms have appeared (2, 3). Therefore, ultimate goal of cancer prevention is preferably to live without cancer or with cancer without suffering from symptoms until the natural termination of life (7, 8).
CANCER CHEMOPREVENTION
Cancer can be prevented by either avoiding life style-related risk factors such as the smoking habit, a high-fat western diet, physical inactivity and carcinogen containing foods, or alternatively by increasing exposure to beneficial influences, including intake of chemopreventive agents (2, 3, 9–13). The latter may be particularly practical because chemopreventive agents can be taken as supplements or by modulation of the current diet status (13). One can envisage subjects for cancer chemoprevention falling into two groups, one at high-risk of cancer because of the presence of precancerous lesions or predisposing conditions, and the other being the apparently healthy general population. Given difficulties in ensuring that the test compound will not have any toxicity besides proven efficacy and convenience for use in the long term, the practical aim of chemoprevention, for the present, should best be focused on ‘high-risk’ groups. Furthermore, there are advantages with application of natural compounds so that regulatory approval can be facilitated. Two basic concepts underlie in cancer chemoprevention: the multi-step nature of cancer development and field carcinogenesis. The development of cancer occurs over years and involves multiple genetic and phenotypic alterations that lead to invasive cancer. Chemoprevention is based on the premise that intervention is possible during the many steps of this process (2, 3. 13, 14).
CANCER: A THREE STEP MODEL
Based on animal model studies, carcinogenesis has been broadly divided into three phases: initiation, promotion, and progression. In initiation, a carcinogen interacts with DNA, producing a fixed mutation. The specific molecular change depends on the carcinogen and can be influenced by a number of factors, including the rate and type of carcinogenic metabolism and the response of the DNA repair function. During promotion, the initiated cells proliferate. This stage occurs over a long period and can be altered by chemopreventive agents and affect growth rates (15). Progression is the phase between a pre-malignant lesion and the development of invasive cancer. During this stage, genetic and phenotypic changes occur, with the rate of progression based on the rate of genetic mutation and cell proliferation (16–19). Carcinogenesis is the concept that, in patients at risk, extensive, multi-focal, genetically distinct pre-malignant and malignant lesions can occur within the whole carcinogen-exposed region. The classical example is exposure of the upper aero-digestive tract and lungs to the carcinogenic effects of tobacco (20). The finding of one neoplasm in the exposed area provides evidence for the presence of multiple pre-malignant lesions of independent origin (21). In this setting, lesion-specific therapy is insufficient; interventions that prevent the promotion and progression of unrecognized lesions are needed. Thus chemopreventive agents aim to directly modulate specific steps in the carcinogenic process, block mutagenic carcinogens, prevent DNA damage by free radicals, suppress epithelial cell hyper-proliferation, and/or modulate epithelial cell differentiation and apoptosis. The molecular targets of these agents include cell signaling, cell-cycle regulators, and survival/apoptotic molecules, which are implicated in uncontrolled cancer growth and progression. Furthermore, angiogenic and metastatic targets, including vascular endothelial growth factor (VEGF), hypoxia-inducing factor (HIF)-1α, matrix metalloproteinases (MMPs), and urokinase-type plasminogen activator (uPa) are also modulated by many chemopreventive agents to suppress the growth and invasive potential of cancer (17, 18). In contrast, chemotherapy is the use of specific drugs that can destroy cancer cells. Chemoprevention differs from cancer treatment in that the individuals most appropriate for chemopreventive intervention are generally healthy people at ‘high-risk’ for developing the specific cancer who have not contracted the disease. However, most healthy individuals never contract the clinically evident disease; thus, for practical reasons (i.e. achievable sample sizes, duration of follow-up) chemoprevention trials have often focused on individuals with precancerous lesions or with history of previously treated cancers (secondary prevention). Such individuals may already have cancer that has not been diagnosed and are actually receiving cancer treatment rather than cancer prevention. Drugs developed to treat cancer are fundamentally different from those developed to prevent it. Because some subjects receiving chemoprevention are both symptom- and disease- free, toxic drugs are not nearly as acceptable in this group compared with cancer patients who are receiving treatment to save or extend their lives. Also, preventive agents are often taken for long periods of time to produce desired effects. An ideal chemopreventive agent should be inexpensive, safe, and well tolerated with long-term administration, and effective in preventing cancer without compromising with the quality of life (3). The main goal of administering a chemopreventive agent is to prevent the development of cancers.
CANCER CHEMOPREVENTION WITH DIETARY AGENTS
The importance of developing totally new classes of chemopreventive agents is stressed, with particular emphasis on the bioactive agents derived from vegetables, fruits, spices, teas, herbs and medicinal plants. The need for new agents with novel mechanisms of action to prevent cancer is perhaps the most urgent need in the entire field of chemoprevention. Although ‘proof of principle’ of chemoprevention has been clearly demonstrated, in both animal and clinical studies, none of the existing chemopreventive agents is ideal, either because of lack of efficacy and potency or because of toxic side effects that preclude widespread, long term use. During the last decade several bioactive agents are identified from the diets which are being developed as chemopreventive agents (9–11). For example, glucobrassicin and glucoraphanin from broccoli, Chinese cabbage, radish and watercress; sinigrin from brussels sprouts, cabbage and cauliflower are being developed as chemopreventive agents. The isothiocynates formed from indole glucosinolates, indole-3-carbinol, indole-acetonitrile, thiocynate and 3, 3′-diindolylmethane and sulforaphane act as chemopreventive agents (22). These agents favorably modifying carcinogen metabolism via selective alteration of cytochrome P450 enzyme involved in carcinogen metabolic activation and induction of phase II enzymes. Flavanoids including flavones (apigenin, luteolin), flavanols (quercetin, kaempferol), flavanones (hesperetin, naringenin), flavanols (epigallocatechin, epigallocatechin-3-gallate), anthocyanins (cyanidin, delphinidin) and isoflavones (geneistein, daidzein) (23) has been shown to possess free radical scavenging properties and modulate COX-2, inhibit EGFR, IGF-RI and NF-κB signaling (24). Phytoestrogens compete with endogenous estrogens for binding to estrogen receptor. Therefore, they may have beneficial effects in prevention of steroid hormone-dependent cancers such as breast and prostate cancer. There are evidences that different berries such as black raspberries, blackberries and strawberries inhibit carcinogen-induced malignancy in animal models. Some of the known chemopreventive agents in berries include vitamin C, E and folic acid; small amount of calcium and selenium, β-and α-carotene, polyphenols such as ellagic acid, ferulic acid, p-coumaric acid, quercetin, several anthocyanins. It has been observed that they could inhibit the growth of pre-malignant cells through down-regulation of COX-2 and also expression of other genes associated with tumor development such as iNOS and VEGF (25). Reverastrol, a phytoalexin present in grapes, berries and peanuts suppress the activity of COX-2, causes cell cycle arrest and pro-apoptotic cell death in many types of cancer cells (26). Tea contains large quantities of polyphenolic compounds known as catechins. The leading catechins in green tea are epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG), and epigallocatechin-3-gallate (EGCG) whereas black tea contains thearubigin and theaflavins. Several studies based on cell culture and animal models have demonstrated the cancer preventive role of tea associated with the inhibition of c-fos and cyclin D1 promoter activity, decrease Bcl-XL, inhibition of VEGF, p53 stabilization and NF-κB activation (27, 28). The bioactive ingredients of different spices such as curcumin, gingerol, paradol, and capsaicin are the members of vanilloid compounds possessing chemopreventive activities (29). Similarly, terpenoids are an extensive group of natural compounds and from the nutritional standpoint, monoterpenes, limonene, menthol, perillyl alcohol, diterpenes, retinoids and tetraterpenes include carotenoids provide health benefits in decreasing the risk of certain diseases including cancer (30). The triterpenoids, oleanolic acid and ursolic acid are commonly present in many plant species and exhibit anti-inflammatory and anti-carcinogenic activities (31). Structure-activity studies among several oleanolic and ursolic acid derivatives have identified 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid and related compounds as potent anti-inflammatory compounds that inhibit growth and induce apoptosis in several cancer cells and tumor types (32).
PROSTATE CANCER CHEMOPREVENTION
The criteria for selecting chemopreventive agents that are ultimately evaluated in clinical trials can rely on leads from several distinct areas of investigation. Epidemiologic, experimental and basic mechanistic carcinogenesis data in all can provide rationale for pursuing the development of a particular pharmaceutical agent, micronutrient, or dietary substance (33, 34). There is strong evidence that high intakes of vegetables, fruits, and whole grains are associated with reduced cancer risk. Comprehensive reviews of case control and prospective cohort studies found that the relationship between high vegetable, fruit, spices, teas, grains intake and reduced cancer risk (35, 36). The epidemiologic data from dietary questionnaires in the Health Professional’s Follow-up Study suggested that consumption of tomato products was one of the few discernable food use patterns to be strongly associated with a decreased risk of prostate cancer development. Other case control studies provide support that use of soy products and green and black teas often decrease the risk of prostate cancer (37). The beneficial effect of vegetables, fruits and whole grains may be due to their individual and combined effects of their constituents, including fiber, micronutrients, and phytochemicals.
TERPENOIDS: A CLASS OF CANCER CHEMOPREVENTIVE AGENT
Terpenoids are natural constituents of plants and of invertebrate and vertebrate animals. The terpenoids are the most diverse group of plant substituents that play a variety of roles in many different plant species. All terpenes are constructed from isoprenoid units by biochemically unusual pathways involving highly reactive intermediates. They mostly occur as monoterpenoids (essential oils), diterpenoids, triterpenoids, sesquiterpenoids (phytosterols, saponins), and tetraterpenoid carotenoids (38). All are related by being derived from a common isopentenyl precursor. The distributional patterns and functions of terpenoids are as diverse as the chemical structures. The importance of terpenes to plants relates to their necessity to fix carbon through photosynthetic reactions using photosensitizing pigments. A monoterpene contains ten carbons (two isoprene units); a sesquiterpene, fifteen carbons (three isoprene units); a diterpene, twenty carbons (four isoprene units), triterpenes (thirty carbons) are important structural components of plant cell membranes. Many plant pigments, including the yellow and red carotenoids, are tetraterpenes (forty carbons). Natural rubber is a polyterpene containing many isoprene units. The monoterpenes and sesquiterpenes are common components of the essential oils of herbs and spices (peppermint, lavender), of flower scents (rose), and of turpentine, derived from the resin of evergreen trees (38, 39). Animals have evolved to utilize these compounds for hormonal growth regulatory functions (vitamin A) and, as it is now being understood, the presence of these molecules in normal tissues also provides a measure of protection from certain diseases, especially those related to chronic damage and growth deregulation. Several hundred terpenoids have been reported to have chemopreventive activity, and promising agents and agent combinations are currently being evaluated clinically for cancer chemoprevention (40, 41). A list of dietary terpenoids which are being evaluated for their anti-cancer activity is listed in table 1.
Table 1.
Dietary terpenoids tested in prostate cancer
| Terpenoids | Chemical Structure | Source | Ref. |
|---|---|---|---|
|
| |||
|
Monoterpenes D-Limonene Perillyl Alcohol |
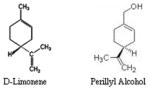
|
Lemons, oranges, grapefruit, caraway, bergamot, peppermint, spearmint, dill, tomatoes | 12 |
|
Diterpenes Retinol Trans-retionic acid |
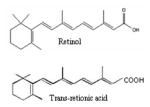
|
Carrot, spinach, pumpkin, broccoli, mango, papaya, cherry, tomato, corn, orange, cabbage, watermelon, lettuce | 61–63 |
|
Triterpenoids Betulinic Acid Lupeol |
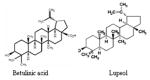
|
Olive, mango, strawberry, grapes, figs | 88, 89, 93, 99 |
|
Terpenoid Chromanols Tocotrienols Tocopherols |
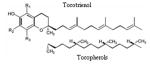
|
Almonds, peanut oil, walnuts, palm oil | 100, 108, 110 |
|
Carotenoids Carotene Lycopene |
|
Tomatoes, orange, carrot, peas, sprouts, green, beans, corn | 125, 128 |
i) Monoterpenes
Monoterpenes are the focus of much investigation in the areas of both cancer prevention and therapy. Cancer prevention, inhibition, and regression are the most noteworthy attributes of the monoterpenes. d-Limonene, the most abundant monocyclic monoterpene present in orange peel oil has shown to inhibit neoplasia in animal models (42, 43) and cell growth in vitro (44). D-limonene and perillyl alcohol (POH) have been shown to be chemopreventive against various types of human cancers (45–48). D-limonene is a monocyclic monoterpene with POH a metabolite of D-limonene, being its hydroxylated form. There are a number of synonyms for limonene, include the following: 1, 8(9)p-Menthadiene; 1-methyl1-4(1-methylethenyl) cycloclohexene; 1-methyl-4-isopropenyl-1-cyclo-hexene; alpha-limonene; dipentene; limonene; pmentha-1,8-diene. They are found in essential oils of many plants including lemons, oranges, grapefruit, caraway, dill, bergamot, peppermint, spearmint, grasses and tomatoes. They are also associated with vegetables and some evergreen trees. POH is often distilled from lavender, found in cherries, mint, celery seeds and can be produced synthetically. It is typically used as flavoring agents, food additive, and fragrance and has been found to be a major volatile component of mother’s milk (49). D-limonene has different metabolites for different animals. In humans, the three major metabolites after an oral dosage are perillic acid, dihydroperillic acid, and limonene-1, 2-diol. It is thought that the metabolic precursors of the first two are perillyl alcohol and perillyl aldehyde (50). D-limonene is found in orange juice at concentrations ranging from 10–100 ppm and chewing gum, which contains up to 2,300 ppm (51). D-Limonene has been shown to be effective anti-cancer agent (52–54). Studies from our laboratory have shown that treatment of human prostate cancer cells with D-limonene cause inhibition of cell growth, decrease in mitochondrial activity leading to cell death which is independent of androgen association and p53 status (55). More recent studies have demonstrated that some derivatives of D-limonene increased the anti-proliferative effects in human prostate cancer cells via activation of ERK pathway and induction WAF1/p21 leading to cell cycle arrest (56). Similar to D-Limonene, perillyl alcohol is effective in prostate cancer. Studies have shown that perillyl alcohol caused sensitization of hormone insenstitive prostate cancer cells and kill them. Exposure of these cells to perillyl alcohol induced a transient G2/M arrest and enhanced the expression of the membrane bound form of the Fas ligand and sensitized the cells to Fas-mediated apoptosis (57). Another recent study has demonstrated that perillyl alcohol can attenuate androgen receptor (AR)-mediated action in androgen sensitive prostate cancer cells by inhibiting AR gene expression and activation of c-Jun, which represses the expression and function of AR (58). Human phase I clinical results for POH used in the treatment of advanced malignancies in humans, have been reported (59). Dosages ranging from 800–2400 mg/m2/dose were assayed for tolerability. The main toxicity was gastrointestinal and included nausea and vomiting, anorexia, unpleasant taste, satiety, and eructation. The main metabolites were perillic acid and dihydroperillic acid. The evidence of the POH efficacy in prostate cancer patients has been reported. POH was administered orally to patients with metastatic androgen-independent prostate cancer at 1200 mg/m2/dose four times daily and continued until disease progression or development of unacceptable toxicity. The disease was shown to stabilize for 6 months, although no objective tumor response was observed (60).
ii) Diterpenes
Among diterpenes, vitamin A or retinol is the most important compound which is found in animals. The term retinoids refers to all analogs of retinol. Retinoids, a class of over 3000 natural derivatives and synthetic analogs of vitamin A, are powerful modulators of epithelial carcinogenesis (61). About 1500 different retinoids have been synthesized by modifying the ring structure, the side chain, or the terminal group of the molecule in attempts to obtain greater anti-carcinogenic activity and less toxicity. The naturally occurring retinoids include: retinol, the alcohol of vitamin A, retinoic acid, the carboxylic acid, retinal, the aldehyde, and 13-cis-retinoic acid, an isomer of retinoic acid. Retinoids, including vitamin A (retinol) and its active metabolite, retinoic acid play important roles in inhibiting cell proliferation, and promoting morphogenesis and differentiation (62, 63). Retinoids may be particularly effective in reversing pre-malignant epithelial lesions in the lung, skin, head and neck (64). Considerable interest has developed in the application of retinoids as chemopreventive agents for human cancer (65, 66). Most actions of retinoids are believed to be mediated by its nuclear receptors, which function as ligand-activated transcription factors (67). There are two types of retinoid receptors, retinoic acid receptors (RARs) and retinoid X receptors (RXRs). Heterodimers of the RARs and RXRs bind to a specific DNA promoter sequence, termed the retinoic acid response element, and regulate gene transcription (68, 69). RXRs can also form homodimers and activate the retinoid X response element or form heterodimers with other members of the steroid receptor superfamily, thus serving in multiple regulatory functions in different signaling pathways (70). Retinoids provide resistance to chemical carcinogenic challenge, while vitamin A deficiency results in increased sensitivity to induce cancer (71). Vitamin A deficiency in humans has been associated with an increased incidence of cancer of anatomical sites (72). A low intake of vitamin A in cohort studies was also associated with an increased risk of cancer in the prostate (73, 74). Numerous studies have also shown that retinoids can prevent or reduce the incidence of prostate cancer in various animal models. For example, both the synthetic retinoid N-(4-hydroxyphenyl) retinamide (4-HPR) and 9-cis RA have been shown to prevent cancer in animal models of prostatic carcinogenesis (75). In human prostate cancer cell lines, RXR protein was decreased relative to noncancer prostate cell lines, and a reduction of cell growth or increased susceptibility to apoptosis was demonstrated with increases in the level of RXR in RXR -transduced prostate cancer cells (76). In a variety of studies using cultured human prostatic cancer cells, retinoids have been shown to inhibit tumor cell growth. For instance, the growth of the androgen-sensitive LNCaP cell line (77) and androgen-independent prostatic cancer cell lines (78), as well as primary cultures from prostatic adenocarcinoma, can be inhibited by retinoids. Retinoids can also inhibit the ability of these tumor cells to invade the extra-cellular matrix and induce apoptosis (79, 80). The mechanisms by which RA can induce growth inhibition of prostate cancer cell lines are unclear, but studies have implicated apoptosis, activation of retinoblastoma protein, and modulation of androgen receptor pathways (79–81). It has also been shown recently that the Bcl-2 protein is down-regulated by RA in prostatic cancer cells (82). The synthetic retinoid, fenretinide has been studied extensively as a chemopreventive agent against prostate cancer and exhibited chemopreventive activity (83). Retinoids used in clinical therapies exhibit potential toxicity (84, 85). All-trans-retinoic acids are relatively non-toxic with few serious adverse reactions compared to anticancer chemotherapy drugs; however, it does share adverse effects common to other retinoids (84–86).
iii) Triterpenoids
Triterpenoids form a group of natural substances which includes steroids and consequently sterols (87–89). Squalene is the immediate biological precursor of all triterpenoids. The large groups of steroids including sterols are present in very small amounts in bacteria but at large amounts in plants and animals while the hopanoids are very abundant in prokaryotes where they replace cholesterol (89). Triterpenoids have shown to possess anti-inflammatory and anti-carcinogenic properties (90, 91). Lup-20(29)-en-3β-ol (Lupeol), a triterpene found in fruits such as olive, mango, strawberry, grapes and figs, in many vegetables and in several medicinal plants, is used in the treatment of various aliments worldwide by native people (92, 93). Lupeol possesses strong antioxidant, anti-inflammatory, anti-arthritic, anti-mutagenic, and anti-malarial activity in vitro and in vivo systems acts as a potent inhibitor of protein kinases and serine proteases, and inhibits the activity of DNA topoisomerases II, a target for anticancer chemotherapy (92–96). It has been shown that Lupeol activates Fas receptor-mediated apoptotic machinery of LNCaP cells and inhibits the tumorigenesis of prostate cancer cells in animal model. A striking observation on the effect of Lupeol was that prostate cancer cells were highly sensitive to Lupeol-mediated loss of viability, and no such effect was observed in normal prostate PrEC cells (97).
Betulinic acid is a pentacyclic triterpene isolated from the stem bark of Betulin alba, and the studies show that Betulinic acid decreases the expression of VEGF and anti-apoptotic protein survivin in both prostate LNCaP cells and tumors (98). Studies from our laboratory has shown that betulinic acid selectively inhibit the proliferation of human prostate cancer LNCaP, DU-145 and PC-3 cells and effectively induces apoptosis associated with the cleavage of PARP and shift in Bax:Bcl-2 ratio in favor of cell death (99).
iv) Terpenoid Chromanols
Tocotrienols and tocophenols (vitamin E) are terpenoid chromanols, tocotrienols possess the ability to stimulate the killing of cancer cells selectively through apoptosis in order to reduce cancer cell proliferation, while leaving normal cells unaffected (100). One of the mechanisms by which tocotrienols are thought to suppress cancer is related to the isoprenoid side-chain that makes them different from tocopherols (101). Isoprenoids are plant compounds that have shown to suppress the initiation, growth and progression of prostate cancer and many types of cancer in experimental studies (102, 103). They are common in almonds, peanut oil and walnuts, which may explain why diets rich in these foods have consistently been shown to reduce the incidence of cancer (104–107). Much of the broad involvement of vitamin E in human metabolism is due to its role as the body’s primary lipid-soluble antioxidant. Tocopherols and tocotrienols are part of the body’s highly effective defense system, without which life as we know it could not exist. This defense system consists of a network of antioxidants, interacting with and supporting each other. Antioxidants such as vitamin C, coenzyme Q10 and glutathione are needed for effective recycling of tocopherols and tocotrienols. The unique power of both tocopherols and tocotrienols is their ability to break the chain reaction of lipid peroxidation by neutralizing peroxyl radicals to prevent the spread of free radical damage in cell membranes. Tocotrienols are more potent scavengers of the peroxy radical than alpha-tocopherol and provide far better protection against lipid peroxidation (108, 109). Vitamin E is a generic term for at least eight structurally related molecules: α-tocopherol (αT), β-tocopherol, γ-tocotrienol (γTE), and δ-tocotrienol. Among them, αT is the predominant form of vitamin E in plasma and tissues and is the form that has drawn most attention in the past. Benefit from αT for cancer prevention has been suggested in some studies (110). Recently, studies have indicated that other forms of vitamin E appear to have unique properties that are not shared by αT but may be important to human health (111). For instance, γT, the major form of vitamin E in US diets, but not αT, exhibits anti-inflammatory activities by inhibiting cyclooxygenase – catalyzed prostaglandin E2 formation in cell cultures and animals (112, 113). γT, unlike αT, is strongly nucleophilic and thus is more efficient than αT in trapping reactive nitrogen species (114–116). Consistently, the administration of γ-enriched tocopherols significantly lowered C-reactive protein, a biomarker of inflammation, in haemodialysis patients (117). Recently, Helzlsouer et al. reported that men in the highest quintile of plasma concentration of γT had a 5-fold reduced risk of prostate cancer compared with those in the lowest quintile. In the same study, significant protective effects of high concentrations of selenium and αT were observed only when γT concentrations were high (118). Consistently, γT and its metabolite, 2,7,8-trimethyl-2-(β-carboxyethyl-6-hydroxychroman), is more potent than αT in inhibiting prostate cancer cell growth by the down regulation of cyclins (119, 120). γT dose-dependently inhibited proliferation of prostate LNCaP and PC-3 but had no effect on prostate epithelial PrEC cells. γT and its combination with δT induced apoptosis in LNCaP by interrupting de novo synthesis of sphingolipids (120). More recently our laboratory has demonstrated that tocotrienol-rich fraction of palm oil is capable of selectively inhibiting cellular proliferation and accelerating apoptotic events in prostate cancer LNCaP, DU-145 and PC-3 cells, in comparison to human normal prostate epithelial PrEC and virally transformed normal human prostate epithelial PZ-HPV-7 cells (121). The tocotrienol-rich fraction offers significant promise as a chemopreventive and/or therapeutic agent against prostate cancer and it is becoming clear that individual vitamin E forms possess different chemical and biological activities and have distinct tissue distribution (122, 123). Combinations of different form of vitamin E may be superior to each alone. Growing evidence implies that selenium and vitamin E may decrease the risk of prostate cancer. The Selenium and Vitamin E Cancer Prevention Trial (SELECT) is a randomized prospective double-blind study designed to determine whether selenium and vitamin E decrease the risk of prostate cancer in healthy men. A total of 32,400 men are planned to be randomized in SELECT. SELECT is the second large-scale, population based, phase III, randomized controlled trial which directly test the effect of these agents alone and combination on the incidence of prostate cancer in North American males study of chemoprevention for prostate cancer with final results anticipated in 2013 (124).
v) Carotenoids
Carotenoid group include beta-carotene, alpha-carotene, lycopene, lutein, astaxanthin, cryptoxanthin and zeaxanthin (125). Carotenoids belong to the category of tetraterpenoids, derived from a 40-carbon polyene chain, which could be considered the backbone of the molecule. The hydrocarbon carotenoids are known as carotenes, while oxygenated derivatives of these hydrocarbons are known as xanthophylls. Beta-carotene is a tetratepenoid distributed widely throughout the plant kingdom and is the predominant pigment in orange-flashed melan (Cucumis melo L) varieties (126). Interest in beta-carotene as a potential anti-cancer agent established in the 1980s from the results of both case-control and cohort studies showing a consistent association for foods high in beta-carotene and reduced risk of prostate cancer (127). They possess anti-oxidant action as one of the mechanism for their cancer preventive effects. Tomatoes are the major source of lycopene commercially. Although lycopene is the most abundant carotenoids in tomatoes, they also contain other potentially beneficial carotenoids such as alpha-carotene, beta-carotene, lutein, phytoene, and phtyofluene (128). Studies confirmed that increased serum levels of lycopene, the carotenoid pigment present in large amounts of tomatoes, were more highly correlated to decreased prostate cancer risk than were levels of other circulating, diet derived carotenoids (129). It has been shown that lycopene is one of the most potent natural antioxidant. A case-control study indicated that the consumption of tomato products reduces the risk of prostate cancer and lycopene is one of the compounds in raw and processed tomato products that may contribute to a reduced risk of prostate cancer, other carotenoids and phytochemicals in tomato products may be synergistically responsible for the health benefits (130, 131). One 6-year, prospective, epidemiological study of approximately 47,000 men, the Health Professional Follow-up study (HPFS), concluded that 2 to 4 servings per week of raw tomatoes significantly reduced the risk of prostate cancer by 26% compared to no servings per week. Additionally, eating tomato products such as pizza and tomato sauce 2–4 times per week significantly reduced the risk by 15% and 34%, respectively, compared to not eating these foods (132). A clinical trial of 26 prostate cancer patients demonstrated supplementation with a tomato oleoresin extract (as Lyc-O-Mato, from LycoRed-Biodar, New York), which contained 30 mg of lycopene, reduced tumor size and made less involvement of surgical margins and/or extra prostate tissues with cancer less than in the control group (133). It has been suggested that the antioxidant carotenoid and that the free radical scavenging activity of these compounds may protect cells against oxidative mutagenesis. Another clinical study which randomly assigned 32 prostate cancer patients to a tomato-based diet for three weeks, noted those in the treatment group had a 15.5% reduction in prostate specific antigen (PSA, a marker of prostate cancer), as well as reductions of several other markers for prostate cancer (134). Although the role of all carotenoids in humans has yet to be fully determined, 25 carotenoids and 9 metabolites have been identified and characterized in human serum and other organs including prostate (135). Tomato and its products contain primarily all-trans-lycopene (79–91%); however, cis-lycopene accounts for 79–88% of total lycopene in malignant and benign prostate tissues (136). In vitro experimentation demonstrated that cis lycopene is absorbed more readily than all-trans-lycopene. The role of cis versus trans lycopene in human physiology has not yet been determined (135, 136). Two other studies concluded that dietary intervention and supplementation with 15 mg lycopene and smaller quantities of other tomato carotenoids, including phtyoene, phytofluene, ζ-carotene, and γ-carotene twice daily positively altered serum markers of prostate cancer progression (137, 138). Serum prostate specific antigen (PSA) levels, a marker of tumor activity, decreased in both trials, and tomato oleoresin supplementation altered biomarkers of cell growth and differentiation in the one study in which it was tested. Cancer cells show a decrease in cellular differentiation, and are sometimes ‘revert back’ to a more undifferentiated, embryonic-type cell. If tomato carotenoids can increase cellular differentiation, they may be important in the treatment of prostate cancer.
MODULATION OF INTERMEDIATE AND ENDPOINT BIOMARKERS BY TERPENOIDS
Study of markers of risk and surrogate end-point biomarkers holds great promise for cancer chemoprevention (139–141). The criteria for biomarker relevance are that they must be differentially expressed in normal and high-risk tissue, be closely linked to the casual pathway for cancer, be modified by the chemopreventive agent and with a shorter latency than cancer, and, finally, be assayed easily and with quantitative reliability. Alterations in the levels of prostate specific antigen (PSA) and the various grades of PIN are considered to be the primary intermediate biomarkers for evaluating the efficacy of an agent and for identifying appropriate cohorts for chemoprevention studies (142). Studies reported in literature have shown that terpenoids have potential to modify certain proteins and transcription factors which could be used as intermediate and endpoint markers to evaluate the efficacy of the test compound. For example, RXRα is the major component of retinoid action whose inactivation leads to the development of preneoplastic lesions in the prostate whereas chemopreventive responses including induction of apoptosis in prostate cancer cells by retinoic acids appear to be transcriptionally regulated by RARβ (143). Further, it has been demonstrated that gap junctional intercellular communication and Cx43 expression levels could be useful intermediate endpoints in prostate cancer chemoprevention trials, because they are decreased in prostate cancer cells (144). Therefore, chemopreventive agents modulating Cx43 expression and/or gap junctional intercellular communication would be of great interest. Retinoids and carotenoids are potent regulators of Cx43 and gap junctional intercellular communication. In particular, lycopene increases gap junctional intercellular communication by increasing expression of the gap junctional gene, Cx43. This action correlates strongly with the ability of lycopene and other carotenoids to suppress neoplastic transformation in cell culture systems. Upon restoring the gap junction proteins, functional communication was restored in human prostatic carcinoma cell lines, increased normal differentiation, reduced proliferation, and suppressed tumorigenecity. In recent epidemiological studies, relatively high plasma IGF-1 and low IGFBP-3 levels have been independently associated with greater risk of prostate cancer (145). Recent data show that lycopene administration to humans with prostate cancer significantly reduces serum IGF-1 levels. Additionally, lycopene produces changes in the expression of many proteins in prostate cancer chemoprevention processes, e.g. cyclins, and phase II detoxification enzymes. Furthermore, γ-tocopherol (γT), the major form of vitamin E in diets, exhibits anti-inflammatory activities by inhibiting cyclooxygenase catalyzed prostaglandin E2 formation in cell cultures and animals (146). γT is strongly nucleophilic and thus is more efficient in trapping reactive nitrogen species. Consistently, administration of γ-tocopherols significantly lowered C-reactive protein, a biomarker of inflammation in dialysis patients (147). Similarly, cell death induced by vitamin E forms is linked to de novo synthesis of sphingolipids. Ceramide is the common intermediate for the formation of complex sphingolipids, including sphingomyelin and glucosphingolipids. Recently, ceramide has been proposed as a mediator in regulating stress response, particularly during apoptosis (148). In addition, other sphingolipid intermediates, such as sphingosine, sphingosine-1-phosphate and dihydrosphingosine, have been shown to mediate cell survival (149). Vitamin E caused a large amount of dihydroceramide accumulation was observed before apoptosis, whereas total ceramide increased only when apoptosis was substantial. An increase in ceramide could be used as a potential biomarker for the vitamin E caused biological effects.
CONCLUSION
The future of terpenoid research remains open to innovation, with a specific need for emphasizing on important beneficial properties for human health. The biological role of terpenoids in the prevention and perhaps treatment of cancer and other chronic diseases are being studied and understood. Although the antioxidant properties of some terpenoids have been extensively studied, their role as an anticancer agent needs further investigation. The simple reason could be that tumors have many molecular targets, which function aberrantly, and therefore requires extensive research. The goal for the success of terpenoids in chemoprevention research is to evaluate bioavailability, metabolism, mechanism of action and safety of test agent in better devised tumor-specific risk model and/or high-risk cohorts, preclinical drug testing models (i.e. gene targeting/knockout models), and developing translational/mechanistic studies. Above all, diet modification, and food or nutritional extracts containing terpenoids with known anti-carcinogenic activity may be more acceptable to healthy populations with known genetic risk based upon gene-environment interactions. Because prostate carcinogenesis may span 20 years or more, clinicians and medical practitioners have potential opportunities to suppress the disease in its early, pre-malignant stages before clinical, invasive disease develops with various dietary terpenoid agents. Although some human clinical trials are beginning to be undertaken there is a great need for well designed human intervention studies that takes into consideration study designs including subject selection, endpoint measurement and the bioavailability of test agent in the target organs. Only through these measures we can increase our understanding and the important role played by dietary terpenoids with an attempt to make a meaningful impact in the prevention, treatment and increased survival of the patients at risk for prostate cancer.
Acknowledgments
The original work from author’s laboratory outlined in this review was supported by United States Public Health Service Grants RO1 CA108512, RO1 AT002709, RO3 CA107806 and funds from Cancer Research and Prevention Foundation to SG.
Abbreviations
- DNA
Deoxyribonucleic acid
- VEGF
vascular endothelial growth factor
- HIF
hypoxia-inducing factor
- MMPs
matrix metalloproteinases
- uPa
urokinase-type plasminogen activator
- IGF
insulin-like growth factor
- COX
cyclooxygenase
- EGFR
epidermal growth factor receptor
- EC
epicatechin
- EGC
epigallocatechin
- ECG
epicatechin-3-gallate
- EGCG
epigallocatechin-3-gallate
- POH
perillyl alcohol
- ERK
extracellular-regulated kinase
- AR
androgen receptor
- 4-HPR
N-(4-hydroxyphenyl) retinamide
- RA
retinoic acid
- PrEC
normal prostate epithelial cells
- PARP
Poly (ADP-ribose) polymerase
- T
tocopherol
- TE
tocotrienol
- SELECT
Selenium and Vitamin E Cancer Prevention Trial
- Cx
connexin
- PIN
prostatic intraepithelial neoplasia
- PSA
prostate specific antigen
- IGFBP
insulin-like growth factor binding protein
References
- 1.American Cancer Society. Cancer Statistics. 2007. [Google Scholar]
- 2.Shukla S, Gupta S. Dietary agents in the chemoprevention of prostate cancer. Nutr Cancer. 2005;53:18–32. doi: 10.1207/s15327914nc5301_3. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S. Prostate cancer chemoprevention: Current status and future prospects. Toxicol Appl Pharmacol. 2006 Nov 15; doi: 10.1016/j.taap.2006.11.008. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.Ichikawa T, Suzuki H, Ueda T. Hormone treatment for prostate cancer: current issues and future directions. Cancer Chemother Pharmacol. 2005;56:58–63. doi: 10.1007/s00280-005-0100-x. [DOI] [PubMed] [Google Scholar]
- 5.Tang DG, Porter AT. Target to apoptosis: a hopeful weapon for prostate cancer. Prostate. 1997;32:284–293. doi: 10.1002/(sici)1097-0045(19970901)32:4<284::aid-pros9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 6.Tannock I, de Wit R, Berry W. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. New England Journal of Medicine. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 7.Kitagawa T, Hara M, Sano T, Sugimura T. The concept of Tenju-gann, or “natural-end cancer”. Cancer. 1998;83:1061–1065. doi: 10.1002/(sici)1097-0142(19980915)83:6<1061::aid-cncr1>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 8.Sugimura T. An overview of cancer prevention. Eur J of Cancer Prev. 1996;5:1–8. doi: 10.1097/00008469-199612002-00001. [DOI] [PubMed] [Google Scholar]
- 9.Wattenberg LW. An overview of chemoprevention: current status and future prospects. Proc Soc Exp Biol Med. 1997;216:133–141. doi: 10.3181/00379727-216-44163. [DOI] [PubMed] [Google Scholar]
- 10.Greenwald P, Milner JA, Anderson DE, McDonald SS. Micronutrients in cancer chemoprevention. Cancer Metastasis Rev. 2002;21:217–230. doi: 10.1023/a:1021202709003. [DOI] [PubMed] [Google Scholar]
- 11.Ames BN, Gold LS, Willett WC. The causes and prevention of cancer. Proc Natl Acad Sci USA. 1995;92:5258–5265. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka T, Kohno H, Mori H. Chemoprevention of colon carcinogenesis by dietary non-nutritive compounds. Asian Pac J Cancer Prev. 2001;2:165–177. [PubMed] [Google Scholar]
- 13.Wattenberg LW. Chemoprevention of cancer. Prev Med. 1996;25:44–45. doi: 10.1006/pmed.1996.0015. [DOI] [PubMed] [Google Scholar]
- 14.Tsuda H, Ohshima Y, Nomoto H, Fujita K, Matsuda E, Iigo M, Takasuka N, Moore MA. Cancer prevention by natural compounds. Drug Metab Pharmacokin. 2004;19:245–263. doi: 10.2133/dmpk.19.245. [DOI] [PubMed] [Google Scholar]
- 15.Anne S, Tsao, Edwards S, Kim, Hong WK. Chemoprevention of cancer. CA Cancer J Clin. 2004;54:150–180. doi: 10.3322/canjclin.54.3.150. [DOI] [PubMed] [Google Scholar]
- 16.Olinski R, Gackowski D, Foksinski M, Rozalski R, Roszkowski K, Jaruga P. Oxidative DNA damage: assessment of the role in carcinogenesis, artherosclerosis, and acquired immunodeficiency syndrome. Free Radic Biol Med. 2002;33:192–200. doi: 10.1016/s0891-5849(02)00878-x. [DOI] [PubMed] [Google Scholar]
- 17.Owuor ED, Kong AN. Antioxidants and oxidants regulated signal transduction pathways. Biochem Pharmacol. 2002;64:765–770. doi: 10.1016/s0006-2952(02)01137-1. [DOI] [PubMed] [Google Scholar]
- 18.Nakae D, Umemura T, Kurokawa Y. Reactive oxygen and Nitrogen Oxide Species-induced stress, a Major Intrinsic Factor involved in Carcinogenesic Processes and a Possible Target for Cancer Prevention. Asian Pac J cancer Prev. 2002;3:313–318. [PubMed] [Google Scholar]
- 19.Oshima H. Genetic and epigenetic damage induced by reactive nitrogen species: implications in carcinogenesis. Toxicol Lett. 2003;140–141:99–104. doi: 10.1016/s0378-4274(02)00506-4. [DOI] [PubMed] [Google Scholar]
- 20.Steinmetz, Kristi A, Potter, John D. Vegetables, fruit, and cancer prevention. J American Diet Assoc. 1996;96:1–18. doi: 10.1016/S0002-8223(96)00273-8. [DOI] [PubMed] [Google Scholar]
- 21.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–530. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 22.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 23.Van Meeteren ME, Hendriks JJ, Dijkstra CD, Van Tol EA. Dietary compounds prevent oxidative damage and nitric oxide production by cells involved in demyelinating disease. Biochem Pharmacol. 2004;67:967–975. doi: 10.1016/j.bcp.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 24.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–2505. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 25.Hou DX. Potential mechanisms of cancer chemoprevention by anthocyanins. Current Molecular Medicine. 2003;3:149–159. doi: 10.2174/1566524033361555. [DOI] [PubMed] [Google Scholar]
- 26.Jannin B, Menzel M, Berlot JP, Delmas D, Lancon A, Latruffe N. Transport of resveratrol, a cancer chemopreventive agent, to cellular targets: Plasmatic protein binding and cell uptake. Biochem Pharmacol. 2004;68:1113–1118. doi: 10.1016/j.bcp.2004.04.028. [DOI] [PubMed] [Google Scholar]
- 27.Masuda M, Suzui M, Lim JT, Deguchi A, Soh JW, Weinstein IB. Epigallocatechin-3-gallate decreases VEGF production in head and neck and breast carcinoma cells by inhibiting EGFR-related pathways of signal transduction. J Exp Ther Oncol. 2002;2:350–359. doi: 10.1046/j.1359-4117.2002.01062.x. [DOI] [PubMed] [Google Scholar]
- 28.Hastak K, Gupta S, Ahmad N, Agarwal MK, Agarwal ML, Mukhtar H. Role of p53 and NF-kappaB in epigallocatechin-3-gallate-induced apoptosis of LNCaP cells. Oncogene. 2003;22:4851–4859. doi: 10.1038/sj.onc.1206708. [DOI] [PubMed] [Google Scholar]
- 29.Surh YJ. Anti-tumor promoting potential of selected spice ingredients with antioxidative and anti-inflammatory activities: A short review. Food Chem Toxicol. 2002;40:1091–1097. doi: 10.1016/s0278-6915(02)00037-6. [DOI] [PubMed] [Google Scholar]
- 30.Johnson EJ. The role of carotenoids in human health. Nutr Clin Care. 2002;5:56–65. doi: 10.1046/j.1523-5408.2002.00004.x. [DOI] [PubMed] [Google Scholar]
- 31.Gner KH, Elmadfa I. Biological relevance of terpenoids. Overview focusing on mono- di- and tetraterpenes. Ann Nutr Metab. 2003;47:95–106. doi: 10.1159/000070030. [DOI] [PubMed] [Google Scholar]
- 32.Honda T, Rounds BV, Gribble GW, Suh N, Wang Y, Sporn MB. Design and synthesis of 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, a novel and highly active inhibitor of nitric oxide production in mouse macrophases. Bioorg Med Chem Lett. 1998;8:271–274. doi: 10.1016/s0960-894x(98)00479-x. [DOI] [PubMed] [Google Scholar]
- 33.Suh N, Wang Y, Honda T. A novel synthetic oleanane triterpenoid 2-cyano-3,12-dioxoolean-1,9-dien-28-oic acid, with potent differentiating antiproliferative and anti-inflammatory activity. Cancer Res. 1999;39:326–341. [PubMed] [Google Scholar]
- 34.Kelloff GJ, Hawk ET, Karp JE. Progress in clinical chemoprevention. Semin Oncol. 1997;24:241–252. [PubMed] [Google Scholar]
- 35.Kirsh VA, Peters U, Mayne ST, Subar AF, Chatterjee N, Johnson CC, Hayes RB. Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Prospective study of fruit and vegetable intake and risk of prostate cancer. J Natl Cancer Inst. 2007;99:1200–1209. doi: 10.1093/jnci/djm065. [DOI] [PubMed] [Google Scholar]
- 36.Steinmetz KA, Potter JD. Vegetables, fruit, and cancer. I. Epidemiology. Cancer Causes Control. 1991;2:325–357. doi: 10.1007/BF00051672. [DOI] [PubMed] [Google Scholar]
- 37.Block G, Patterson B, Subar A. Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer. 1992;18:129. doi: 10.1080/01635589209514201. [DOI] [PubMed] [Google Scholar]
- 38.Grassmann J. Terpenoids as plant antioxidants. Vitam Horm. 2005;72:505–535. doi: 10.1016/S0083-6729(05)72015-X. [DOI] [PubMed] [Google Scholar]
- 39.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 40.Govannucci E. Tomatoes, tomato-based products, lycopene, and cancer: review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:317–331. doi: 10.1093/jnci/91.4.317. [DOI] [PubMed] [Google Scholar]
- 41.Campbell J, Canene-Adams K, Lindshield B, Boileau T, Clinton S, Erdman JW. Tomato phytochemicals and prostate cancer risk. J Nutr. 2004;134:3486S–3493S. doi: 10.1093/jn/134.12.3486S. [DOI] [PubMed] [Google Scholar]
- 42.Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S–778S. doi: 10.1093/jn/129.3.775S. [DOI] [PubMed] [Google Scholar]
- 43.Egbede JA, Elson CE, Qureshi A, Tanner MA, Gould MN. Inhibition of DMBA-induced mammary cancer by the monoterpene d-limonene. Carcinogenesis. 1984;5:661–664. doi: 10.1093/carcin/5.5.661. [DOI] [PubMed] [Google Scholar]
- 44.Bardon S, Picard K, Martel P. Monoterpenes inhibit cell growth, cell cycle progression, and cyclin D1 gene expression in human breast cancer cell lines. Nutr Cancer. 1998;32:1–7. doi: 10.1080/01635589809514708. [DOI] [PubMed] [Google Scholar]
- 45.Elegbede JA, Elson CE, Tanner MA, Qureshi A, Gould MN. Regression of rat mammary tumors following dietary d-limonene. J Natl Cancer Inst. 1986;76:323–325. [PubMed] [Google Scholar]
- 46.Crowell PL, Kennan WS, Haag JD. Identification of metabolites of the antitumor agent d-limonene capable of inhibiting protein isoprenylation and cell growth. Cancer Chemother Pharmacol. 1992;31:205–212. doi: 10.1007/BF00685549. [DOI] [PubMed] [Google Scholar]
- 47.Chang BH, Lee HY, Lee JS, Young CY. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2006;236:222–228. doi: 10.1016/j.canlet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 48.Kawata S, Nagas T, Yamasaki E, Ishiguro H, Matsuzawa Y. Modulation of the mevalonate pathway and cell growth by pravastatin and d-limonene in a human hepatoma cell line (Hep G2) Br J Cancer. 1994;69:1015–1020. doi: 10.1038/bjc.1994.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belanger JT. Perillyl alcohol: applications in oncology. Altern Med Rev. 1998;3:448–457. [PubMed] [Google Scholar]
- 50.Brudnak M. Cancer preventing properties of essential oil monoterpenes D-Limonene and Perillyl alcohol. Complementary Medicine Magazine. :1–8. [Google Scholar]
- 51.Von Burg R. Limonene. J Appl Toxicol. 1995;15:495–499. doi: 10.1002/jat.2550150611. [DOI] [PubMed] [Google Scholar]
- 52.Nakaizumi A, Baba M, Uehara H, Iishi H, Tatsuta M. d-Limonene inhibits N-nitrosobis (2-oxoprenyl) amine induced hamster pancreatic carcinogenesis. Cancer Lett. 1997;117:99–103. doi: 10.1016/s0304-3835(97)00207-3. [DOI] [PubMed] [Google Scholar]
- 53.Jde SF, Carvalho PC, Gattass CR, Paschoal EM, da Mota MS, Silva e, Carvalho Mda G. Effects of perillyl alcohol and heat shock treatment in gene expression of human lung adenocarcinoma cell line A549. J Exp Ther Oncol. 2006;5(4):301–307. [PubMed] [Google Scholar]
- 54.Kaji I, Tatsuta M, Ishi H, Baba M, Inoue A, Kasuga H. Inhibition by D-limonene of experimental hepatocarcinogenesis in Sprague-Dawley rats does not involve p21 RAS plasma membrane association. Int J Cancer. 2001;93:441–444. doi: 10.1002/ijc.1353. [DOI] [PubMed] [Google Scholar]
- 55.Trokhan S, Gupta S. Anti-proliferative effects of d-Limonene on human prostate carcinoma cells. Proc Amer Asso Cancer Res. 2003;44:1094. [Google Scholar]
- 56.Chen J, Lu M, Jing Y, Dong J. The synthesis of L-carvone and limonene derivatives with increased antiproliferative effect and activation of ERK pathway in prostate cancer cells. Bioorganic & Medicinal Chemistry. 2006;14:6539–6547. doi: 10.1016/j.bmc.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 57.Rajesh D, Howard S. Perillyl alcohol mediated radiosensitization via augmentation of the Fas pathway in prostate cancer cells. Prostate. 2003;57:14–23. doi: 10.1002/pros.10269. [DOI] [PubMed] [Google Scholar]
- 58.Chung BH, Lee HY, Lee JS, Young CYF. Perillyl alcohol inhibits the expression and function of the androgen receptor in human prostate cancer cells. Cancer Lett. 2006;236:222–228. doi: 10.1016/j.canlet.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 59.Ripple GH, Gould MN, Arzoomanian RZ, Alberti D, Feierabend C, Simon K, Binger K, Tutsch KD, Pomplun M, Wahamaki A, Marnocha R, Wilding G, Bailey HH. Phase I clinical and pharmacokinetic study of Perillyl alcohol administered four times a day. Clin Cancer Res. 2000;6:390–396. [PubMed] [Google Scholar]
- 60.Liu G, Oettel K, Bailey H, Ummersen LV, Tutsch K, Staab MJ, Horvath D, Arzoomanian R, Rezazadeh H, McGovern J, Robinson E, DeMets D, Wilding G. Phase II trial of perillyl alcohol (NSC 641066) administered daily in patients with metastatic androgen independent prostate cancer. Invest New Drugs. 2003;21:367–72. doi: 10.1023/a:1025437115182. [DOI] [PubMed] [Google Scholar]
- 61.del Bano MJ, Lorente J, Castillo J, Benavente-Garcia O, del Rio JA, Ortuno A, Quirin KW, Gerard D. Phenolicditerpenes, flavones, and rosmarinic acid distribution during the development of leaves, flowers, stems, and roots of Rosmarinus officinalis. Antioxidant activity. J Agric Food Chem. 2003;51:4247–4253. doi: 10.1021/jf0300745. [DOI] [PubMed] [Google Scholar]
- 62.Lippman SM, Kessle JF, Meyskens FL. Retinoids as preventive and therapeutic anticancer agents. Cancer Treat Rep. 1987;71:391–405. [PubMed] [Google Scholar]
- 63.Goodman DS. Overview of current knowledge of metabolism of vitamin A and carotenoids. J Natl Cancer Inst. 1984;73:1375–1380. [PubMed] [Google Scholar]
- 64.Sporn MB, Roberts AB. The role of retinoids in differentiation and carcinogenesis. Cancer Res. 1983;43:3034–3040. [PubMed] [Google Scholar]
- 65.Hong WK, Lippman SM, Itri LL. Prevention of second primary tumors with isotretinoin in squamous cell carcinoma of the head and neck. N Engl J Med. 1990;323:795–801. doi: 10.1056/NEJM199009203231205. [DOI] [PubMed] [Google Scholar]
- 66.Sporn MB, Dunlop NM, Newton DL, Smith JM. Prevention of chemical carcinogenesis by vitamin A and its synthetic analogues (retinoids) Fed Proceed. 1976;35:1332–1338. [PubMed] [Google Scholar]
- 67.Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- 68.Glass CK, DiRenzo J, Kurokawa R, Han ZH. Regulation of gene expression by retinoic acid receptors. DNA Cell Biol. 1991;10:623–638. doi: 10.1089/dna.1991.10.623. [DOI] [PubMed] [Google Scholar]
- 69.de The H, Vivanco-Ruiz MM, Triollais P, Stunnerberg H, Dejean A. Identification of a retinoic acid responsive element in the retinoic acid receptor β gene. Nature. 1990;343:177–180. doi: 10.1038/343177a0. [DOI] [PubMed] [Google Scholar]
- 70.Mangelsdorf DJ, Evans RM. The RXR heterodimers and orphan receptors. Cell. 1995;83:841–850. doi: 10.1016/0092-8674(95)90200-7. [DOI] [PubMed] [Google Scholar]
- 71.Schuman LM, Mandell JS, Radke A, Seal U, Halberg F. some selected features of the epidemiology of prostatic cancer. Minneapolis-St. Paul, Minnesota case-control study, 1976–1979. In: Magnus K, editor. Trends in Cancer Incidence: Causes and Practical Implications. Hemisphere Publishing; Washngton, D.C: 1982. pp. 345–354. [Google Scholar]
- 72.Yong LC, Brown CC, Schatzkin A, Dresser CM, Slesinski MJ, Cox CS, Taylor PR. Intake of vitamins E, C, and A and risk of lung cancer. The NHANES I epidemiologic followup study. First National Health and Nutrition Examination Survey. Am J Epidemiol. 1997;146:231–243. doi: 10.1093/oxfordjournals.aje.a009258. [DOI] [PubMed] [Google Scholar]
- 73.Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Intake of carotenoids and retinol in relation to risk of prostate cancer. J Natl Cancer Inst. 1995;87:1767–1776. doi: 10.1093/jnci/87.23.1767. [DOI] [PubMed] [Google Scholar]
- 74.Hirayama T. Epidemiology of prostate cancer with special reference to the role of diet. Natl Cancer Inst Monogr. 1979;53:149–155. [PubMed] [Google Scholar]
- 75.Lippman SM, Kessler JF, Meyskens FL. Retinoids as preventive and therapeutic anticancer agents (Part I) Cancer Treat Rep. 1987;71:391–405. [PubMed] [Google Scholar]
- 76.Zhong C, Yang S, Huang J, Cohen MB, Roy-Burman P. Aberration in the expression of the retinoid receptor, RXRα, in prostate cancer. Cancer Biol Ther. 2003;2:179–184. doi: 10.4161/cbt.2.2.281. [DOI] [PubMed] [Google Scholar]
- 77.Gediya LK, Chopra P, Purushotta Machar P, Maheshwari N, Njar VC. A new simple and high-yield synthesis of suberoylanilide hydroxamic acid and its inhibitory effect alone or in combination with retinoids on proliferation of human prostate cancer cells. J Med Chem. 2005;28:5047–5051. doi: 10.1021/jm058214k. [DOI] [PubMed] [Google Scholar]
- 78.Jin F, Liu X, Zhou Z, Yue P, Lotan R, Khuri FR, Chung LW, Sun SY. Activation of nuclear factor-kappaB contributes to induction of death receptors and apoptosis by the synthetic retinoid CD437 in DU145 human prostate cancer cells. Cancer Res. 2005;65:6354–6363. doi: 10.1158/0008-5472.CAN-04-4061. [DOI] [PubMed] [Google Scholar]
- 79.Webber MM, Waghray A. Urokinase-mediated extracellular matrix degradation by human prostatic carcinoma cells and its inhibition by retinoic acid. Clin Cancer Res. 1995;1:755–761. [PubMed] [Google Scholar]
- 80.Liang JY, Fontana JA, Rao JN, Ordonez JV, Dawson MI, Shroot B, Wilber JF, Feng P. Synthetic retinoid CD437 induces S-phase arrest and apoptosis in human prostate cancer cells LNCaP and PC-3. Prostate. 1999;38:228–236. doi: 10.1002/(sici)1097-0045(19990215)38:3<228::aid-pros7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 81.Buletic Z, Soprano KJ, Soprano DR. Retinoid targets for the treatment of cancer. Crit Rev Eukaryot Gene Expr. 2006;16:193–210. doi: 10.1615/critreveukargeneexpr.v16.i3.10. [DOI] [PubMed] [Google Scholar]
- 82.Cheema SK, Mishra SK, Rangnekar VM, Tari AM, Kumar R, Lopez-Berestein G. Par-4 transcriptionally regulates Bcl-2 through a WT1-binding site on the bcl-2 promoter. J Biol Chem. 2003;278:19995–20005. doi: 10.1074/jbc.M205865200. [DOI] [PubMed] [Google Scholar]
- 83.Pollard M, Luckert P, Sporn M. Prevention of primary prostate cancer in Loblund-Wistar rats by N-(4-hydroxyphenyl)retinamide. Cancer Res. 1991;51:3610–3611. [PubMed] [Google Scholar]
- 84.Wang Q, Weider R. All-trans retinoic acid potentiates Taxotere-induced cell death mediated by Jun N-terminal kinase in breast cancer cells. Oncogene. 2004;23:426–433. doi: 10.1038/sj.onc.1207040. [DOI] [PubMed] [Google Scholar]
- 85.Costa A, Formelli F, Chiesa F, Decensi A, De Palo G, Veronesi U. Prospects of chemoprevention of human cancers with the synthetic retinoid fenretinide. Cancer Res (Suppl) 1994;54:2032s–2037s. [PubMed] [Google Scholar]
- 86.Kamm JJ, Ashenfelter KO, Ehmann CW. Preclinical and clinical toxicity of selected retinoids. In: Sporn MB, Roberts AB, Goodman D, editors. The Retinoids. Academic Press; NY: 1984. pp. 287–326. [Google Scholar]
- 87.Young CW, Warrell RP. Differentiating Agents. In: Devita VT, Hellman S, Rosenberg SA, editors. Cancer Principles and Practive of Oncology. 4. J. B. Lippincott; Philadelphia: 1993. pp. 2636–2646. [Google Scholar]
- 88.Beveridge TH, Li TS, Drover JC. Phytosterol content in American ginseng seed oil. J Agric Food Chem. 2002;50:744–750. doi: 10.1021/jf010701v. [DOI] [PubMed] [Google Scholar]
- 89.Kakuda R, Iijima T, Yaolta Y, Machida K, Kikuchi M. Triterpenoids from. Gentiana scabra Phytochem. 2002;59:791–794. doi: 10.1016/s0031-9422(02)00021-3. [DOI] [PubMed] [Google Scholar]
- 90.Hasmeda M, Kweifio-Okai G, Macrides T, Polya GM. Selective inhibition of eukaryote protein kinases by anti-inflammatory triterpenoids. Planta Med. 1999;65:14–8. doi: 10.1055/s-1999-13954. [DOI] [PubMed] [Google Scholar]
- 91.Rajic A, Kweifio-Okai G, Macrides T, Sandeman RM, Chandle DS, Polya GM. Inhibition of serine proteases by anti-inflammatory triterpenoids. Planta Med. 2000;66:206–210. doi: 10.1055/s-2000-8657. [DOI] [PubMed] [Google Scholar]
- 92.Geetha T, Varalakshmi P. Anti-inflammatory activity of lupeol and lupeol linoleate in rats. J Ethnopharmacol. 2001;76:77–80. doi: 10.1016/s0378-8741(01)00175-1. [DOI] [PubMed] [Google Scholar]
- 93.Moriarity DM, Huang J, Yancey CA. Lupeol is the cytotoxic principle in the leaf extract of Dendropanax cf. quercetin. Planta Med. 1998;64:370–372. doi: 10.1055/s-2006-957454. [DOI] [PubMed] [Google Scholar]
- 94.Saleem M, Alam A, Arifin S, Shah MS, Ahmed B, Sultana S. Lupeol, a triterpene, inhibits early responses of tumor promotion induced by benzoyl peroxide in murine skin. Pharmacol Res. 2001;43:127–134. doi: 10.1006/phrs.2000.0710. [DOI] [PubMed] [Google Scholar]
- 95.Hodges LD, Kweifio-Okai G, Macrides TA. Antiprotease effect of anti-inflammatory lupeol esters. Mol Cell Biochem. 2003;252:97–101. doi: 10.1023/a:1025569805468. [DOI] [PubMed] [Google Scholar]
- 96.Wada S, Iida A, Tanaka R. Triterpene constituents from the stem barks of Pinus luchuensis and their DNA topoisomerase II inhibitory effect. Planta Med. 2001;67:659–664. doi: 10.1055/s-2001-17360. [DOI] [PubMed] [Google Scholar]
- 97.Saleem M, Kweon MH, Yun JM, Adhami VM, Khan N, Syed DN, Mukhtar H. A novel dietary triterpene Lupeol induces Fas-mediated apoptotic death of androgen-sensitive prostate cancer cells and inhibits tumor growth in a xenograft model. Cancer Res. 2005;65:11203–11213. doi: 10.1158/0008-5472.CAN-05-1965. [DOI] [PubMed] [Google Scholar]
- 98.Chintharipalli S, Papineni S, Ramiah SK, Safe S. Betulinic acid inhibits prostate cancer growth through inhibition of specificity protein transcription factors. Cancer Res. 2007;67:2816–2823. doi: 10.1158/0008-5472.CAN-06-3735. [DOI] [PubMed] [Google Scholar]
- 99.Rabi T, Zhang A, Gupta S. Research ShowCASE. Case Western Reserve University; 2007. Betulinic acid: A new potential agent against human prostate cancer; p. 378. [Google Scholar]
- 100.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sci. 2006;78:2088–2098. doi: 10.1016/j.lfs.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goh SH, Hew NF, Norhanom AW, Yadav M. inhibition of tumor promotion by various palm-oil tocotrienols. Int J Cancer. 1994;57:529–531. doi: 10.1002/ijc.2910570415. [DOI] [PubMed] [Google Scholar]
- 102.Conte C, Floridi A, Aisa C, Piroddi M, Floridi A, Galli F. Gamma-tocotrienol metabolism and aqntiproliferative effect in prostate cancer cells. Ann NY Acad Sci. 2004;1031:391–394. doi: 10.1196/annals.1331.054. [DOI] [PubMed] [Google Scholar]
- 103.Gunawardena K, Murray DK, Meikle AW. Vitamin E and other antioxidants inhibit human prostate cancer cells through apoptosis. Prostate. 2000;44:287–295. doi: 10.1002/1097-0045(20000901)44:4<287::aid-pros5>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 104.Rodrigo R, Guichard C, Charles R. Clinical pharmacology and therapeutic use of antioxidant vitamins. Fundam Clin Pharmacol. 2007;21:111–127. doi: 10.1111/j.1472-8206.2006.00466.x. [DOI] [PubMed] [Google Scholar]
- 105.Sung L, Greenberg ML, Koren G, Tomilinson GA, Tong A, Malkin D, Feldman BM. Vitamin E: the evidence for multiple roles in cancer. Nutr Cancer. 2003;46:1–14. doi: 10.1207/S15327914NC4601_01. [DOI] [PubMed] [Google Scholar]
- 106.Brigelius-Flohe R, Kelly FJ, Salomen JT, Neuzil J, Zingg JM, Azzi A. The European perspective on vitamin E: current knowledge and future research. Am J Clin Nutr. 2002;76:703–716. doi: 10.1093/ajcn/76.4.703. [DOI] [PubMed] [Google Scholar]
- 107.Fleshner E, Fair WR, Huryk R. Vitamin E inhibits the high fat diet promoted growth of established human prostate LNCaP tumors in nude mice. J Urol. 1999;161:1651–1654. [PubMed] [Google Scholar]
- 108.Meydani M. Vitamin E. Lancet. 1995;345:170–175. doi: 10.1016/s0140-6736(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 109.Eicholzer M, Stalielin HB, Gey KF, Ludin E, Bernasconi F. Prediction of male cancer mortality by plasma levels of interacting vitamins: 17-year follow-up of the prospective Basel study. Int J Cancer. 1996;66:145–150. doi: 10.1002/(SICI)1097-0215(19960410)66:2<145::AID-IJC1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 110.Halliwell B, Rafter J, Jenner A. Health promotion by flavonoids, tocopherols, tocotrienols, and other phenols: direct or indirect effects? Antioxidant or not? Am J Clin Nutr. 2005;81:268S–276S. doi: 10.1093/ajcn/81.1.268S. [DOI] [PubMed] [Google Scholar]
- 111.Jiang Q, Christen S, Shigenaga MK, Ames BN. gamma-tocopherol, the major form of vitamin E in the US diet, deserves more attention. Am J Clin Nutr. 2001;74:714–722. doi: 10.1093/ajcn/74.6.714. [DOI] [PubMed] [Google Scholar]
- 112.Jiang Q, Elson-Schwah I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Jiang Q, Ames BN. Gamma-tocopherol, but not alpha-tocopherol, decreases proinflammatory eicosanoids and inflammation damage in rats. FASEB J. 2003;17:816–822. doi: 10.1096/fj.02-0877com. [DOI] [PubMed] [Google Scholar]
- 114.Cooney RV, Franke AA, Harwood PJ, Hatch-Pigott V, Custe LJ, Mordan LJ. Gamma-tocopherol detoxification of nitrogen dioxide: superiority to alpha-tocopherol. Proc Natl Acad Sci USA. 1993;90:1771–1775. doi: 10.1073/pnas.90.5.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Cooney RV, Harwood PJ, Franke AA, Narala K, Sundstrom AK, Berggren PO, Mordan LJ. Products of gamma-tocopherol reaction with NO2 and their formation in rat insulinoma (RINm5F) cells. Free Radical Biol Med. 1995;19:259–269. doi: 10.1016/0891-5849(95)00019-t. [DOI] [PubMed] [Google Scholar]
- 116.Christen S, Woodall AA, Shigenaga MK, Southwell-Keely PT, Duncan MW, Ames BN. gamma-tocopherol traps mutagenic electrophiles such as NO(X) and complements alpha-tocopherol: physiological implications. Proc Natl Acad Sci USA. 1997;94:3217–3222. doi: 10.1073/pnas.94.7.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Himmelfarb J, Kane J, McMonogle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 118.Helzlsouer KJ, Huang HY, Allerg AJ, Hoffman S, Burke A, Norkus EP, Morris JS, Comstick GW. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–2023. doi: 10.1093/jnci/92.24.2018. [DOI] [PubMed] [Google Scholar]
- 119.Galli F, Stable AM, Berti M, Conte C, Pistilli A, Rende M, Floridi A, Azzi A. The effect of alpha- and gamma-tocopherol and their carboxyethyl hydroxychroman metabolites on prostate cancer cell proliferation. Arch Biochem Biophys. 2004;423:97–102. doi: 10.1016/j.abb.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 120.Gysin R, Azzi A, Visarius T. Gamma-tocopherol inhibits human cancer cell cycle progression and cell proliferation by down-regulation of cyclins. FASEB J. 2002:1952–1954. doi: 10.1096/fj.02-0362fje. [DOI] [PubMed] [Google Scholar]
- 121.Srivastava JK, Gupta S. Tocotrienol-rich fraction of palm oil induces cell cycle arrest and apoptosis selectively in human prostate cancer cells. Biochem Biophys Res Commun. 2006;346:447–453. doi: 10.1016/j.bbrc.2006.05.147. [DOI] [PubMed] [Google Scholar]
- 122.Okabe M, Oji M, Ikeda I, Tachbana H, Yamada K. Tocotrienol levels in various tissues of Sprague-Dawley rats after intragastric administration of tocotrienols. Biosc Bioctechnol Biochem. 2002;66:1768–1771. doi: 10.1271/bbb.66.1768. [DOI] [PubMed] [Google Scholar]
- 123.Burton GW, Traber MG, Acuff RV, Walters DN, Kayden L, Hughes L, Ingold KU. Human plasma and tissue alpha-tocopherol concentrations in response to supplementation with deuterated natural and synthetic vitamin E. Am J Clin Nutr. 1998;67:669–684. doi: 10.1093/ajcn/67.4.669. [DOI] [PubMed] [Google Scholar]
- 124.Klein EA, Thompson IM, Lippman SM, Goodman PJ, Albanes D, Taylor PR, Coltman C. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–1315. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 125.Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. doi: 10.1016/j.mam.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 126.Keijer J, Bunschoten A, Palou A, Franssen-van Hal NL. Beta-carotene and the application of transcriptomics in risk-benefit evaluation of natural dietary components. Biochim Biophys Acta. 2005;1740:139–146. doi: 10.1016/j.bbadis.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 127.Peters U, Leitzmann ME, Chatterjee N, Wang Y, Albanes D, Gelmann EP, Friesen MD, Riboli E, Hayes RB. Serum lycopene, other carotenoids and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal and ovarian cancer screening trial. Cancer Epidemiol Biomarkers Rev. 2007;16:962–968. doi: 10.1158/1055-9965.EPI-06-0861. [DOI] [PubMed] [Google Scholar]
- 128.Gerster H. The potential role of lycopene for human health. J Am Coll Nutr. 1997;16:109–126. doi: 10.1080/07315724.1997.10718661. [DOI] [PubMed] [Google Scholar]
- 129.Stahl W, Seis H. Lycopene: a biologically important carotenoid for humans? Arch Biochem Biophys. 1996;336:1–9. doi: 10.1006/abbi.1996.0525. [DOI] [PubMed] [Google Scholar]
- 130.Clinton SK, Emenhiser C, Schwartz SJ. Cis-trans lycopene isomers, carotenoids, and retinol in the human prostate cancer patients. Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- 131.Vogt TM, Mayne ST, Graubard BI, Swanson CA, Sowell AL, Schoenberg JB, Swanson GM, Greensberg RS. Serum lycopene, other serum carotenoids, and risk of prostate cancer in US blacks and whites. Am J Epidemiol. 2002;155:1023–1032. doi: 10.1093/aje/155.11.1023. [DOI] [PubMed] [Google Scholar]
- 132.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–398. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 133.Kucuk O, Sarkar FH, Djuric Z, Sakr W, Pollak MN, Khachik F, Banerjee M, Bertram JS, Wood DP., Jr Effects of lycopene supplementation in patients with localized prostate cancer. Exp Biol Med (Maywood) 2002;227:881–885. doi: 10.1177/153537020222701007. [DOI] [PubMed] [Google Scholar]
- 134.Bowen P, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Sharifi R, Ghosh L, Kim HS, Christov-Tzelkov K, van Breemen RB. Tomato sauce supplementation and prostate cancer: lycopene accumulation and modulation of biomarkers of carcinogenesis. Exp Biol Med (Maywood) 2002;227:886–893. doi: 10.1177/153537020222701008. [DOI] [PubMed] [Google Scholar]
- 135.van Breemen RB, Xu X, Viana MA, Chen L, Stacewicz-Sapuntzakis M, Duncan C, Bowen PE, Sharifi R. Liquid chromatography-mass spectrometry of cis- and all-trans-lycopene in human serum and prostate tissue after dietary supplementation with tomato sauce. J Agric Food Chem. 2002;50:2214–2219. doi: 10.1021/jf0110351. [DOI] [PubMed] [Google Scholar]
- 136.Clinton SK, Emenhiser C, Schwartz SJ, Bostwick DG, Williams AW, Moore BJ, Erdman JW., Jr cis-trans lycopene isomers, carotenoids, and retinol in the human prostate. Cancer Epidemiol Biomarkers Prev. 1996;5:823–833. [PubMed] [Google Scholar]
- 137.Clark PE, Hall MC, Borden LS, Jr, Miller AA, Hu JJ, Lee WR, Stindt D, D’Agostino R, Jr, Lovato J, Harmon M, FM Phase I–II prospective dose-escalating trial of lycopene in patients with biochemical relapse of prostate cancer after definitive local therapy. Urology. 2006;67:1257–1261. doi: 10.1016/j.urology.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 138.Kucuk O, Sarkar FH, Sakr W, Djuric Z, Pollak MN, Khachik F, Li YW, Banerjee M, Grignon D, Bertram JS, Crissman JD, Pontes EJ, Wood DP., Jr Phase II randomized clinical trial of lycopene supplementation before radical prostatectomy. Cancer Epidemiol Biomarkers Prev. 2001;10:861–868. [PubMed] [Google Scholar]
- 139.Greenwald P, Wikin KM, Malone WF, Byar DP, Freedman LS, Stern HR. The study of markers of biological effects in cancer prevention research trials. Int J Cancer. 1992;82:555–560. doi: 10.1002/ijc.2910520206. [DOI] [PubMed] [Google Scholar]
- 140.Stadtzkin A, Freedman LS, Stern HR. Surrogate end points in cancer research: a critique. Cancer Epidemiol Biomarkers Prev. 1996;5:947–953. [PubMed] [Google Scholar]
- 141.Bostwick DG, Burke HB, Wheeler TM. The most promising surrogate endpoint biomarkers for screening candidate chemopreventive compounds for prostate adenocarcinoma in short-term phase II clinical trials. J Cell Biochem. 1994;19:283–289. [PubMed] [Google Scholar]
- 142.Bostwick DG. Prostatic intraepithelial neoplasia is a risk factor for cancer. Semin Urol Oncol. 1999;17:187–198. [PubMed] [Google Scholar]
- 143.Lippman SM, Lotan R. Advances in the development of retinoids as chemopreventive agents. J Nutr. 2000;130:479S–482S. doi: 10.1093/jn/130.2.479S. [DOI] [PubMed] [Google Scholar]
- 144.Tsai H, Werber M, Davia O, Edelman KE, Tanaka E, Melman A, Christ J, Geliebtor J. Reduced connexin 43 expression in high grade, human prostatic adenocarcinoma cells. Biochem Biophys Res Commun. 1996;22:64–69. doi: 10.1006/bbrc.1996.1468. [DOI] [PubMed] [Google Scholar]
- 145.Pollak M, Beamer W, Zhang JC. Insulin-like growth factors and prostate cancer. Cancer Met Rev. 1998;17:383–390. doi: 10.1023/a:1006154108619. [DOI] [PubMed] [Google Scholar]
- 146.Jiang Q, Elson-Schwab I, Courtemanche C, Ames BN. gamma-tocopherol and its major metabolite, in contrast to alpha-tocopherol, inhibit cyclooxygenase activity in macrophages and epithelial cells. Proc Natl Acad Sci USA. 2000;97:11494–11499. doi: 10.1073/pnas.200357097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Himmelfarb J, Kane J, McMonagle E, Zaltas E, Bobzin S, Boddupalli S, Phinney S, Miller G. Alpha and gamma tocopherol metabolism in healthy subjects and patients with end-stage renal disease. Kidney Int. 2003;64:978–991. doi: 10.1046/j.1523-1755.2003.00151.x. [DOI] [PubMed] [Google Scholar]
- 148.Kolesnick R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J Clin Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Solomon JC, Sharma K, Wei L, Fugita T, Shi YF. A novel role for sphingolipid intermediates in activation-induced cell death in T cells. Cell death Differ. 2003;10:193–202. doi: 10.1038/sj.cdd.4401136. [DOI] [PubMed] [Google Scholar]


