Abstract
The constitutive androstane receptor (CAR) and pregnane X receptor (PXR) are important nuclear receptors involved in the regulation of cellular responses from exposure to many xenobiotics and various physiological processes. Phenobarbital (PB) is a non-genotoxic indirect CAR activator, which induces cytochrome P450 (CYP) and other xenobiotic metabolizing enzymes and is known to produce liver foci/tumors in mice and rats. From literature data, a mode of action (MOA) for PB-induced rodent liver tumor formation was developed. A MOA for PXR activators was not established owing to a lack of suitable data. The key events in the PB-induced liver tumor MOA comprise activation of CAR followed by altered gene expression specific to CAR activation, increased cell proliferation, formation of altered hepatic foci and ultimately the development of liver tumors. Associative events in the MOA include altered epigenetic changes, induction of hepatic CYP2B enzymes, liver hypertrophy and decreased apoptosis; with inhibition of gap junctional intercellular communication being an associative event or modulating factor. The MOA was evaluated using the modified Bradford Hill criteria for causality and other possible MOAs were excluded. While PB produces liver tumors in rodents, important species differences were identified including a lack of cell proliferation in cultured human hepatocytes. The MOA for PB-induced rodent liver tumor formation was considered to be qualitatively not plausible for humans. This conclusion is supported by data from a number of epidemiological studies conducted in human populations chronically exposed to PB in which there is no clear evidence for increased liver tumor risk.
Keywords: Constitutive androstane receptor, dose–response assessment, human relevance framework, key and associative events, liver cancer, mode of action, modulating factors, phenobarbital, pregnane X receptor
Introduction
Background and rationale for evaluation
Many non-genotoxic chemicals such as phenobarbital (phenobarbitone; PB) produce liver tumors in rats and mice (Gold et al., 2001; Grasso & Hinton, 1991; Huff et al., 1991). In recent years, frameworks for analyzing the modes of action (MOAs) by which such chemicals produce liver tumors have been developed. For non-genotoxic rodent liver carcinogens, proposed MOAs include cytotoxicity, activation of the constitutive androstane receptor (CAR), activation of the peroxisome proliferator-activated receptor alpha (PPARα), hormonal perturbation, immunosuppression and porphyria (Boobis et al., 2009; Holsapple et al., 2006; Klaunig et al., 2003; Meek et al., 2003; Williams, 1997a).
Liver tumor formation in rodents resulting from exposure to non-genotoxic agents is often associated with the selective induction of hepatic microsomal cytochrome P450 (CYP) enzymes. These induction responses are normally mediated through the activation of receptor-mediated mechanisms that lead to enhanced gene transcription (Dickins, 2004; Pelkonen et al., 2008). For example, important xenobiotic-sensing transcriptional factors that induce CYP1A, CYP2B, CYP3A and CYP4A enzymes comprise, respectively, the aryl hydrocarbon receptor (AHR) and the nuclear receptors CAR, pregnane X receptor (PXR) and PPARα. Several other receptors are also involved in the regulation of hepatic CYPs, and there can be considerable cross-talk between receptors in their regulation of CYP enzymes (Dickins, 2004; Omiecinski et al., 2011a; Stanley et al., 2006; Yoshinari et al., 2008).
For induction of CYP2B and CYP3A enzymes (which occur largely via activation of CAR and PXR, respectively), both CAR and PXR heterodimerize with the retinoid X receptor (RXR) prior to binding of the complex to specific transcriptional enhancer response elements in DNA. Although CYP inducers normally bind as ligands to the nuclear receptor, CAR in particular can be activated without direct ligand binding by an indirect or ligand-independent mechanism (Hosseinpour et al., 2006; Pelkonen et al., 2008; Yoshinari et al., 2008). The activation of CAR by PB is indirect and involves a dephosphorylation reaction at serine 202 (mouse) or threonine 38 (human), apparently signalling through the epidermal growth factor receptor, leading to the nuclear translocation of CAR (Hosseinpour et al., 2006; Mutoh et al., 2009, 2013; Yoshinari et al., 2008). For the purposes of this article, compounds will be referred to as either CAR or PXR activators, without any consideration of the precise mechanism of receptor activation. CAR activation results in changes in the expression of a wide range of genes, including genes involved in phase I and II xenobiotic metabolism; induction of phase IIl transporters and regulation of genes associated with various physiological processes such as cell proliferation, apoptosis and metabolism (Pelkonen et al., 2008; Tien & Negishi, 2006; Yoshinari et al., 2008). PXR activation also leads to increased expression of specific genes including xenobiotic metabolizing enzymes, many of which are also CAR-responsive (Maglich et al., 2002; Moore et al., 2000, 2003). Many of the molecules that can activate CAR may also activate PXR, producing a combined response pattern of gene expression and functional changes (Moore et al., 2000).
PB is a prototypical inducer of hepatic microsomal CYP2B subfamily enzymes. Other known CYP2B inducers include 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene (TCPOBOP), 1,1,1,-trichloro-2,2-bis(4-chlorophenyl)ethane (DDT), chlordane, dieldrin, α-hexachlorocyclohexane, oxazepam, phenytoin, and 2, 4, 5, 2′, 4′, 5′-hexachlorobiphenyl (Lake, 2009; Martignoni et al., 2006; Nims & Lubet, 1996; Omiecinski et al., 2011b). Treatment with PB induces a range of phase I (e.g. CYP2B, CYP2C and CYP3A subfamily enzymes) and phase II (e.g. microsomal epoxide hydrolase, some microsomal UDPglucuronosyltransferase enzymes and some cytosolic sulfotransferase and GSH S-transferase enzymes) xenobiotic metabolizing enzymes, as well as phase III transporters (e.g. the efflux transporter MRP2) (Martignoni et al., 2006; Nims & Lubet, 1996; Omiecinski et al., 2011a). Rodent CYP3A inducers include pregnenolone-16α-carbonitrile (PCN), dexamethasone, clotrimazole and troleandomycin (Dickins, 2004; Martignoni et al., 2006).
A workshop entitled “Dose–Response Approaches for Nuclear Receptor-Mediated Modes of Action” evaluated the roles of the activation of CAR and PXR in establishing MOAs for rodent liver tumor development (Andersen et al., in press). However, examination of the literature revealed that while an extensive database is available on the effects of CAR activators on liver tumor formation in rodents, comparatively little information is available on the corresponding effects of compounds that specifically activate PXR. For example, many studies have investigated the hepatocarcinogenicity of the CAR activator PB in rodents (IARC, 2001; Whysner et al., 1996), whereas there appears to be little information on the tumorigenic effects of non-genotoxic PXR activators (e.g. PCN). Thus, to focus the workshop discussions, the CAR/PXR panel considered only CAR activation and examined the CAR activator PB as a model compound to facilitate the evaluation of the current knowledge of the CAR activation MOA for rodent liver tumor development. A deeper understanding of the molecular mechanisms and the dose-responsive key events involved in this MOA can help inform a model that is most appropriate for use in risk assessment. CAR activation is a well-documented key event for rodent liver tumor development and specifically for PB-induced mouse liver tumor development, such that mice lacking the CAR gene do not display the biological responses seen in PB-treated wild-type mice (Huang et al., 2005; Wei et al., 2000; Yamamoto et al., 2004). In fact CAR activation is the molecular initiating event for the cellular pathway ultimately leading to the apical outcome of liver tumors in PB treated rodents.
In addition to the extensive data on the effects of PB in rodents, there are data in humans. PB has been used as a sedative, hypnotic and antiepileptic drug in humans for many years (IARC, 2001). PB induces CYP2B6, CYP3A4 and other CYP enzymes including CYP2A6, CYP2C9 and CYP2C19 in human liver (Martignoni et al., 2006; Pelkonen et al., 2008). Moreover, data are available from a number of epidemiological studies where PB has been administered to human subjects for extended periods (Friedman et al., 2009; IARC, 2001; La Vecchia & Negri, 2013; Olsen et al., 1989, 1995; Selby et al., 1989; Whysner et al., 1996).
Liver tumor formation in rodents
Many studies have shown that PB and/or its sodium salt (sodium phenobarbital; NaPB) can promote liver tumors in rats and mice (IARC, 2001; Whysner et al., 1996). Chronic treatment with PB or NaPB has been reported to produce liver tumors in a number of mouse strains. Unfortunately, in many of these studies PB or NaPB was administered only at a single dose level, hence dose–response relationships for liver tumor formation were not evaluated. For example, PB or NaPB produced liver tumors in C3H and CF-1 strain mice when administered either at 500 ppm (0.05%) in the drinking water or at 500 ppm in the diet, giving daily intakes of ~65–70 mg/kg day (Peraino et al., 1973; Ponomarkov et al., 1976; Thorpe & Walker, 1973). Although marked mouse strain differences in susceptibility to spontaneous liver tumor formation are known to exist (Becker, 1982), PB has been shown to produce liver tumors in both high (C3H/He) and low (C57BL/6) spontaneous incidence strains when administered in the diet to provide a daily intake level of 85 mg/kg day (Evans et al., 1986, 1992).
Changes in pathologic diagnostic criteria for liver lesions and liver tumors complicate the interpretation of some of the earlier studies performed with PB, which have been reviewed by IARC (2001) and Whysner et al. (1996). For example, in some early publications, lesions were sometimes described as neoplastic or hyperplastic nodules, as eosinophilic and basophilic nodules, or as type A and B nodules or tumors. Some of the lesions formerly diagnosed as various types of nodules would now be characterized as hepatocellular adenomas or foci of cellular alteration (Wolf & Mann, 2005). Hepatocellular adenomas are considered to be benign neoplasms (tumors). Although changes in diagnostic terminology have identified lesions that were formerly classified as nodules to be re-classified as foci of cellular alteration, these lesions are still considered preneoplastic lesions, and thus their presence is consistent with a tumorigenic response. More recently, PB has been shown to produce both hepatocellular adenomas and carcinomas in mice. This was demonstrated in a study with the low spontaneous incidence C57BL/10J mouse strain where currently accepted pathological diagnostic criteria were employed and tumors were noted in males but not in females (Jones et al., 2009). This study also demonstrated a threshold for PB-induced tumor formation with liver adenomas and carcinomas being observed in male mice at a dietary level of 1000 ppm (mean intake of 113 mg/kg day), but not at 200 ppm (22 mg/kg day).
Generally, the rat appears to be more resistant than the mouse to PB-induced tumor formation. In one study, the treatment of Wistar rats with 500 ppm PB in the drinking water was reported to produce hepatocellular adenomas (Rossi et al., 1977), whereas in some other rat studies PB has been reported to increase only the incidence of altered hepatic foci (Butler, 1978; Hagiwara et al., 1999; Whysner et al., 1996). However, for some other CYP2B enzyme inducers which appear to have a similar MOA for liver tumor formation to PB, such as pyrethrins and metofluthrin, liver tumors have been observed in the rat and not in the mouse (Osimitz & Lake, 2009; Yamada et al., 2009). Thus both the rat and mouse are susceptible species to liver tumor formation induced by non-genotoxic CAR activators.
The hepatic effects of PB are more pronounced in old rather than in young rats and mice. Compared to 42-day-old male F344 rats, the treatment of 2.4-year-old male F344 rats with 500 ppm PB in the drinking water resulted in an increased incidence of some types of foci and adenomas (Ward, 1983). Compared to 6-week-old male C3H/He mice, the treatment of 1-year-old male C3H/He mice with 500 ppm PB in the drinking water produced an increased incidence of foci, adenomas and carcinomas (Ward et al., 1988). The increased susceptibility with age suggests that liver tumor formation may involve the promotion of spontaneously initiated preneoplastic lesions that are more commonly observed in the livers of old rats and mice (Schulte-Hermann et al., 1983).
Methods
Literature identification and selection process
There is extensive literature on the hepatic effects of PB and related compounds (i.e. CAR activators) in rodents and other species, particularly for effects on CYP and other xenobiotic metabolizing enzymes. Data are also available on the hepatic effects of PXR activators and on the structure and functions of the CAR and PXR receptors. The hepatocarcinogenicity and/or promotional effects of PB and related compounds have been investigated in studies conducted in the mouse and rat and to a lesser extent in other species.
Pre-meeting materials were made available so that all CAR/PXR panel members were aware of the rigorous, stepwise criteria to be followed in the International Programme on Chemical Safety (IPCS) framework (see below) for establishing an animal MOA and for subsequently assessing the relevance of the MOA for humans. All CAR/PXR panel members were asked to provide data and references to assist in the development of the MOA framework at the workshop. The data submitted by the panel members, together with that from literature searches including previous reviews on the effects of PB (IARC, 2001; Lake, 2009; Whysner et al., 1996; Williams, 1997a), were used to compile a number of tables. One table presented the application of modified Bradford Hill considerations of causality to a number of possible key events, associative events and modulating factors for a MOA for PB-induced rodent liver tumor formation (Boobis et al., 2006; Hill, 1965; U.S. EPA, 2005). Other tables of possible key events, associative events and modulating factors focused on time, dose and species concordances. The collated tables were provided to the participants at the workshop for review and discussion. The completed tables helped to frame the extent and direction of discussion for the weight of evidence approach to establish key and associative events in the MOA and for human relevance analysis.
Mode of action (MOA) frameworks
A framework for analyzing the MOAs by which chemicals induce tumors in laboratory animals and the relevance of those tumors for human risk assessment has been developed through the efforts of the IPCS and the International Life Sciences Institute (Boobis et al., 2006; Cohen et al., 2003; Meek et al., 2003; Sonich-Mullin et al., 2001). For the present case study the features of a CAR-mediated mode of tumorigenic action were reviewed and discussed. A weight of evidence approach was used to describe the key and associative events based on traditional toxicology studies as well as molecular and genomic data. In addition, dose–response information was evaluated in order to determine its value for developing a quantitative biological model.
Prior to the workshop the Steering Committee had agreed upon definitions of key events, associative events and modulating factors. These definitions and the data evaluation framework to be applied for the MOA evaluation are shown below and are described in detail in the first manuscript in this series (Andersen et al., 2013).
Key event
An empirically observable causal precursor step to the adverse outcome that is itself a necessary element of the MOA. Key events are required events for the MOA, but often are not sufficient to induce the adverse outcome in the absence of other key events.
Associative event
Biological processes that are themselves not causal necessary key events for the MOA, but are reliable indicators or markers for key events. Associative events can often be used as surrogate markers for a key event in a MOA evaluation or as indicators of exposure to a xenobiotic that has stimulated the molecular initiating event or a key event.
Modulating factor
There are many factors or biological responses that are not necessary to induce the adverse outcome, but could modulate the dose–response behaviour or probability of inducing one or more key events or the adverse outcome. Such biological factors are considered modulating factors.
CAR/PXR case study panel procedures
In order to apply the framework methodology, the panel relied on a multi-disciplinary approach. Panel members were selected in order to cover all areas of CAR and PXR receptor biology, molecular biology, biochemical toxicology, pathology, dose–response assessment and risk assessment. The composition of the CAR/PXR panel is shown in Table 1. The overall panel selection process is described elsewhere (Andersen et al., 2013). It should be noted that not all the CAR/PXR panel decisions were unanimous and that in some instances votes were taken and a majority decision taken as the panel conclusion. Conclusions based on a majority decision of the panel are indicated in the text.
Table 1.
CAR/PXR case study panel members and affiliations.
| Participant names | Affiliations | |
|---|---|---|
| Co-Chairs | Cliff Elcombe, PhD | CXR Biosciences |
| Douglas Wolf, DVM, PhD, | U.S. EPA | |
| Rapporteurs | Jillian McEwan, PhD | CXR Biosciences |
| Audrey Vardy, PhD | CXR Biosciences | |
| Panel Members | Jason Bailey, PhD | Dow Agrosciences |
| Remi Bars, PharmD, PhD | Bayer CropScience | |
| David Bell, PhD | European Chemicals Agency | |
| Russell Cattley, DVM, PhD | Auburn University | |
| Rory Conolly, ScD | U.S. EPA | |
| Kenny Crump, PhD | Louisiana Tech University | |
| Stephen Ferguson, PhD | CellzDirect/Life Technologies | |
| David Geter, PhD | Dow Chemical Company | |
| Amber Goetz, PhD | Syngenta Crop Protection Inc. | |
| Jay Goodman, PhD | Michigan State University | |
| Susan Hester, PhD | U.S. EPA | |
| Abigail Jacobs, PhD | U.S. FDA-CDER | |
| Brian Lake, DSc | Centre for Toxicology, University of Surrey | |
| Curtis Omiecinski, PhD | Molecular Toxicology and Carcinogenesis, Penn State University | |
| Richard Peffer, PhD | Syngenta Crop Protection, LLC | |
| Rita Schoeny, PhD | U.S. EPA | |
| Wen Xie, MD, PhD | Center for Pharmacogenetics, University of Pittsburgh |
Prior to the meeting, the steering committee provided discussion questions to the CAR/PXR panel (Table 2). These questions were crafted to allow for a similar approach by all three workshop panels (i.e. the AHR, CAR/PXR and PPARα panels). Members of the CAR/PXR panel gave overview presentations in selected areas (available at http://www.TERA.org/peer/nuclearreceptor/) including the current state of knowledge and role in biology/physiology of CAR; a review of key events for PB-induced rodent liver tumor formation; morphological criteria for proliferative lesions in rodent liver; epigenetic effects of PB; effects on oxidative stress; species differences; results of recent PB microarray studies; and biologically based dose-response modeling. The panel considered the IPCS framework (Boobis et al., 2006) for analysis of the MOA for PB-induced rodent liver tumors and focused on questions in three major areas:
Is the existing biological knowledge for liver tumors induced through the receptor sufficiently understood to identify the MOA and its component key events, associative events and modulating factors? What are the key data gaps?
Is the existing biological knowledge of the MOA for liver tumors sufficiently understood to reasonably exclude, on a qualitative or quantitative basis, the human relevance of rodent liver tumors induced through this receptor? What are the key data gaps?
Are the data sufficient to identify a MOA? What is the relevance to humans? What are the dose–response implications of the key events in the MOA, including associative events and modulating factors? Are the data adequate to develop biologically based dose–response or other biological-informed models for this receptor? Is linear low-dose modeling appropriate based on the underlying science of nuclear receptor biology?
Table 2.
Primary charge questions and concluding comments from the CAR/PXR case study panel rapporteur report.
| Discussion questions (Q) and panel overall conclusions (A) for each | |
|---|---|
| Key events in MOA for nuclear receptor-mediated hepatomegaly and tumorigenicity | |
| Q1. | What is the Mode of Action for CAR-mediated mouse liver tumors for a model CAR activator (e.g. phenobarbital or related compounds), as evaluated using the IPCS Framework for Human Relevance and the modified Hill Criteria applied to mode of action (IPCS and EPA MOA Framework)? |
| A1. | See Figure 1 for phenobarbital-induced rodent liver tumor MOA. |
| Q2. | Which events are key events, which are associated events and which are neither? |
| A2. | For CAR activators there are five key events: |
| |
| Q3. | Are there key events that are not mediated via nuclear receptor activation? |
| A3. | There are no key events not mediated by CAR activation. |
| Q4. | Using the IPCS Framework, what is the human relevance of each key event? |
| A4. | The overall MOA for rodent liver tumor formation is likely not qualitatively plausible for humans. |
| Q5. | What are the fundamental biological steps in ligand-activation of the specific receptor(s) necessary to affect gene expression? |
| A5. | CAR heterodimerizes with the retinoid X receptor (RXR) prior to the binding of the complex to specific response elements in DNA. CAR can be activated without direct ligand binding by an indirect or ligand-independent mechanism (Hosseinpour et al., 2006; Pelkonen et al., 2008; Yoshinari et al., 2008). |
| Q6. | Is the existing molecular biology for gene regulation sufficiently understood to define it as a key event in the MOA? Does this event meet the requirements of the IPCS Human Relevance and MOA Frameworks to be supported as a key event? What are the key data needs to support receptor activation as a key event; what are the data needs to establish or exclude human relevance of this key event? |
| A6. | |
| |
| Q7. | Are the existing data sufficient to determine a dose-response relationship for this biological response? Are the existing descriptions of mathematical and statistical models for characterizing the fundamental biological steps complete? Is the existing description of concentration or dose-response data for these steps sufficient for dose-response modeling? What are the data needs, if any, for dose—response characterization and modeling? |
| A7. | The existing mathematical and statistical models for characterizing the key events are not complete due to lack of kinetic data. |
| Q8. | Is there an amount of ligand that would be insufficient for activating the specific receptor for induction of changes in gene expression? Are there empirical data that show an amount of ligand that is insufficient to activate a specific nuclear receptor such that there is no observable change in gene expression? Has a no effect level been demonstrated? |
| A8. | |
| |
| Additional biological responses | |
| Q9. | Subsequent to ligand activation of the specific nuclear receptor, what are the fundamental biological changes necessary to cause biological responses? |
| A9. | Increased or decreased gene expression. |
| Q10. | Is there sufficient understanding of these biological responses that lead to the adverse outcome (liver tumor) by the described mode of action sufficiently understood? Does the proposed sequence of these biological responses meet the criteria for establishing a mode of action and its human relevance within the IPCS Framework? If not, what are the key data needs to determine the mode of action and/or the Human Relevance? |
| A10. | The human relevance of the animal MOA can be reasonably excluded on the basis of fundamental qualitative differences. |
| Q11. | If these biological changes are key events, are there sufficient data to determine a dose-response relationship? Is the existing description of mathematical and statistical models for characterizing these key events complete? Is the existing description of concentration or dose—response data for these key events sufficient for dose-response modeling? If not, what are the key data needed to characterize the dose-response relationship? |
| A11. | There are sufficient data to establish no observable effect levels for phenobarbital. However, there are insufficient data at higher dose levels to describe dose-response relationships. |
| Q12. | Is there an amount of ligand that would be insufficient for activating the specific nuclear receptor such that there would be no induction of these key events or associated biological responses? Does a no effect level exist? |
| A12. | |
| |
| Outcome | |
| Q13. | Does knowledge from the above questions support the choice of an appropriate dose-response model for either precursor events or the adverse outcome of concern? If not, what are the missing data and what is needed to determine these data? |
| A13. | As mentioned earlier, there are insufficient data to establish dose-response relationships above the no observable effect levels. |
| Forward-looking questions | |
| Q14. | If data indicates a compound induces liver tumors, or could be reasonably expected to induce liver tumors, based on a likelihood that it is a nuclear receptor ligand, what framework or guidance can be suggested that describe a minimum series of assays, tests, experiments, or studies that would specifically confirm a nuclear receptor mediated mode of action and rule out other modes. |
| A14. | |
| |
| Q15. | If more than one nuclear receptor is activated, how does one describe the relative contribution and interactions? |
| A15. | |
| |
| Q16. | What would be the most appropriate data to generate to inform future risk assessments for nuclear receptor activators? |
| A16. | |
| |
Q, Discussion questions; A, CAR/PXR case study panel remarks, conclusions and referenced figures.
Results
The results of the workshop were presented in a formal rapporteurs’ report on the third day of the overall workshop. The summary statements for each of the discussion questions are presented in Table 2. The data and analyses supporting these conclusions are described in this section.
MOA hypothesis for PB-induced rodent liver tumor formation
A MOA and description of the human relevance of PB-like rodent liver carcinogens has been published previously (Holsapple et al., 2006). The key events in the MOA for rodent liver tumor formation by PB and related compounds described by Holsapple et al. (2006) included activation of CAR, increased hepatocyte proliferation, inhibition of apoptosis, liver hypertrophy and development of altered hepatic foci.
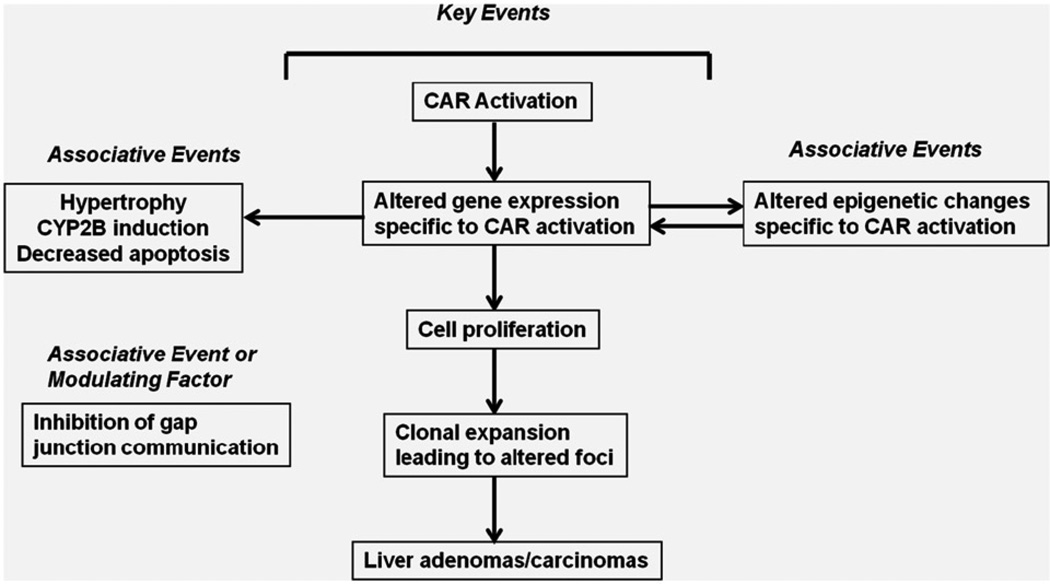
The workshop panel identified a CAR activation MOA for PB-induced rodent liver tumor formation, which is shown in Figure 1. PB-induced rodent liver tumor formation involves activation of the CAR nuclear receptor, which results in a pleiotropic response including altered gene expression specific to CAR activation and increased cell proliferation. Associative events include altered epigenetic changes specific to CAR activation, CYP2B enzyme induction, liver hypertrophy and inhibition of apoptosis. Prolonged PB treatment results in the formation of altered hepatic foci and subsequently in the development of hepatocellular adenomas and carcinomas.
Figure 1.
MoA for Phenobarbital-induced rodent liver tumour formation. Proposed key events, associative events and modulating factors for the mode of action (MOA) for PB-induced rodent liver tumor formation. The initial key event is CAR activation which results in altered gene expression, increased cell proliferation, clonal expansion? leading to altered foci and subsequently in the formation of liver tumors. Associative events which can serve as reliable biomarkers of key events include epigenetic changes, induction of CYP2B enzymes and liver hypertrophy and decreased apoptosis; whereas inhibition of gap junctional intercellular communication constitutes an associative event or modulating factor.
Key events as part of the overall MOA
In identifying a MOA for PB-induced rodent liver tumor formation, the CAR/PXR panel assessed the observable biological steps in the MOA as key events, associative events and modulating factors. Distinct from the key events shown in Figure 1, CYP2B enzyme induction, liver hypertrophy and inhibition of apoptosis were identified as associative events; with inhibition of gap junctional intercellular communication being considered an associative event or a modulating factor. The available evidence for each of the identified key and other events is reviewed below.
Key event 1 – CAR activation
The activation of CAR is the molecular initiating event, the initial key event, for PB-induced liver tumor formation. Studies in knockout mice lacking CAR have demonstrated that, unlike wild-type mice, PB does not increase liver weight, does not induce CYP2B enzymes and does not stimulate replicative DNA synthesis (Huang et al., 2005; Wei et al., 2000; Yamamoto et al., 2004). Moreover, although PB promoted liver tumors in wild-type mice initiated with the genotoxic carcinogen diethylnitrosamine (DEN), no liver adenomas or carcinomas were observed in CAR knockout mice (Huang et al., 2005; Yamamoto et al., 2004). In a study with the potent and highly selective mouse CAR activator TCPOBOP, liver tumors were observed in wild-type mice with or without DEN initiation, whereas no liver tumors were observed in CAR knockout mice with or without DEN initiation (Huang et al., 2005).
Key event 2 – altered gene expression specific to CAR activation
Activation of CAR in both the mouse and rat results in changes in the expression of a large number of genes involved in phase I and II xenobiotic metabolism, cell proliferation, apoptosis and energy metabolism (Hamadeh et al., 2002; Ross et al., 2009, 2010; Tien & Negishi, 2006; Ueda et al., 2002; Waterman et al., 2010; Yoshinari et al., 2008). Studies in wild-type mice and CAR-knockout mice have demonstrated that not all genes affected by PB are CAR-dependent (Ueda et al., 2002). While PB and the related compound chlordane produced effects on a large number of genes in wild-type mice (Ross et al., 2010), both PB and chlordane only produced effects on a more limited set of genes in humanized mice where mouse CAR/PXR was genetically replaced with the human counterpart genes (hCAR/hPXR).
CAR and PXR regulate overlapping but distinct sets of genes in mouse and human liver (Maglich et al., 2002). Studies in mice and rats have demonstrated that while some hepatic genes are affected by both the CAR activator PB and peroxisome proliferators (which activate PPARα), many other genes are only affected by either PB or peroxisome proliferators (Hamadeh et al., 2002; Ross et al., 2009).
Key event 3 – increased cell proliferation
In assessing the roles of increased cell proliferation and inhibition of apoptosis (see below) as key and associative events, attention needs to be given to the experimental design, the methods employed and the time points examined. For example, although replicative DNA synthesis may be determined by administration of a DNA precursor (e.g. 5-bromo-2′-deoxyuridine or radiolabelled thymidine) given as a single dose; this is not as sensitive in detecting low levels of cell proliferation as continuous administration of the DNA precursor via the drinking water or via a subcutaneously implanted osmotic pump. Other methods include immunocytochemistry for markers such as proliferating cell nuclear antigen (PCNA) or Ki-67. Several studies have demonstrated that PB can induce replicative DNA synthesis in rat and mouse hepatocytes. The stimulation of cell proliferation by PB, assessed as the labelling index (i.e. the percentage of hepatocyte nuclei undergoing replicative DNA synthesis), in rat and mouse liver is transient and not sustained. It is observed at early time points such as up to 7 days and perhaps also after 14 or 28 days of PB treatment, but generally not after longer treatment times (IARC, 2001; Kolaja et al., 1996a; Orton et al., 1996; Phillips et al., 1997; Whysner et al., 1996). However, while the hepatocyte labelling index returns to control levels with sustained PB treatment, overall cell proliferation is still enhanced due to the increase in the total number of hepatocytes per animal. Employing a stereological technique, an increase in the total number of hepatocytes was observed in rats treated with PB for 12 weeks (Carthew et al., 1998). At longer treatment times, rates of cell proliferation are enhanced in altered hepatic foci. For example, in promotion studies in F344 rats and B6C3F1 mice where altered hepatic foci were produced by initiation with DEN, PB was found to increase replicative DNA synthesis in the foci (Kolaja et al., 1996b,c).
Key event 4 – clonal expansion leading to altered foci
Altered liver foci are the precursor lesions for subsequent tumor formation (Williams, 1997b). The chronic administration of PB results in the development of altered hepatic foci (IARC, 2001; Jones et al., 2009; Thorpe & Walker, 1973; Whysner et al., 1996). In studies in aged rats (Ward, 1983), in C3H/He and C57BL/6 mice (Evans et al., 1986, 1992) and in B6C3F1 mice after DEN initiation (Kolaja et al., 1996c), the liver lesions produced by PB were predominantly eosinophilic in nature.
Key event 5 – liver adenomas/carcinomas
The chronic administration of PB has been shown to produce both hepatocellular adenomas and carcinomas in the mouse, with liver lesions and liver tumors also reported in some studies conducted in the rat (IARC, 2001; Jones et al., 2009; Whysner et al., 1996).
Associative events as part of the overall MOA
Associative events in the MOA for PB-induced liver tumor formation include induction of CYP enzymes, liver hypertrophy and inhibition of apoptosis. In addition, changes are also observed in DNA methylation.
Altered epigenetic changes specific to CAR activation
Recent studies of rodent hepatocarcinogenesis demonstrate that the exposure of rats and mice to genotoxic and non-genotoxic hepatocarcinogens leads to alterations in the epigenome. An altered pattern of hepatic DNA methylation has been observed in mice exposed to PB, especially in the livers of the tumor-prone B6C3F1 and C3H mice (Bachman et al., 2006; Phillips et al., 2007; Phillips and Goodman, 2008). The treatment of mice with PB for 2 or 4 weeks results in progressive changes in the methylation status of hepatic DNA, predominantly an accumulation of hypomethylated regions. Of note, these changes are more pronounced in the livers of tumor-susceptible (tumor prone) B6C3F1 mice as compared with the relatively more resistant C57BL/6 mice (Phillips & Goodman, 2008). Furthermore, PB induces unique alterations in the methylation status of hepatic DNA of CAR wild-type mice as compared with CAR knockout mice (Phillips et al., 2007). Such observations have led to the suggestion that strain- and species-dependent sensitivity to hepatocarcinogenesis may be inversely related to the ability to maintain normal profiles of DNA methylation (Goodman & Watson, 2002).
CYP2B induction
A characteristic effect of PB in rodent liver is the induction of CYP enzymes, particularly of CYP2B subfamily enzymes (IARC, 2001; Martignoni et al., 2006; Nims & Lubet, 1996). The induction of CYP2B enzymes can serve as a surrogate indicator for the pleiotropic effects of PB in rodent liver. However, while induction of CYP2B is an indicator of CAR activation, PXR activation can also produce an induction of CYP2B enzymes, along with a greater induction of CYP3A enzymes.
Liver hypertrophy
PB-induced liver enlargement is due to both hepatocyte hypertrophy and hyperplasia, with ultrastructural examination revealing a proliferation of the smooth endoplasmic reticulum (IARC, 2001; Lake, 2009; Nims & Lubet, 1996; Whysner et al., 1996). In the rat and mouse, PB-induced hypertrophy is normally observed in the centrilobular region of the liver lobule, although some related compounds may either produce a diffuse hypertrophy or hypertrophy in other regions of the liver lobule. The treatment of both rats and mice with PB results in dose-dependent increases in relative liver weight (i.e. hepatomegaly).
Inhibition of apoptosis
Studies in rat liver and in isolated hepatocytes have demonstrated that PB can inhibit apoptosis (Foster, 2000; James & Roberts, 1996). PB was shown to inhibit both transforming growth factor-β- and bleomycin-induced apoptosis in mouse hepatocytes, but not to affect the basal rate of apoptosis in untreated mouse hepatocytes (Christensen et al., 1998). Mouse hepatocytes do not appear to enter apoptosis as readily as rat hepatocytes following withdrawal of PB, as no increase in rates of apoptosis was seen when C3H/He, C57BL/6, or B6C3F1 mice were given PB for 7 days followed by subsequent withdrawal (Bursch et al., 2005b). In promotion studies in F344 rats and B6C3F1 mice following DEN initiation, PB was reported to produce an inhibition of apoptosis in altered liver foci (Kolaja et al., 1996b,c). However, other studies suggest that the inhibition of apoptosis appears to be only a minor determinant of tumor promotion in the mouse (Bursch et al., 2005a; Goldsworthy and Fransson-Steen, 2002). The potent direct CAR activator TCPOBOP was shown to produce a marked inhibition of apoptosis in liver cells from wild-type mice, but did not suppress apoptosis in CAR knockout mice (Huang et al., 2005). The lack of effect on apoptosis observed in some studies may be due to the technique employed or to inadequate group sizes that fail to account for substantial inter-animal variability. An inhibition of apoptosis in altered liver foci may act as an associative event by enhancing lesion growth and subsequent tumor formation.
Associative events or modulating factors
The panel also considered inhibition of gap junctional intercellular communication and oxidative stress as potential associative events or modulating factors.
Gap junctional intercellular communication
Gap junctions are intercellular membrane channels that allow the direct exchange of small molecules between adjacent cells and the sustained down regulation of such intercellular communication confers a loss of tumor-suppressive action (Chipman et al., 2003). A number of non-genotoxic hepatocarcinogens including PB have been shown to inhibit gap junctional intercellular communication in rodent liver (Chipman et al., 2003; Klaunig et al., 1990; Neveu et al., 1994). In one study, PB was shown to promote DEN-induced liver lesions only in wild-type mice and not in mice lacking connexin32, the major gap junction forming protein in liver (Moennikes et al., 2000). As the CAR-dependency of the disruption of gap junctional intercellular communication by PB has not been demonstrated, this process was not considered to be a key event. However, the disruption of gap junctional intercellular communication by PB may contribute to the process of tumor formation and hence this was considered an associative event or a modulating factor.
Oxidative stress
Oxidative damage to macromolecules due to the increased production of reactive oxygen species can play a role in chemically induced carcinogenesis (Klaunig et al., 2010). Some studies have reported that prolonged administration of PB may be associated with effects on oxidative stress. For example, CYP2B enzyme induction in rodent liver by PB can result in the enhanced production of reactive oxygen species (Dostalek et al., 2007; Imaoka et al., 2004) and the over expression of rat CYP2B1 in mouse liver was associated with enhanced spontaneous tumor formation (Lehman-McKeeman et al., 1999). However, an evaluation of the available literature did not reveal any clear role for oxidative stress in the MOA for PB-induced rodent liver tumor formation. Overall, oxidative stress was not considered by the majority of the panel to constitute a key or associative event or a modulating factor.
Application of Bradford Hill criteria for evaluation of causality for the proposed MOA and key and associative events
Concordance of dose–response relationships
Most carcinogenicity studies with PB have been performed at one dose level, unlike some other chemicals for which MOA studies have been performed for submission to regulatory agencies. However, an examination of the literature demonstrates that PB produces dose-dependent effects on key and associative events including replicative DNA synthesis and increased liver weight in the rat and mouse (Jones et al., 2009; Kolaja et al., 1996a; Orton et al., 1996). Evidence that the hepatic effects of PB are dose-dependent is also provided by data from promotion studies in the rat and mouse following DEN initiation (Kitano et al., 1998; Kolaja et al., 1996b).
Dose–response relationships for PB-induced liver tumors in CD-1 and B6C3F1 mice have been reported in unpublished studies reviewed by Whysner et al. (1996), where animals were given diets containing PB to provide intakes of 0 (control), 10, 75 and 150 mg/kg day. Treatment with 10 mg/kg day PB did not increase the incidence of liver adenomas and carcinomas in CD-1 mice, whereas an increase in liver tumors was observed at dose levels of 75 and 150 mg/kg day and at all PB dose levels in B6C3F1 mice. The lowest observed adverse effect level (LOAEL) for neoplasia for CD-1 mice in this study was thus 75 mg/kg day. In a recent study, reported in an abstract, the effects of PB on a number of key events and on global gene expression analysis was investigated in CD-1 mice given diets containing PB to provide intakes of 0 (control), 0.15, 1.5, 15, 75 and 150 mg/kg day for periods of 2 and 7 days (Gollapudi et al., 2011). Increased cell proliferation was observed in male mice at doses of ≥15 mg/kg day, whereas gene expression analysis revealed little or no pathway alterations at doses of ≤1.5 mg/kg day. A comparison of the LOAELs in these two studies with CD-1 mice indicates that changes in gene expression were observed at low doses (~1.5 mg/kg day), followed by increased cell proliferation (at doses at or above 15 mg/kg day), with neoplasia being observed only at higher doses (75 mg/kg day and above). The data in Table 3 also demonstrate a no effect level for these key events of 0.15 mg/kg day. These dose–response relationships are consistent with the proposed MOA.
Table 3.
Dose-response and temporality concordance table for PB-induced liver tumors.
| Temporal |
||||||
|---|---|---|---|---|---|---|
| Dose (mg/kg day) |
Key event 1 CAR activationa (Immediate) |
Key event 2 Altered gene expressionb (Days) |
Key event 3 Increased cell proliferation (Days) |
Key event 4 Clonal expansion leading to altered foci (Months) |
Liver adenomas/ carcinomas (Years) |
|
| Male CD-1 Micec | 0.15 | − | − | − | nd | nd |
| 1.5–10 | ± | ± | − | nd | − | |
| 15 | + | + | + | nd | nd | |
| 75–150 | + | + | + | nd | + | |
| Male C57B1/10J Miced | 22 | nd | nd | − | − | − |
| 113 | nd | nd | + | + | + | |
CAR activation inferred in vivo based on surrogate markers of increased CYP enzyme activities and Cyp2b10 mRNA levels.
Altered gene expression based on results of gene expression analysis.
Data are from CD-1 mouse studies (Gollapudi et al., 2011; Whysner et al., 1996). Measurements of enzyme activities, gene expression and replicative DNA synthesis were made after 2 and 7 days of treatment (Gollapudi et al., 2011) and bioassay dose levels were 0, 10, 75 and 150 mg/kg/day (Whysner et al., 1996).
Data are from C57B1/10J mouse studies (Jones et al., 2009). Replicative DNA synthesis was increased after 3, 8 and 15 days of treatment.
+, key event observed; −, key event not present; ±, equivocal; nd, not determined.
PB was found to produce liver tumors in male C57BL/10J mice at a dietary level of 1000 ppm but not at 200 ppm (Jones et al., 2009). The dietary levels of 200 and 1000 ppm produced achieved dose levels of 22 and 113 mg/kg day, respectively. The treatment of male mice with 200 and 1000 ppm PB for periods up to one month resulted in an increase in relative liver weight, whereas replicative DNA synthesis was increased after 3, 8 and 15 days of treatment with 1000 ppm PB, but not after treatment with 200 ppm PB. After 99 weeks of treatment, significant increases in relative liver weight and centrilobular hepatocyte hypertrophy were observed at both dose levels, but altered hepatic foci and liver tumors were observed only at 1000 ppm PB.
The available data from the CD-1 and C57BL/10J studies in male mice described earlier are shown in Table 3. These data demonstrate that the effects of PB on a number of the key and associative events are dose-dependent, with very low doses of PB having no effect and with liver tumors only being observed at high doses in the presence of effects on the key and associative events. In some instances effects on key and associative events may be observed at lower than carcinogenic dose levels.
Temporal association
If a key event (or events) is an essential element for carcinogenesis, it must precede the appearance of tumors. As shown in Table 3, CAR activation, altered gene expression and increased cell proliferation are early events, whereas clonal expansion leading to altered foci is only observed after chronic treatment with PB. While effects on some key and associative events including CAR activation, altered gene expression, CYP induction and hypertrophy are observed throughout the period of PB treatment, the stimulation of cell proliferation in normal hepatocytes is only observed at early time points. However, increased cell proliferation is also important in the growth of altered hepatic foci. In promotion studies where altered hepatic foci were produced by initiation with DEN, PB was found to increase replicative DNA synthesis within the foci (Kolaja et al., 1996b,c). Increases in adenomas and carcinomas appear only after chronic treatment, typically at the end of lifetime studies in rats and mice.
Strength, consistency and specificity of association of the tumor response with key events
A part of the Hill criteria requires that, for the key events to be causally related to the formation of tumors, they must clearly be shown to be required steps that lead to the tumors and that the findings are reproducible. The key events observed in mice receiving PB occurred in a logical temporal sequence, in a dose-dependent manner and occurred at dose levels that were concordant with the doses that produce liver tumors (Table 3). Multiple investigations have demonstrated that PB can stimulate cell replication in rodent liver (IARC, 2001; Kolaja et al., 1996a; Orton et al., 1996; Phillips et al., 1997; Whysner et al., 1996) and the requirement for CAR activation to produce tumors has been shown in two promotion studies (Huang et al., 2005; Yamamoto et al., 2004). The specificity of the association between the key events of increased cell proliferation and liver tumor formation is also demonstrated by data on species differences in the hepatic effects of PB (see below and Table 4). For example, while PB increases cell proliferation and produces liver foci and tumors in mice and rats, such effects are not observed in the Syrian hamster.
Table 4.
Species concordance and human relevance of key and associative events.
Effects observed either in studies conducted in vivo and/or in vitro in cultured hepatocytes.
By inference from increased gene expression.
No increase observed in studies with cultured human hepatocytes. However, while no increase in cell proliferation was observed in a hCAR/hPXR mouse model (Ross et al., 2010), increased cell proliferation was observed in a hCAR mouse model (Huang et al., 2005).
Observed in some, but not all, studies.
Inhibition of gap junctional intercellular communication was considered to be an associative event or a modulating factor.
The relationship between the observed hepatic effects to PB treatment has been demonstrated in recovery studies in the rat where the effects of PB on liver weight, xenobiotic metabolizing enzymes and inhibition of gap junctional intercellular communication were reversible on cessation of treatment (Crampton et al., 1977; Isenberg et al., 2001; Lake et al., 1978). In addition, the liver lesions produced by PB in the mouse appear to be at least partially reversible. The treatment of male C3H/He mice for 91 weeks and male C57BL/6 mice for 100 weeks with diet containing PB to produce an intake of 85 mg/kg day increased liver tumors in both mouse strains (Evans et al., 1992). However, liver tumor incidence was reduced in both mouse strains given PB for 60 weeks followed by control diet compared to mice that were given PB throughout the 91 or 100 weeks of the study.
Biological plausibility and coherence
The proposed MOA for PB-induced rodent liver tumor formation is biologically plausible and consistent with our current understanding of liver tumor formation by non-genotoxic mitogenic agents that can activate nuclear receptors. As an example, the proposed MOA shares many similarities with the postulated MOA for PPARα-activating compounds, including activation of a nuclear receptor, selective CYP induction, an increase in cell proliferation and suppression of apoptosis (Klaunig et al., 2003). This creates an environment in the liver where spontaneously initiated cells have a greater chance to survive and divide, eventually leading to tumor formation.
Consideration of alternative MOAs
Liver tumors can be produced in rodents by both genotoxic and non-genotoxic MOAs (Boobis et al., 2009; Holsapple et al., 2006; Klaunig et al., 2003; Meek et al., 2003). In terms of a genotoxic MOA, PB has been shown to be negative in a wide range of genotoxicity tests and does not form adducts with DNA (IARC, 2001; Whysner et al., 1996, 1998). Liver tumors can be induced in rodents by cytotoxic agents such as chloroform (Meek et al., 2003). Examination of the literature reveals that PB-induced rodent liver tumors are observed at dose levels that are not associated with any evidence of marked hepatotoxicity. In terms of nuclear receptor activation, PB does not appear to be a PPARα activator. While there can be considerable crosstalk between CAR and PXR receptors, the key and associative events for PB-induced liver tumor formation appear to be predominantly CAR-dependent as such effects are absent in mice lacking CAR (Huang et al., 2005; Wei et al., 2000; Yamamoto et al., 2004). The effects of PB in mice and rats are well documented (IARC, 2001; Whysner et al., 1996). Based on such data other possible MOAs including hormonal perturbation, immunosuppression and porphyria can also be excluded.
Species differences in the hepatic effects of PB
As described earlier, there is direct experimental evidence in rodents for the effects of PB on the key and associative events identified in the MOA for PB-induced rodent liver tumor formation. However, a number of in vitro and in vivo studies have demonstrated species differences in the hepatic effects of PB among experimental animal models and between rodents and humans. Table 4 lists the effects of PB on the key and associative events in mice, rats, Syrian hamsters, non-human primates and humans. While for some key and associative events the effects of PB are similar in all species examined, important species differences have been identified for others. Some of the observed species differences in the hepatic effects of PB, including a lack of stimulation of cell proliferation, have also been observed in studies with PPARα activators (Klaunig et al., 2003; Lake, 2009).
A number of studies have shown that CAR can be activated in mice, rats, Syrian hamsters, primates and humans resulting in altered gene expression, hypertrophy and CYP2B enzyme induction (Table 4). In contrast, while PB enhances cell proliferation and decreases apoptosis in the mouse and rat, other species appear to be refractory to the proliferative and anti-apoptotic responses. For example, PB has been reported not to stimulate DNA synthesis and not to inhibit apoptosis in cultured Syrian hamster and guinea pig hepatocytes (James & Roberts, 1996). In addition, while PB has been shown to inhibit gap junctional intercellular communication in mouse and rat hepatocytes, such effects have not been observed in primate hepatocytes (Baker et al., 1995; Klaunig & Ruch, 1987).
In keeping with the lack of effect of PB on cell proliferation in the Syrian hamster, chronic PB treatment does not produce liver tumors in this species when given in the drinking water at 500 ppm (Diwan et al., 1986). Many studies have demonstrated that PB and related compounds are efficient promoters of genotoxin-induced tumors in mouse and rat liver (IARC, 2001; Whysner et al., 1996). In contrast, a number of studies have shown that the Syrian hamster is resistant to the promoting effects of PB and related compounds after initiation with genotoxic carcinogens (Diwan et al., 1986; Stenbäck et al., 1986; Tanaka et al., 1987). PB was administered at a dose level of 500 ppm in either the drinking water (Diwan et al., 1986) or diet (Stenbäck et al., 1986; Tanaka et al., 1987).
A summary of the literature shown in Table 4 demonstrates that while some effects of PB including CAR activation and CYP2B induction are observed in a number of animal species, other effects such as cell proliferation appear to be confined to the mouse and rat. In addition, while PB can produce liver tumors and act as a promoter of genotoxic carcinogen-initiated lesions in the mouse and rat, such effects are not observed in the Syrian hamster.
CAR is expressed in human liver (Moore et al., 2000, 2003). Through effects on CAR, PXR and other receptors, PB has been shown to induce a number of CYPs in human liver including CYP2B6 and CYP3A4 (Martignoni et al., 2006; Pelkonen et al., 2008). Apart from CYP induction, some of the other effects produced by PB and related compounds in rodent liver have also been observed in human liver. Thus prolonged treatment with PB and other anticonvulsant agents has been shown to increase liver size in humans, which is associated with swelling of the hepatocytes without any evidence of necrosis, inflammation, fibrosis, or disturbance of hepatic architecture; with ultrastructural examination revealing a proliferation of the smooth endoplasmic reticulum without any other cellular alterations (Aiges et al., 1980; Pirttiaho et al., 1978, 1982).
Although PB can increase liver size in both rodents and humans, significant species differences in the mitogenic and anti-apoptotic properties of PB and related compounds have been demonstrated. In contrast to effects in cultured rodent hepatocytes, PB does not induce replicative DNA synthesis and does not inhibit apoptosis in human hepatocytes (Hasmall and Roberts, 1999; Hirose et al., 2009; Parzefall et al., 1991). PB has also been reported not to inhibit gap junctional intercellular communication in human hepatocytes (Baker et al., 1995).
The similarities and differences between human CAR and PXR and mouse CAR and PXR have been compared in studies performed with transgenic mice, in which the mouse receptors have been replaced with their human counterparts. One study employed male wild-type mice, CAR/PXR receptor double-knockout mice and humanized mice in which mouse CAR and PXR have been genetically replaced with their human counterpart genes (hCAR/hPXR mice). Mice were given 0 (vehicle only) and 80 mg/kg day PB by intraperitoneal injection for 4 days (Ross et al., 2010). Other groups of mice were given corn oil (control) and 10 mg/kg day chlordane by oral gavage for 4 days. Like PB, chlordane is known to induce CYP enzymes, stimulate cell proliferation and produce liver tumors in the mouse (Barrass et al., 1993). The treatment of wild-type mice with PB or chlordane increased liver weight, produced hepatocellular hypertrophy, increased replicative DNA synthesis and induced Cyp2b and Cyp3a enzymes, whereas no such effects were observed in the PXR/CAR receptor knockout mice. However, while liver hypertrophy and CYP induction were also observed after PB and chlordane treatment of hCAR/hPXR mice, replicative DNA synthesis was not increased.
The effect of PB was examined in another study (Huang et al., 2005) employing a different transgenic model that expressed only human CAR in the liver (hCAR mice). Mice were fed diets containing either 0 or 500 ppm PB for 1 week. Treatment of the hCAR mice with PB resulted in increases in relative liver weight, Cyp2b10 mRNA levels and cell proliferation. PB also suppressed UV-induced apoptosis in hepatocytes from hCAR mice.
The observation that neither PB nor chlordane can induce cell proliferation in hCAR/hPXR mice (Ross et al., 2010) is mirrored by in vitro data that PB does not stimulate cell proliferation in cultured primary human hepatocytes. However, the study of Huang et al. (2005) did demonstrate a stimulation of cell replication following PB treatment in hCAR mice. At present there is no clear explanation for the apparent discrepancy of the reported lack of effect of PB and chlordane on cell proliferation in hCAR/hPXR mice by Ross et al. (2010) and the effect of PB in hCAR mice reported by Huang et al. (2005). Possibly, the difference between the results obtained may lie in the nature of the mouse models developed for these studies (Omiecinski et al., 2011a) or, alternatively, may reflect the time concentration plasma profile of PB following the two different treatment regimens. Thus 500 ppm in the diet for 1 week would result in a higher exposure of the animals to PB than administration by intraperitoneal injection at 80 mg/kg day for 4 days. Overall, it is considered that more studies with humanized CAR and CAR/PXR mouse models are required and that no firm conclusions can be drawn from these two studies (Huang et al., 2005; Ross et al., 2010).
Human Relevance of the proposed MOA
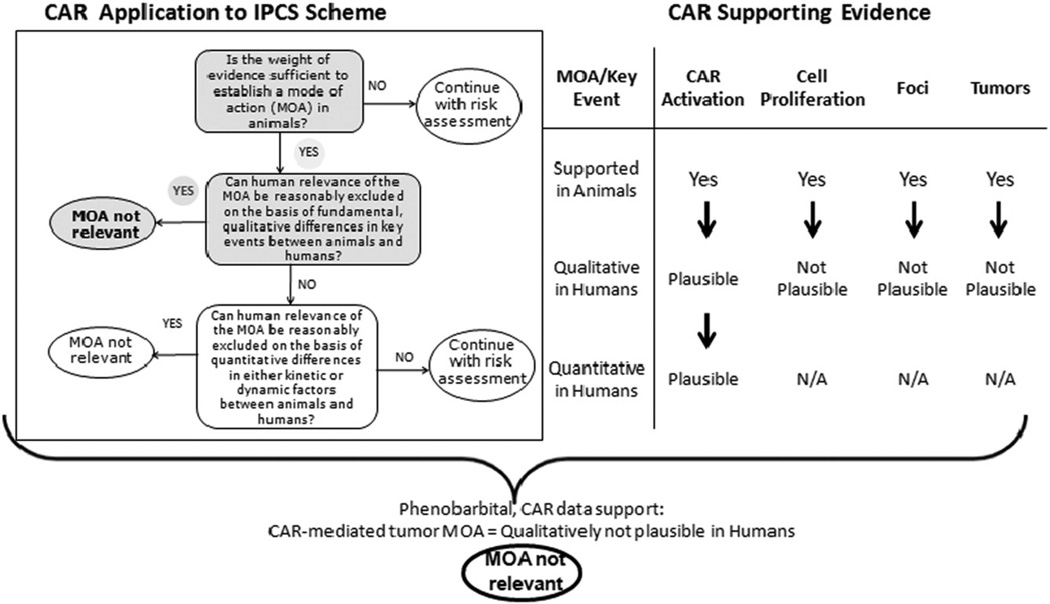
In terms of the human relevance of an animal carcinogenic MOA there are three key questions to answer (Boobis et al., 2006; Figure 2). These are as follows:
Is the weight of evidence sufficient to establish a MOA in animals?
Can human relevance of the MOA be reasonably excluded on the basis of fundamental, qualitative differences in key events between animals and humans?
Can human relevance of the MOA be reasonably excluded on the basis of quantitative differences in either kinetic or dynamic factors between animals and humans?
Figure 2.
Human Relevance Framework Analysis for Phenobarbital-induced rodent liver tumour formation. Evaluation of the human relevance of the proposed mode of action (MOA) for PB-induced rodent liver tumor formation. A robust MOA has been developed for PB-induced liver tumor formation in rodents. By reference to the IPCS scheme (Boobis et al., 2006), the animal MOA is considered to be likely not qualitatively plausible for humans. While PB can result in CAR activation in both rodents and humans, the key events of increased cell proliferation, clonal expansion leading to altered foci and subsequently to liver tumors are considered not plausible in humans.
Once the question concerning the sufficiency of the weight of evidence for the animal MOA is determined, then one may address the other two questions posed by the Human Relevance Framework (Boobis et al., 2006). A plausible MOA for PB-induced rodent liver tumor formation was previously described by Holsapple et al. (2006) and an updated MOA has now been developed by the CAR/PXR panel (Figure 1). As discussed in this publication, the rodent MOA consists of key events that are dose-concordant, occur in a logical temporal sequence, and are reproducible and consistent across multiple studies. Other potential MOAs have been considered and have been excluded. Thus, the weight of evidence is sufficient to establish a MOA in animals. This leads to the second question as to whether the human relevance of the MOA can be reasonably excluded on the basis of fundamental, qualitative differences in key events between animals and humans.
A number of effects of PB that are produced in the rodent liver can also be observed in humans. Such effects include activation of CAR, the induction of CYP2B and other CYP forms and liver hypertrophy. However, some clear species differences have been reported. For example, while PB stimulates replicative DNA synthesis in cultured rodent hepatocytes, as well as after in vivo administration, PB does not appear to increase replicative DNA synthesis in cultured human hepatocytes (Hirose et al., 2009; Parzefall et al., 1991). In addition, unlike in rodent liver, PB does not inhibit apoptosis in human hepatocytes (Hasmall & Roberts, 1999).
As described earlier, the present data for effects on cell proliferation and apoptosis obtained with transgenic mice expressing either hCAR/hPXR or just hCAR is equivocal. Hence no firm conclusions for the human relevance of the animal MOA for PB-induced liver tumor formation can be drawn at present from such data and additional studies are required.
Overall, based on the species differences data, the panel concluded that the animal MOA for rodent liver tumour formation is likely not qualitatively plausible for humans. As the data suggest that there are sufficient qualitative differences between the species that develop liver tumors after PB treatment (rats and mice) and humans, it was not necessary to answer the third question regarding quantitative differences. Thus as illustrated in Figure 2, the key events of CAR activation, cell proliferation, production of altered foci and production of tumors are well characterized in animals that develop liver tumors. However, while CAR activation does occur in humans, certain other key events do not appear to occur in humans. PB has been shown not to increase cell proliferation in cultured human hepatocytes and the development of altered hepatic foci has not been reported in the literature. It is, therefore, not likely that liver tumors would occur through this MOA as a consequence of PB exposure in humans.
As a further weight of evidence, while PB can act as a non-genotoxic carcinogen and tumor promoter in the rat and mouse, it does not appear to produce liver tumors in humans. Because of the therapeutic uses of PB, a number of epidemiological and other human studies are available. Reports of PB-induced liver injury are rare and many factors can contribute to drug-induced liver injury in humans (Navarro & Senior, 2006). In one study, PB and/or diphenylhydantoin were reported to produce liver hypertrophy with no evidence of necrosis, inflammation and fibrosis (Aiges et al., 1980), whereas in other studies treatment with PB and/or other anticonvulsant (antiepileptic) drugs hepatotoxicity was reported, suggesting that chronic anticonvulsant therapy may cause liver damage (Di Mizio et al., 2007; Foster et al., 1991). In a paediatric case report from a 13-year-old female subject, Cerminara et al. (2012) speculated that treatment with PB for 7 years may have contributed to the development of hepatocellular adenoma observed in this study. A number of epidemiological studies with PB and other anticonvulsant drugs have been performed (Friedman et al., 2009; IARC, 2001; Lamminpää et al., 2002; Olsen et al., 1989, 1995; Selby et al., 1989; White et al., 1979; Whysner et al., 1996) and the data for PB has been recently reviewed by La Vecchia & Negri (2013). In one study, with patients with severe epilepsy, treatment with anticonvulsant drugs was found not to increase the incidence of liver cancer (White et al., 1979), whereas in another study with PB and other anticonvulsant drugs an increase in liver tumors was reported (Lamminpää et al., 2002), although other factors such as alcohol and smoking may have contributed to the increase in liver tumors (La Vecchia & Negri, 2013). Other epidemiological studies have demonstrated that PB does not increase the incidence of liver tumors in humans (Friedman et al., 2009; Olsen et al., 1989, 1995; Selby et al., 1989). Based on the available data in the literature, La Vecchia & Negri (2013) concluded that there was no evidence for a specific role of PB in human liver cancer risk. Moreover, in these studies showing no evidence of increased liver tumor risk, the subjects received PB for many years at doses producing plasma concentrations similar to those that are carcinogenic in rodents. For example, plasma levels of PB in three strains of mice given 500 ppm PB in the drinking water ranged from 5–29 µg/ml, whereas plasma concentrations of PB in human subjects given therapeutic doses of 3–6 mg/kg ranged from 10–25 µg/ml (Munro, 1993). Overall, the human epidemiological studies support the conclusion that the MOA for PB-induced rodent liver tumors is not relevant to humans.
Hepatic effects of PXR activators
The panel also examined the available data for the hepatic effects of PXR activators in order to ascertain if there were any suitable compounds that could be used to develop a MOA for liver tumor formation by such compounds. PXR activators appear to produce similar effects in rodent liver to those produced by CAR activators, in that they can increase liver weight, stimulate replicative DNA synthesis and induce CYP forms. For example, PCN has been shown to increase liver weight, stimulate replicative DNA synthesis and induce Cyp3a11 mRNA levels and enzyme activity in the mouse (Staudinger et al., 2001, 2003). These effects were PXR-dependent, as no increase in liver weight, replicative DNA synthesis and Cyp3a11 mRNA levels and enzyme activity were observed in PXR knockout mice. A number of CYP3A inducers including PCN, clotrimazole and troleandomycin have been shown to increase liver weight and stimulate replicative DNA synthesis in rat liver (Lake et al., 1998). While all three compounds induced hepatic microsomal CYP3A protein levels, PCN and clotrimazole also increased CYP2B protein levels or enzyme activity.
In terms of establishing a MOA for rodent liver tumor formation by PXR activators, by analogy with the MOAs for CAR activators and PPARα agonists, key and associative events are likely to include PXR activation, increased cell proliferation, hypertrophy, CYP3A induction and clonal expansion leading to altered foci. However, studies of liver tumor formation by suitable non-genotoxic CYP3A inducers, which could be employed as model compounds for MOA studies, have not been reported. Although a number of studies have been performed with the mitogenic CYP3A activator cyproterone acetate, which produces liver tumors in the rat and mouse, tumor formation in female rats from cyproterone treatment is probably due to a combination of both genotoxic and non-genotoxic effects (Kasper, 2001; Tucker et al., 1996).
Species differences in PXR activation have been studied in wild-type mice, PXR knockout mice and humanized PXR mice (where mouse PXR has been genetically replaced with human PXR) (Ma et al., 2007; Scheer et al., 2008, 2010; Xie et al., 2000). While PCN did not induce Cyp3a11 in PXR knockout mice or in humanized PXR mice, rifampicin induced Cyp3a11 in humanized PXR mice but not in wild-type mice (Ma et al., 2007; Xie et al., 2000). The similarities and differences between mouse and human PXR have recently been investigated employing an improved humanized PXR line, in which mouse PXR has been replaced with human PXR (Scheer et al., 2010). Treatment with rifampicin produced a greater induction of Cyp3a11 in humanized PXR mice compared to wild-type mice and also induced Cyp2b10 in humanized PXR mice. In contrast, dexamethasone produced a greater induction of Cyp3a11 in wild-type than in humanized PXR mice, with no induction being observed in PXR knockout mice. The induction of Cyp2b10 by dexamethasone was comparable in wild-type mice, PXR knockout mice and humanized PXR mice, thus suggesting that Cyp2b10 induction is independent of PXR for this compound.
Overall, the panel could not identify a suitable non-genotoxic PXR activator for which carcinogenicity data were available and hence a MOA was not developed for liver tumor formation by PXR activators.
Discussion
A robust MOA was developed for PB-induced rodent liver tumor formation. Figure 1 illustrates the key and associative events for this MOA. The key and associative events for this MOA identified by the CAR/PXR panel are consistent with those previously proposed by Holsapple et al. (2006).
As would be expected for a non-genotoxic rodent liver carcinogen, the hepatic effects of PB are dose-dependent and exhibit a threshold (Table 3). Thus at low doses PB produces little or no effect on the key and associative events, with higher doses producing effects on the key and associative events through activation of CAR, with even higher doses being required to produce liver tumors.
The human relevance of the proposed MOA for PB-induced rodent liver tumor formation was assessed by considering data on the effects of PB in various species, including humans. PB has been shown to activate CAR in a number of species including mice, rats, Syrian hamsters, primates and humans (Table 4). Activation of CAR leads to altered gene expression including the induction of CYP forms. A number of important species differences were identified between the hepatic effects of CAR activation induced by PB in the rat and mouse and other species including humans (Table 4). For example, a number of in vivo and in vitro studies have identified that while CAR activation by PB stimulates cell proliferation, decreases apoptosis and inhibits gap junctional intercellular communication in mouse and rat hepatocytes, these effects are not observed in other species, including the guinea pig, Syrian hamster, primates, or humans. While PB can produce liver tumors in the mouse and rat and act as a tumor promoter, PB does not induce liver tumors in the Syrian hamster after long term exposure or when used as a tumor promoter after initiation with a mutagenic rodent liver carcinogen.
PB has been used as a sedative, hypnotic and antiepileptic drug in humans for many decades, with therapeutic doses for anticonvulsant therapy being in the range 3–6 mg/kg/day (IARC, 2001; Munro, 1993; Whysner et al., 1996). PB has been shown to induce a number of human hepatic CYP forms including CYP2A6, CYP2B6, CYP2C9, CYP2C19 and CYP3A4 (IARC, 2001; Martignoni et al., 2006; Pelkonen et al., 2008). In addition, prolonged treatment with PB and other anticonvulsant drugs has been shown to increase liver size in humans, which is due to hepatocyte hypertrophy and proliferation of the smooth endoplasmic reticulum (Aiges et al., 1980; Pirttiaho et al., 1978, 1982). However, while the hypertrophic effects of PB can be observed in human liver as well as in the livers of mice and rats, the hyperplastic effects of PB appear to be present only in mice and rats. Although PB can stimulate replicative DNA synthesis in the mouse and rat both after in vivo administration and in vitro in cultured hepatocytes, studies with cultured human hepatocytes have demonstrated that PB and related compounds do not stimulate replicative DNA synthesis.
The available data on species differences suggest significant cellular dynamic differences such that the MOA for PB-induced rodent liver tumor formation would not occur in humans. Although the key events of CAR activation, cell proliferation, production of altered foci and production of tumors occur in rodents, and CAR activation can occur in humans, there is no evidence that subsequent effects on other key events would occur in humans. PB has been shown not to increase cell proliferation in cultured human hepatocytes and the development of altered hepatic foci has not been reported in the literature. This conclusion is consistent with the data from a number of epidemiological studies in patients after extended treatment with PB that report no evidence of increased liver tumor risk (Friedman et al., 2009; IARC, 2001; La Vecchia & Negri, 2013; Olsen et al., 1989, 1995; Selby et al., 1989; Whysner et al., 1996).
The panel determined there were no specific data gaps. However, additional data that would strengthen the proposed rodent MOA and the evaluation of human relevance could be performed. With respect to human hepatocytes, a number of laboratories possess unpublished data on the effects of PB and related compounds on replicative DNA synthesis in mouse and/or rat hepatocytes compared to effects in human hepatocytes. It would be most valuable to compile such data from these various sources for subsequent publication in order to enhance the current data sets showing that while PB and related compounds can stimulate replicative DNA synthesis in cultured mouse and rat hepatocytes, no mitogenic effects are observed in human hepatocyte preparations. Additional studies with humanized mice to investigate differences in the molecular pathways stimulated by mouse CAR and PXR compared to human CAR and PXR could also be performed and would likely strengthen the conclusions of the panel. Furthermore, the conflicting data on the effect of PB on cell proliferation between the hCAR/hPXR mouse model of Ross et al. (2010) and the hCAR model used by Huang et al. (2005) needs to be explained.
The nuclear receptors CAR, PXR and PPARα all heterodimerize with RXR for activation followed by binding to response elements in DNA. While there is much information on liver tumor formation in rodents by CAR activators such as PB and for PPARα activators (peroxisome proliferators), less is known about PXR activators. An examination of the literature demonstrates that CAR and PPARα activators have many common key/associative events in their MOAs for rodent liver tumor formation (Holsapple et al., 2006; Klaunig et al., 2003; Lake, 2009). Moreover, similar species differences in effects on cell proliferation and other endpoints have been observed for CAR and PPARα activators. Hence, while a MOA was not developed for rodent liver tumor formation by PXR activators, it is likely that the MOA for non-genotoxic activators of PXR would be similar to the MOAs established for CAR and PPARα activators.
In conclusion, from an evaluation of literature data a robust MOA based on CAR activation for PB-induced rodent liver tumor formation has been developed. The data on species differences was considered by the majority of the panel to be sufficient to determine that this MOA would be qualitatively not plausible for humans. Thus compounds that cause rat or mouse liver tumors through this CAR-mediated MOA, similar to PB, would not be expected to increase the risk of liver tumor development in humans.
Acknowledgements
The authors would like to thank the Nuclear Receptor Workshop Steering Committee members (Dr Melvin Andersen, Dr Richard Becker, Dr Michael Cunningham, Dr Vicki Dellarco, Dr Michael Dourson, Dr Michael Honeycutt and Dr Julian Preston) for their input in the workshop development, along with the Case Study Leaders (Dr Chris Corton, Dr Cliff Elcombe, Dr James Klaunig, Dr Richard Peffer and Dr Douglas Wolf). The authors would also like to thank Ms. Alison Willis and Dr Andrew Maier for their contribution and review of this manuscript and for their help in coordination and compiling drafts prior to submission.
Footnotes
Declaration of interest
This manuscript, including all analyses, interpretations and opinions expressed are exclusively those of the authors and are not necessarily those of their employers or respective institutions, as indicated on the cover page. This article reflects the views of the authors and does not necessarily represent views or polices of the U. S. EPA, the U.S. FDA, the European Chemicals Agency or any of the other authors’ affiliations, as indicated on the cover page. Funding and in-kind support for the workshop and this manuscript were provided by the Alliance for Risk Assessment (ARA), American Chemistry Council (ACC) Center for Advancing Risk Assessment, CropLife America, CXR Biosciences, The Dow Chemical Company, DuPont, The Hamner Institute for Health, Indiana University Department of Environmental Health, Society for Risk Analysis (SRA), Society of Toxicology (SOT), 3M Company, Toxicology Excellence for Risk Assessment (TERA), U.S. EPA National Health and Environmental Effects Research Laboratory (NHEERL), U.S. EPA Office of Chemical Safety and Pollution Prevention (OCSPP) and U.S. EPA Office of Water (OW). The National Institute of Environmental Health Sciences (NIEHS) for provided the workshop venue. Many of these organizations have active research and vested interests in the outcome of this workshop. Several of the manuscript authors are employed by the institutions that provided funding for the workshop. Many authors have worked in consulting for government and industry on receptor-specific related projects, have been or are funded through grants and by organizations mentioned earlier and have served as reviewers or on committees for related topics. Toxicology Excellence for Risk Assessment (TERA) was provided funding to organize and run the workshop. All case study panel members were offered funding for travel and lodging to and from the workshop, of which some accepted and received. Funding remaining after the workshop was utilized by TERA to begin coordination and drafting of workshop manuscripts. Honoraria were offered to all case study leaders and manuscript lead authors for their time drafting the manuscript, of which some accepted and received. This manuscript has been reviewed by multiple institutions; however, none of these internal reviews made substantive changes to the scientific conclusions or technical approaches and outcomes and these were made as a matter of protocol.
References
- Aiges HW, Daum F, Olson M, et al. The effects of phenobarbital and diphenylhydantoin on liver function and morphology. J Pediatr. 1980;97:22–26. doi: 10.1016/s0022-3476(80)80123-5. [DOI] [PubMed] [Google Scholar]
- Andersen ME, et al. Dose-response approaches for nuclear receptor-mediated modes of action for liver carcinogenicity: results of a workshop. Crit Rev Toxicol. 2013 doi: 10.3109/10408444.2013.835785. In press. [DOI] [PubMed] [Google Scholar]
- Bachman AN, Kamendulis LM, Goodman JI. Diethanolamine and Phenobarbital produce an altered pattern of methylation in GC-rich regions of DNA in B6C3F1 mouse hepatocytes similar to that resulting from choline deficiency. Toxicol Sci. 2006;90:317–325. doi: 10.1093/toxsci/kfj091. [DOI] [PubMed] [Google Scholar]
- Baker TK, Bachowski S, Stevenson DE, et al. Modulation of gap junctional intercellular communication in rodent, monkey and human hepatocyte by nongenotoxic compounds. Prog Clin Biol Res. 1995;391:71–780. [PubMed] [Google Scholar]
- Barrass N, Stewart M, Warburton S, et al. Cell proliferartion in the liver and thyroid of C57B1/10J mice after dietary administration of chlordane. Environ Health Perspect. 1993;101:219–223. doi: 10.1289/ehp.93101s5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker FF. Morphological classification of mouse liver tumors based on biological characteristics. Cancer Res. 1982;42:3918–3923. [PubMed] [Google Scholar]
- Boobis AR, Cohen SM, Dellarco V, et al. IPCS framework for analyzing the relevance of a cancer mode of action for humans. Crit Rev Toxicol. 2006;36:781–792. doi: 10.1080/10408440600977677. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Cohen SM, Doerrer NG, et al. A data-based assessment of alternative strategies for identification of potential human cancer hazards. Toxicol Pathol. 2009;37:714–732. doi: 10.1177/0192623309343779. [DOI] [PubMed] [Google Scholar]
- Bullock P, Pearce R, Draper A, et al. Induction of liver microsomal cytochrome P450 in Cynomolgus monkeys. Drug Metab Dispos. 1995;23:736–748. [PubMed] [Google Scholar]
- Bursch W, Chabicovsky M, Wastl U, et al. Apoptosis in early stages of mouse hepatocarcinogenesis: failure to counterbalance cell proliferation and to account for strain differences in tumor susceptibility. Toxicol Sci. 2005a;85:515–529. doi: 10.1093/toxsci/kfi129. [DOI] [PubMed] [Google Scholar]
- Bursch W, Wastl U, Hufnagl K, Schulte-Hermann R. No increase in apoptosis in regressing mouse liver after withdrawal of growth stimuli or food restriction. Toxicol Sci. 2005b;85:507–514. doi: 10.1093/toxsci/kfi128. [DOI] [PubMed] [Google Scholar]
- Butler WH. Long-term effects of phenobarbitone-Na on male Fischer rats. Brit J Cancer. 1978;37:418–423. doi: 10.1038/bjc.1978.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew P, Edwards RE, Nolan BM. The quantitative distinction of hyperplasia from hypertrophy in hepatomegaly in the rat liver by phenobarbital. Toxicol Sci. 1998;44:46–51. doi: 10.1006/toxs.1998.2473. [DOI] [PubMed] [Google Scholar]
- Cerminara C, Bagnolo V, De Leonardis F, et al. Hepatocellular adenoma associated with long-term exposure to phenobarbital: a paediatric case report. Childs Nerv Syst. 2012;28:939–941. doi: 10.1007/s00381-011-1636-1. [DOI] [PubMed] [Google Scholar]
- Chiang JY, Steggles AW. Identification and partial purification of hamster microsomal cytochrome P-450 isoenzymes. Biochem Pharmacol. 1983;32:1389–1397. doi: 10.1016/0006-2952(83)90452-5. [DOI] [PubMed] [Google Scholar]
- Chipman JK, Mally A, Edwards GO. Disruption of gap junctions in toxicity and carcinogenicity. Toxicol Sci. 2003;71:146–153. doi: 10.1093/toxsci/71.2.146. [DOI] [PubMed] [Google Scholar]
- Christensen JG, Gonzales AJ, Cattley RC, Goldsworthy TL. Regulation of apoptosis in mouse hepatocytes and alteration of apoptosis by nongenotoxic carcinogens. Cell Growth Differ. 1998;9:815–825. [PubMed] [Google Scholar]
- Cohen SM, Meek ME, Klaunig JE, et al. The human relevance of information on carcinogenic modes of action: overview. Crit Rev Toxicol. 2003;33:581–589. doi: 10.1080/713608371. [DOI] [PubMed] [Google Scholar]
- Crampton RF, Gray TJB, Grasso P, Parke DV. Long-term studies on chemically induced liver enlargement in the rat. I. Sustained induction of microsomal enzymes with absence of liver damage on feeding phenobarbitone or butylated hydroxytoluene. Toxicology. 1977;7:289–306. doi: 10.1016/0300-483x(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Dickins M. Induction of cytochromes P450. Curr Topics Med Chem. 2004;4:1745–1766. doi: 10.2174/1568026043387115. [DOI] [PubMed] [Google Scholar]
- Di Mizio G, Gambardella A, Labate A, et al. Hepatonecrosis and cholangitis related to long-term phenobarbital therapy: an autopsy report of two patients. Seizure. 2007;16:653–656. doi: 10.1016/j.seizure.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Diwan BA, Ward JM, Anderson LM, et al. Lack of effect of phenobarbital on hepatocellular carcinogenesis initiated by N-nitrosodiethylamine or methylazoxymethanol acetate in male Syrian golden hamsters. Toxicol Appl Pharmacol. 1986;86:298–307. doi: 10.1016/0041-008x(86)90060-8. [DOI] [PubMed] [Google Scholar]
- Dostalek M, Brooks JD, Hardy KD, et al. In vivo oxidative damage in rats is associated with barbiturate response but not other cytochrome P450 inducers. Mol Pharmacol. 2007;72:1419–1424. doi: 10.1124/mol.107.040238. [DOI] [PubMed] [Google Scholar]
- Evans JG, Collins MA, Lake BG, Butler WH. The histology and development of hepatic nodules and carcinoma in C3H/He and C57BL/6 mice following chronic phenobarbitone administration. Toxicol Pathol. 1992;20:585–594. doi: 10.1177/019262339202000405. [DOI] [PubMed] [Google Scholar]
- Evans JG, Collins MA, Savage SA, et al. The histology and development of hepatic nodules in C3H/He mice following chronic administration of phenobarbitone. Carcinogenesis. 1986;7:627–631. doi: 10.1093/carcin/7.4.627. [DOI] [PubMed] [Google Scholar]
- Foster JR. Cell death and cell proliferation in the control of normal and neoplastic tissue growth. Toxicol Pathol. 2000;28:441–446. doi: 10.1177/019262330002800314. [DOI] [PubMed] [Google Scholar]
- Foster GR, Goldin RD, Freeth CJ, et al. Liver damage in long-term anticonvulsant therapy: a serological and histological study. Quart J Med. 1991;79:315–322. [PubMed] [Google Scholar]
- Friedman GD, Jiang S-F, Udaltsova N, et al. Epidemiological evaluation of pharmaceuticals with limited evidence of carcinogenicity. Int J Cancer. 2009;125:2173–2178. doi: 10.1002/ijc.24545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold LS, Manley NB, Slone TH, Ward JM. Compendium of chemical carcinogens by target organ: results of chronic bioassays in rats, mice, hamsters, dogs, and monkeys. Toxicol Pathol. 2001;29:639–652. doi: 10.1080/019262301753385979. [DOI] [PubMed] [Google Scholar]
- Goldsworthy TL, Fransson-Steen R. Quantitation of the cancer process in C57BL/6J, B6C3F1 and C3H/HeJ mice. Toxicol Pathol. 2002;20:97–105. doi: 10.1080/01926230252824770. [DOI] [PubMed] [Google Scholar]
- Gollapudi B, Kan H, Wood A, et al. Dose response characterization of dietary phenobarbital-induced gene expression, enzyme induction, and cell proliferation in the livers of male and female mice. Toxicol Sci. 2011;120:565–566. [Google Scholar]
- Goodman JI, Watson RE. Altered DNA methylation: a secondary mechanism involved in carcinogenesis. Annu Rev Pharmacol Toxicol. 2002;42:501–525. doi: 10.1146/annurev.pharmtox.42.092001.141143. [DOI] [PubMed] [Google Scholar]
- Grasso P, Hinton RH. Evidence for and possible mechanisms of non-genotoxic carcinogenesis in rodent liver. Mutat Res. 1991;248:271–290. doi: 10.1016/0027-5107(91)90062-s. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Miyata E, Tamano S, et al. Non-carcinogenicity, but dose-related increase in preneoplastic hepatocellular lesions, in a two-year feeding study of phenobarbital sodium in male F344 rats. Food Chem Toxicol. 1999;37:869–879. doi: 10.1016/s0278-6915(99)00072-1. [DOI] [PubMed] [Google Scholar]
- Hamadeh HK, Bushel PR, Jayadev S, et al. Gene expression analysis reveals chemical-specific profiles. Toxicol Sci. 2002;67:219–231. doi: 10.1093/toxsci/67.2.219. [DOI] [PubMed] [Google Scholar]
- Hasmall SC, Roberts RA. The perturbation of apoptosis and mitosis by drugs and xenobiotics. Pharmacol Ther. 1999;82:63–70. doi: 10.1016/s0163-7258(98)00058-8. [DOI] [PubMed] [Google Scholar]
- Hill AB. The environment and disease: association or causation? President’s Address. Proc Royal Soc Med. 1965;9:295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose Y, Nagahori N, Yamada T, et al. Comparison of the effects of the synthetic pyrethroid Metofluthrin and phenobarbital on CYP2B form induction and replicative DNA synthesis in cultured rat and human hepatocytes. Toxicology. 2009;258:64–69. doi: 10.1016/j.tox.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Holsapple MP, Pitot HC, Cohen SH, et al. Mode of action in relevance of rodent liver tumors to human cancer risk. Toxicol Sci. 2006;89:51–56. doi: 10.1093/toxsci/kfj001. [DOI] [PubMed] [Google Scholar]
- Hosseinpour F, Moore R, Negishi M, Sueyoshi T. Serine 202 regulates the nuclear translocation of constitutive active/androstane receptor. Mol Pharmacol. 2006;69:1095–1102. doi: 10.1124/mol.105.019505. [DOI] [PubMed] [Google Scholar]
- Huang W, Zhang J, Wahington M, et al. Xenobiotic stress induces hepatomegaly and liver tumors via the nuclear receptor constitutive androstane receptor. Mol Endocrinol. 2005;19:1646–1653. doi: 10.1210/me.2004-0520. [DOI] [PubMed] [Google Scholar]
- Huff J, Cirvello J, Haseman J, Bucher J. Chemicals associated with site-specific neoplasia in 1394 long-term carcinogenesis experiments in laboratory rodents. Environ Health Perspect. 1991;93:247–270. doi: 10.1289/ehp.9193247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer) IARC monographs on the evaluation of carcinogenic risks to humans. Vol. 79. Lyon: Some Thyrotropic Agents, IARC Press; 2001. [Google Scholar]
- Imaoka S, Osada M, Minamiyama Y, et al. Role of phenobarbital-inducible cytochrome P450s as a source of active oxygen species in DNA-oxidation. Cancer Lett. 2004;203:117–125. doi: 10.1016/j.canlet.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Isenberg JS, Kamendulis LM, Ackley DC, et al. Reversibility and persistence of di-2-ethylhexyl phthalate (DEHP)- and phenobarbital-induced hepatocellular changes in rodents. Toxicol Sci. 2001;64:192–199. doi: 10.1093/toxsci/64.2.192. [DOI] [PubMed] [Google Scholar]
- James NH, Roberts RA. Species differences in response to peroxisome proliferators correlate in vitro with induction of DNA synthesis rather than suppression of apoptosis. Carcinogenesis. 1996;17:1623–1632. doi: 10.1093/carcin/17.8.1623. [DOI] [PubMed] [Google Scholar]
- Jones CR, Lubet RA. Induction of a pleiotropic response by phenobarbital and related compounds. Response of various inbred strains of rats, response in various species and the induction of aldehyde dehydrogenase in Copenhagen rats. Biochem Pharmacol. 1992;44:1651–1660. doi: 10.1016/0006-2952(92)90483-y. [DOI] [PubMed] [Google Scholar]
- Jones HB, Orton TC, Lake BG. Effect of chronic phenobarbitone administration on liver tumour formation in the C57BL/10J mouse. Food Chem Toxicol. 2009;47:1333–1340. doi: 10.1016/j.fct.2009.03.014. [DOI] [PubMed] [Google Scholar]
- Kasper P. Cyproterone acetate: a genotoxic carcinogen? Pharmacol Toxicol. 2001;88:223–231. [PubMed] [Google Scholar]
- Kitano M, Ichihara T, Matsuda T, et al. Presence of a threshold for promoting effects of phenobarbital on diethylnitrosamine-induced hepatic foci in the rat. Carcinogenesis. 1998;19:1475–1480. doi: 10.1093/carcin/19.8.1475. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Babich MA, Baetcke KP, et al. PPAR-alpha agonist-induced rodent tumors: modes of action and human relevance. Crit Rev Toxicol. 2003;33:655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. doi: 10.1177/0192623309356453. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Ruch RJ. Strain and species effects on the inhibition of hepatocyte intercellular communication by liver tumor promoters. Cancer Lett. 1987;36:161–168. doi: 10.1016/0304-3835(87)90087-5. [DOI] [PubMed] [Google Scholar]
- Klaunig JE, Ruch RJ, Weghorst CM. Comparative effects of phenobarbital, DDT, and lindane on mouse hepatocyte gap junctional intercellular communication. Toxicol Appl Pharmacol. 1990;102:553–563. doi: 10.1016/0041-008x(90)90050-5. [DOI] [PubMed] [Google Scholar]
- Kolaja KL, Stevenson DE, Johnson JT, et al. Subchronic effects of dieldrin and phenobarbital on hepatic DNA synthesis in mice and rats. Fundam Appl Toxicol. 1996a;29:219–228. doi: 10.1006/faat.1996.0025. [DOI] [PubMed] [Google Scholar]
- Kolaja KL, Stevenson DE, Johnson JT, et al. Dose dependence of phenobarbital promotion of preneoplastic hepatic lesions in F344 rats and B6C3F1 mice: effects on DNA synthesis and apoptosis. Carcinogenesis. 1996b;17:947–954. doi: 10.1093/carcin/17.5.947. [DOI] [PubMed] [Google Scholar]
- Kolaja KL, Stevenson DE, Walborg EF, Jr, Klaunig JE. Reversibility of promoter induced hepatic focal lesion growth in mice. Carcinogenesis. 1996c;17:1403–1409. doi: 10.1093/carcin/17.7.1403. [DOI] [PubMed] [Google Scholar]
- Kostka G, Urbanek K, Ludwicki JK. The effect of phenobarbital on the methylation level of the p16 promoter region in rat liver. Toxicology. 2007;239:127–135. doi: 10.1016/j.tox.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Lake BG. Species differences in the hepatic effects of inducers of CYP2B and CYP4A subfamily forms: relationship to rodent liver tumour formation. Xenobiotica. 2009;39:582–596. doi: 10.1080/00498250903098184. [DOI] [PubMed] [Google Scholar]
- Lake BG, Longland RC, Harris RA, et al. The effect of prolonged sodium phenobarbitone treatment on hepatic xenobiotic metabolism and the urinary excretion of metabolites of the D-glucuronic acid pathway in the rat. Biochem Pharmacol. 1978;27:2357–2361. doi: 10.1016/0006-2952(78)90144-2. [DOI] [PubMed] [Google Scholar]
- Lake BG, Renwick AB, Cunningham ME, et al. Comparison of the effects of some CYP3A and other enzyme inducers on replicative DNA synthesis and some cytochrome P450 isoforms in rat liver. Toxicology. 1998;131:9–20. doi: 10.1016/s0300-483x(98)00085-7. [DOI] [PubMed] [Google Scholar]
- Lamminpää A, Pukkala E, Teppo L, Neuvonen PJ. Cancer incidence among patients using antiepileptic drugs: a long-term follow-up of 28,000 patients. Eur J Clin Pharmacol. 2002;58:137–141. doi: 10.1007/s00228-002-0429-6. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Negri E. A review of epidemiological data on epilepsy, phenobarbital, and risk of liver cancer. Eur J Cancer Prevent. 2013 doi: 10.1097/CEJ.0b013e32836014c8. in press. [DOI] [PubMed] [Google Scholar]
- Lehman-McKeeman LD, Caudill D, Vassallo JD, Fix AS. Increased spontaneous liver tumor susceptibility in cytochrome P450 2B1 (CYP2B1) transgenic mice. Toxicol Sci. 1999;48:253. [Google Scholar]
- Ma X, Shah Y, Cheung C, et al. The pregnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007;35:194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- Maglich JM, Stoltz CM, Goodwin BM, et al. Nuclear pregnane X receptor and consititutive androstane receptor regulate overlapping but distinct sets of genes involved in xenobiotic metabolism. Mol Pharmacol. 2002;62:638–646. doi: 10.1124/mol.62.3.638. [DOI] [PubMed] [Google Scholar]
- Martignoni M, Groothuis GMM, de Kanter R. Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin Drug Metab Toxicol. 2006;2:875–894. doi: 10.1517/17425255.2.6.875. [DOI] [PubMed] [Google Scholar]
- Meek ME, Bucher JR, Cohen SM, et al. A framework for human relevance analysis of information on carcinogenic modes of action. Crit Rev Toxicol. 2003;33:591–653. doi: 10.1080/713608373. [DOI] [PubMed] [Google Scholar]
- Moennikes O, Buchmann A, Romualdi A, et al. Lack of phenobarbital-mediated promotion of hepatocarcinogenensis in Connexin32-null mice. Cancer Res. 2000;60:5087–5091. [PubMed] [Google Scholar]
- Moore JT, Moore LB, Maglich JM, Kliewer SA. Functional and structural comparison of PXR and CAR. Biochim Biophys Acta. 2003;1619:235–238. doi: 10.1016/s0304-4165(02)00481-6. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, et al. Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem. 2000;275:15122–15127. doi: 10.1074/jbc.M001215200. [DOI] [PubMed] [Google Scholar]
- Munro A. The paradoxical lack of interspecies correlation between plasma concentrations and chemical carcinogenicity. Regul Toxicol Pharmacol. 1993;18:115–135. doi: 10.1006/rtph.1993.1048. [DOI] [PubMed] [Google Scholar]
- Mutoh S, Osabe M, Inoue K, et al. Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR113) J Biol Chem. 2009;284:34785–34792. doi: 10.1074/jbc.M109.048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S, Sobhany M, Moore R, et al. Phenobarbital indirectly activates the constitutive androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal. 2013;6:ra31. doi: 10.1126/scisignal.2003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VJ, Senior JR. Drug-related hepatotoxicity. New Eng J Med. 2006;354:731–739. doi: 10.1056/NEJMra052270. [DOI] [PubMed] [Google Scholar]
- Neveu MJ, Babcock KL, Hertzberg EL, et al. Colocalized alterations in connexin32 and cytochrome P450IIB1/2 by phenobarbital and related liver tumor promoters. Cancer Res. 1994;54:3145–3152. [PubMed] [Google Scholar]
- Nims RW, Lubet RA. The CYP2B subfamily. In: Ioannides C, editor. Cytochromes P450: metabolic and toxicological aspects. Boca Raton: CRC Press; 1996. pp. 135–160. [Google Scholar]
- Olsavsky KM, Page JL, Johnson MC, et al. Gene expression profiling and differentiation assessment in primary human hepatocyte cultures, established hepatoma cell lines, and human liver tissues. Toxicol Appl Pharmacol. 2007;222:42–56. doi: 10.1016/j.taap.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen JH, Boice JD, Jr, Jensen JPA, Fraumeni JF., Jr Cancer among epileptic patients exposed to anticonvulsant drugs. J Natl Cancer Inst. 1989;81:803–808. doi: 10.1093/jnci/81.10.803. [DOI] [PubMed] [Google Scholar]
- Olsen JH, Schulgen G, Boice JD, Jr, et al. Antiepileptic treatment and risk for hepatobiliary cancer and malignant lymphoma. Cancer Res. 1995;55:294–297. [PubMed] [Google Scholar]
- Omiecinski CJ, Vanden Heuvel JP, Perdew GH, Peters JM. Xenobiotic metabolism, disposition, and regulation by receptors: from biochemical phenomenon to predictors of major toxicities. Toxicol Sci. 2011a;120:9–75. doi: 10.1093/toxsci/kfq338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omiecinski CJ, Coslo DM, Chen T, et al. Multi-species analyses of direct activators of the constitutive androstane receptor. Toxicol Sci. 2011b;123:550–562. doi: 10.1093/toxsci/kfr191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orton TC, Doughty SE, Kalinowski AE, et al. Expression of growth factors and growth factor receptors in the liver of C57BL/10J mice following administration of phenobarbitone. Carcinogenesis. 1996;17:973–981. doi: 10.1093/carcin/17.5.973. [DOI] [PubMed] [Google Scholar]
- Osimitz TG, Lake BG. Mode-of-action analysis for induction of rat liver tumors by pyrethrins: relevance to human cancer risk. Crit Rev Toxicol. 2009;39:501–511. doi: 10.1080/10408440902914014. [DOI] [PubMed] [Google Scholar]
- Parzefall W, Erber E, Sedivy R, Schulte-Hermann R. Testing for induction of DNA synthesis in human hepatocyte primary cultures by rat liver tumor promoters. Cancer Res. 1991;51:1143–1147. [PubMed] [Google Scholar]
- Pelkonen O, Turpeinen M, Hakkola J, et al. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol. 2008;82:667–715. doi: 10.1007/s00204-008-0332-8. [DOI] [PubMed] [Google Scholar]
- Peraino C, Fry RJM, Staffeldt E. Enhancement of spontaneous hepatic tumorigenesis in C3H mice by dietary phenobarbital. J Natl Cancer Inst. 1973;51:1349–1350. doi: 10.1093/jnci/51.4.1349. [DOI] [PubMed] [Google Scholar]
- Phillips JC, Price RJ, Cunninghame ME, et al. Effect of piperonyl butoxide on cell replication and xenobiotic metabolism in the livers of CD-1 mice and F344 rats. Fundam Appl Toxicol. 1997;38:64–74. doi: 10.1006/faat.1997.2326. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Goodman JI. Identification of genes that may play critical roles in phenobarbital (PB)-induced liver tumorigenesis due to altered DNA methylation. Toxicol Sci. 2008;104:86–99. doi: 10.1093/toxsci/kfn063. [DOI] [PubMed] [Google Scholar]
- Phillips JM, Yamamoto Y, Negishi M, et al. Orphan nuclear receptor constitutive active/androstane receptor-mediated alterations in DNA methylation during phenobarbital promotion of liver tumorigenesis. Toxicol Sci. 2007;96:72–82. doi: 10.1093/toxsci/kfl188. [DOI] [PubMed] [Google Scholar]
- Pirttiaho HI, Sotaniemi EA, Ahokas JT, Pitkänen U. Liver size and indices of drug metabolism in epileptics. Brit J Clin Pharmacol. 1978;6:273–278. doi: 10.1111/j.1365-2125.1978.tb04597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirttiaho HI, Sotaniemi EA, Pelkonen RO, Pitkänen U. Hepatic blood flow and drug metabolism in patients on enzyme-inducing anticonvulsants. Eur J Clin Pharmacol. 1982;22:441–445. doi: 10.1007/BF00542550. [DOI] [PubMed] [Google Scholar]
- Plant NJ, Horley NJ, Dickins M, et al. The coordinate regulation of DNA synthesis and suppression of apoptosis is differentially regulated by the liver growth agents, phenobarbital and methylclofenapate. Carcinogenesis. 1998;22:441–445. doi: 10.1093/carcin/19.9.1521. [DOI] [PubMed] [Google Scholar]
- Ponomarkov V, Tomatis L, Turusov V. The effect of long-term administration of phenobarbitone in CF-1 mice. Cancer Lett. 1976;1:165–172. [Google Scholar]
- Ross J, Plummer SM, Scheer N, et al. Human constitutive androstane receptor (CAR) and pregnane X receptor (PXR) support the hypertrophic but not the hyperplastic response to the murine nongenotoxic hepatocarcinogens phenobarbital and chlordane in vivo. Toxicol Sci. 2010;116:452–466. doi: 10.1093/toxsci/kfq118. [DOI] [PubMed] [Google Scholar]
- Ross PK, Woods CG, Bradford BU, et al. Time-course comparison of xenobiotic activators of CAR and PPARα in mouse liver. Toxicol Appl Pharmacol. 2009;235:199–207. doi: 10.1016/j.taap.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi L, Ravera M, Repetti G, Santi L. Long-term administration of DDT or phenobarbital-Na in Wistar rats. Int J Cancer. 1977;19:179–185. doi: 10.1002/ijc.2910190207. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Klaunig JE. Kinetics of phenobarbital inhibition of intercellular communication in mouse hepatocytes. Cancer Res. 1988;48:2519–2523. [PubMed] [Google Scholar]
- Scheer N, Ross J, Kapelyukh Y, et al. In vivo responses of the human and murine pregnane X receptor to dexamethasone in mice. Drug Metab Dispos. 2010;38:1046–1053. doi: 10.1124/dmd.109.031872. [DOI] [PubMed] [Google Scholar]
- Scheer N, Ross J, Rode A, et al. A novel panel of mouse models to evaluate the role of human pregnane X receptor and constitutive androstane receptor in drug response. J Clin Invest. 2008;118:3228–3239. doi: 10.1172/JCI35483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Hermann R, Timmermann-Trosiener I, Schuppler J. Promotion of spontaneous preneoplastic cells in rat liver as a possible explanation of tumor production by nonmutagenic compounds. Cancer Res. 1983;43:839–844. [PubMed] [Google Scholar]
- Selby JV, Friedman GD, Fireman BH. Screening prescription drugs for possible carcinogenicity: eleven to fifteen years of follow-up. Cancer Res. 1989;49:5736–5747. [PubMed] [Google Scholar]
- Sonich-Mullin C, Fielder R, Wiltse J, et al. IPCS conceptual framework for evaluating a mode of action for chemical carcinogenesis. Regul Toxicol Pharmacol. 2001;34:146–152. doi: 10.1006/rtph.2001.1493. [DOI] [PubMed] [Google Scholar]
- Stanley L, Horsburgh B, Ross J, et al. PXR and CAR: nuclear receptors which play a pivotal role in drug disposition and chemical toxicity. Drug Metabolism Reviews. 2006;38:515–597. doi: 10.1080/03602530600786232. [DOI] [PubMed] [Google Scholar]
- Staudinger J, Lui Y, Madan A, et al. Coordinate regulation of xenobiotic and bile acid homeostasis by pregnane X receptor. Drug Metab Dispos. 2001;29:1467–1472. [PubMed] [Google Scholar]
- Staudinger JL, Madan A, Carol KM, Parkinson A. Regulation of drug transporter gene expression by nuclear receptors. Drug Metab Dispos. 2003;31:523–527. doi: 10.1124/dmd.31.5.523. [DOI] [PubMed] [Google Scholar]
- Stenbäck F, Mori H, Furuya K, Williams GM. Pathogenesis of dimethylnitrosamine-induced hepatocellular cancer in hamster liver and lack of enhancement by phenobarbital. J Natl Cancer Inst. 1986;76:327–333. [PubMed] [Google Scholar]
- Tanaka T, Mori H, Williams GM. Enhancement of dimethylnitrosamine-initiated hepatocarcinogenesis in hamsters by subsequent administration of carbon tetrachloride but not by phenobarbital or p,p′-dichlorodiphenyltrichoroethane. Carcinogenesis. 1987;8:1171–1178. doi: 10.1093/carcin/8.9.1171. [DOI] [PubMed] [Google Scholar]
- Thorpe E, Walker AIT. The toxicology of dieldrin (HEOD). II. Comparative long-term oral toxicity studies in mice with dieldrin, DDT, phenobarbitone, β-BHC and γ-BHC. Food Chem Toxicol. 1973;11:433–432. doi: 10.1016/0015-6264(73)90008-4. [DOI] [PubMed] [Google Scholar]
- Tien ES, Negishi M. Nuclear receptors CAR and PXR in the regulation of hepatic metabolism. Xenobiotica. 2006;36:1152–1163. doi: 10.1080/00498250600861827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier E, Belzil A, Stoltz C, Anderson A. Localization of a phenobarbital-responsive element (PBRE) in the 5′-flanking region of the rat CYP2B2 gene. Gene. 1995;158 doi: 10.1016/0378-1119(94)00916-g. 263-2688. [DOI] [PubMed] [Google Scholar]
- Tucker MJ, Kalinowski AE, Orton TC. Carcinogenicity of cyproterone acetate in the mouse. Carcinogenesis. 1996;17:1473–1476. doi: 10.1093/carcin/17.7.1473. [DOI] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, et al. Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol. 2002;61:1–6. doi: 10.1124/mol.61.1.1. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (United States Environmental Protection Agency) Guidelines for carcinogen risk assessment and supplemental guidance for assessing susceptibility from early-life exposure to carcinogens. Fed Reg. 2005;70:17765–17817. [Google Scholar]
- Ward JM. Increased susceptibility of livers of aged F344/NCr rats to the effects of phenobarbital on the incidence, morphology, and histochemistry of hepatocellular foci and neoplasms. J Natl Cancer Inst. 1983;71:815–823. [PubMed] [Google Scholar]
- Ward JM, Lynch P, Riggs C. Rapid development of hepatocellular neoplasms in aging male C3H/HeNCr mice given phenobarbital. Cancer Lett. 1988;39:9–18. doi: 10.1016/0304-3835(88)90035-3. [DOI] [PubMed] [Google Scholar]
- Waterman CL, Currie RA, Cottrell LA, et al. An integrated functional genomic study of acute phenobarbital exposure in the rat. BMC Genomics. 2010;11:1–17. doi: 10.1186/1471-2164-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver RJ, Thompson S, Smith G, et al. A comparative study of constitutive and induced alkoxyresorufin O-dealkylation and individual cytochrome P450 forms in Cynomolgus monkey (Macaca fascicularis), human, mouse, rat and hamster liver microsomes. Biochem Pharmacol. 1994;47:763–773. doi: 10.1016/0006-2952(94)90475-8. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, et al. The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature. 2000;407:920–923. doi: 10.1038/35038112. [DOI] [PubMed] [Google Scholar]
- White SJ, McLean AEM, Howland C. Anticonvulsant drugs and cancer. A cohort study in patients with severe epilepsy. Lancet. 1979;ii:458–461. doi: 10.1016/s0140-6736(79)91505-8. [DOI] [PubMed] [Google Scholar]
- Whysner J, Montandon F, McClain RM, et al. Absence of DNA adduct formation by phenobarbitol, polychlorinated biphenols, and chlordane in mouse liver using the 32P-postlabelling assay. Toxicol Appl Pharmacol. 1998;148:14–23. doi: 10.1006/taap.1997.8311. [DOI] [PubMed] [Google Scholar]
- Whysner J, Ross PM, Williams GM. Phenobarbital mechanistic data and risk assessment: enzyme induction, enhanced cell proliferation, and tumor promotion. Pharmacol Ther. 1996;71:153–191. doi: 10.1016/0163-7258(96)00067-8. [DOI] [PubMed] [Google Scholar]
- Williams GM. Chemicals with carcinogenic activity in the rodent liver; mechanistic evaluation of human risk. Cancer Lett. 1997a;117:175–188. doi: 10.1016/s0304-3835(97)00229-2. [DOI] [PubMed] [Google Scholar]
- Williams GM. Carcinogenic responses to toxic liver injury in rodents. In: McCuskey RS, Earnest DL, editors. Comprehensive Toxicology, Volume 9, Hepatic and Gastrointestinal Toxicology. Oxford: Pergamon Press; 1997b. pp. 165–179. [Google Scholar]
- Wolf DC, Mann PC. Confounders in interpreting pathology for safety and risk assessment. Toxicol Appl Pharmacol. 2005;202:302–308. doi: 10.1016/j.taap.2004.06.022. [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Simon CM, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000;406:435–439. doi: 10.1038/35019116. [DOI] [PubMed] [Google Scholar]
- Yamada T, Uwagawa S, Okuno Y, et al. Case study: an evaluation of the human relevance of the synthetic pyrethroid metofluthrin-induced liver tumors in rats based on mode of action. Toxicol Sci. 2009;108:59–68. doi: 10.1093/toxsci/kfp007. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Goldsworthy TL, et al. The orphan nuclear receptor constitutive active/androstane receptor is essential for liver tumor promotion by phenobarbital in mice. Cancer Res. 2004;64:7197–7200. doi: 10.1158/0008-5472.CAN-04-1459. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Tien E, Negishi M, Honkakoski P. Receptor-mediated regulation of cytochromes P450. In: Ioannides C, editor. Cytochromes P450: role in the metabolism and toxicity of drugs and other xenobiotics. Cambridge: RSC Publishing; 2008. pp. 417–448. [Google Scholar]