Abstract
Traditionally, placebo has been associated with using an inert substance, in part so the subsequent response could be attributed to the target treatment, controlling for the confound of a “placebo effect.” 15,19,26 Placebo’s link with inert substances is so strong that “sham treatment” is a common synonym, and widespread placebo use is discouraged—even when there is supporting evidence for its effectiveness.15,19,26 Recent research has helped to redefine placebo, and this editorial will highlight key information supporting a contemporary view of placebo.
Description of neuropsychological and neurophysiologic mechanisms has confirmed the complex and dynamic nature of the placebo response. Treatment expectation and desire for pain relief, as well as classical conditioning, have been confirmed as important cognitive factors in a placebo response for analgesia.26 Studies have also confirmed involvement of the endogenous opioid system by demonstrating that the placebo response is naloxone reversible.2,3,20 That is, the reduction of pain from a placebo response can be reversed by using a pharmacological antagonist for opioid receptors. During placebo response, functional imaging studies have confirmed that the response is a measurable neurobiological event, as activity has been documented in cortical areas directly associated with pain inhibition and affective, cognitive, and evaluative centers.10,11,25 Furthermore, studies highlighting immediate spinal cord activity have challenged the notion that the placebo response is only defined by cortical activity.12,16,21
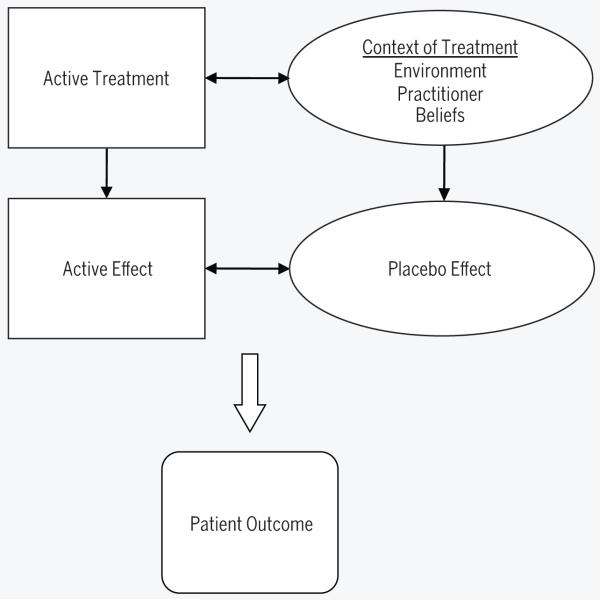
Focus on the inert substance is a dated conceptual model of placebo. Current placebo models capture the psychosocial context of treatment delivery, including the interaction between the patient, clinician, treatment, and environment.15,19,26 A recent study also demonstrated that the placebo response can be enhanced without direct experience through behavioral and social influences.7 Therefore, these factors may also be considered for their role in the placebo response.27 An important implication of this model is that the placebo effect is inherent in every clinician-patient encounter. Furthermore, the repeated contact with the context of treatment is likely to serve as a potent conditioning process in the establishment and maintenance of placebo. In routine clinical practice, placebo effects are always present and cannot be separated from active effects (FIGURE).
FIGURE.
Depiction of routine clinical practice: combination of active and placebo effects. Adapted from Colloca et al.8
A placebo response can be enhanced through conditioning or use of an instructional set. A very interesting example of an instructional set comes from a visceral pain model. Patients with irritable bowel syndrome receiving lidocaine or inert gel were compared to a natural history condition for pain experience during rectal distention.30,31 When patients received an instructional set indicating that they might “receive an active pain reducing medication or an inert placebo agent,” the pain relief they received during the inert gel was greater than natural history but less than when receiving lidocaine. When patients received an instructional set indicating that “the agent you have just been given is known to significantly reduce pain in some patients,” the pain relief they received during the inert gel was indistinguishable from lidocaine. These studies clearly show the an influence of suggestion on pain relief, providing further evidence of the dynamic nature of placebo and also potential models for use of instructional sets in studies of musculoskeletal pain.
A placebo response can also be diminished through conditioning or use of an instructional set. When this negative effect occurs, it is referred to as “nocebo.” An example of an instructional set creating a nocebo effect comes from a spinal manipulation study in healthy subjects.5 Typically, there is measurable pain inhibition immediately following spinal manipulation. However, when the spinal manipulation was paired with the instructional set (“The spinal manipulation you are about to receive is an ineffective form of manipulation used to treat low back pain and we expect it to temporarily worsen your perception of heat pain”), pain perception was not inhibited, and, in fact, greater pain perception was reported after the manipulation. This study and others reporting a nocebo effect9 demonstrate that the influence of instructional sets is bidirectional and specific to the nature of the instructional set. These data provide even more compelling evidence that placebo effects are not inert.
Separation of the placebo effect involves a specific study design. In randomized clinical trials with parallel arms, a control group (one not receiving treatment) is necessary to describe the placebo effect.14,26 Otherwise, the placebo effect could be confounded by natural history, symptom variation, regression to the mean, response bias, or receiving treatment.14,15,26 This stipulation effectively means that, if the goal is to distinguish between active treatment effects and placebo effects, the study should have a minimum of 3 treatment arms (active treatment, placebo group, and control group). In our experience, these designs are not commonly reported, so description of actual placebo effects is lacking in the rehabilitation literature.
It is more common for a randomized clinical trial to include 2 parallel arms comparing active treatment and placebo groups.14 This design allows for determination of whether the active treatment outperforms the placebo, without direct estimation of the size of the placebo effect. For example, in a recently published study in the JOSPT, Bialosky et al4 reported that an active neural dynamic technique inhibited temporal summation of thermal stimuli more than a sham neural dynamic technique. These data suggested better pain inhibition from the active technique, without indicating how much inhibition came from the general act of interacting with a skilled manual therapy practitioner. Depending on the particulars of the research question, this methodology may be entirely appropriate; however, it is stressed that the actual placebo effect cannot be determined from this more commonly implemented randomized trial design.14,26
Open-hidden treatment designs offer a potentially novel approach in determining the placebo effect.15,26 In this paradigm the treatment of interest is received under 2 conditions: once with knowledge of the treatment delivery (open condition) and once without knowledge of the treatment delivery (hidden condition). This design has definite application for pharmacological studies, but the approach may be limited for rehabilitation studies because of the difficulty in offering a hidden condition. However, there may be some potential for investigation of open-hidden designs for application of modalities.
Evidence for the placebo effect is strongest from mechanistic studies that are specifically designed to elicit these effects.15,26 Proponents of the mechanistic studies indicate a concern that randomized clinical trials may not mimic routine clinical practice and therefore may not include the same psychosocial context required to effectively elicit placebo effects. Despite these concerns, systematic reviews of the placebo effect show positive effects, but not for all outcome measures and all medical conditions.17,18 Strongest placebo effects were noted for continuous outcome measures in studies of pain, nausea, asthma, and phobia.18
The placebo effect is variable in both the number of responders and the size of the effect. The percentage of placebo responders was first estimated at 35%1; but, since then, various response ranges have been reported.19 Magnitude of response also differs, as the aforementioned systematic reviews reported both positive and negative placebo effects,17,18 while a review focused on mechanistic studies reported a range of effects, though all were positive.28,29 Recent interest in this area is on a priori identification of subgroups likely to respond favorably to placebo, although nothing definitive has been reported in the literature.19
Ethical concerns remain over the clinical use of the placebo,22-24 with the primary concern being deception to the patient when used in clinical settings.13,19 While ethical debates cannot be directly resolved with empirical studies, the limited data available in this area indicate that deception may not be a key impediment to using placebo. For example, a study of healthy volunteers reported that deception was not necessary to elicit a placebo response, and those who were deceived did not report a worsening of emotion or provider relations.6
Acknowledgments
The primary goal of this editorial was to provide a concise update on the placebo for JOSPT readers. Certainly some of these findings have potential application for rehabilitation settings, and we hope this information spurs consideration of updated placebo concepts in future practice or research.
REFERENCES
- 1.Beecher HK. The powerful placebo. J Am Med Assoc. 1955;159:1602–1606. doi: 10.1001/jama.1955.02960340022006. [DOI] [PubMed] [Google Scholar]
- 2.Benedetti F, Amanzio M. The neurobiology of placebo analgesia: from endogenous opioids to cholecystokinin. Prog Neurobiol. 1997;52:109–125. doi: 10.1016/s0301-0082(97)00006-3. [DOI] [PubMed] [Google Scholar]
- 3.Benedetti F, Arduino C, Amanzio M. Somatotopic activation of opioid systems by target-directed expectations of analgesia. J Neurosci. 1999;19:3639–3648. doi: 10.1523/JNEUROSCI.19-09-03639.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bialosky JE, Bishop MD, Price DD, Robinson ME, Vincent KR, George SZ. A randomized sham-controlled trial of a neurodynamic technique in the treatment of carpal tunnel syndrome. J Orthop Sports Phys Ther. 2009;39:709–723. doi: 10.2519/jospt.2009.3117. http://dx.doi.org/10.2519/jospt.2009.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bialosky JE, Bishop MD, Robinson ME, Barabas JA, George SZ. The influence of expectation on spinal manipulation induced hypoalgesia: an experimental study in normal subjects. BMC Musculoskelet Disord. 2008;9:19. doi: 10.1186/1471-2474-9-19. http://dx.doi.org/10.1186/1471-2474-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chung SK, Price DD, Verne GN, Robinson ME. Revelation of a personal placebo response: its effects on mood, attitudes and future placebo responding. Pain. 2007;132:281–288. doi: 10.1016/j.pain.2007.01.034. http://dx.doi.org/10.1016/j.pain.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144:28–34. doi: 10.1016/j.pain.2009.01.033. http://dx.doi.org/10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- 8.Colloca L, Lopiano L, Lanotte M, Benedetti F. Overt versus covert treatment for pain, anxiety, and Parkinson’s disease. Lancet Neurol. 2004;3:679–684. doi: 10.1016/S1474-4422(04)00908-1. http://dx.doi.org/10.1016/S1474-4422(04)00908-1. [DOI] [PubMed] [Google Scholar]
- 9.Colloca L, Sigaudo M, Benedetti F. The role of learning in nocebo and placebo effects. Pain. 2008;136:211–218. doi: 10.1016/j.pain.2008.02.006. http://dx.doi.org/10.1016/j.pain.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Craggs JG, Price DD, Perlstein WM, Verne GN, Robinson ME. The dynamic mechanisms of placebo induced analgesia: evidence of sustained and transient regional involvement. Pain. 2008;139:660–669. doi: 10.1016/j.pain.2008.07.025. http://dx.doi.org/10.1016/j.pain.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craggs JG, Price DD, Verne GN, Perlstein WM, Robinson MM. Functional brain interactions that serve cognitive-affective processing during pain and placebo analgesia. Neuroimage. 2007;38:720–729. doi: 10.1016/j.neuroimage.2007.07.057. http://dx.doi.org/10.1016/j.neuroimage.2007.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eippert F, Finsterbusch J, Bingel U, Buchel C. Direct evidence for spinal cord involvement in placebo analgesia. Science. 2009;326:404. doi: 10.1126/science.1180142. http://dx.doi.org/10.1126/science.1180142. [DOI] [PubMed] [Google Scholar]
- 13.Fassler M, Meissner K, Schneider A, Linde K. Frequency and circumstances of placebo use in clinical practice--a systematic review of empirical studies. BMC Med. 2010;8:15. doi: 10.1186/1741-7015-8-15. http://dx.doi.org/10.1186/1741-7015-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fillingim RB, Price DD. What is controlled for in placebo-controlled trials? Mayo Clin Proc. 2005;80:1119–1121. doi: 10.4065/80.9.1119. [DOI] [PubMed] [Google Scholar]
- 15.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686–695. doi: 10.1016/S0140-6736(09)61706-2. http://dx.doi.org/10.1016/S0140-6736(09)61706-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goffaux P, Redmond WJ, Rainville P, Marchand S. Descending analgesia--when the spine echoes what the brain expects. Pain. 2007;130:137–143. doi: 10.1016/j.pain.2006.11.011. http://dx.doi.org/10.1016/j.pain.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 17.Hrobjartsson A, Gotzsche PC. Is the placebo powerless? Update of a systematic review with 52 new randomized trials comparing placebo with no treatment. J Intern Med. 2004;256:91–100. doi: 10.1111/j.1365-2796.2004.01355.x. http://dx.doi.org/10.1111/j.1365-2796.2004.01355.x. [DOI] [PubMed] [Google Scholar]
- 18.Hrobjartsson A, Gotzsche PC. Placebo interventions for all clinical conditions. Cochrane Database Syst Rev. 2010 doi: 10.1002/14651858.CD003974.pub3. CD003974. http://dx.doi.org/10.1002/14651858.CD003974.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koshi EB, Short CA. Placebo theory and its implications for research and clinical practice: a review of the recent literature. Pain Pract. 2007;7:4–20. doi: 10.1111/j.1533-2500.2007.00104.x. http://dx.doi.org/10.1111/j.1533-2500.2007.00104.x. [DOI] [PubMed] [Google Scholar]
- 20.Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet. 1978;2:654–657. doi: 10.1016/s0140-6736(78)92762-9. [DOI] [PubMed] [Google Scholar]
- 21.Matre D, Casey KL, Knardahl S. Placebo-induced changes in spinal cord pain processing. J Neurosci. 2006;26:559–563. doi: 10.1523/JNEUROSCI.4218-05.2006. http://dx.doi.org/10.1523/JNEUROSCI.4218-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller FG, Brody H. What makes placebo-controlled trials unethical? Am J Bioeth. 2002;2:3–9. doi: 10.1162/152651602317533523. http://dx.doi.org/10.1162/152651602317533523. [DOI] [PubMed] [Google Scholar]
- 23.Miller FG, Colloca L. The legitimacy of placebo treatments in clinical practice: evidence and ethics. Am J Bioeth. 2009;9:39–47. doi: 10.1080/15265160903316263. http://dx.doi.org/10.1080/15265160903316263. [DOI] [PubMed] [Google Scholar]
- 24.Miller FG, Wendler D, Swartzman LC. Deception in research on the placebo effect. PLoS Med. 2005;2:e262. doi: 10.1371/journal.pmed.0020262. http://dx.doi.org/10.1371/journal.pmed.0020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. http://dx.doi.org/10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 26.Price DD, Finniss DG, Benedetti F. A comprehensive review of the placebo effect: recent advances and current thought. Annu Rev Psychol. 2008;59:565–590. doi: 10.1146/annurev.psych.59.113006.095941. http://dx.doi.org/10.1146/annurev.psych.59.113006.095941. [DOI] [PubMed] [Google Scholar]
- 27.Robinson ME, Price DD. Placebo analgesia: widening the scope of measured influences. Pain. 2009;144:5–6. doi: 10.1016/j.pain.2009.03.004. http://dx.doi.org/10.1016/j.pain.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vase L, Petersen GL, Riley JL, 3rd, Price DD. Factors contributing to large analgesic effects in placebo mechanism studies conducted between 2002 and 2007. Pain. 2009;145:36–44. doi: 10.1016/j.pain.2009.04.008. http://dx.doi.org/10.1016/j.pain.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 29.Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–452. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 30.Vase L, Robinson ME, Verne GN, Price DD. The contributions of suggestion, desire, and expectation to placebo effects in irritable bowel syndrome patients. An empirical investigation. Pain. 2003;105:17–25. doi: 10.1016/s0304-3959(03)00073-3. [DOI] [PubMed] [Google Scholar]
- 31.Verne GN, Robinson ME, Vase L, Price DD. Reversal of visceral and cutaneous hyperalgesia by local rectal anesthesia in irritable bowel syndrome (IBS) patients. Pain. 2003;105:223–230. doi: 10.1016/s0304-3959(03)00210-0. [DOI] [PubMed] [Google Scholar]