Abstract
Objective
To assess the efficacy and tolerability of everolimus in advanced urothelial carcimoma (UC).
Patients and Methods
The present study comprised a single-arm, non-randomized study in which all patients received everolimus 10 mg orally once daily continuously (one cycle = 4 weeks).
In total, 45 patients with metastatic UC progressing after one to four cytotoxic agents were enrolled between February 2009 and November 2010 at the Memorial Sloan-Kettering Cancer Center.
The primary endpoints were 2-month progression-free survival (PFS) and the safety of everolimus, with the secondary endpoint being the response rate.
A Simon minimax two-stage design tested the null hypothesis that the true two month PFS rate was ≤50%, as opposed to the alternative hypothesis of ≥70%.
Results
The most common grade 3/4 toxicities were fatigue, infection, anaemia, lymphopaenia, hyperglycaemia and hypophosphataemia.
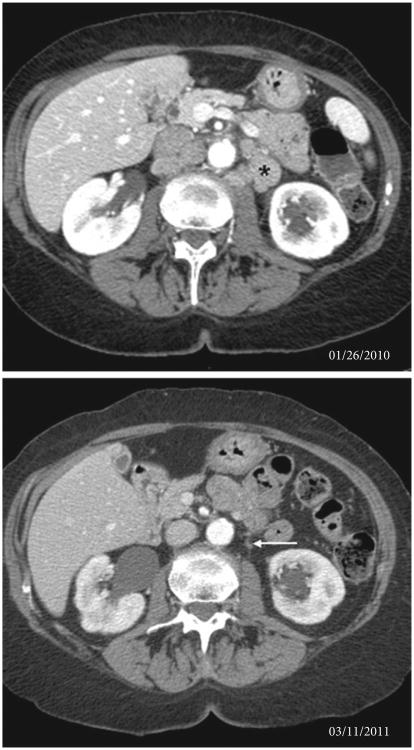
There were two partial responses in nodal metastases, with one patient achieving a 94% decrease in target lesions and remaining on drug at 26 months.
An additional 12 patients exhibited minor tumour regression.
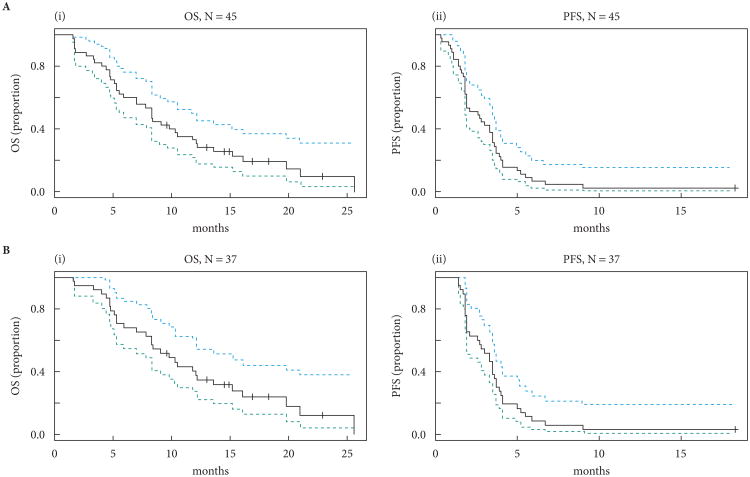
There were 23 of 45 (51%) patients who were progression-free at 2 months with a median (95% CI) PFS of 2.6 (1.8–3.5) months and a median (95% CI) overall survival of 8.3 (5.5–12.1) months.
No clear association was observed between mammalian target of rapamycin pathway marker expression and 2-month PFS.
Conclusions
Although everolimus did not meet its primary endpoint, one partial response, one near-complete response and twelve minor regressions were observed.
Everolimus possesses meaningful anti-tumour activity in a subset of patients with advanced UC.
Studies aiming to define the genetic basis of everolimus activity in individual responders are ongoing.
Keywords: bladder cancer, everolimus, mTOR, transitional cell carcinoma, urothelial cancer
Introduction
In the USA, urothelial carcinoma (UC) of the bladder is a common malignancy, with over 70 000 cases and 14 000 deaths occurring in 2010 [1]. Although representing a chemosensitive malignancy, the median survival of patients with metastatic UC is ≈ 15 months [2]. Second-line chemotherapy trials have yielded discouraging response rates in the range 10–30% with a progression-free survival (PFS) of between 2 and 3 months and a median survival of 6–9 months [3]. In Europe, vinflunine, a novel fluorinated vinca alkaloid, is approved for previously treated patients with advanced UC based on a modest improvement in survival compared to best supportive care alone seen in the eligible (6.9 vs 4.3 months; P = 0.040) but not in the intent-to-treat population [4]. No agent approved by the Food and Drug Administration exists for this setting because conventional chemotherapy has shown no survival benefit in patients with metastatic UC progressing after first-line platinum-based chemotherapy. The development of novel agents for advanced UC is desperately needed.
Targeted therapies have shown activity in multiple tumour types, including genitourinary cancers. Two agents that inhibit mammalian target of rapamycin (mTOR), temsirolimus and everolimus, are approved for treating patients with advanced kidney cancer [5,6]. mTOR is a ubiquitous serine-threonine kinase and a downstream component of the phosphoinositol 3-kinase (PI3K)/Akt/phosphatase and tensin homologue (PTEN) signalling pathway, playing a critical role in the regulation of protein synthesis, cell growth, proliferation, survival and angiogenesis [7]. mTOR is comprised of two structurally and functionally distinct multiprotein signalling complexes: mTOR-complex 1 (mTORC1) and mTOR-complex 2 (mTORC2) [8]. mTORC1 is predominantly activated through the PI3K/Akt pathway [9] and mTORC1-mediated signalling is sensitive to rapamycin and its derivatives, including everolimus [10]. A major function of mTOR relates to its regulation of mRNA translation through the downstream intermediates: eukaryotic initiation factor 4E binding protein (4E-BP1) and the 40S ribosomal S6 kinase (p70S6K1) [10]. To gauge the potential for mTOR-targeted therapy in UC, we performed an immunohistochemical analysis of phospho-S6 and phospho-4E-BP1 expression in a tissue microarray prepared from 92 cases of ≥ pT2 UC of the bladder [11]. Grade 2–3+ expression of phospho-4E-BP1 and phospho-S6 was shown in 58% and 34% of the tumours, respectively, including a moderate correlation with immunoreactivity observed between mTOR pathway markers, suggesting that the pathway is active in UC and providing the rationale for a trial targeting mTOR in this disease.
Everolimus is an oral derivative of rapamycin that selectively inhibits mTOR and has shown activity in multiple malignancies, leading to its approval by the Food and Drug Administration for advanced kidney cancer, advanced pancreatic neuroendocrine tumours and subependymal giant cell astrocytomas [6,12,13]. Everolimus inhibits the growth of bladder cancer cell lines in vitro and has activity in nude mouse models in vivo, including the inhibition of protein synthesis through S6K and 4E-BP1, as well as inhibition of angiogenesis [14,15]. Based on the above observations supporting a potential role for mTOR inhibition in UC, the present trial evaluated everolimus in patients with advanced UC who have progressed after first-line therapy.
Patients and Methods
Eligibility Criteria
Patients with previously treated progressive metastatic UC of the bladder, renal pelvis, ureter or urethra, histologically confirmed at the Memorial Sloan-Kettering Cancer Center, were eligible for the present study. Progressive disease was defined as new or progressing unidimensionally measurable lesions on cross-sectional imaging. Previous therapy included one to four cytotoxic agents administered in the peri-operative or metastatic setting because conventional chemotherapy ranges from one drug (e.g. gemcitabine) to regimens containing four agents (e.g. methotrexate, vinblastine, doxorubicin and cisplatin). The previous therapy must have included at least one of the agents: cisplatin, carboplatin, paclitaxel, docetaxel or gemcitabine. At least 4 weeks must have elapsed subsequent to radiotherapy or chemotherapy. Pretreatment tumour tissue was required for mTOR pathway marker analysis. Additional inclusion criteria included: Karnofsky performance status ≥60; age ≥18 years; total serum bilirubin ≤1.5; serum aspartate transaminase and serum alanine transaminase ≤2.5 × upper limit of normal (ULN), or serum aspartate transaminase and serum alanine transaminase ≤5 × ULN if liver function abnormalities were related to malignancy; absolute neutrophil count ≥1.5 × 109/L; platelets >100 × 109/L; haemoglobin >9.0 g/dL; creatinine ≤1.5 × ULN; international normalized ratio <1.3 (or <3 on anticoagulants); fasting serum cholesterol ≤300 mg/dL; fasting triglycerides ≤2.5 × ULN.
Patient exclusion criteria included major surgery within 4 weeks; symptomatic central nervous system metastases; any other investigational drugs; chronic treatment with systemic corticosteroids or immunosuppressive agents; another active cancer; uncontrolled diabetes mellitus; severely impaired lung function as indicated by total lung capacity <50% predicted, forced vital capacity <50% predicted, or diffusion capacity of the lung for carbon monoxide <40% predicted; active liver disease; known HIV or severe infection; pregnancy or breast-feeding. The Institutional Review Board of the Memorial Sloan-Kettering Cancer Center approved this protocol and written informed consent was obtained from all patients.
Treatment
Everolimus was administered orally at 10 mg daily continuously (one cycle = 4 weeks). Therapy was continued until progression of disease (POD) or unacceptable toxicity. Dose reductions for toxicity included: dose level 1 = 5 mg daily and dose level 2 = 5 mg every other day. Any patient requiring dose reduction below dose level 2 was removed from the study. Prespecified dose modification criteria were included for haematological and non-haematological toxicities, including stomatitis, hyperlipidaemia, hyperglycaemia and non-infectious pneumonitis.
Patient Evaluation
Baseline evaluations included a complete history and physical examination; Karnofsky performance status; complete blood count and comprehensive metabolic panel; fasting lipid profile; hepatitis B and C testing for patients meeting specific criteria; pregnancy test in women of childbearing age; and electrocardiogram. Studies required within 30 days were: abdominal and pelvic CT scans or MRI; bone X-rays and/or bone scan for patients with osseous lesions; chest X-ray or chest CT; and pulmonary function tests.
Tumour measurement was assessed at baseline, after every two cycles and after completion/withdrawal. Response was assessed using the Response Evaluation Criteria in Solid Tumors (version 1.0) [16] by a reference radiologist (S.R.G.). Safety was assessed on day 1 of each cycle and on day 14 of cycle 1.
mTOR Pathway Marker Expression and Mutation Detection
Markers of mTOR pathway activation, including p-S6 (phospho-ribosomal protein S6 [Ser240/Ser244]; Cell Signaling,, Beverly, MA, USA; dilution 1:120), p-4E-BP1 (phospho-4E-BP1 [Thr37/46]; Cell Signaling; dilution 1:400) and PTEN (clone 6H2.1; Dako, Glostrup, Denmark; dilution 1:50), were assessed by immunohistochemistry (IHC) in pretreatment tumour samples. Immunoreactivity was semi-quantitatively assessed for the percentage of tumour cells expressing the marker and graded from 0 to 3+ (0, 0–5%; 1+, 6–25%; 2+, 26–50%; 3+, >50% tumour cell positivity).
In 26 patients, formalin-fixed, paraffin-embedded pretreatment tumour specimens were macro-dissected and genomic DNA was extracted using the DNeasy Tissue kit (Qiagen, Valencia, CA, USA). Tumours were screened for FGFR3, PIK3CA, HRAS and BRAF hotspot mutations using a mass spectrometry-based iPLEX assay (Sequenom, San Diego, CA, USA), as described previously [17]. Primer sequences are available upon request.
Biostatistics
The primary endpoints included 2-month PFS and safety of everolimus. A Simon minimax two-stage design was used to test the null hypothesis that the true 2-month PFS rate was ≤50%, as opposed to the alternative hypothesis of ≥70% [18]. Assuming a type I error rate of 5% and power of 80%, ≥13 of 23 patients were required to be progression-free at 2 months to proceed to stage two, in which an additional 14 patients would be enrolled, giving a total of 37 patients. If ≥24 patients were progression-free at 2 months at the end of stage two, the agent would be considered promising. PFS and overall survival (OS) were calculated from treatment initiation until progressive disease or death and analyzed using the Kaplan–Meier method. They were correlated with risk group and mTOR pathway markers using the log-rank test. A protocol amendment deemed patients unevaluable for the primary PFS endpoint if they received one cycle or less of everolimus and were discontinued from study for rapid clinical deterioration related to POD or adverse events unrelated to everolimus. These unevaluable patients were replaced for the purpose of the primary PFS endpoint; however, PFS and OS analyses were performed and reported for all patients (n = 45) and the evaluable patient population (n = 37). Evaluable patients discontinued from the study for toxicity or other complicating diseases were considered as events for the calculation of PFS. All patients were included for the toxicity assessment. Toxicity frequencies were tabulated in accordance to Common Terminology Criteria for Adverse Events, version 3.0.
The secondary endpoint of response rate was estimated as the proportion of patients meeting the criteria for a complete response plus partial response using the Response Evaluation Criteria in Solid Tumors (version 1.0). mTOR pathway marker expression was determined by IHC in pretreatment tumour specimens and correlated with 2-month PFS using Fisher's exact test. The immunoreactivity grade was dichotomized into ≤ 1+ vs ≥2+. Samples with adequate pretreatment tumour tissue underwent targeted mutation detection. No research-directed biopsies were performed.
Results
Patient Characteristics and Efficacy
Forty-five patients were enrolled between February 2009 and November 2010, and 37 patients were evaluable for the primary PFS endpoint (Table 1). Seven patients were deemed unevaluable after receiving one cycle or less of everolimus and discontinuing treatment for either rapid clinical deterioration related to POD or adverse events unrelated to everolimus. One patient withdrew from the study after 1 day of therapy to pursue alternative treatment. Twenty-three of 45 (51%) patients were progression-free at 2 months. The median (95% CI) PFS for all 45 patients and the 37 evaluable patients was 2.6 (1.8–3.5) months and 3.3 (2.1–3.7) months, and the median (95% CI) OS was 8.3 (5.5–12.1) months and 9.8 (7.8–15.2) months, respectively (Fig. 1). Two (5%) patients achieved a partial response in nodal metastases, one of whom achieved a 94% decrease in target lesions and remains on everolimus for >26 months (Fig. 2). The other responder experienced a 36% decrease in target lesions with a 5.9-month PFS. The patient came off study after an admission for back pain and fatigue with high serum creatinine and creatine phosphokinase levels consistent with rhabdomyolysis, which improved with intravenous hydration. The patient was taking atorvastatin, which may also be associated with rhabdomyolysis. This event was considered possibly related to everolimus and the patient was discontinued from study treatment. In addition to these two patients, twelve patients exhibited some degree of tumour regression (range 1–24% best response). In total, 37 (82%) of forty-five patients had undergone primary tumour removal before enrollment in the present study. Of the evaluable patients, 21 (66%) of 32 patients who had undergone primary tumour removal were progression-free at 2 months, whereas two (40%) of five patients who had not undergone surgical resection were progression-free at 2 months. All patients had received at least one platinum agent before enrollment in the trial. Twenty-five patients received one previous regimen, 17 received two previous regimens, two received three previous regimens and one had received four previous regimens.
Table 1.
Patient demographics and clinical characteristics (n = 45).
| Characteristic | Value |
|---|---|
| Age (years), median (range) | 66 (32–84) |
| KPS, median (range) | 90 (70–100) |
| Sex, n (%) | |
| Male | 31 (69) |
| Female | 14 (31) |
| Previous chemotherapy, number of agents (%) | |
| 2 | 30 (67) |
| 3 | 13 (29) |
| 4 | 2 (4) |
| Primary site of disease, n (%) | |
| Bladder | 32 (71) |
| Ureter | 2 (4) |
| Renal pelvis | 11 (24) |
| Visceral metastases | 36 (80) |
| Lung | 23 (51) |
| Liver | 19 (42) |
| Bone | 6 (13) |
| Node-only disease | 9 (20) |
| Risk group, n* | |
| 0 | 14 |
| 1 | 18 |
| 2 | 10 |
| 3 | 3 |
KPS, Karnofsky performance status.
Adverse risk factors include Eastern Cooperative Oncology Group performance status >0, haemoglobin level <10 g/dL and the presence of liver metastasis, which can be categorized into four risk groups (zero, one, two or three prognostic factors present) [18].
Fig. 1.
Kaplan–Meier plots of progression-free survival (PFS) and overall survival (OS) for (A) all 45 patients and (B) the 37 evaluable patients.
Fig. 2.
CT scan showing near-complete response in para-aortic lymphadenopathy in a major responder treated with everolimus. Baseline imaging shows moderate para-aortic adenopathy (*); follow-up imaging shows a remaining sub-centimetre node (arrow). The change in appearance of the inferior liver and right kidney on the follow-up scan is the result of a combination of different inspiratory effort and interval mild right hydronephrosis with cortical scarring and atrophy.
A previously described, recently validated model using three adverse risk factors (Eastern Cooperative Oncology Group performance status >0, haemoglobin level <10 g/dL and the presence of liver metastasis) organized into four risk groups (zero, one, two or three prognostic factors) to predict OS in patients with platinum-refractory disease on second-line chemotherapy was applied to the 37 evaluable patients [19,20]. The median OS for patients in groups 0,1, 2 and 3 was 11.8, 10.5, 4.7 and 4.4 months, respectively (P < 0.001).
Safety and Tolerability
All toxicities are described in Table 2. Grade 3/4 everolimus-related toxicities were observed in 29 (64%) patients. A dose reduction was necessary in nine (20%) patients. The most common grade 3/4 toxicities were fatigue, infection, anaemia, lymphopaenia, hyperglycaemia and hypophosphataemia. There were six patients who experienced everolimus-related pneumonitis (one grade 3) and six who experienced grade 3 infections, including perianal abscess (n = 1), bronchitis (n = 1), UTI (n = 2), pneumonia (n = 1) and infected hip prosthesis (n = 1). Treatment was discontinued as a result of everolimus-related toxicity in eight patients. Four patients died within 30 days of study discontinuation from POD.
Table 2.
Toxicity assessment according to the Common Terminology Criteria for Adverse Events, version 3.0 (adverse events, n = 45).
| Adverse event* | Grade | |
|---|---|---|
|
| ||
| 1–2 | 3–4 | |
|
|
|
|
| n (%) | n (%) | |
| Non-haematological | ||
| Fatigue | 39 (87) | 5 (11) |
| Weight loss | 29 (64) | 1 (2) |
| Mucositis | 24 (53) | 3 (7) |
| Rash | 22 (49) | 0 (0) |
| Constipation | 21 (47) | 0 (0) |
| Neuropathy | 21 (47) | 0 (0) |
| Oedema | 20 (44) | 1 (2) |
| Urinary frequency/urgency | 15 (33) | 0 (0) |
| Nausea | 14 (31) | 0 (0) |
| Dyspnoea | 11 (24) | 1 (2) |
| Vomiting | 10 (22) | 0 (0) |
| Fever | 9 (20) | 0 (0) |
| Diarrhoea | 7 (16) | 0 (0) |
| Pain | 6 (13) | 1 (2) |
| Infection | 0 (0) | 6 (13) |
| Cough | 6 (13) | 0 (0) |
| Pneumonitis | 5 (11) | 1 (2) |
| Dysgeusia | 4 (9) | 0 (0) |
| Haemorrhage | 2 (4) | 1 (2) |
| Dehydration | 0 (0) | 2 (4) |
| Pruritis | 2 (4) | 0 (0) |
| Confusion | 1 (2) | 0 (0) |
| Dry mouth | 1 (2) | 0 (0) |
| Dry skin | 1 (2) | 0 (0) |
| Enteritis | 1 (2) | 0 (0) |
| Flushing | 1 (2) | 0 (0) |
| Heartburn | 1 (2) | 0 (0) |
| Hypoxia | 1 (2) | 1 (2) |
| Muscle weakness | 1 (2) | 0 (0) |
| Gastroesophageal reflux disease | 1 (2) | 0 (0) |
| Myositis | 0 (0) | 1 (2) |
| Renal failure | 0 (0) | 1 (2) |
| Arrhythmia | 0 (0) | 1 (2) |
| Laboratory | ||
| Anemia | 39 (87) | 9 (20) |
| Thrombocytopaenia | 21 (47) | 0 (0) |
| Leukopaenia | 16 (36) | 1 (2) |
| Neutropaenia | 7 (16) | 1 (2) |
| Lymphopaenia | 0 (0) | 5 (11) |
| Elevated aspartate aminotransferase | 30 (67) | 3 (7) |
| Elevated alanine aminotransferase | 27 (60) | 2 (4) |
| Elevated alkaline phosphatase | 22 (49) | 1 (2) |
| Hypoalbuminaemia | 27 (60) | 0 (0) |
| Hyperbilirubinaemia | 1 (2) | 1 (2) |
| Hyponatraemia | 11 (24) | 3 (7) |
| Hypernatraemia | 7 (16) | 0 (0) |
| Hypokalaemia | 7 (16) | 1 (2) |
| Hyperkalaemia | 7 (16) | 0 (0) |
| Hypophosphataemia | 16 (36) | 4 (9) |
| Hypomagnesaemia | 6 (13) | 0 (0) |
| Hyperglycaemia | 42 (93) | 5 (11) |
| Hypercholesterolaemia | 29 (64) | 0 (0) |
| Hypertriglyceridaemia | 28 (62) | 1 (2) |
| Elevated creatinine | 26 (58) | 1 (2) |
| International normalized ratio, abnormal | 18 (40) | 7 (16) |
| Partial thromboplastin time, abnormal | 12 (27) | 2 (4) |
All adverse events with a suspected relationship with everolimus are listed, in addition to all adverse events that occurred in >10% of the study population, regardless of causality.
mTOR Pathway Marker Expression and Mutation Detection
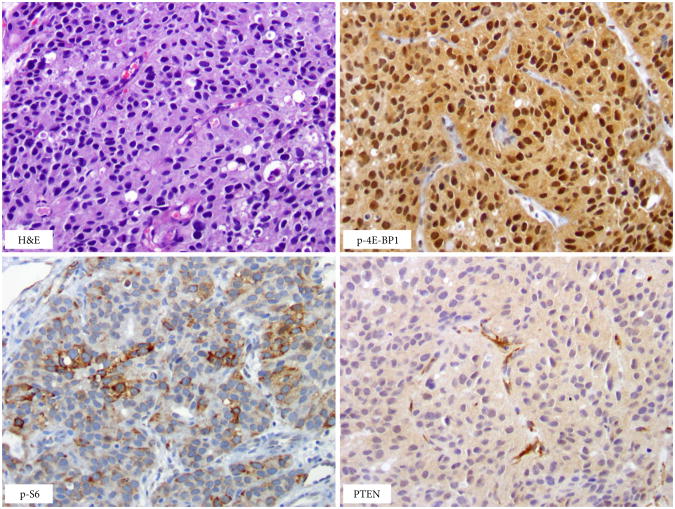
mTOR pathway marker expression, including phospho-S6, phospho-4E-BP1 and PTEN, was assessed by IHC in 43 of 45 pretreatment tumour samples obtained from the primary tumour site (Fig. 3). No significant association was observed between mTOR pathway markers and PFS (P = 0.62, 0.85 and 0.69 for phospho-S6, phospho-4E-BP1 and PTEN, respectively). There was a borderline significant association between phospho-4E-BP1 expression (≥2+ vs ≤ 1+) and the binary variable, progression-free at 2 months (P = 0.08), for 35 patients who were evaluable for the primary endpoint and for whom IHC analysis was performed.
Fig. 3.
Pretreatment tumour sample with over-expression of phospho-S6 and phospho-4E-BP1 and an absence of phosphatase and tensin homologue (PTEN) staining in tumour cells (note retained PTEN staining in blood vessels – positive internal control). H&E, haematoxylin and eosin.
In 26 pretreatment tumour specimens (24 primary site and two metastatic lymph node sites) for which adequate tumour tissue was available to screen for hotspot mutations in FGFR3, HRAS, PIK3CA and BRAF, two mutations were detected (one FGFR3 S249C and one PIK3CA E542K), with no mutations detected in the two responders.
Discussion
The present study establishes mTOR as a therapeutic target in patients with advanced UC. Although the trial did not meet its primary endpoint, the median (95% CI) PFS and OS for the evaluable patients of 3.3 (2.1–3.7) months and 9.8 (7.8–15.2) months, along with the two observed partial responses, shows activity for everolimus in UC. In an unselected patient population with advanced UC progressing after platinum-based chemotherapy, everolimus has single-agent activity similar to that observed with standard chemotherapy [3]. However, a subset of patients exhibited minor regressions, suggesting that everolimus possesses both a biologically and clinically relevant anti-tumour effect in certain individuals. Defining the genetic basis for such minor regressions is imperative for enabling the better selection of patients with the greatest probability of deriving clinical benefit.
Everolimus-related toxicities were more frequently seen in this patient population compared to RCC, with grade 3/4 toxicities being observed in 29 (64%) patients, including fatigue, infection, anaemia, lymphopaenia, hyperglycaemia and hypophosphataemia. Everolimus-related pneumonitis (one grade 3) and grade 3 infection were observed in six patients. Treatment was discontinued as a result of drug-related toxicity in eight patients. This spectrum of toxicities is similar to that seen in everolimus-treated patients with advanced RCC. In the RECORD-1 trial, a double-blind, randomized, placebo-controlled phase III study of everolimus in 410 patients with advanced RCC, there was a low incidence of grade 3/4 adverse events, with the most commonly reported being stomatitis, asthaenia, fatigue, rash and diarrhoea [6,21]. Pneumonitis was detected in 22 (8%) everolimus-treated patients (eight with grade 3 severity). In an international expanded-access programme of everolimus in 1367 patients with metastatic RCC, the most commonly reported serious adverse events were dyspnoea, pneumonia and anaemia, and the most commonly reported grade 3/4 adverse events were anaemia (13.4%), fatigue (6.7%) and dyspnoea (6.5%) [22]. The higher incidence of grade 3/4 events in UC compared to RCC may reflect the older age and increased co-morbidities of UC patients, as well as the impact of previous multi-agent chemotherapy employed in metastatic UC compared to the targeted therapies used in advanced RCC. The older age and complex co-morbidities observed in patients with advanced UC, including tobacco use, cardiovascular disease, diabetes mellitus and chronic obstructive pulmonary disease, make this a particularly challenging patient population to manage [23,24]. Together with the lack of effective therapies to control the underlying cancer, therapeutic clinical trials have been hampered by issues related to patient ineligibility and rapid clinical deterioration [25]. In the present study, seven of the initial 28 patients received one cycle or less of everolimus secondary to either rapid clinical deterioration as a result of POD or adverse events unrelated to everolimus. The protocol was subsequently amended to exclude all such patients for the primary PFS endpoint. We acknowledge the statistical bias inherent in removing these patients and have presented the outcome analysis for the intent-to-treat population, including all 45 patients who received any drug on study (median [95% CI]: PFS, 2.6 [1.8–3.5] months; OS, 8.3 [5.5–12.1] months). Although the small number of enrolled and evaluable patients in the present study, as well as the lack of prospective molecular screening (see below), limit our ability to accurately gauge response to everolimus therapy, these results are similar to the modest outcomes observed in trials evaluating standard chemotherapy, and thus this does not change the conclusion that the outcome in unselected patients treated with everolimus is poor.
In the present study, we evaluated a previously described, recently validated prognostic model using three adverse risk factors organized into four risk groups to predict OS in patients with platinum-refractory disease in the 37 evaluable patients treated with everolimus, and found a statistically significant correlation between median OS and the number of risk factors present (P < 0.001) [19,20]. These results are similar to the decline in OS seen in first-line platinum refractory patients treated in the phase III vinflunine trial with a median OS for the four groups in that study of 14.2, 7.3, 3.8, and 1.7 months (P < 0.001) [4,19].
The requirement for pretreatment tumour tissue for immunohistochemical and mutation analysis provided an opportunity to retrospectively identify predictive biomarkers that condition the response to everolimus. This approach has been successfully employed in other malignancies (e.g. metastatic colorectal cancer) to identify biomarkers such as K-Ras mutations that predict for cetuximab resistance [26]. There are no established predictive biomarkers associated with the response to everolimus. We performed an exploratory analysis using IHC for activated mTOR pathway marker expression, including phospho-S6, phospho-4E-BP1 and PTEN in pretreatment tumour samples. A positive association was seen with phospho-S6 expression and the response to the rapamycin prodrug, temsirolimus, in patients with advanced RCC [27]. In the present study, no association was seen between PFS and phospho-S6 expression; however, a borderline significant association was observed between phospho-4E-BP1 expression and the primary endpoint, PFS at 2 months (P = 0.08), in 35 evaluable patients for whom IHC analysis was performed. PI3K pathway activation leads to downstream stimulation of mTOR with both PTEN loss and PIK3CA mutations sensitizing tumours to mTOR inhibition [28,29]. The present study did not identify an association between PTEN loss and PFS as determined by IHC. Additionally, we analyzed 26 pretreatment tumour samples for mutations in FGFR3, HRAS, PIK3CA and BRAF; one sample possessed an FGFR3 S249C alteration and a second sample contained a PIK3CA E542K mutation. Both samples were derived from primary cystectomy specimens. Notably, no mutations were detected in the two partial responders. Most samples were derived from the primary tumour site and it is possible that the genetic drivers of primary vs metastatic tumours may be significantly different. This exploratory biomarker analysis is limited by the retrospective design, small sample size and limited number of genes that were screened. However, we have embarked on an outlier analysis of the durable, near-complete responder to everolimus using array comparative genomic hybridization, methylation analysis, and targeted and whole genome sequencing, with the aim of identifying the genetic basis for this dramatic response. Our hope is that this analysis will detect novel biomarkers for prospectively identifying the subsets of patients with the greatest probability of deriving benefit from mTOR pathway inhibition. Additional genotyping of patients who showed minor tumour regression is also underway aiming to identify candidates for combination therapies based upon tumour genotype.
In conclusion, advanced UC represents an orphan disease with limited effective therapies; more than two decades of research have not led to a significant improvement in outcome. An older patient population with multiple medical co-morbidities has contributed to the difficulty in designing, accruing to and completing clinical trials of therapeutic agents. We have now entered a new era of molecular medicine with targeted therapeutics offering more promise compared to traditional chemotherapy. The genotypic stratification of patients based upon the presence of predictive and prognostic molecular biomarkers of such agents in clinical trials will ultimately improve the outcome for patients with advanced UC [30]. Future phase II clinical trials of targeted agents should incorporate the molecular screening of patient tumours to identify biomarkers of response as part of the pre-enrollment eligibility criteria for selecting those patients with the greatest probability of exhibiting a response to such therapies.
What's known on the subject? and What does the study add?
No recent advances have been made in the treatment of patients with advanced bladder cancer and, to date, targeted therapies have not resulted in an improvement in outcome. The mammalian target of rapamycin pathway has been shown to be up-regulated in bladder cancer and represents a rational target for therapeutic intervention.
In the present phase II study of everolimus, one near-complete response, one partial response and several minor responses suggest that everolimus possesses biological activity in a subset of patients with bladder cancer. To maximize benefit from targeted agents such as everolimus, the preselection of patients based on molecular phenotype is required.
Acknowledgments
Everolimus (RAD001) in Metastatic Transitional Cell Carcinoma of the Urothelium; http://www.clinicaltrials.gov; identifier: NCT00805129. The present study was funded in part by Novartis Pharmaceuticals Corporation.
Abbreviations
- 4E-BP1
eukaryotic initiation factor 4E binding protein
- IHC
immunohistochemistry
- OS
overall survival
- PFS
progression-free survival
- PI3K
phosphoinositol 3-kinase
- POD
progression of disease
- PTEN
phosphatase and tensin homologue
- UC
urothelial carcimoma
- ULN
upper limit of normal
Footnotes
Conflict of Interest: Matthew I. Milowsky received research funding from Novartis Pharmaceuticals Corporation.
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.von der Maase H, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol. 2000;18:3068–77. doi: 10.1200/JCO.2000.18.17.3068. [DOI] [PubMed] [Google Scholar]
- 3.Gallagher DJ, Milowsky MI, Bajorin DF. Advanced bladder cancer: status of first-line chemotherapy and the search for active agents in the second-line setting. Cancer. 2008;113:1284–93. doi: 10.1002/cncr.23692. [DOI] [PubMed] [Google Scholar]
- 4.Bellmunt J, Theodore C, Demkov T, et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol. 2009;27:4454–61. doi: 10.1200/JCO.2008.20.5534. [DOI] [PubMed] [Google Scholar]
- 5.Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 7.Garcia JA, Danielpour D. Mammalian target of rapamycin inhibition as a therapeutic strategy in the management of urologic malignancies. Mol Cancer Ther. 2008;7:1347–54. doi: 10.1158/1535-7163.MCT-07-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–84. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 9.Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335–48. doi: 10.1038/nrc1362. [DOI] [PubMed] [Google Scholar]
- 10.Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423–35. doi: 10.1038/sj.onc.1209887. [DOI] [PubMed] [Google Scholar]
- 11.Tickoo SK, Milowsky MI, Dhar N, et al. Hypoxia-inducible factor and mammalian target of rapamycin pathway markers in urothelial carcinoma of the bladder: possible therapeutic implications. BJU Int. 2011;107:844–9. doi: 10.1111/j.1464-410X.2010.09517.x. [DOI] [PubMed] [Google Scholar]
- 12.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krueger DA, Care MM, Holland K, et al. Everolimus for subependymal giant-cell astrocytomas in tuberous sclerosis. N Engl J Med. 2010;363:1801–11. doi: 10.1056/NEJMoa1001671. [DOI] [PubMed] [Google Scholar]
- 14.Chiong E, Lee IL, Dadbin A, et al. Effects of mTOR inhibitor everolimus (RAD001) on bladder cancer cells. Clin Cancer Res. 2011;17:2863–73. doi: 10.1158/1078-0432.CCR-09-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansure JJ, Nassim R, Chevalier S, Rocha J, Scarlata E, Kassouf W. Inhibition of mammalian target of rapamycin as a therapeutic strategy in the management of bladder cancer. Cancer Biol Ther. 2009;8:2339–47. doi: 10.4161/cbt.8.24.9987. [DOI] [PubMed] [Google Scholar]
- 16.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 17.Pratilas CA, Hanrahan AJ, Halilovic E, et al. Genetic predictors of MEK dependence in non-small cell lung cancer. Cancer Res. 2008;68:9375–83. doi: 10.1158/0008-5472.CAN-08-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–5. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 20.Niegisch G, Fimmers R, Siener R, Park SI, Albers P. Prognostic factors in second-line treatment of urothelial cancers with gemcitabine and paclitaxel (German Association of Urological Oncology trial AB20/99) Eur Urol. 2011;60:1087–96. doi: 10.1016/j.eururo.2011.07.063. [DOI] [PubMed] [Google Scholar]
- 21.Porta C, Osanto S, Ravaud A, et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47:1287–98. doi: 10.1016/j.ejca.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Grunwald V, Karakiewicz PI, Bavbek SE, et al. An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer. 2012;48:324–32. doi: 10.1016/j.ejca.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 23.Balar A, Bajorin DF, Milowsky MI. Management of invasive bladder cancer in patients who are not candidates for or decline cystectomy. Ther Adv Urol. 2011;3:107–17. doi: 10.1177/1756287211407543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shariat SF, Milowsky M, Droller MJ. Bladder cancer in the elderly. Urol Oncol. 2009;27:653–67. doi: 10.1016/j.urolonc.2009.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of patients with metastatic urothelial cancer ‘unfit’ for Cisplatin-based chemotherapy. J Clin Oncol. 2011;29:2432–8. doi: 10.1200/JCO.2011.34.8433. [DOI] [PubMed] [Google Scholar]
- 26.Lievre A, Bachet JB, Le Corre D, et al. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–5. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 27.Cho D, Signoretti S, Dabora S, et al. Potential histologic and molecular predictors of response to temsirolimus in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2007;5:379–85. doi: 10.3816/CGC.2007.n.020. [DOI] [PubMed] [Google Scholar]
- 28.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene. 2011;30:3222–33. doi: 10.1038/onc.2011.42. [DOI] [PubMed] [Google Scholar]
- 30.McDermott U, Settleman J. Personalized cancer therapy with selective kinase inhibitors: an emerging paradigm in medical oncology. J Clin Oncol. 2009;27:5650–9. doi: 10.1200/JCO.2009.22.9054. [DOI] [PubMed] [Google Scholar]