Abstract
Background
Macular edema is the most common cause of visual loss among patients with diabetes.
Objective
To determine the cost-effectiveness of different treatments of diabetic macular edema (DME).
Design
Markov model.
Data Sources
Published literature and expert opinion.
Target Population
Patients with clinically significant DME.
Time Horizon
Lifetime.
Perspective
Societal.
Intervention
Laser treatment, intraocular injections of triamcinolone or a vascular endothelial growth factor (VEGF) inhibitor, or a combination of both.
Outcome Measures
Discounted costs, gains in quality-adjusted life-years (QALYs), and incremental cost-effectiveness ratios (ICERs).
Results of Base-Case Analysis
All treatments except laser monotherapy substantially reduced costs, and all treatments except triamcinolone monotherapy increased QALYs. Laser treatment plus a VEGF inhibitor achieved the greatest benefit, gaining 0.56 QALYs at a cost of $6975 for an ICER of $12 410 per QALY compared with laser treatment plus triamcinolone. Monotherapy with a VEGF inhibitor achieved similar outcomes to combination therapy with laser treatment plus a VEGF inhibitor. Laser monotherapy and triamcinolone monotherapy were less effective and more costly than combination therapy.
Results of Sensitivity Analysis
VEGF inhibitor monotherapy was sometimes preferred over laser treatment plus a VEGF inhibitor, depending on the reduction in quality of life with loss of visual acuity. When the VEGF inhibitor bevacizumab was as effective as ranibizumab, it was preferable to because of its lower cost.
Limitation
Long-term outcome data for treated and untreated diseases are limited.
Conclusion
The most effective treatment of DME is VEGF inhibitor injections with or without laser treatment. This therapy compares favorably with cost-effective interventions for other conditions.
Primary Funding Source
Agency for Healthcare Research and Quality.
Diabetes affects approximately 26 million patients in the United States, accounts for $1 in $10 spent on health care, and is the leading cause of new-onset blindness among adults (1). Dilated eye examinations identify diabetic retinopathy, which ranges from mild (retinal hemorrhages) to severe (ischemia-induced neovascularization and fibrovascular proliferation, with potential hemorrhage, retinal detachment, or glaucoma). These examinations also identify diabetic macular edema (DME)—central retinal edema resulting from increased vascular permeability of the retina.
Diabetic macular edema affects central vision and is the most common cause of visual loss in patients with diabetes (2). Its prevalence is 9% among these patients (3), with approximately 75 000 new cases annually (4). Untreated DME can cause progressive visual decline (5) and medical costs that are 29% higher than those for unaffected patients with diabetes (6).
The goal of DME treatment is to stop decline and, ideally, to recover vision. Successful treatment can enable a patient to resume driving, depending on the degree of visual impairment. Standard therapy has been macular laser treatment, which targets leaking microaneurysms and mildly stimulates subretinal cells to decrease edema. More recently, treatment of this condition has included intravitreal (intraocular) injections of triamcinolone acetonide or vascular endothelial growth factor (VEGF) inhibitors, which reduce vascular permeability and allow fluid reabsorption.
Unlike laser treatment, which has potentially long-lasting effects, injections require periodic retreatment. Vascular endothelial growth factor inhibitors include bevacizumab, ranibizumab, and the newer VEGF Trap-Eye (aflibercept). In contrast to monotherapy, combination therapies of laser treatment and intravitreal injections aim to provide the long-term benefits of laser treatment and short-term benefits of fluid reabsorption. Treatment costs per injection differ substantially, from approximately $50 (off-label bevacizumab) to $1200 (U.S. Food and Drug Administration–approved ranibizumab) (7–11).
Each strategy is well-studied, but no trial compares all therapies and few compare costs. Evidence on cost-effectiveness is conflicting. The U.K.’s National Institute for Health and Clinical Excellence evaluated an industry-conducted modeling analysis of ranibizumab, and it found the cost-effectiveness of VEGF inhibitors to be unconvincing relative to that of laser treatment (12). Two published studies reached the opposite conclusion; however, they did not consider lifetime costs and benefits nor compare all major treatments (13, 14).
Our study compares 6 strategies for lifetime management of DME. By comparing therapy with no treatment, we evaluate the societal effect of undiagnosed DME—particularly important given the increasing prevalence of diabetes. Diagnosis and proper management of DME depends on appropriate referral from primary care clinicians, who also play an important role in shared decision making with patients. Building on a relationship of long-standing trust with their primary care providers, patients often seek advice and second opinions about treatments offered by specialists. Understanding the options, visual benefits, and tradeoffs in therapy for DME will support and inform such conversations as part of comprehensive diabetes care.
METHODS
Overview
We developed a decision-analytic Markov cohort model of the natural history and treatment of DME, integrating mortality, visual acuity, treatment costs, complications, and societal costs. The model compared the effect on health, longevity, and costs of the following management strategies: no treatment; monotherapy with a VEGF inhibitor (ranibizumab, 0.3 mg); monotherapy with triamcinolone, 4 mg; laser monotherapy; combination therapy with laser treatment plus a VEGF inhibitor; and combination therapy with laser treatment plus triamcinolone. We translated vision and complications into utility-based quality-of-life measures and analyzed these from a societal perspective, broadly considering all lifetime costs and benefits regardless of who benefited (15–20).
Patient Cohort
The main cohort included patients with type 1 or 2 diabetes and clinically significant DME. Fifty percent were men, age was 63 years, and visual acuity of the better eye was 20/63. Patients had no previous cataract surgery and did not receive treatment of DME within 4 months. These characteristics were similar to those of the baseline populations of major clinical trials of DME (21–33).
Disease Progression
Health states of DME reflected vision (visual acuity categories 1 through 6 [Table 1]), treatment status, and complications (Figure 1). Because longitudinal data on the natural history of progression of DME are limited, we used expert opinion and calibration to the DRCR.net (Diabetic Retinopathy Clinical Research Network) study cohort to estimate long-term changes without treatment (transition probabilities were calculated using a Markov model with constant progression rates [Supplement, available at www.annals.org]) (34). Table 2 shows input values (see Table 4 of the Supplement for visual acuity outcomes with and without treatment and Table 23 of the Supplement for uncertainty ranges).
Table 1.
Definition of Visual Acuity Categories Used in the Model
| Visual Acuity Category* |
Corresponding Snellen Acuity† |
Corresponding ETDRS Acuity† |
Change in ETDRS Letters Relative to Starting Acuity of 60 |
|---|---|---|---|
| 1 | 20/30 or better | ≥75 | ≥15-letter gain |
| 2 | 20/40 | 70–74 | 10- to 14-letter gain |
| 3 | 20/50 | 65–69 | 5- to 9-letter gain |
| 4 | 20/60–20/100 | 51–64 | 9-letter loss to 4-letter gain |
| 5 | 20/125 | 46–50 | 10- to 14-letter loss |
| 6 | 20/200 or worse | ≤45 | ≥15-letter loss |
ETDRS = Early Treatment Diabetic Retinopathy Study.
Visual acuity categories were selected to correspond to measured clinical trial outcomes (change in ETDRS letters) (21–33).
The Snellen and ETDRS charts are standardized methods of measuring visual acuity. The Snellen chart is used most in practice, and the ETDRS chart is a research-quality visual acuity used for more standardized measurement in clinical trials (32, 33). These conversions are standard and well-accepted.
Figure 1.
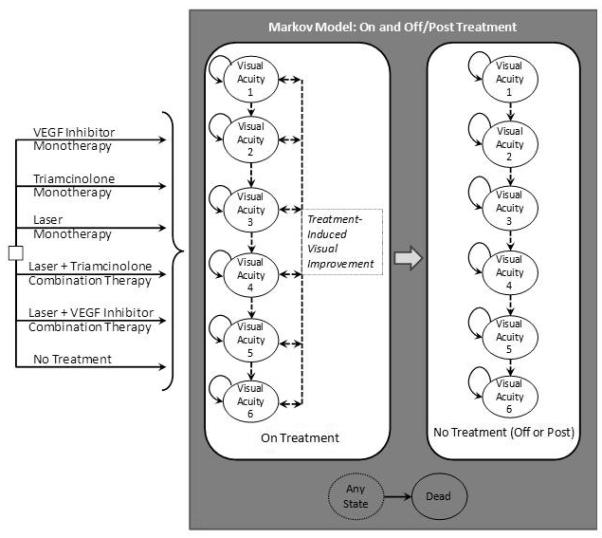
Markov Model Schematic
VEGF=Vascular Endothelial Growth Factor
The six strategy alternatives to the right of the decision node (square box) represent the six strategies for comparison, each progressing within the Markov model.
The gray box represents the Markov model transitions for diabetic macular edema progression. Visual Acuity categories 1-6 represent visual acuity states (Table 1). Solid arrows represent possible worsening (Progression) within a given month, and dashed arrows represent the potential for improvement or progression within a given month while on treatment. On-treatment states are subject to risk of complications (arterial thromboembolic events, glaucoma, cataract, and other major or minor complications). In the base case, treatment was stopped after one year for all strategies, sooner if an arterial thromboembolic event or severe glaucoma.
Table 2.
Base-Case Model Inputs*
| Characteristic | Base- Case Value |
Range for Sensitivity Analysis |
Distribution for Probabilistic Sensitivity Analysis |
References |
|---|---|---|---|---|
| Starting age, y | 63 | 40 to 70 | Normal | 21–31, 37 |
|
Hazard ratio (survival in patients with
diabetic retinopathy) |
2.20 | 1.36 to 3.60 | Truncated normal (minimum of 1) |
69 – 71 |
| Complication risks | ||||
| Arterial thromboembolic event | ||||
| VEGF inhibitor (annual probability) | 0.016 | 0.004 to 0.033 | β | 21–31, 38–48, 72, 73 |
| Triamcinolone (annual probability) | 0.014 | 0.004 to 0.054 | β | 21–31, 38–48, 72, 73 |
| Laser (annual probability) | 0.014 | 0.006 to 0.054 | β | 21–31, 38–48, 72, 73 |
| Not receiving treatment (annual probability) |
0.014 | 0.006 to 0.054 | β | 21–31, 38–48, 72, 73 |
| Mild to moderate glaucoma | ||||
| VEGF inhibitor (annual probability) | 0.050 | 0.04 to 0.06 | β | 21–31, 38–48, 72–74 |
| Triamcinolone (annual probability) | 0.450 | 0.40 to 0.50 | β | 21–31, 38–48, 72–74 |
| Laser (annual probability) | 0.046 | 0.03 to 0.06 | β | 21–31, 38–48, 72–74 |
| Not receiving treatment (annual probability) |
0.046 | 0.03 to 0.06 | β | 21–31, 38–48, 72–74 |
| Severe/uncontrolled glaucoma (from mild to moderate glaucoma) |
||||
| VEGF inhibitor (annual probability) | 0.050 | 0.00 to 0.20 | β | Clinical assumption |
| Triamcinolone (annual probability) | 0.050 | 0.00 to 0.20 | β | Clinical assumption |
| Laser (annual probability) | 0.050 | 0.00 to 0.20 | β | Clinical assumption |
| Not receiving treatment (annual probability) |
0.050 | 0.00 to 0.20 | β | Clinical assumption |
| Cataract | ||||
| VEGF inhibitor (annual probability) | 0.067 | 0.05 to 0.07 | β | 21–31, 38–48, 72–74 |
| Triamcinolone (annual probability) | 0.314 | 0.15 to 0.33 | β | 21–31, 38–48, 72–74 |
| Laser (annual probability) | 0.062 | 0.05 to 0.07 | β | 21–31, 38–48, 72–74 |
| Not on treatment (annual probability) |
0.062 | 0.05 to 0.07 | β | 21–31, 38–48, 72–74 |
| Other major complications | ||||
| VEGF inhibitor (probability per injection) |
0.004 | 0.000 to 0.004 | β | 21–31, 38–48, 72–74† |
| Triamcinolone (probability per injection) |
0.004 | 0.000 to 0.004 | β | 21–31, 38–48, 72–74† |
| Laser (probability per treatment) | 0.020 | 0.000 to 0.050 | β | 21–31, 38–48, 72–74† |
| Not receiving treatment (probability per month) |
0.000 | By definition | NA | NA |
| Other minor complications | ||||
| VEGF inhibitor (probability per injection) |
0.059 | 0.00 to 0.08 | β | 21–31, 38–48, 72–74 |
| Triamcinolone (probability per injection) |
0.100 | 0.00 to 0.15 | β | 21–31, 38–48, 72–74 |
| Laser (probability per treatment) | 0.090 | 0.00 to 0.15 | β | 21–31, 38–48, 72–74 |
| Not receiving treatment (probability per month) |
0.000 | By definition | NA | NA |
| Direct costs, $ | ||||
| Examinations and testing | ||||
| Initial office visit | 141.2 7 |
103.80 to 141.27 |
Normal | 7, 8 |
| Follow-up office visit | 87.21 | 87.21 to 105.28 |
Normal | 7, 8 |
| OCT | 99.34 | 90.00 to 110.00 |
Normal | 7, 8 |
| Fundus photography | 133.1 9 |
115.00 to 145.00 |
Normal | 7, 8 |
| FA | 252.9 3 |
225.00 to 275.00 |
Normal | 7, 8 |
| Treatment (per-treatment cost) | ||||
| VEGF inhibitor | ||||
| Ranibizumab (0.3 mg) | 1194 00 |
1194.00 to 2437.00 |
Normal | 7, 9, 10 |
| Bevacizumab (1.25 mg) | 42.58 .00 |
40.00 to 60.00 | Normal | 10 |
| VEGF Trap-Eye (2 mg) | 1961. 00 |
1800.00 to 2000.00 |
Normal | 36 |
| VEGF Trap-Eye (0.5 mg) | 490.0 0 |
400.00 to 600.00 |
Normal | 36 |
| Triamcinolone (4 mg) | 136.7 4 |
120.00 to 150.00 |
Normal | 11 |
| Laser | 393.3 5 |
360.00 to 420.00 |
Normal | 8 |
| Complications | ||||
| Arterial thromboembolic events | ||||
| Acute cost, $ | 13 263.0 0 |
4900.00 to 49 000.00 |
Truncated normal‡ | 75 † |
| Recurrent cost, $/mo | 4514 00 |
130.00 to 21 600.00 |
Truncated normal‡ | 75 † |
| Endophthalmitis, $ | ||||
| Intravitreal injection | 309.8 9 |
250.00 to 350.00 |
Truncated normal‡ | 7, 8 |
| Antibiotics | 5.37 | 5.37 to 50.00 | Truncated normal‡ | 7, 9 |
| Vitrectomy | 3300. 00 |
3300.00 to 3500.00 |
Truncated normal‡ | 7, 8 |
| Major complications, $ | ||||
| Average for injection | 2000. 00 |
0.00 to 3700.00 |
Truncated normal‡ | 7, 8 |
| Average for laser | 500.0 0 |
0.00 to 600.00 | Truncated normal‡ | 7, 8 |
| Glaucoma, $ | ||||
| Eye drops | 50.00 | 20.00 to 300.00 |
Truncated normal‡ | 7, 9 |
| Trabeculectomy | 2194. 00 |
2000.00 to 2400.00 |
Normal | 7, 8 |
| Selective laser trabeculoplasty | 503.5 9 |
400.00 to 600.00 |
Normal | 7, 8 |
| Cataract, $ | 2021. 00 |
1820 to 2220 | Normal | 7, 8 |
| Minor complication, $ | 50.00 | 0.00 to 100.00 | γ | 7, 8 |
| Indirect (time) costs, $ | ||||
| Annual costs of blindness§ | ||||
| Visual acuity 1 | 0.00 | 0.00 | NA | 56, 59, 60 |
| Visual acuity 2 | 0.00 | 0.00 to 500.00 | Truncated normal‡ | 56, 59, 60 |
| Visual acuity 3 | 0.00 | 0.00 to 1000.00 |
Truncated normal‡ | 56, 59, 60 |
| Visual acuity 4 | 2867. 00 |
1000.00 to 5000.00 |
Truncated normal‡ | 56, 59, 60 |
| Visual acuity 5 | 2867. 00 |
1000.00 to 5500.00 |
Truncated normal‡ | 56, 59, 60 |
| Visual acuity 6 | 4315. 00 |
1500.00 to 6000.00 |
Truncated normal‡ | 56, 59, 60 |
| Annual caregiver costs∥ | ||||
| Visual acuity 1 | 267.4 1 |
0.00 to 500.00 | Truncated normal‡ | 61, 62 |
| Visual acuity 2 | 1621. 00 |
0.00 to 2000.00 |
Normal | 61, 62 |
| Visual acuity 3 | 4308. 00 |
2000.00 to 6000.00 |
Normal | 61, 62 |
| Visual acuity 4 | 4308. 00 |
2000.00 to 7000.00 |
Normal | 61, 62 |
| Visual acuity 5 | 13 727.0 0 |
8000.00 to 15 000.00 |
Normal | 61, 62 |
| Visual acuity 6 | 19 991.0 0 |
10 000.00 to 25 000.00 |
Normal | 61, 62 |
| Utility estimates ¶ | ||||
| DME alone | ||||
| Visual acuity 1 | 0.80 | 0.76 to 0.92 | β | 58 |
| Visual acuity 2 | 0.70 | 0.69 to 0.92 | β | 58 |
| Visual acuity 3 | 0.66 | 0.66 to 0.92 | β | 58 |
| Visual acuity 4 | 0.61 | 0.59 to 0.84 | β | 58 |
| Visual acuity 5 | 0.57 | 0.57 to 0.78 | β | 58 |
| Visual acuity 6 | 0.53 | 0.55 to 0.71 | β | 58 |
| Arterial thromboembolic event | ||||
| Acute utility decrement | −0.42 | −0.24 to −0.90 | β | 75 † |
| Recurrent utility decrement | −0.29 | −0.04 to −1.00 | β | 75 † |
| Other major complications | ||||
| Acute decrement | −0.60 | −0.30 to −0.70 | β | 76†, clinical assumption |
| Glaucoma (recurrent decrement) | ||||
| Controlled/mild | −0.05 | 0.00 to −0.20 | β | 77 † |
| Uncontrolled/severe | −0.10 | 0.00 to −0.35 | β | 77 † |
| Cataract | ||||
| Acute decrement | −0.20 | 0.00 to −0.50 | β | 77 † |
| Other minor complications | ||||
| Acute decrement | −0.05 | 0.00 to −0.20 | β | Clinical assumption |
DME = diabetic macular edema; FA= fluorescein angiography; NA = not applicable; OCT = optical coherence tomography; VEGF = vascular endothelial growth factor.
Costs are reported in 2010 U.S. dollars
Additional references in Supplement (available at www.annals.org).
In cases where values on a normal distribution would fall below 0, the distribution was truncated to a minimum value of 0.
Costs of blindness were obtained from reference 60. Estimates were derived from the costs of any Medicare service not related to eye care or performed by an ophthalmologist/optometrist on the basis of Medicare claims data. Costs were adjusted for potential confounders via multivariate regression modeling and calculated as the amount in excess of non–eye-related costs with normal vision. Highlighted costs of blindness included injury, depression, and skilled nursing/long-term care facility utilization.
Caregiver costs were obtained from reference 61. Estimated hours per week of caregiver assistance were based on results of the Age-Related Macular Degeneration Health and Impact Questionnaire administered to a sample of age-related patients with macular degeneration. The cost of caregiver time was calculated as forfeited wages, from Consumer Price Index–adjusted U.S. Bureau of Labor Statistics hourly nonfarm, nonsupervisory seasonally adjusted wages (62).
Utility decrements represent the amount by which the quality-of-life utility in a given state is lessened because of a complication. In the case of an acute decrement, the amount shown is subtracted from quality of life only in the cycle (month) in which the complication occurs; a recurrent decrement is applied every month (for complications with chronic effects, such as glaucoma or stroke).
Treatment Effectiveness and Complications
Primary data sources were the multicenter, randomized, double-masked clinical trials RESTORE (Ranibizumab Monotherapy or Combined With Laser Versus Laser Monotherapy for Diabetic Macular Edema) (21), DRCR.net (22–26), READ-2 (Two-Year Outcomes of the Ranibizumab for Edema of the Macula in Diabetes) (27, 28), RISE (A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus) (29, 30), RIDE (A Study of Ranibizumab Injection in Subjects With Clinically Significant Macular Edema With Center Involvement Secondary to Diabetes Mellitus) (29, 30), RESOLVE (Safety and Efficacy of Ranibizumab in Diabetic Macular Edema) (31), and ETDRS (Early Treatment Diabetic Retinopathy Study) (32, 33), totaling 5009 patients. Patient characteristics reasonably reflected those in general clinical practice; however, patients in clinical trials had more homogeneous visual impairment and better-controlled diabetes. Dosage, treatment frequency, and follow-up also vary in practice. We used smaller studies in sensitivity analyses to explore these differences (35–48).
We modeled key complications individually: arterial thromboembolic events, glaucoma (controlled and severe or uncontrolled), and cataracts. We divided complications into major (for example, endophthalmitis) and minor (for example, eye irritation) categories (Supplement). We captured 1-time costs and quality-of-life decrements for acute complications, and monthly costs and quality-of-life decrements for chronic complications (Table 2).
In the main analysis, we assumed treatment of the better eye for a direct and predictable effect on vision-related quality of life. Treatment was received for 1 year except when stopped earlier because of complications (arterial thromboembolic events or uncontrolled glaucoma). We calibrated the model to match the average and the distribution of number of treatments for each strategy in clinical trials (Supplement).
We selected a 1-year treatment period for consistency with primary trial end points and because greater crossover among treatment groups occurred in extended 2- and 3-year follow-up. For progression of DME after treatment, we modeled visual decline at a slower rate in strategies involving laser treatment (because clinical benefit from this therapy can be long-standing, in contrast to the short-term effect of injections) (49). We evaluated each assumption in sensitivity analyses.
Additional simplifying assumptions included no crossover among treatments and no treatment resumption once a treatment had been discontinued (because of an arterial thromboembolic event or uncontrolled glaucoma or—in the main analysis—after 1 year of therapy). We made these assumptions to keep the modeling tractable and because of limited data (Supplement). We validated clinical outcomes by comparing the outcomes of the no-treatment group with the expected cumulative risk for blindness with untreated DME (Table 2 of the Supplement).
Mortality
We obtained age- and sex-specific death rates, adjusted for diabetes or diabetic retinopathy, from U.S. life tables (Table 2) (50). We assumed DME and its treatments did not directly affect mortality and validated life-expectancy projections against clinical trials. We evaluated alternative mortality scenarios in sensitivity analyses (Supplement).
Quality of Life
Our primary health outcome was quality-adjusted life-years (QALYs) gained, allowing comparison with other treatments that extend life and improve health in various domains (51). Previous studies have correlated quality-of-life weights with visual acuities, using time-tradeoff and standard gamble methods (52–55). We selected the most commonly cited utility values in the ophthalmology cost-effectiveness literature (56, 57) for our main analysis, computed from the following equation (58):
This was the best-fit equation from multiple linear regression modeling of time-tradeoff utilities for patients across a spectrum of eye diseases, including diabetic retinopathy, age-related macular degeneration, and retinal dystrophies (58). Our inputs (Table 2) correlated well with other published quality-of-life estimates. We varied utilities in sensitivity analyses (Tables 7 and 8 of the Supplement).
Costs
We obtained costs from published Medicare reimbursement and expenditures (7, 8) and average wholesale prices (9–11). We adjusted costs to 2010 U.S. dollars by using Consumer Price Index deflators (59). Our main analysis included direct and indirect (time) costs (Table 2). We obtained time costs, which comprised costs from blindness and caregiver costs, from the literature. Costs from blindness represented non–eye-related medical costs in excess of those in patients with normal vision (for example, from falls or depression) (56, 59, 60). Caregiver costs represented caregiver time: lost potential wages based on U.S. Bureau of Labor Statistics data, up to $19 991 annually (visual acuity category 6 in our main analysis) (61, 62).
Statistical Analysis
All analyses used TreeAge Pro 2009 (Williamstown, Massachusetts). Additional technical details are available in the Supplement.
Cost-Effectiveness Analysis
Our primary outcome was the incremental cost-effectiveness ratio (ICER) of cost per QALY gained. Secondary outcomes included treatments, visual changes, complications, and life expectancy. We discounted costs and benefits 3% annually over a lifetime horizon (63).
Sensitivity Analyses
Sensitivity analyses assessed whether the main findings depended critically on specific assumptions or inputs. We conducted more than 250 deterministic sensitivity analyses. Probabilistic sensitivity analysis to quantify total uncertainty involved randomly sampling from independent distributions for each model input 1000 times, repeating the main analysis with each set of samples. Generally, we assumed β distributions for probabilities and utilities and normal distributions for costs (Table 2). Table 23 of the Supplement shows uncertainty ranges.
Role of the Funding Source
The Agency for Healthcare Research and Quality had no role in the analysis or reporting of the data or the design or conduct of the study.
RESULTS
Visual Acuity Outcomes and Complications
Table 3 shows the effect of each strategy on visual acuity outcomes, complications, costs, and QALYs. Combination therapy with laser treatment plus triamcinolone yielded the most time with visual acuity of 20/30 or better. However, VEGF inhibitor treatment, as monotherapy or combined with laser treatment, yielded the longest length of life with any improvement in visual acuity over baseline. Outcomes depended on the use of laser treatment, such that VEGF inhibitor strategies resulted in either a slightly higher or a lower lifetime risk for blindness than combination therapy with laser treatment plus triamcinolone after 1 year of treatment (Table 3; Table 2 of the Supplement). All strategies that included triamcinolone had the highest risk for nonminor complications because of a greater risk for glaucoma and cataract. Patients received approximately 8 VEGF inhibitor injections, 2 to 3 triamcinolone injections, or 2 to 3 laser treatments during the first year for each strategy.
Table 3.
Model Results: 1-Year Treatment and Lifetime Treatment
| Treatment | Average Procedures, n |
Complications, Excluding Minor Complications, n* |
Total Time Spent With Blindness (Visual Acuity 20/200 or worse), y†‡ |
Total Time Spent With Stable or Improved Visual Acuity, y‡ |
Total Time Spent With ≥5 Letters of Visual Acuity Improvement, y‡ |
Total Time Spent With Visual Acuity 20/30 or better, y‡ |
Lifetime Costs, $ |
Lifetime QALYs |
ICER, $/QALY§ |
|---|---|---|---|---|---|---|---|---|---|
| 1-y treatment∥ | |||||||||
| Laser treatment plus triamcinolone |
2.9 laser treatments and 2.9 injections |
0.32 | 0.10 | 13.63 | 13.06 | 10.61 | 97 998 | 5.63 ¶ |
(Reference
strategy) ** |
| Laser treatment plus a VEGF inhibitor |
1.8 laser treatments and 8.2 injections |
0.11 | 0.03 | 13.76 | 13.28 | 10.57 | 104 973 | 6.19 | 12 410 |
| Monotherapy with a VEGF inhibitor |
8.3 injections | 0.05 | 0.02 | 13.74 | 13.17 | 9.64 | 106 213 | 6.16 | (Dominated) †† |
| Triamcinolone monotherapy | 1.8 injections | 0.13 | 0.12 | 11.94 | 3.21 | 0.66 | 167 162 | 4.33 ¶ | (Dominated) †† |
| No treatment | None | 0.00‡‡ | 0.12 | 12.42 | 0.00 | 0.00 | 169 469 | 4.58 | (Dominated) †† |
| Laser monotherapy | 1.9 laser treatments |
0.04 | 0.12 | 11.75 | 3.47 | 0.75 | 170 027 | 4.66 | (Dominated) †† |
| Lifetime treatment §§ | |||||||||
| Laser treatment plus triamcinolone |
2.9 laser treatments and 13.7 injections |
0.59 | 0.10 | 13.66 | 13.09 | 12.52 | 97 493 | 5.16 ¶ |
(Reference
strategy) ** |
| Laser treatment plus a VEGF inhibitor |
1.8 laser treatments and 40.6 injections |
0.33 | 0.03 | 13.79 | 13.33 | 12.46 | 126 082 | 6.24 | 26 477 |
| Monotherapy with a VEGF inhibitor |
40.7 injections | 0.27 | 0.01 | 13.78 | 13.27 | 12.26 | 133 126 | 6.23 | (Dominated) †† |
| Triamcinolone monotherapy | 12.6 injections | 0.45 | 0.12 | 12.70 | 3.44 | 0.85 | 162 831 | 3.73 ¶ | (Dominated) †† |
| Laser monotherapy | 6.3 laser treatments | 0.15 | 0.12 | 12.23 | 3.64 | 0.89 | 167 341 | 4.70 | (Dominated) †† |
| No treatment | None | 0.00‡‡ | 0.12 | 12.42 | 0.00 | 0.00 | 169 469 | 4.58 | (Dominated) †† |
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year; VEGF = vascular endothelial growth factor.
Number of complications occurring during treatment, in excess of baseline.
Values were normalized to the no-treatment group to adjust for starting visual acuity distributions.
Undiscounted, over lifetime follow-up.
Relative to the next best alternative.
Treatment administered over 1 y, with subsequent lifetime follow-up.
Unlike in other strategies, the lifetime QALYs associated with triamcinolone therapies decrease in going from the 1-y treatment model to the lifetime model. This is due to an increased likelihood of glaucoma and cataract with lifetime treatment (see Complications section in the Supplement, available at www.annals.org, for further details).
Baseline comparator for calculation of ICER. Represents the reference point on the cost-effectiveness frontier (Figure 2).
Costs more and is less effective than another strategy.
Baseline defined by the number of complication events in the untreated (clinical observation) group.
Treatment administered over lifetime.
QALYs
Combination therapy with laser treatment plus a VEGF inhibitor achieved the largest overall gains in QALYs, closely followed by monotherapy with a VEGF inhibitor; this finding is consistent with clinical outcomes (Table 3 and Figure 2). No treatment, laser monotherapy, and triamcinolone monotherapy achieved substantially fewer QALYs gained than the other management strategies. Because of complications, triamcinolone monotherapy was inferior to no treatment.
Figure 2.
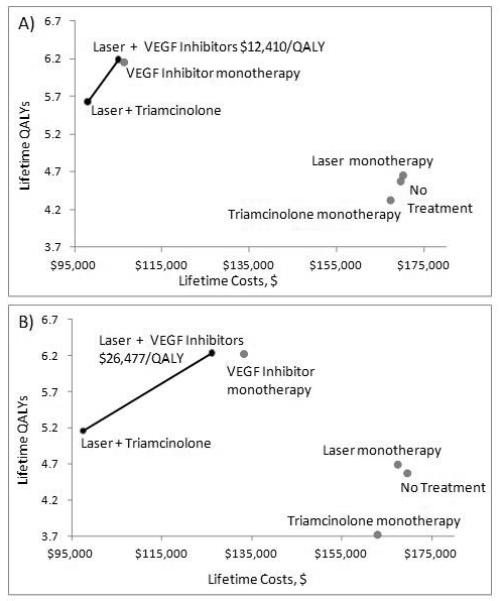
One-Year Treatment and Lifetime Treatment Cost-Effectiveness Frontiers
Representing the discounted lifetime costs and QALYs associated with the six strategies.
(A) represents the main analysis, one year of treatment with lifetime followup
(B) represents lifetime treatment with 3 VEGF-inhibit or injections per year (monotherapy or combination therapy), 1 triamcinolone injection per year (monotherapy or combination therapy), 1 laset treatment every other year in laser montherapy, and no additional laser treatments in combination therapy strategies.
The solid black line indicates the cost-effectiveness fronties, which represents the most cost-effective series of strategies (achieving the greatest relative benefit for the lowest cost). Incremental cost-effectiveness ratios (ICERs) are indicated in $/QALY, representing the cost of additional effectiveness relative to the next best strategy (here, the preferred strategy of laser + VEGF-inhibitors is compared to laser + triamcinolone, with an ICER of $12,410/QALY with on year of treatment, and an ICER of $26,477 with treatment over a lifetime). The strategies that form the cost-effectiveness frontier (laser + triamcinolone and laser + VEGF-inhibitors, each depicted with a black dot) dominate those to the right of the frontier (gray dots) because they are either more effective and costless (strong dominance), or have better cost-effectiveness ratio (weak dominace).
VEGF=Vascular Endothelial Growth Factor
QALY=Quality-Adjusted Life Year
Cost-Effectiveness
All therapies were cost-saving compared with no treatment, except for laser monotherapy, which had lifetime costs similar to no treatment in the main analysis (Figure 2 [top] and Table 3). Combination therapy with laser treatment plus a VEGF inhibitor cost $12 410 per QALY gained over combination therapy with laser treatment plus triamcinolone with 1 year of treatment (Figure 2, top) and $24 477 per QALY gained with lifetime treatment (Figure 2, bottom). Injections with a VEGF inhibitor yielded more health benefits at lower cost than laser monotherapy, triamcinolone monotherapy, and no treatment and seemed more cost-effective in combination with laser treatment than as monotherapy because monotherapy was slightly less effective and slightly more expensive (Table 3 and Figure 2).
Sensitivity Analyses
The most important clinical uncertainties surrounding treatment of DME include whether combination therapy with laser treatment plus a VEGF inhibitor is superior to monotherapy with a VEGF inhibitor and which types of patients may be better suited for monotherapy; the choice of VEGF inhibitor, given their vastly different costs and adverse effect profiles; and the benefits and costs that may accrue if treatment is continued over a patient’s lifetime or extended to include the patient’s other eye.
Choosing Between Monotherapy With a VEGF Inhibitor and Combination Therapy With Laser Treatment Plus a VEGF Inhibitor
Although the main analysis clearly indicates that combination therapy and VEGF inhibitors are cost-effective for DME, the advantage of combination therapy with laser treatment over monotherapy with VEGF inhibitors is less certain. The lifetime differences in costs and benefits of combination therapy versus monotherapy are only 0.03 QALYs (11 quality-adjusted life-days) and $1240. Monotherapy with a VEGF inhibitor becomes cost-effective in patients with DME who experience smaller quality-of-life decrements in progressing from moderate to severe vision loss, who are less likely to lose vision, or whose rate of visual decline is unlikely to be slowed by laser treatment (Tables 5 to 8 of the Supplement) (11, 12). Furthermore, monotherapy with a VEGF inhibitor becomes cost-effective if the cost of a laser treatment exceeds that of a VEGF inhibitor injection (Supplement).
However, combination therapy with laser treatment plus a VEGF inhibitor performs better than monotherapy with a VEGF inhibitor even if 0.5 mg of ranibizumab is used (an alternative dose in clinical practice, as opposed to 0.3 mg in our base case). Our main analysis may actually understate the benefits and cost-effectiveness of combination therapy with a VEGF inhibitor because the RESTORE trial provided our estimates of the effectiveness of monotherapy (21) and many RESTORE patients had received laser treatment prior to the trial, potentially augmenting the benefit from VEGF injections. This finding effectively made monotherapy outcomes closer to those of combination therapy in the DRCR.net trial, narrowing the apparent difference in effectiveness between monotherapy and combination therapy—a potential source of bias in favor of higher effectiveness from monotherapy with a VEGF inhibitor. Even so, we believe that the effect estimate from the RESTORE trial is appropriate for use in the main analysis because it better reflects clinical practice in which many patients have received laser treatment.
Choice of a VEGF Inhibitor
Bevacizumab
Bevacizumab is also effective for DME at approximately 4% of the cost of ranibizumab, although it is not currently approved by the U.S. Food and Drug Administration for treatment of DME and concerns have been raised about increased risks for endophthalmitis or vascular complications related to systemic absorption (Supplement). Given noninferiority to ranibizumab in CATT (Comparison of Age-Related Macular Degeneration Treatments Trial) (64, 65) and results of smaller trials with bevacizumab in patients with DME (35, 66, 67), we repeated analyses using bevacizumab prices with and without doubled risk for endophthalmitis, recognizing recent cases of endophthalmitis attributed to contaminated bevacizumab (68). Even after doubling the risk for endophthalmitis with bevacizumab, laser treatment plus ranibizumab cost more than $3.5 million per QALY gained compared with laser treatment plus bevacizumab (Table 15 of the Supplement).
Data from CATT and other trials have raised the question of whether bevacizumab has a higher mortality rate than ranibizumab (possibly from greater systemic absorption of bevacizumab). Laser treatment plus ranibizumab may be more cost-effective (Table 15 of the Supplement) if bevacizumab yields a hazard ratio for death greater than 2.6 (vs. 2.2 with all other strategies). However, bevacizumab-associated mortality is unlikely to be this marked (64, 65).
VEGF Trap-Eye
Although not approved by the U.S. Food and Drug Administration for DME, VEGF Trap-Eye is another VEGF-targeted agent used clinically. Its cost-effectiveness depends on dose, cost, and frequency of treatment (Tables 18 to 20 of the Supplement). Relative to laser treatment plus triamcinolone, VEGF Trap-Eye yielded an ICER of $23 519 when 2 mg was administered and $3616 when 0.5 mg was administered (over 1 year as in the DA VINCI (DME And VEGF Trap-Eye: Investigation of Clinical Impact) trial (36). Just as no dose-related difference in outcomes was seen in the DA VINCI trial, alternative 1-year treatment outcomes did not change our conclusions. With lifetime treatment, lower-dose and less-frequent VEGF Trap-Eye may be more cost-effective than ranibizumab if efficacy is the same.
Bilateral Disease
Diabetic macular edema often affects both eyes, although it may be asymmetric. However, data are not available to estimate utility gains from treating both eyes (such benefits as improved stereopsis or wider visual field). Although our main analysis assumed treatment of the eye with the better visual acuity, we used 2 approaches to consider treatment of both eyes.
Assuming symmetric disease, bilateral treatment, and doubled treatment costs, combination therapy with laser treatment plus a VEGF inhibitor cost $28 236 per QALY gained over combination therapy with laser treatment plus triamcinolone. Alternatively, if the need for therapy to the second eye is assumed to be more modest by using a method accepted by the National Institute for Health and Care Excellence (multiplying the 1-eye ICER by 1.5) (12), combination therapy with laser treatment plus a VEGF inhibitor costs $18 615 per additional QALY.
Lifetime Treatment
Because most patients will receive treatment for longer than 1 year, we also considered different treatment frequencies over a lifetime. Vision was assumed to remain stable while receiving treatment (extrapolating from the DRCR.net and READ-2 trials, in which patients maintained benefit at 24 and 36 months despite declining frequency of treatment) (23–28).
On the basis of data from the DRCR.net trial, we assumed 3 VEGF inhibitor injections and 1 triamcinolone injection per year, 1 laser treatment every other year when laser was given as monotherapy, and no additional laser treatments with combination therapy (Supplement) (23–26). In this scenario, combination therapy with laser treatment plus a VEGF inhibitor was preferred, costing $26 477 per QALY gained over combination therapy with laser treatment plus triamcinolone (Table 3 and Figure 2 [bottom]). However, if additional laser treatments are required to maintain vision in patients receiving combination therapy, monotherapy with a VEGF inhibitor may be superior, reflecting laser-associated complications and costs (Table 16 and Figure 1 of the Supplement). Strategies involving VEGF inhibitors were consistently preferred even when continuing treatment at the same frequency as in the first year (Tables 16 and 17 of the Supplement).
Additional Deterministic Sensitivity Analyses
The preferred strategy remained unchanged from the main analysis in scenarios examining alternative assumptions about treatment outcomes, complications, glycemic control, cataract, glaucoma, and mortality (Table 2; Tables 4 to 22 of the Supplement).
Probabilistic Sensitivity Analysis
Allowing for uncertainty of all model variables simultaneously, VEGF inhibitor injections, with or without laser treatment, were cost-effective in 97.1% of simulations using a willingness-to-pay threshold of $50 000 per QALY (59.5% probability of combination therapy with laser treatment plus a VEGF inhibitor, 37.6% probability of monotherapy with a VEGF inhibitor, and 2.9% probability of combination therapy with laser treatment plus triamcinolone being most cost-effective, respectively). With a $100 000 willingness-to-pay threshold, VEGF inhibitor strategies were cost-effective in 99.7% of simulations (58.4% probability of combination therapy with laser treatment plus a VEGF inhibitor, 42.3% probability of monotherapy with a VEGF inhibitor, and 0.3% probability of combination therapy with laser treatment plus triamcinolone being most cost-effective, respectively) (Figure 2 of the Supplement).
Discussion
Diabetic macular edema is a major cause of diabetes-related morbidity, and diagnosis and treatment depend on timely referral from primary care clinicians. Our analysis indicates that treatment is cost-saving (except for laser monotherapy, which has costs similar to no treatment in our main analysis) and that all treatments other than triamcinolone monotherapy increase quality of life and lifetime QALYs. With lifetime treatment, all treatment strategies were cost-saving compared with no treatment. A visual “review of systems” and regular referrals to an ophthalmologist are important, because patients may not volunteer symptoms of DME (mild visual decline, distortion, or diminished contrast sensitivity). Dilated ophthalmologic examinations are necessary to identify DME, and early treatment has the best prognosis for visual recovery.
Other recent cost-effectiveness analyses addressing DME lack consensus about preferred treatment (12–14). In evaluating industry-modeled analysis of DME treatments, the National Institute for Health and Clinical Excellence did not recommend covering ranibizumab, citing model limitations and insufficient evidence of cost-effectiveness (12). Our analysis explicitly addressed these limitations— including treatment beyond 1 year, disease progression after treatment, and uncertainty about bilateral disease—by relying on assumptions, including regular treatment, maintenance of treatment-induced visual gain, and estimation techniques for bilateral disease. We ultimately found that combination therapy with laser treatment plus a VEGF inhibitor provides reasonable value.
The most cost-effective treatment in our analysis was combination therapy with laser treatment plus injections of a VEGF inhibitor, with other reasonable options being monotherapy with a VEGF inhibitor and combination therapy with laser treatment plus triamcinolone. However, laser monotherapy and triamcinolone monotherapy were not cost-effective (lifetime costs and quality of life were similar to those of no treatment). The superiority of combination therapy to laser monotherapy or triamcinolone monotherapy reflects better visual outcomes in clinical trials (Table 4 of the Supplement).
Our analysis is limited by outcomes for strategies obtained from different publications; however, most relevant trials were part of the DRCR.net study. Furthermore, laser treatment and triamcinolone act via different mechanisms and it is clinically reasonable to expect that combining the 2 treatments yields a better result than monotherapy with each. For example, reduction of acute edema from triamcinolone may improve laser uptake and efficacy.
Combination therapy with laser treatment plus a VEGF inhibitor remained cost-effective in many sensitivity analyses that reflected clinical uncertainties. However, because of lack of available data, we had limited ability to model treatment outcomes for some clinical uncertainties, including treatment for both eyes, crossover from 1 treatment to another, and lifelong treatment. We aimed to make clinically realistic assumptions to evaluate these scenarios. For example, we used 2 methods to estimate the cost-effectiveness of treating both eyes and found that VEGF inhibitors remained cost-effective regardless of the method chosen. Combination therapy with laser treatment plus a VEGF inhibitor also remained cost-effective in most lifelong treatment scenarios assuming treatment with 0.3 mg of ranibizumab. However, if treatment is more frequent or less effective than in the main analysis, lifetime combination therapy with laser treatment may not be superior to monotherapy with a VEGF inhibitor.
Monotherapy with a VEGF inhibitor achieved outcomes and had costs similar to those of combination therapy with laser treatment plus a VEGF inhibitor. The modest additional benefit of laser treatment may be understated in our model because effectiveness estimates for monotherapy with a VEGF inhibitor were based on trials in which many patients had received laser treatment before—therefore being less likely to show large benefits from combination therapy relative to VEGF inhibitor monotherapy. The benefits of combination therapy may vary among patients, according to their relative disutility from visual decline versus risks for adverse effects. Furthermore, these modest additional benefits are achieved at a relatively small additional cost, which reflects the low cost of laser treatment relative to the cost of injections with a VEGF inhibitor.
Choosing between monotherapy with a VEGF inhibitor and combination therapy with laser treatment plus a VEGF inhibitor may ultimately depend on patient preferences. Laser treatment has potential complications and adds small incremental benefit to VEGF inhibitor therapy; thus, persons with different relative values for complications, blindness, and alternative outcome measures for improvement of visual acuity may prefer monotherapy with a VEGF inhibitor. For example, patients may be better suited to injections alone if more frequent injections and/or less visual improvement are preferred to the risk for laser complications. Incorporating patient preferences via shared decision making is an area in which primary care clinicians can play an especially valuable role. An understanding of treatment risks, benefits, and tradeoffs can help primary care providers respond to patients’ questions.
Choice of specific VEGF inhibitor remains controversial. If less-expensive, off-label bevacizumab is available and is as efficacious as ranibizumab, it is preferred because of its substantially lower price. We believe that it is a reasonable and highly cost-effective option. The cost-effectiveness of VEGF Trap-Eye, by comparison, varies with dose, cost, and relative frequency of treatment (36). Meta-analysis or other guidance about VEGF inhibitors would be valuable.
Our analysis ultimately indicates that VEGF inhibitors with or without laser treatment provide important health benefits with favorable cost-effectiveness, costing less per QALY gained than many accepted therapies. Appropriate treatment reduces societal costs and substantially improves quality of life compared with undiagnosed or untreated DME. This finding highlights the need for referral and coordination between primary care providers and ophthalmologists, enabling diagnosis and early management of this visually disabling condition.
From Stanford Health Policy, Center for Health Policy and Center for Primary Care and Outcomes Research, Stanford University, Stanford, California; Byers Eye Institute at Stanford University, Stanford University School of Medicine, and Veterans Affairs Palo Alto Health Care System, Palo Alto, California; and University of Minnesota School of Public Health, Minneapolis, Minnesota.
Supplementary Material
Acknowledgment
The authors thank Dr. Mark S. Blumenkranz for guidance and manuscript review and Dr. John Wong for statistical review.
Financial Support: By grant T32-HS000028 from the Agency for Healthcare Research and Quality and a National Institutes of Health National Institute on Aging Career Development Award (K01 AG037593-01A1; Dr. Goldhaber-Fiebert, principal investigator). Dr. Owens was supported by the U.S. Department of Veterans Affairs. This research was also supported in part by the Office of the Dean, Stanford Medical School, Stanford Society of Physician Scholars.
Footnotes
This material was presented at the 34th Annual Meeting of the Society of Medical Decision Making, Phoenix, Arizona, 17–19 October 2012.
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the views of the National Institutes of Health, Agency for Healthcare Research and Quality, or U.S. Department of Veterans Affairs. Discussion of DRCR.net data, as cited in this publication, is by the authors and is in no way affiliated or endorsed by DRCR.net. Additional data provided by DRCR.net is not an indication that DRCR.net has made any statement as to the validity of these analyses or interpretations.
Potential Conflicts of Interest: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M13-0768.
Current Author Addresses: Dr. Pershing: 2452 Watson Court, Palo Alto, CA 94303. Dr. Enns: University of Minnesota, Division of Health Policy and Management, MMC 729 Mayo, Campus Mail Code 8729A, 420 Delaware Street SE, Minneapolis, MN 55455. Mr. Matesic: 316 Grant Avenue, Palo Alto, CA 94306. Dr. Owens and Goldhaber-Fiebert: Stanford University, Center for Health Policy and Center for Primary Care and Outcomes Research, 117 Encina Commons, Stanford, CA 94305.
Author Contributions: Conception and design: S. Pershing, J.D. Goldhaber-Fiebert, D.K. Owens.
Analysis and interpretation of the data: S. Pershing, E.A. Enns, J.D. Goldhaber-Fiebert.
Drafting of the article: S. Pershing, Critical revision of the article for important intellectual content: S. Pershing, E.A. Enns, J.D. Goldhaber-Fiebert, D.K. Owens.
Final approval of the article: S. Pershing, E.A. Enns, J.D. Goldhaber-Fiebert, D.K. Owens, B. Matesic.
Provision of study materials or patients:
Statistical expertise: S. Pershing, E.A. Enns, J.D. Goldhaber-Fiebert.
Obtaining of funding:
Administrative, technical, or logistic support:
Collection and assembly of data: S. Pershing.
Context Current therapies for diabetic macular edema include laser treatment, intraocular injections of triamcinolone or drugs that inhibit vascular endothelial growth factor (VEGF), and combinations of laser treatment plus injections of triamcinolone or a VEGF inhibitor.
Contribution The investigators compared the lifetime costs and effectiveness of alternative treatments by using mathematical models that incorporated what was already known about each treatment.
Caution Long-term outcome data are limited.
Implication The most effective treatment of diabetic macular edema is VEGF inhibitor injection with or without laser treatment. This therapy is as cost-effective as acceptable treatments for many other conditions. —The Editors
References
- 1.Centers for Disease Control and Prevention . National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States, 2011. Centers for Disease Control and Prevention; Atlanta, GA: Dec 8, 2011. Accessed at www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf. 2011. [Google Scholar]
- 2.Williams R, Airey M, Baxter H, Forrester J, Kennedy-Martin T, Girach A. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond) 2004;18:963–83. doi: 10.1038/sj.eye.6701476. [PMID: 15232600] [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII: the twenty-five-year incidence of macular edema in persons with type 1 diabetes. Ophthalmology. 2009;116:497–503. doi: 10.1016/j.ophtha.2008.10.016. [PMID: 19167079] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Moss SE. The epidemiology of ocular problems in diabetes mellitus. In: Ferman SS, editor. Ocular Problems in Diabetes Mellitus. Blackwell Scientific; Boston: 1991. pp. 1–51. [Google Scholar]
- 5.Gangnon RE, Davis MD, Hubbard LD, Aiello LM, Chew EY, Ferris FL, 3rd, et al. Early Treatment Diabetic Retinopathy Study Research Group. A severity scale for diabetic macular edema developed from ETDRS data. Invest Ophthalmol Vis Sci. 2008;49:5041–7. doi: 10.1167/iovs.08-2231. [PMID: 18539929] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shea AM, Curtis LH, Hammill BG, Kowalski JW, Ravelo A, Lee PP, et al. Resource use and costs associated with diabetic macular edema in elderly persons. Arch Ophthalmol. 2008;126:1748–54. doi: 10.1001/archopht.126.12.1748. [PMID: 19064859] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Medicare & Medicaid Services Medicare ASP Drug Pricing Files, October 2010 Update. Accessed at www.cms.gov/McrPartBDrugAvgSalesPrice/ on 18 September 2012.
- 8.Centers for Medicare & Medicaid Services Medicare Outpatient Addendum A and B Updates. Accessed at www.cms.gov/HospitalOutpatientPPS/AU/list.asp#TopOfPage on 8 December 2011.
- 9.Physicians’ Desk Reference . Red Book: Pharmacy’s Fundamental Reference. Thomson-Reuters PDR Network; Montvale, NJ: 2010. Edition. 2010. [Google Scholar]
- 10.Levinson DR. [Last accessed 11/5/2013];Department of Health and Human Services Review of Medicare Part B Avastin and Lucentis Treatments for Age-Related Macular Degeneration (A-01-10-00514) 2011 Sep 6; Accessed at http://oig.hhs.gov/oas/reports/region10/11000514.pdf.
- 11.Alcon [Last accessed 11/5/2013];Triesence Suspension (triamcinolone acetonide injectable suspension) 40 mg/mL Coding and Reimbursement Fact Sheet. Accessed at https://www.myalcon.com/docs/ars-TRIESENCE-fact-sheet-single.pdf.
- 12.National Institute for Health and Clinical Excellence National Institute for Health and Clinical Excellence Final Appraisal Determination: Ranibizumab for the Treatment of Macular Oedema. 2011 Jul; Accessed at www.nice.org.uk/nicemedia/live/13125/55324/55324.pdf on 30 June 2012.
- 13.Mitchell P, Annemans L, Gallagher M, Hasan R, Thomas S, Gairy K, et al. Cost-effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. Br J Ophthalmol. 2012;96:688–93. doi: 10.1136/bjophthalmol-2011-300726. [PMID: 22399690] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dewan V, Lambert D, Edler J, Kymes S, Apte RS. Cost-effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2012;119:1679–84. doi: 10.1016/j.ophtha.2012.01.049. [PMID: 22503301] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frazier AL, Colditz GA, Fuchs CS, Kuntz KM. Cost-effectiveness of screening for colorectal cancer in the general population. JAMA. 2000;284:1954–61. doi: 10.1001/jama.284.15.1954. [PMID: 11035892] [DOI] [PubMed] [Google Scholar]
- 16.Ruiz MS, Committee on HIV Prevention Strategies in the United States . No Time to Lose: Getting More from HIV Prevention. National Academies Pr; Washington, DC: 2001. [PubMed] [Google Scholar]
- 17.Gaspoz JM, Coxson PG, Goldman PA, Williams LW, Kuntz KM, Hunink MG, et al. Cost effectiveness of aspirin, clopidogrel, or both for secondary prevention of coronary heart disease. N Engl J Med. 2002;346:1800–6. doi: 10.1056/NEJM200206063462309. [PMID: 12050341] [DOI] [PubMed] [Google Scholar]
- 18.Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005;352:570–85. doi: 10.1056/NEJMsa042657. [PMID: 15703422] [DOI] [PubMed] [Google Scholar]
- 19.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Alliance for Cervical Cancer Prevention Cost Working Group. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [PMID: 16291985] [DOI] [PubMed] [Google Scholar]
- 20.Sanders GD, Hlatky MA, Owens DK. Cost-effectiveness of implantable cardioverter-defibrillators. N Engl J Med. 2005;353:1471–80. doi: 10.1056/NEJMsa051989. [PMID: 16207849] [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. RESTORE study group. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–25. doi: 10.1016/j.ophtha.2011.01.031. [PMID: 21459215] [DOI] [PubMed] [Google Scholar]
- 22.Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, et al. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–1077. doi: 10.1016/j.ophtha.2010.02.031. [PMID: 20427088] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL, 3rd, Friedman SM, et al. Diabetic Retinopathy Clinical Research Network. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118:609–14. doi: 10.1016/j.ophtha.2010.12.033. [PMID: 21459214] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL, 3rd, et al. Diabetic Retinopathy Clinical Research Network. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312–8. doi: 10.1016/j.ophtha.2012.08.022. [PMID: 22999634] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diabetic Retinopathy Clinical Research Network A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–9. doi: 10.1016/j.ophtha.2008.06.015. [PMID: 18662829] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, et al. Diabetic Retinopathy Clinical Research Network (DRCR.net). Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245–51. doi: 10.1001/archophthalmol.2008.610. [PMID: 19273785] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. READ-2 Study Group. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–51. doi: 10.1016/j.ophtha.2010.08.016. [PMID: 20855114] [DOI] [PubMed] [Google Scholar]
- 28.Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. READ-2 Study Group. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2009;116:2175–81. doi: 10.1016/j.ophtha.2009.04.023. [PMID: 19700194] [DOI] [PubMed] [Google Scholar]
- 29.Bandello F, Lang GE, Schlingemann RO, Leclair D, Weichselberger A. [Last accessed 11/5/2013];Pooled safety analysis in patients with visual impairment due to diabetic macular edema treated with 0.5 mg ranibizumab in RESOLVE and RESTORE trials [Abstract] Presented at 47th European Association for the Study of Diabetes Annual Meeting in Lisbon, Portugal, 12–16 September 2011. Abstract 1122. Accessed at http://www.abstractsonline.com/Plan/ViewAbstract.aspx?sKey=3af4f519-dbbd-4b2d-8fe2-9e5360e8b8fd&cKey=e4bdbd0c-750d-40b7-9753-b1c0ea0bef7f&mKey=%7bBAFB2746-B0DD-4110-8588-E385FAF957B7%7d.
- 30.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [PMID: 22330964] [DOI] [PubMed] [Google Scholar]
- 31.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [PMID: 20980427] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796–806. [PMID: 2866759] [PubMed] [Google Scholar]
- 33.Photocoagulation for diabetic macular edema Early Treatment Diabetic Retinopathy Study Report no. 4. The Early Treatment Diabetic Retinopathy Study Research Group. Int Ophthalmol Clin. 1987;27:265–72. doi: 10.1097/00004397-198702740-00006. [PMID: 3692708] [DOI] [PubMed] [Google Scholar]
- 34.Enns EA, Pershing S, Wang Y, Goldhaber-Fiebert JD. [Last accessed 11/5/2013];Calibration methods for inferring transition probabilities from cross-sectional studies [Abstract] Presented at 34th Annual Meeting of the Society for Medical Decision Making, Phoenix, Arizona, 17–20 October 2012. Abstract I-4. Accessed at https://smdm.confex.com/smdm/2012az/webprogram/Paper7077.html.
- 35.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Pan-American Collaborative Retina Study Group. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [PMID: 17398322] [DOI] [PubMed] [Google Scholar]
- 36.Do DV, Nguyen QD, Boyer D, Schmidt-Erfurth U, Brown DM, Vitti R, et al. da Vinci Study Group. One-year outcomes of the da Vinci Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology. 2012;119:1658–65. doi: 10.1016/j.ophtha.2012.02.010. [PMID: 22537617] [DOI] [PubMed] [Google Scholar]
- 37.Michaelides M, Kaines A, Hamilton RD, Fraser-Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117:1078–1086. doi: 10.1016/j.ophtha.2010.03.045. [PMID: 20416952] [DOI] [PubMed] [Google Scholar]
- 38.Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology. 2009;116:1142–50. doi: 10.1016/j.ophtha.2009.01.011. [PMID: 19376585] [DOI] [PubMed] [Google Scholar]
- 39.Fong DS, Strauber SF, Aiello LP, Beck RW, Callanan DG, Danis RP, et al. Writing Committee for the Diabetic Retinopathy Clinical Research Network. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch Ophthalmol. 2007;125:469–80. doi: 10.1001/archopht.125.4.469. [PMID: 17420366] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, Elman MJ, et al. Diabetic Retinopathy Clinical Research Network. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology. 2007;114:1860–7. doi: 10.1016/j.ophtha.2007.05.062. [PMID: 17698196] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faghihi H, Roohipoor R, Mohammadi SF, Hojat-Jalali K, Mirshahi A, Lashay A, et al. Intravitreal bevacizumab versus combined bevacizumab-triamcinolone versus macular laser photocoagulation in diabetic macular edema. Eur J Ophthalmol. 2008;18:941–8. doi: 10.1177/112067210801800614. [PMID: 18988166] [DOI] [PubMed] [Google Scholar]
- 42.Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo-controlled, randomized clinical trial. Graefes Arch Clin Exp Ophthalmol. 2008;246:483–9. doi: 10.1007/s00417-007-0688-0. [PMID: 17917738] [DOI] [PubMed] [Google Scholar]
- 43.Solaiman KA, Diab MM, Abo-Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina. 2010;30:1638–45. doi: 10.1097/IAE.0b013e3181e1ed07. [PMID: 20838357] [DOI] [PubMed] [Google Scholar]
- 44.Goyal S, Lavalley M, Subramanian ML. Meta-analysis and review on the effect of bevacizumab in diabetic macular edema. Graefes Arch Clin Exp Ophthalmol. 2011;249:15–27. doi: 10.1007/s00417-010-1452-4. [PMID: 20665044] [DOI] [PubMed] [Google Scholar]
- 45.Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, Antoszyk A, et al. Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating short-term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina. 2011;31:1009–27. doi: 10.1097/IAE.0b013e318217d739. [PMID: 21394052] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ockrim ZK, Sivaprasad S, Falk S, Roghani S, Bunce C, Gregor Z, et al. Intravitreal triamcinolone versus laser photocoagulation for persistent diabetic macular oedema. Br J Ophthalmol. 2008;92:795–9. doi: 10.1136/bjo.2007.131771. [PMID: 18420749] [DOI] [PubMed] [Google Scholar]
- 47.Gillies MC, Sutter FK, Simpson JM, Larsson J, Ali H, Zhu M. Intravitreal triamcinolone for refractory diabetic macular edema: two-year results of a double-masked, placebo-controlled, randomized clinical trial. Ophthalmology. 2006;113:1533–8. doi: 10.1016/j.ophtha.2006.02.065. [PMID: 16828501] [DOI] [PubMed] [Google Scholar]
- 48.Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, et al. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: a systematic review. Ophthalmology. 2009;116:902–11. doi: 10.1016/j.ophtha.2009.02.002. [PMID: 19410949] [DOI] [PubMed] [Google Scholar]
- 49.Browning DJ. Diabetic Retinopathy: Evidence-Based Management. Springer; New York: 2010. Diabetic macular edema; p. 158. [Google Scholar]
- 50.Arias E. United States life tables, 2006. Natl Vital Stat Rep. 2010;58:1–7. [PMID: 21043319] [PubMed] [Google Scholar]
- 51.Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA. 1996;276:1253–8. [PMID: 8849754] [PubMed] [Google Scholar]
- 52.Sharma S, Brown GC, Brown MM, Hollands H, Shah GK. The cost-effectiveness of grid laser photocoagulation for the treatment of diabetic macular edema: results of a patient-based cost-utility analysis. Curr Opin Ophthalmol. 2000;11:175–9. doi: 10.1097/00055735-200006000-00004. [PMID: 10977223] [DOI] [PubMed] [Google Scholar]
- 53.Brown MM, Brown GC, Sharma S, Shah G. Utility values and diabetic retinopathy. Am J Ophthalmol. 1999;128:324–30. doi: 10.1016/s0002-9394(99)00146-4. [PMID: 10511027] [DOI] [PubMed] [Google Scholar]
- 54.Lloyd A, Nafees B, Gavriel S, Rousculp MD, Boye KS, Ahmad A. Health utility values associated with diabetic retinopathy. Diabet Med. 2008;25:618–24. doi: 10.1111/j.1464-5491.2008.02430.x. [PMID: 18346157] [DOI] [PubMed] [Google Scholar]
- 55.Sharma S, Hollands H, Brown GC, Brown MM, Shah GK, Sharma SM. The cost-effectiveness of early vitrectomy for the treatment of vitreous hemorrhage in diabetic retinopathy. Curr Opin Ophthalmol. 2001;12:230–4. doi: 10.1097/00055735-200106000-00016. [PMID: 11389353] [DOI] [PubMed] [Google Scholar]
- 56.Hurley SF, Matthews JP, Guymer RH. Cost-effectiveness of ranibizumab for neovascular age-related macular degeneration. Cost Eff Resour Alloc. 2008;6:12. doi: 10.1186/1478-7547-6-12. [PMID: 18573218] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma S, Bakal J, Sharma SM, Covert D, Shah GK. Drug pricing for a novel treatment for wet macular degeneration: using incremental cost-effectiveness ratios to ensure societal value. Can J Ophthalmol. 2005;40:369–77. doi: 10.1016/S0008-4182(05)80079-1. [PMID: 15947806] [DOI] [PubMed] [Google Scholar]
- 58.Sharma S, Brown GC, Brown MM, Shah GK, Snow K, Brown H, et al. Converting visual acuity to utilities. Can J Ophthalmol. 2000;35:267–72. doi: 10.1016/s0008-4182(00)80077-0. [PMID: 10959467] [DOI] [PubMed] [Google Scholar]
- 59.U.S. Census Bureau Table 725: Consumer Price Indexes (CPIO) by Major Groups: 1990 to 2010. U.S. Census Bureau, Statistical Abstract of the United States: 2012. Accessed at www.census.gov/compendia/statab/2012/tables/12s0725.pdf on 8 December 2011.
- 60.Javitt JC, Zhou Z, Willke RJ. Association between vision loss and higher medical care costs in Medicare beneficiaries costs are greater for those with progressive vision loss. Ophthalmology. 2007;114:238–45. doi: 10.1016/j.ophtha.2006.07.054. [PMID: 17270673] [DOI] [PubMed] [Google Scholar]
- 61.Schmier JK, Halpern MT, Covert D, Delgado J, Sharma S. Impact of visual impairment on use of caregiving by individuals with age-related macular degeneration. Retina. 2006;26:1056–62. doi: 10.1097/01.iae.0000254890.48272.5a. [PMID: 17151494] [DOI] [PubMed] [Google Scholar]
- 62.U.S. Bureau of Labor Statistics [Last accessed 11/5/2013];Employment and Earnings. 2009 Table B-11 page 74. Accessed at http://www.bls.gov/opub/ee/empearn200912.pdf.
- 63.Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. Oxford Univ Pr; New York: 1996. [Google Scholar]
- 64.Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ, CATT Research Group Ranibizumab and bevacizumab for neovascular age-related macular degeneration. N Engl J Med. 2011;364:1897–908. doi: 10.1056/NEJMoa1102673. [PMID: 21526923] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, et al. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–98. doi: 10.1016/j.ophtha.2020.01.029. [PMID: 22555112] [DOI] [PubMed] [Google Scholar]
- 66.van der Reis MI, La Heij EC, De Jong-Hesse Y, Ringens PJ, Hendrikse F, Schouten JS. A systematic review of the adverse events of intravitreal anti-vascular endothelial growth factor injections. Retina. 2011;31:1449–69. doi: 10.1097/IAE.0b013e3182278ab4. [PMID: 21817960] [DOI] [PubMed] [Google Scholar]
- 67.Curtis LH, Hammill BG, Schulman KA, Cousins SW. Risks of mortality, myocardial infarction, bleeding, and stroke associated with therapies for age-related macular degeneration. Arch Ophthalmol. 2010;128:1273–9. doi: 10.1001/archophthalmol.2010.223. [PMID: 20937996] [DOI] [PubMed] [Google Scholar]
- 68.Pollack A. Avastin injections are reported to cause blindness. New York Times. 2011 Aug 30; Accessed at www.nytimes.com/2011/08/31/health/31drug.html on 8 December 2011.
- 69.Lövestam-Adrian M, Hansson-Lundblad C, Torffvit O. Sight-threatening retinopathy is associated with lower mortality in type 2 diabetic subjects: a 10-year observation study. Diabetes Res Clin Pract. 2007;77:141–7. doi: 10.1016/j.diabres.2006.10.026. [PMID: 17178168] [DOI] [PubMed] [Google Scholar]
- 70.Xu L, Wang YX, Xie XW, Jonas JB. Retinopathy and mortality. The Beijing Eye Study. Graefes Arch Clin Exp Ophthalmol. 2008;246:923–5. doi: 10.1007/s00417-008-0773-z. [PMID: 18299875] [DOI] [PubMed] [Google Scholar]
- 71.Knudtson MD, Klein BE, Klein R. Age-related eye disease, visual impairment, and survival: the Beaver Dam Eye Study. Arch Ophthalmol. 2006;124:243–9. doi: 10.1001/archopht.124.2.243. [PMID: 16476894] [DOI] [PubMed] [Google Scholar]
- 72.Genentech . Lucentis [package insert] Genentech; San Francisco: [Last accessed 11/5/2013]. 2013. Accessed at www.gene.com/gene/products/information/pdf/lucentis-prescribing.pdf. [Google Scholar]
- 73.Genentech, Inc. Lucentis medical information for diabetic macular edema. [Received January 31, 2012].
- 74.Day S, Acquah K, Mruthyunjaya P, Grossman DS, Lee PP, Sloan FA. Ocular complications after anti-vascular endothelial growth factor therapy in Medicare patients with age-related macular degeneration. Am J Ophthalmol. 2011;152:266–72. doi: 10.1016/j.ajo.2011.01.053. [PMID: 21664593] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Freeman JV, Zhu RP, Owens DK, Garber AM, Hutton DW, Go AS, et al. Cost-effectiveness of dabigatran compared with warfarin for stroke prevention in atrial fibrillation. Ann Intern Med. 2011;154:1–11. doi: 10.7326/0003-4819-154-1-201101040-00289. [PMID: 21041570] [DOI] [PubMed] [Google Scholar]
- 76.Clark A, Ng JQ, Morlet N, Tropiano E, Mahendran P, Spilsbury K, et al. Quality of life after postoperative endophthalmitis. Clin Experiment Ophthalmol. 2008;36:526–31. doi: 10.1111/j.1442-9071.2008.01827.x. [PMID: 18954314] [DOI] [PubMed] [Google Scholar]
- 77.Brown MM, Brown GC, Sharma S, Kistler J, Brown H. Utility values associated with blindness in an adult population. Br J Ophthalmol. 2001;85:327–31. doi: 10.1136/bjo.85.3.327. [PMID: 11222340] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


