Abstract
Background
High-fat diet (HFD) is considered as a major risk factor for benign prostatic diseases and cancer in the Western world. Studies have shown an association between oxidative stress and prostatic diseases. NF-κB has been implicated in stress response and is deregulated in prostrate disorders; therefore, we sought to determine whether HFD could induce oxidative stress in the prostate which could contribute to prostatic diseases.
Methods
Transgenic NF-κB-Luc-Tag mice were either fed with regular diet (RD) or HFD for 12 weeks. Serial, non-invasive molecular imaging was performed to study NF-κB activation in the whole body, and in various organs including thymus, spleen, and prostate. Western blotting was used to determine the expression of NF-κB, its upstream and downstream targets in the prostate.
Results
Two-fold increase in whole body NF-κB activity in vivo and 2–3 fold up-regulated prostate NF-κB activity ex vivo were observed after HFD intake compared with RD controls. HFD-induced NF-κB activity was elevated remarkably in the abdominal cavity, thymus, spleen, and prostate with increase in prostrate weight. In the prostrate, an increase in the protein expression of gp91phox, p22phox, and p47phox NADPH oxidase subunits was observed suggesting the involvement of HFD in causing oxidative stress. Nuclear extracts from the prostrate tissue showed an increased expression of p65/RelA that corresponded with elevated cytosolic levels of p-IκBα, along with increased expression of downstream targets of NF-κB, nitric oxide synthase and cyclooxygenase-2.
Conclusions
Our findings suggest that HFD-mediated oxidative stress and deregulation of NADPH oxidase leads to NF-κB activation in the prostrate.
Keywords: oxidative stress, high-fat diet, prostate, obesity, NF-κB
INTRODUCTION
Several risk factors attribute towards prostatic diseases including prostrate cancer. These are ethnicity, age, family history and lifestyle factors. Diet is considered as one of the most important lifestyle factor that has potential influence in determining the risk of prostate diseases [1]. Studies ascertaining the risk of prostate diseases have shown that high intake of total fat, saturated fat and cholesterol were positively associated with an increased risk of prostate cancer and benign prostatic hyperplasia (BPH) after adjustment of confounding factors [2, 3]. Saturated fat, in particular, omega-6 fatty acids and cholesterol have been shown to be a risk factor for prostate cancer [4]. Consistent with this possibility, health professional studies have shown that the long-term use of cholesterol-lowering drugs, ‘statins’ reduces prostate cancer risk by 20–50% [5, 6]. Levels of circulating cholesterol have been reported to cause relapse after treatment of localized prostate cancer and promote prostate cancer cell proliferation [7].
A number of intriguing hypothesis for the association between fatty acids and prostatic diseases have been proposed [8, 9]. These include mechanisms involving inflammation, oxidative stress, peroxidation of lipids and accumulation of 8-hydroxy-2′ –deoxyguanosine and androgen synthesis driving the growth of the prostate [10–13]. However, most of these existing studies assume that the effects of high-dietary fat on prostate growth results primarily from lipogenesis, inflammation and oxidative stress, [11] and evidence of direct effects of diet on prostate is still lacking. Chronic inflammation and oxidative stress are thought to play an essential role in prostatic diseases as well as aging [10 and 14], so the possibility exists that high fat diet could induce intraprostatic inflammation and oxidative stress to increase vulnerability of the prostate to numerous prostatic diseases and to age-associated deficits. Therefore, it is important to determine whether high dietary fat can induce oxidative stress and inflammation directly in the prostate.
Nuclear Factor-kappa B (NF-κB), a family of transcription factors is activated as a result of inflammatory and stress responses in most mammalian cell types. It regulates various cellular processes like apoptosis, cell growth, angiogenesis, cell survival and its involvement in cancer is well recorded [15, 16]. In fact, NF-κB has been reported to be constitutively upregulated in prostate cancer [17, 18]. Apart from cancer, NF-κB is also associated with wide range of human diseases like rheumatism, inflammatory bowel diseases, neurodegenerative conditions, asthma and chronic obstructive pulmonary disease [19–22]. Over-expression of NF-κB in these inflammatory diseases has been associated with increase presence of reactive oxygen species (ROS) and NADPH oxidase activity [23]. Upregulation of NF-κB during inflammation also results in the recruitment of inflammatory cells leading to the production of various pro-inflammatory cytokines, such as IL-1, IL-6, IL-8 at the site of inflammation [24, 25]. Studies have shown an association between prostrate inflammation with BPH and prostrate cancer [26]. Studies from our laboratory have reported that IL-1β-induced NF-κB activity contributes in chemoattractant urging initial phase of prostatic inflammation that might have a capability for maintaining the chronic inflammation and proliferative inflammatory atrophy (PIA) in the prostrate, which are recognized as putative precursor lesions in the development of prostrate cancer [27]. Recently it has been shown that high-fat diet (HFD) is a stimulator for NF-κB responses in diet-induced obese mice [28].
In the present study we have investigated the consequence of HFD on the prostate using NF-κB-Luciferase transgenic mice. We observed that HFD increased the activation of NF-κB along with elevated levels of NADPH oxidase components. We report for the first time that HFD could initiate oxidative stress of the prostate that involves both NF-κB and NADPH oxidase activity which might lead to intraprostatic inflammation and be a potential cause of prostatic diseases including BPH and prostate cancer.
MATERIALS AND METHODS
Animals
Forty eight males from the colony of NF-κB-Luciferase transgenic mice (Jackson Laboratory, Bar Harbor, Maine) were used for these experiments. The animals were bred and maintained at the AAA-LAC accredited Animal Resource Facility of Case Western Reserve University. All animal experiments were performed according to institutional guidelines for animal care. The mice were matched according to age and divided into two groups. One group was fed with regular diet (RD) and the other was subjected to high fat diet (HFD) typical of ‘Western style’ high-fat diet. The HFD formula consisted of rodent chow containing of 23% fat, 43% carbohydrate, and 20% of protein. In contrast, RD consisted of standard rodent chow containing 4.5% fat, 56% complex carbohydrate, and 23% protein (all from Harlan Labs Inc., Madison, WI). The animals were kept on these diets for 4, 8 and 12 weeks respectively, sacrificed and used for in vivo and ex vivo experiments.
Materials
Antibodies for gp91phox, p22 phox, and p47 phox, p65/RelA, IκBα, iNOS, COX-2 Oct-1 and β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Phospho-IκBα (Ser32/36) and phospho-IKKα/β (p-IKKα, Ser180; IKKβ, Ser181) were purchased from Cell Signaling Technologies (Danvers, MA). Secondary antibodies for mouse and rabbit (horseradish peroxidase conjugates) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Enhanced Chemiluminescence Kit (ECL) for chemiluminescence was purchased from GE Healthcare Biosciences (Piscataway, NJ).
Imaging of NF-κB, in vivo luciferase activity
Imaging of transgenic mice was performed according to our previous publication [29]. The images of mice were taken after each time interval of feeding. The transgenic mice NF-κB-dependent luciferase activity both in vivo and ex vivo was measured as described previously with some modifications [29].
Western blotting analysis
Prostrate tissue from NF-κB-Luc transgenic male mice fed with RD and HFD were processed for total, cytosolic and nuclear lysates. The whole prostrate was sliced into two portions. The smaller portion was used for total lysate. 200μL of buffer [(50 mM TRIS-HCl (pH7.4), 150 mM Nacl, 1mM EDTA, 1mM EGTA, 20mM NaF, 1mM DTT, 0.5mM PMSF 100mM Na3VO4, and protease inhibitor mixture (Roche Molecular Biochemicals)] was added to the sliced tissue stored in eppendorff tubes and incubated for 30 min on ice, homogenized, followed by incubation for additional 30 min and centrifuged at 14000Xg for 15 min at 4°C. The supernatant was collected in pre-cooled micro-centrifuged tubes and stored at −80 °C.
Cytosolic and nuclear extract were obtained from the larger portion of the minced prostrate tissue. The tissue was suspended in 400μL of ice-cold hypotonic buffer [10mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1mM DTT, 0.5mM PMSF 100mM Na3VO4, and protease inhibitor mixture (Roche Molecular Biochemicals)]. The samples were incubated for 30 min on ice, homogenized, with 30 min additional incubation followed by the addition of 10% NP-40. The samples were vortexed and centrifuged for 1min at 14000Xg at 4°C and the supernatant constituted the cytosolic fraction. The pellet constituted the nuclear fraction and was extracted with 100μL of buffer containing, 200mM HEPES, pH 7.9, 400mM NaCl, 1mM EDTA, 1mM EGTA, 1mM DTT, 0.5mM PMSF and protease inhibitor (Roche Molecular Biochemicals). After 30 minute incubation with intermittent vortex to ensure that the pellet was suspended uniformly, the suspension was centrifuged at 14000Xg at 4°C for 5 minutes. The collected supernatant constituted the nuclear fraction. All the fractions were stored at −80°C and were used for Western blotting.
Total tissue lysate, cytosolic and nuclear fractions were used for Western blotting by loading 40μg proteins resolved over 4–20% Tris-glycine polyacrylamide gel and then transferred onto the nitrocellulose membrane. The blots were blocked using 5% nonfat dry milk and probed using appropriate primary antibodies overnight at 4°C. The membrane was then incubated with appropriate secondary antibody horseradish peroxidase conjugate (Santa Cruz Biotech) followed by detection using chemiluminescence ECL kit (GE Healthcare Biosciences). For equal loading of proteins, the membrane was probed with appropriate loading controls. Densitometric measurements of the bands in Western blot analysis were done using digitalized scientific software program using Kodak 2000R imaging system.
Statistical Analysis
The values are expressed as mean ± SD. The significance of measured values between the groups with RD and HFD were performed by Student’s t-test for Western blotting densitometric analysis and by one-way analysis of variance (ANOVA) with 95% confidence limits for bio-luminescence data analysis. The p values less than 0.05 were taken as significant in experiments.
RESULTS
HFD increases whole body NF-κB activity in NF-κB-Luc mice
Recent reports showed that HFD stimulate NF-κB in obese mice [28]. In our study we used non-obese NF-κB-Luc transgenic mice fed with HFD for 4, 8, and 12 weeks, and compared with mice fed on RD, respectively. In order to know if there was a change in the NF-κB-dependent luminescence in vivo we accessed the photon flux of the whole body from animals with different diets. Following this procedure, we found that in vivo imaging at each time period revealed significant difference in NF-κB-dependent luminescence between the animals kept on RD and HFD, especially in organs located in abdominal cavity (Figure 1A). Unexpectedly, a twofold increase of NF-κB-dependent luminescence of whole body of HFD mice was noted after 4 weeks of HFD. This up-regulated NF-κB dependent luminescence in the mice fed with HFD was steady at 8 week with an increase at 12 week as compared to the animals with RD (Figure 1B). Though an increase in the body weight was observed in both the groups over time, mice fed with HFD gained weight that were markedly higher than the individuals in the control group (Figure 1C). These results suggest that HFD feeding increases NF-κB activity by fourth week that remained elevated during the 8 and 12 weeks.
Figure 1.
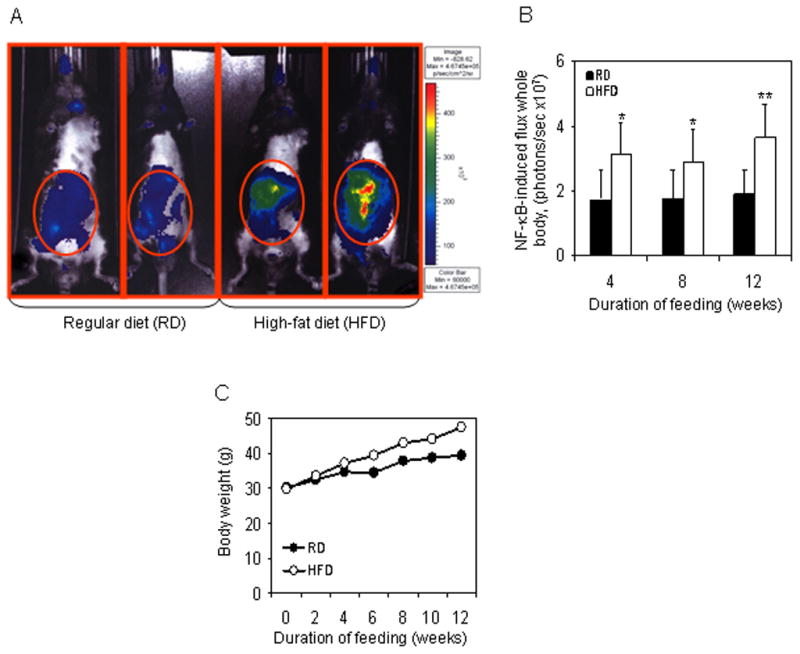
Imaging of NF-κB-Luc mice after treatment with regular (RD) and high-fat diet (HFD). Mice fed with RD or HFD for 4, 8 and 12 weeks were imaged at the end of each time interval. Imaging was performed using an ultra-sensitive camera on anesthetized mice that were placed in a light-sealed chamber connected to an image intensifier coupled to a CCD camera of IVIS 150 System Xenogen (Alameda, CA). Gray scale images were obtained before luminescence imaging for reference. (A) Representative figure of mice imaged for NF-κB-dependent luminescence after 12 weeks of RD and HFD feeding. (B) Data of NF-κB induced total proton flux in the whole body of mice, fed with RD or HFD after 4, 8 and 12 weeks. Black bars indicate RD and white bars indicate HFD. The SD is indicated, *p< 0.05, **p<0.01, represents the significant changes in NF-κB activation by HFD compared to its respective RD treated controls. (C) Body weight changes in mice after RD and HFD feeding. NF-κB-Luc mice were either fed with RD (black circle) or HFD (white circle) and increase in body weight was recorded after the indicated time intervals.
HFD augments NF-κB activity in various organs
In order to determine how HFD affected the NF-κB activity in various organs or body regions of the mice we measured the luminescence photon flux from the abdominal cavity (consisting of the alimentary canal and GU apparatus), thymus and the spleen. The total photon flux was significantly increased (P<0.01) by 12 weeks from the organ of abdominal cavity, but no change was observed in the 4 or 8 weeks of HFD feeding. In the thymus, the NF-κB activity increased by 4 weeks, but showed no change during the 8 week feeding. Interestingly, 12 week HFD feeding caused a significant increase in the total flux of NF-κB-dependent luminescence (P<0.01) in the thymus. In the spleen, HFD feeding caused a modest increase in flux by 4 weeks, compared to RD mice spleen, however showed a marked increase in the photon flux by 8 and 12 weeks (Figure 2).
Figure 2.
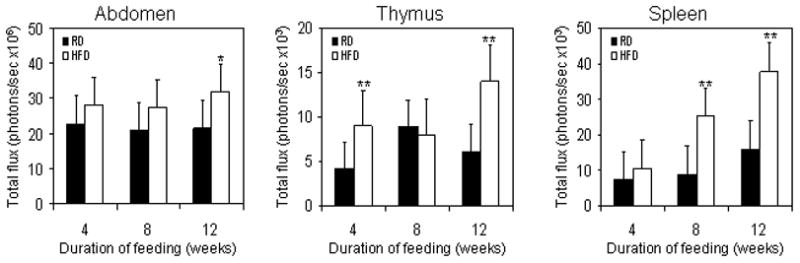
Changes in NF-κB-induced luminescence in the abdominal cavity, thymus and spleen of mice after RD and HFD feeding. NF-κB-Luc mice fed with RD or HFD for 4, 8 and 12 weeks were imaged at the end of each time interval. Total proton flux was measured to determine the extent of NF-κB activation. Data represents the mean value of total photon flux from the abdominal cavity, thymus and spleen after 4, 8 and 12 weeks of RD and HFD feeding. Black bars represent RD and white bars represent HFD. The SD is indicated, *p<0.05, ** p<0.01 represents the significant changes in HFD compared to its respective RD fed controls.
HFD increases prostrate weight and NF-κB activity
We evaluated the effect of HFD on the prostrate weight and NF-κB activity. HFD intake caused a significant elevation of the prostate weight by 4 week which further increase at 8 week with a plateau till 12 week, as compared to the prostate of RD mice. As a result of this, no significant change was observed in the weight of prostrate at 12 weeks of age between the HFD and RD mice. In ex vivo studies, we observed that HFD feeding increases NF-κB-dependent luminance in the prostrate gland that led to an increased total photon flux which peaked at 8 weeks, and correlated with increase in prostate weight at same time interval (Figure 3).
Figure 3.
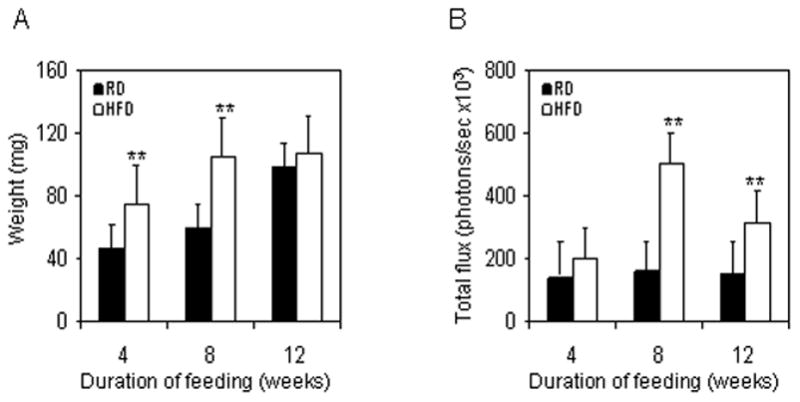
HFD induced changes in the prostrate of NF-κB-Luc mice. (A) Changes in the weight of prostrates after RD and HFD feeding at the end of the indicated time intervals. Data represents the mean value of prostrate weight compared between RD and HFD after each time interval. Black bars represent RD and white bars represent HFD. The SD is indicated, ** p<0.01 represents the significant changes in the weight of prostrates from mice fed with HFD compared to its respective RD treated controls. (B) Data of NF-κB-induced total proton flux from the prostrate of mice, fed with RD or HFD after 4, 8 and 12 weeks. Black bars indicate RD and white bars indicate HFD. The SD is indicated, **p<0.01, represents the significant changes in HFD compared to its respective RD fed controls.
HFD increases intraprostatic expression of NADPH oxidase components
Studies have demonstrated that high-fat diet induces NADPH-oxidase associated oxidative stress in the cerebral cortex of rats that led to inflammation and NF-κB activation [30]. Therefore, we investigated the effect of HFD on the levels of NADPH oxidase components in the prostrate since we observed an increase in NF-κB activation. We used prostrate samples from mice that were treated with HFD for 4 and 8 week and compared them with the RD. Using Western blotting we probed for gp91phox, p22phox and p47phox expression. The protein levels of gp91phox were increased after 4 weeks of HFD by 3.0 fold (P<0.01) which further increased by 6.0 folds (P<0.01) at 8 weeks. Similarly, expression of p47phox were significantly increased by 2.25 fold (P<0.005), and p22phox by 2.6 folds (P<0.005) after 8 weeks for HFD feeding (Figure 4).
Figure 4.
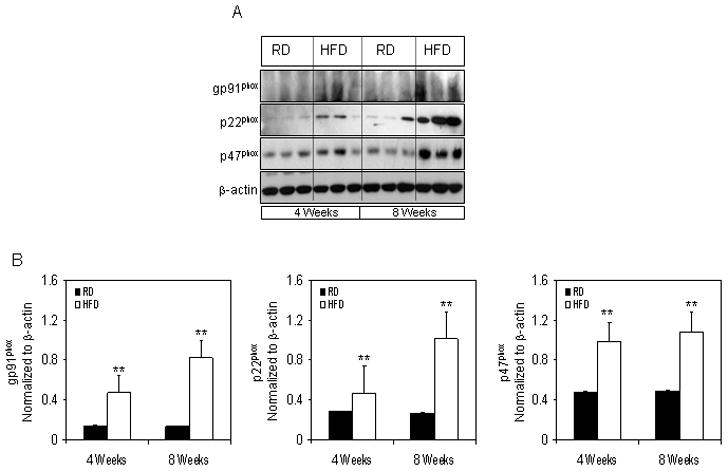
Protein expressions of NADPH oxidase subunits in the prostrate of NF-κB-Luc mice fed with RD and HFD. (A) Total cell lysates were prepared as described in the ‘materials and methods’ from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for the indicated antibodies and β-actin was used as the loading control. (B) Densitometric quantification represents the changes in the levels of gp91phox, p22phox and p47phox. Black bars indicate RD and white bars indicate HFD. Data are a mean of ± SD and corrected for loading. The asterisk (**) indicates the significant changes (p<0.01) in HFD compared to its respective RD fed controls.
HFD causes intraprostatic NF-κB activation
To examine changes in nuclear levels of NF-κB, we performed Western blotting using the nuclear extracts from prostrate tissues from mice fed with RD and HFD. Our data showed that the nuclear levels of p65/RelA significantly increased to two fold (p<0.05) by four weeks of HFD feeding which persisted to the same levels at 8 weeks (p<0.01), suggesting increased translocation of p65/RelA into the nucleus as a result of HFD intake. Since inactivated p65/RelA is bound to IκBα in the cytosol along with p50 subunit and upon activation IκBα is phosphorylated at Ser32/36 releasing p65/RelA that translocates into the nucleus, therefore we examined the total and phosphorylated of IκBα in the cytosol of various groups. Feeding HFD caused a significant increase in the phosphorylation of IκBα after 4 and 8 weeks of feeding (4 weeks, P<0.01; 8 weeks, P<0.05). Furthermore, HFD intake resulted in increased p-IKK expression, the upstream kinase that phosphorylates the NF-κB complex, at 4 and 8 weeks (P<0.01), respectively.
HFD modulates intraprostatic NF-κB-regulated genes
Next we probed for iNOS and COX-2 to find out if HFD caused any alterations in the levels of these proteins. Interestingly, iNOS levels were significantly increased to 4-fold (P<0.001) after 4 weeks of HFD intake, and 2-fold increase (P<0.05) was observed at 8 weeks. Similarly, COX-2 levels also increased (2-fold) at 4 weeks and 1.6-fold increase at 8 weeks of HFD intake.
DISCUSSION
The present study provides evidence that high dietary fat intake promoted oxidative stress and inflammation in the prostate gland. Our major findings revealed that HFD intake enhanced ROS generation through elevated expression of NADPH oxidase subunits causing NF-κB activation and induction of NF-κB regulated genes COX-2 and iNOS. These results demonstrate that NF-κB is important downstream effectors of both oxidative stress and inflammatory pathways and can be activated by consuming HFD. Our studies strengthen the link between the ‘Western style’ high-fat diet and increased risk of prostatic diseases including BPH and prostate cancer.
An increasing scientific literature provides abundant evidence that overproduction of ROS can induce cellular damage via oxidation of critical cellular components such as membrane lipids, proteins and DNA. ROS are generated by both mitochondrial electron transport and by cytosolic enzymes such as the NOX family of NADPH oxidases [31, 32]. NADPH oxidases consists of at least six subunits, the membrane bound gp91phox and p22phox homodimer, the cytoplasmic complex of p40phox, p47phox and p67phox and small GTPase Rac1 & 2, respectively in non-phagocytic cells as well as in various epithelial cells [33, 34]. In prostrate cancer cells, NADPH oxidase system is a major source of production of reactive oxygen species where NOX1 (homolog of the catalytic subunit of the superoxide-generating NADPH oxidase of phagocytes, gp91phox) and NOX5 (Ca2+ concentration-responsive gp91phox homolog) exert major effects on prostate tumor growth and angiogenesis [35]. In the present study, we determined the effect of HFD on the NADPH-oxidase components that are considered to be upstream of NF-κB. Our findings suggests that HFD intake elevated the levels of gp91phox significantly at 4 and 8 weeks of feeding, while p22phox and p47phox were elevated at 8 weeks, suggesting that high levels of dietary fatty acids might induce oxidative stress in the prostate via the NADPH oxidase pathway. However, precise signaling mechanism after HFD intake in the prostate needs further investigation.
Researchers have linked an association between obesity, HFD and NF-κB. Recent studies connect the role of HFD in potentiating NF-κB activity in obese mice, and the activity is possibly derived from the abdominal tissue fat deposits [28]. HFD is suggested to increase the risk of aggressive prostrate tumors in older males [36] and high content of saturated fat in the HFD has been linked to increased mortality in prostate cancer patients [37]. In the present study, we hypothesized that HFD augmented NF-κB activation leading to inflammatory condition of the prostrate in NF-κB-Luc mice. We observed that HFD caused an increased NF-κB dependent luminance which was not uniform in all the regions of the body of mice that harbor the three binding sites for NF-κB coupled to the luciferase gene. We further observed that HFD caused the activation of NF-κB in the abdominal cavity, and lymphoid organs such as spleen and thymus. In the abdominal cavity, the NF-κB activity was significantly higher at 12 weeks of HFD feeding, while in the thymus activity increased after 4 weeks and 12 weeks of feeding. In the spleen, HFD intake caused an increase in NF-κB activity at 8 and 12 weeks. This is in concurrence with previous report that shows that HFD-evoked NF-κB activity was non-uniform and sex-dependent. In the males the NF-κB signals were localized to the abdomen and in the females the activity was more in the thoracic region [28].
NF-κB is a family of transcription factors that play an important role in regulating the expression of genes involved in inflammation, stress response, proliferation and apoptosis [20, 38]. NF-κB is a heterodimer composed of p50 and p65 subunits and, under resting conditions, the dimmer is retained in the cytoplasm in an inactive state through interaction with IκB. NF-κB can be rapidly activated in response to a variety of inflammatory stimuli that lead to degradation of IκB [39]. Earlier reports suggest that HFD induces neural oxidative stress, inflammation and NF-κB activation in rat cerebral cortex that leads to dementia [48]. Our results demonstrate that high-fat diet intake enhanced IκB phosphorylation and p65/RelA subunit activation in the prostate, which supports our observation of HFD-induced oxidative stress and inflammation in the prostate.
HFD is considered as one of the risk factors for prostrate cancer in the United States and other Western nations and has been shown to induce low-grade inflammation in adipose tissue [40]. Importantly, HFD intake typically induced NF-κB activity levels to about 2–3 fold, which is much lower than 10–100 fold activation typically of acute inflammatory reaction. In fact, increase in low NF-κB activity might contribute to intraprostatic inflammation with increased susceptibility to develop prostatic diseases including cancer. Furthermore, it has been reported that a high-fat, high-cholesterol diet induced considerably greater hepatic expression of several inflammatory and oxidative stress-responsive genes and significant NF-κB activation that might cause longstanding inflammation in the prostate. We have previously reported that IL-1β-induced NF-κB has ability to maintain chronic inflammation and proliferative inflammatory atrophic lesions in the prostrate [27]. Further studies are required to precisely understand the role of high-fat diet in intraprostatic inflammation.
It is well documented that NF-κB plays a prominent role inflammation [41] and inflammation driven cancers [16]. In inflammation-associated liver cancer models, tumor development progresses from chronic liver inflammation to dysplasia, carcinoma, and metastasis where NF-κB plays a critical role during malignant conversion [38 and 42]. We have previously demonstrated that NF-κB/p65/RelA is constitutively over-expressed in human prostate adenocarcinoma and correlates with disease progression affecting the downstream target genes related to inflammation, angiogenesis and cancer progression [17]. Therefore, next we determined whether HFD intake has an effect on the downstream targets of NF-κB. We studied the levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2), which are known downstream targets of NF-κB. The level of iNOS and COX-2 were increased as a result of NF-κB activation after HFD intake. Inflammation is reported to induce expression and enzyme activity of iNOS and COX-2, which produces pro-inflammatory mediators such as prostaglandin E2 (PGE2) and nitric oxide [43, 44, 45, 46 and 47]. We have previously demonstrated increased levels of iNOS, prostaglandins and COX-2 in prostate tumor tissue compared with non-cancerous benign tissue, suggesting the role of these two molecules in prostate tumorigenesis [17 and 48].
In summary, HFD might be an important risk factor for benign diseases and prostate cancer in the Western world. Our in-vivo molecular imaging studies suggest that HFD increased NADPH oxidase-related oxidative stress and NF-κB activity with increased inflammatory response due to augmentation of COX-2 and iNOS. In the prostrate, HFD appears to trigger growth signal causing an increase weight of the prostrate. We suggest HFD to be an important risk factor in causing inflammatory and oxidative stress that could be linked with the benign prostatic disease and cancer of the prostrate gland.
Figure 5.
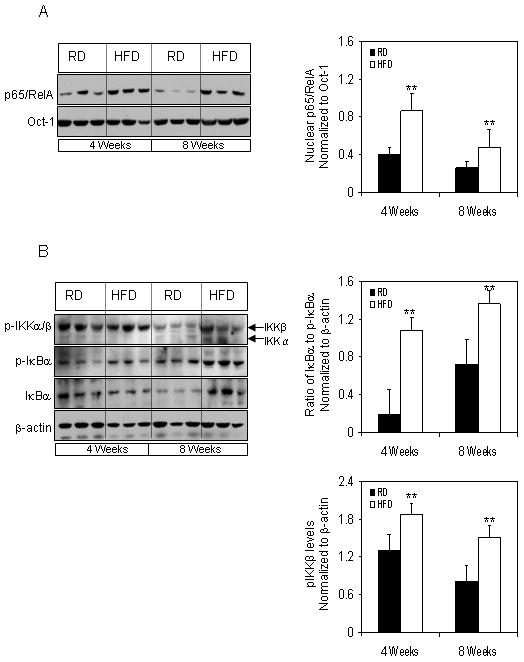
Protein expression of NF-κB in the nuclear and cytosolic fractions extracted from the prostrate of RD and HFD fed NF-κB-Luc mice. (A) Nuclear extract were prepared as described in the ‘materials and methods’ from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for p65/RelA; Oct-1 was used as the loading control. The densitometric quantification for p65/RelA was normalized with Oct-1. Black bars indicate RD and white bars indicate HFD. Data are a mean of ± SD and corrected for loading. The asterisk (**) indicates the significant changes (p<0.01) in HFD compared to its respective RD fed controls. (B) Cytosolic fractions prepared as described in the ‘materials and methods’ from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for the indicated antibodies and β-actin was used as the loading control. Densitometric quantification represents the ratio of IκBα to p-IκBα and p-IKKβ levels. Black bars indicate RD and white bars indicate HFD. Data are a mean of ± SD and corrected for loading. The asterisk (**) indicates the significant changes (p<0.01) in HFD compared to its respective RD fed controls.
Figure 6.
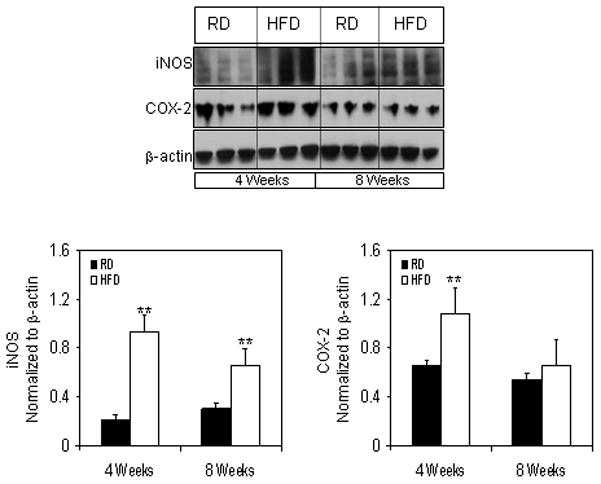
Protein expression of iNOS and COX-2 levels in the total cell lysate extracted from the prostrate of RD and HFD fed NF-κB-Luc mice. (A) Total cell lysates were prepared as described in the ‘materials and methods’ from the prostrate of mice fed with RD and HFD. The samples were subjected to SDS-PAGE gel electrophoresis. The blots were analyzed for the indicated antibodies and β-actin was used as the loading control. Densitometric quantification represents the changes in the levels of iNOS and COX-2 after HFD. Black bars indicate RD and white bars indicate HFD. Data are a mean of ± SD and corrected for loading. The asterisk (**) indicates the significant changes (p<0.01) in HFD compared to its respective RD fed controls.
Acknowledgments
Financial Support: This work was supported by grants from United States Public Health Services RO1 CA108512, RO1 AT002709 and the Sullivan Foundation for the Study of Prostatitis
Abbreviations
- HFD
High Fat Diet
- RD
Regular Diet
- NF-κB
Nuclear Factor-kappa B
- iNOS
nitric oxide synthase
- COX-2
cyclooxygenase-2
- ROS
reactive oxygen species
- PIA
proliferative inflammatory atrophy
References
- 1.Ma RW-L, Chapam K. A systemic review of the effect of diet in prostrate cancer prevention and treatment. Journal of Human Nutrition and Dietetics. 2009;22:187–199. doi: 10.1111/j.1365-277X.2009.00946.x. [DOI] [PubMed] [Google Scholar]
- 2.Barnard RJ, Aronson WJ. Benign prostatic hyperplasia: does lifestyle play a role? Phys Sportsmed. 2009;37:141–146. doi: 10.3810/psm.2009.12.1752. [DOI] [PubMed] [Google Scholar]
- 3.Gann PH. Risk Factors for Prostrate Cancer. Reviews in Urology. 2002;4(Suppl 5):S4–S10. [PMC free article] [PubMed] [Google Scholar]
- 4.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. British Journal of Cancer. 2006;94:842–853. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bañez LL, Klink JC, Jayachandran J, Lark AL, Gerber L, Hamilton RJ, Masko EM, Vollmer RT, Freedland SJ. Association between Statins and Prostate Tumor Inflammatory Infiltrate in Men Undergoing Radical Prostatectomy. Cancer Epidemiol Biomarkers Prev. 2010 Feb 16; doi: 10.1158/1055-9965.EPI-09-1074. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Murtola TJ, Tammela TL, Määttänen L, Huhtala H, Platz EA, Ala-Opas M, Stenman UH, Auvinen A. Prostate cancer and PSA among statin users in the finnish prostate cancer screening trial. Int J Cancer. 2010 Jan 13; doi: 10.1002/ijc.25165. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 7.Vizio DiD, Solomon KR, Freeman MR. Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori. 2008;94:633–639. doi: 10.1177/030089160809400501. [DOI] [PubMed] [Google Scholar]
- 8.Kolonel LN, Nomura AM, Cooney RV. Dietary fat and prostate cancer: current status. J Natl Cancer Inst. 1999;91:414–428. doi: 10.1093/jnci/91.5.414. [DOI] [PubMed] [Google Scholar]
- 9.Christensen JH, Fabrin K, Borup K, Barber N, Poulsen J. Prostate tissue and leukocyte levels of n-3 polyunsaturated fatty acids in men with benign prostate hyperplasia or prostate cancer. BJU Int. 2006;97:270–273. doi: 10.1111/j.1464-410X.2006.05951.x. [DOI] [PubMed] [Google Scholar]
- 10.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 11.Homma Y, Kondo Y, Kaneko M, Kitamura T, Nyou WT, Yanagisawa M, Yamamoto Y, Kakizoe T. Promotion of carcinogenesis and oxidative stress by dietary cholesterol in rat prostate. Carcinogenesis. 2004;25:1011–1014. doi: 10.1093/carcin/bgh105. [DOI] [PubMed] [Google Scholar]
- 12.Miyake H, Hara I, Gleave ME, Eto H. Protection of androgen-dependent human prostate cancer cells from oxidative stress-induced DNA damage by overexpression of clusterin and its modulation by androgen. Prostate. 2004;61:318–323. doi: 10.1002/pros.20087. [DOI] [PubMed] [Google Scholar]
- 13.Savas M, Verit A, Ciftci H, Yeni E, Aktan E, Topal U, Erel O. Oxidative Stress in BPH. JNMA J Nepal Med Assoc. 2009;48:41–45. [PubMed] [Google Scholar]
- 14.Sciarra A, Mariotti G, Salciccia S, Gomez AA, Monti S, Toscano V, Di Silverio F. Prostate growth and inflammation. J Steroid Biochem Mol Biol. 2008;108:254–260. doi: 10.1016/j.jsbmb.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 15.Richmond A. NF-kappa B, chemokine gene transcription and tumor growth. Nat Rev Immunol. 2002;2:664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karin M. Nuclear factor-κB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 17.Shukla S, MacLennan GT, Fu P, Patel J, Marengo SR, Resnick MI, Gupta S. Nuclear factor-kappaB/p65 (Rel A) is constitutively activated in human prostate adenocarcinoma and correlates with disease progression. Neoplasia. 2004;6:390–400. doi: 10.1593/neo.04112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sweeney C, Li L, Shanmugam R, Bhat-Nakshatri P, Jayaprakasan V, Baldridge LA, Gardner T, Smith M, Nakshatri H, Cheng L. Nuclear factor-κB is constitutively activated in prostate cancer in vitro and is overexpressed in prostatic intraepithelial neoplasia and adenocarcinoma of the prostate. Clin Cancer Res. 2004;10:5501–5507. doi: 10.1158/1078-0432.CCR-0571-03. [DOI] [PubMed] [Google Scholar]
- 19.Caramori G, Adcock IM, Ito K. Anti-inflammatory inhibitors of IkappaB kinase in asthma and COPD. Curr Opin Investig Drugs. 2004;5:1141–1147. [PubMed] [Google Scholar]
- 20.Kaltschmidt B, Widera D, Kaltschmidt C. Signaling via NF-kappaB in the nervous system. Biochim Biophys Acta. 2005;1745:287–299. doi: 10.1016/j.bbamcr.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto T. NF-kappaB and rheumatic diseases. Endocr Metab Immune Disord Drug Targets. 2006;6:359–372. doi: 10.2174/187153006779025685. [DOI] [PubMed] [Google Scholar]
- 22.Schottelius AJ, Dinter H. Cytokines, NF-kappaB, microenvironment, intestinal inflammation and cancer. Cancer Treat Res. 2006;130:67–87. doi: 10.1007/0-387-26283-0_3. [DOI] [PubMed] [Google Scholar]
- 23.Maloney E, Sweet IR, Hockenbery DM, Pham M, Rizzo NO, Tateya S, Handa P, Schwartz MW, Kim F. Activation of NF-kappaB by palmitate in endothelial cells: a key role for NADPH oxidase-derived superoxide in response to TLR4 activation. Arterioscler Thromb Vasc Biol. 2009;29:1370–1375. doi: 10.1161/ATVBAHA.109.188813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koay MA, Christman JW, Segal BH, Venkatakrishnan A, Blackwell TR, Holland SM, Blackwell TS. Impaired pulmonary NF-kappaB activation in response to lipopolysaccharide in NADPH oxidase-deficient mice. Infection and Immunity. 2001;69:5991–5996. doi: 10.1128/IAI.69.10.5991-5996.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 26.Abdel-Meguid TA, Mosli HA, Al-Maghrabi JA. Prostrate inflammation. Association with benign prostrate hyperplasia and prostrate cancer. Saudi Med J. 2009;12:1563–1567. [PubMed] [Google Scholar]
- 27.Vykhovanets EV, Shukla S, MacLennan GT, Vykhovanets OV, Bodner DR, Gupta S. Il-1 beta-induced post-transition effect of NF-kappaB provides time-dependent wave of signals for initial phase of intrapostatic inflammation. Prostate. 2009;69:633–643. doi: 10.1002/pros.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr. 2009;4:215–222. doi: 10.1007/s12263-009-0133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vykhovanets EV, Shukla S, MacLennan GT, Resnick MI, Carlsen H, Blomhoff R, Gupta S. Molecular imaging of NF-kappaB in prostate tissue after systemic administration of IL-1 beta. Prostate. 2008;68:34–41. doi: 10.1002/pros.20666. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High Dietry Fat induces NADPH oxidase-associated stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 32.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore. Trends Biochem Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 33.Babior BM. NADPH oxidase: Current opinion in Immunology. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kumar B, Koul S, Khandrika L, Meacham RB, Koul HK. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 36.West DW, Slattery ML, Robison LM, French TK, Mahoney AW. Adult dietary intake and prostate cancer risk in Utah: a case-control study with special emphasis on aggressive tumors. Cancer Causes Control. 1991;2:85–94. doi: 10.1007/BF00053126. [DOI] [PubMed] [Google Scholar]
- 37.Bairati I, Meyer F, Fradet Y, Moore L. Dietary fat and advanced prostate cancer. J Urol. 1998;159:1271–1275. [PubMed] [Google Scholar]
- 38.O’Neill LA, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 39.Gosh S, May MJ, Kopp EB. NF-κB and Rel proteins evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 40.Clement K, Langin D. Regulation of Inflammation –related genes in human adipose tissue. J Intern Med. 2007;262:422–430. doi: 10.1111/j.1365-2796.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 41.Li Q, Verma IM. NF-κB regulation in the immune system. Nature Rev Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 42.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, Gutkovich-Pyest E, Urieli-Shoval S, Galun E, Ben-Neriah Y. NF-kB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004;431:461–466. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 43.Buskens CJ, Van Rees BP, Sivula A, Reitsma JB, Haglund C, Bosma PJ, Offerhaus GJ, Van Lanschot JJ, Ristimäki A. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology. 2002;122:1800–1807. doi: 10.1053/gast.2002.33580. [DOI] [PubMed] [Google Scholar]
- 44.Hur GM, Ryu YS, Yun HY, Jeon BH, Kim YM, Seok JH, Lee JH. Hepatic Ischemia/reperfusionin rats induces iNOS gene expression by activation of NF-κB. Biochem Biophys Res Commun. 1999;261:917–922. doi: 10.1006/bbrc.1999.1143. [DOI] [PubMed] [Google Scholar]
- 45.Hussain, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Letters. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 46.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, Lee SS. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappaB activation. Mutat Res. 2001;480–481:243–268. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 47.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II) Journal of National Cancer Institute. 1998;90:1609–1620. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- 48.Gupta S, Srivastava M, Ahmad N, Bostwick DG, Mukhtar H. Over-expression of cyclooxygenase-2 in human prostate adenocarcinoma. Prostate. 2000;42:73–78. doi: 10.1002/(sici)1097-0045(20000101)42:1<73::aid-pros9>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]


