Abstract
Ethnopharmacological Relevance
The Chinese have used Artemisia annua as a tea infusion to treat fever for > 2,000 yrs. The active component is artemisinin. Previously we showed that when compared to mice fed an equal amount of pure artemisinin, a single oral dose of dried leaves of Artemisia annua (pACT) delivered to Plasmodium chabaudi-infected mice reduced parasitemia at least fivefold. Dried leaves also delivered >40 times more artemisinin in the blood with no toxicity. The pharmacokinetics (PK) of artemisinin delivered from dried plant material has not been adequately studied.
Material and Methods
Healthy and P. chabaudi-infected mice were oral gavaged with pACT to deliver a 100 mg kg−1 body weight dose of artemisinin. Concentrations of serum artemisinin and one of its liver metabolites, deoxyartemisinin, were measured over two hours by GCMS.
Results
The first order elimination rate constant for artemisinin in pACT-treated healthy mice was estimated to be 0.80 hr−1 with an elimination half-life (T½) of 51.6 min. The first order absorption rate constant was estimated at 1.39 hr−1. Cmax and Tmax were 4.33 mg L−1 and 60 min, respectively. The area under the curve (AUC) was 299.5 mg·min L−1. In contrast, the AUC for pACT-treated infected mice was significantly greater at 435.6 mg·min L−1. Metabolism of artemisinin to deoxyartemisinin was suppressed in infected mice over the period of observation. Serum levels of artemisinin in the infected mice continued to rise over the 120 min of the study period, and as a result, the elimination T½ was not determined; the Cmax and Tmax were estimated at ≥ 6.64 mg L−1 and ≥ 120 min, respectively. Groups of healthy mice were also fed either artemisinin or artemisinin mixed in mouse chow. When compared at 60 min, artemisinin was undetectable in the serum of mice fed 100 mg AN kg−1 body weight. When plant material was present either as mouse chow or A. annua pACT, artemisinin levels in the serum rose to 2.44 and 4.32 mg L−1, respectively, indicating that the presence of the plant matrix, even that of mouse chow, had a positive impact on the appearance of artemisinin in the blood.
Conclusions
These results showed that artemisinin and one of its drug metabolites were processed differently in healthy and infected mice. The results have implications for possible therapeutic use of pACT in treating malaria and other artemisinin-susceptible diseases.
Keywords: Plasmodium chabaudi, malaria, Artemisia annua, artemisinin, deoxyartemisinin, pACT
1.0 INTRODUCTION
Malaria is one of the worst vector-borne infectious diseases in the developing world and the World Health Organization (WHO) estimated that 215 million cases of malaria occurred, with >655,000 deaths; half the world’s population is at risk of contracting the disease (WHO, 2012). Artemisinin, delivered in combination with an alternate drug as Artemisinin-based Combination Therapy (ACT), is currently the preferred treatment against the disease in order to prevent emergence of drug resistance.
Artemisinin (AN; Fig. 1) is produced, extracted, and purified from the “generally regarded as safe” (GRAS; Duke, 2001) plant Artemisia annua L. (Asteraceae). The drug also has shown promise against a wide variety of human (Efferth, 2009) and livestock diseases (Ferreira et al., 2011); many are common to the developing world. Although new sources of the drug are emerging from chemical synthesis (Zhu and Cook, 2012) and engineered microbes (Paddon et al., 2013), artemisinin is currently only commercially available from A. annua with insufficient supply to treat malaria, let alone other artemisinin-susceptible diseases. To obviate emergence of drug resistance, ACT co-drugs are also needed, which usually increases cost (O’Connell et al., 2011).
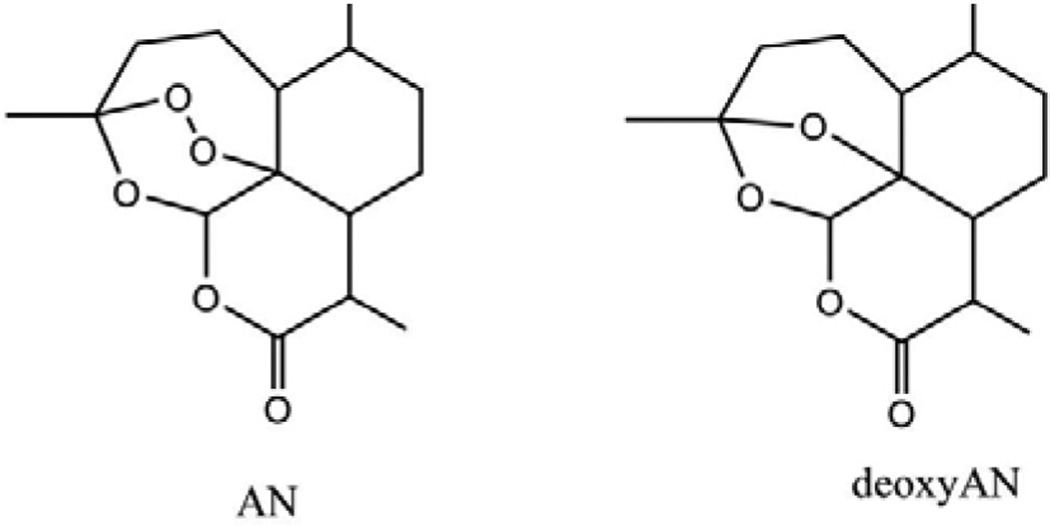
Figure 1.
Artemisinin (left) and deoxyartemisinin (right).
In an effort to improve the efficacy of artemisinin therapy and lower cost, we recently showed that when delivered orally to Plasmodium chabaudi-infected mice, a single dose of dried leaves of A. annua quelled parasitemia at least fivefold more than an equal amount of the pure drug (Elfawal et al., 2012). Beyond the abnormalities associated with infection, analysis of blood toxicology showed no toxicity, results consistent with some human trials using dried A. annua leaves (Onimus et al., 2013; ICIPE, 2005). We also previously showed that healthy mice fed dried A. annua leaves had > 40 times more artemisinin in their bloodstream than mice fed a corresponding amount of pure drug (Weathers et al., 2011). The measured serum levels exceeded by eight fold the minimum concentration of artemisinin (~10 µg L−1) required for lethality against P. falciparum (Alin and Bjorkman, 1994). Together these results suggested that more artemisinin was delivered from whole plant treatments than from the pure drug treatment. Indeed, in a recent simulated digestion study, > 50% of dry leaf-delivered AN was still available in the intestinal digestate (Weathers et al., 2014a). It is thought that besides artemisinin, the combination of other parasite-killing substances normally present in the plant (flavonoids, monoterpenes etc.; Liu et al., 1992; Elford et al., 1987; Lehane and Saliba, 2008; van Zyl et al., 2006) may be responsible for the observed responses either by improving bioavailability and/or improving therapeutic efficacy. In short, the plant may itself be providing endogenous combination drugs with its artemisinin. We thus termed this a plant-based artemisinin combination therapy, hereafter referred to as pACT.
To better assess the potential of pACT, we conducted a longer pharmacokinetic study to answer two main questions: were the pharmacokinetics of pACT different between healthy and infected mice, and was the plant matrix critical to the appearance of artemisinin in the blood? These results will further our understanding of how the drug moves into the blood when orally delivered as dried leaves of the whole plant.
2.0 MATERIALS AND METHODS
2.1 Plant material
Artemisia annua L. (SAM clonal cultivar; voucher MASS 00317314; vernacular names: annual wormwood; qīnghāo; sweet annie, sweet wormwood) containing 1.48 ± 0.06% artemisinin (dry weight) as determined by GCMS was used in this study. Dried leaves of the SAM cultivar also contain monoterpenes (e.g., 0.21% (w/w) camphor, 0.007% eucalyptol, 0.037% α-pinene) and flavonoids (0.37% total) (unpublished). Plants were grown under controlled conditions, harvested, dried, and leaves sieved and pulverized as previously described (Elfawal et al., 2012). Homogenized dried leaf biomass was assayed for artemisinin and deoxyartemisinin using GCMS as described in Elfawal et al. (2012). Identification was via NIST library and purchased standards of artemisinin (Sigma-Aldrich Chemical, St. Louis, MO) and deoxyartemisinin (Toronto Research Chemicals Inc.).
2.2 Mouse infection, feeding and drug delivery
Plasmodium chabaudi ASS (MRA-429) was obtained through the Malaria Research and Reference Reagent Resource Center (MR4) as a part of the BEI Resources Repository, NIAID, NIH. Infection of 20 inbred male C57BL/6 mice weighing an average of 23 g was established by intraperitoneal (i.p.) injection with 107 P. chabaudi-infected red blood cells as detailed in Elfawal et al. (2012). A second group of 20 of these same mice served as healthy controls. Infected (n=20) and healthy (n=20) mice received oral-gastric gavage of pACT treatment consisting of dried, powdered A. annua leaves in a water slurry, prepared fresh, and administered 72 hours post-infection (when parasitemia reached 2–3%) at an amount corresponding to 100 mg AN kg−1 live body weight as detailed in Elfalwal et al. (2012). Blood was collected by cardiac puncture from five infected and five healthy mice at each of 4 time intervals (15, 30, 60, and 120 min post-gavage). For additional serum comparisons, two groups of three healthy mice were individually treated with 100 mg kg−1 of pure AN in DMSO and water per kg live body weight with or without mouse chow (Elfawal et al., 2012) and blood collected at 60 min post gavage. Because a preliminary study showed that serum AN was at best barely detectable when mice were fed the pure drug, blood from each group of mice was pooled prior to extraction and analysis.
2.3 Blood extraction and analysis
Serum and red blood cells were separated by centrifugation at 5,000 × g for 10 min and both fractions were immediately stored at −80°C until extraction. Samples were extracted overnight at room temperature with an equal volume of methylene chloride. Where necessary, extracts were filtered through a 0.45 µm filter to remove particulates. Extracts were dried under nitrogen gas at room temperature and the dried residue resuspended in a known volume of methylene chloride for analysis of artemisinin and deoxyartemisinin by GCMS. Analysis and identification using authentic standards was as detailed above for analysis of plant extracts.
2.4 Ethics Statement
This study was conducted in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Massachusetts (Protocol# 2011-0015). All efforts were made to minimize suffering of animals during experimental procedures.
2.5 Statistical analysis
The locally weighted scatterplot smoothing (LOWESS) function was employed to generate a polynomial regression trend line for each of the kinetics plots (Cleveland, 1981). Similar results were obtained using the R PK package (Jaki and Wolfsegger, 2011), which was used to compute the area under the serum concentration versus time curve (AUC). Specifically, functions relevant to the serial sampling design employed in this study were applied. Based on mean concentrations at the various time points, the method of residuals was used to estimate a first order elimination rate constant (and half-life) as well as a first order absorption rate constant.
3.0 THEORY
Oral consumption of dried leaves of A. annua is showing therapeutic promise (ICIPE, 2005; Elfawal et al., 2013; Onimus et al., 2013). In contrast to what is known for pure artemisinin, little is known about how the drug moves from orally ingested plant leaves (e.g. pACT) into the blood stream. Comparing the pharmacokinetics of the plant-delivered drug and one of its most common metabolic products in the serum of healthy and infected animals will improve our understanding of artemisinin metabolism and help clarify how such a therapeutic could eventually be implemented.
4.0 RESULTS AND DISCUSSION
4.1 Artemisinin pharmacokinetics differs between healthy and infected mice
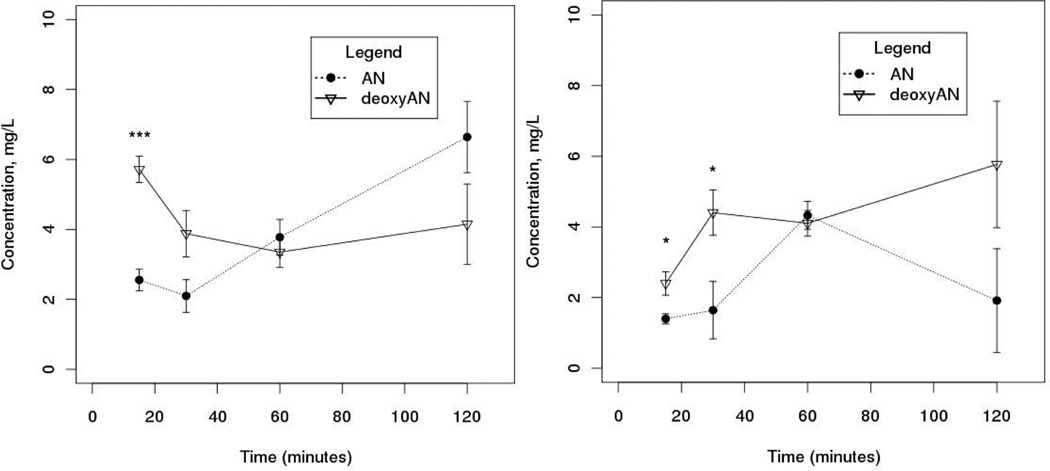
Similar to a preliminary study that compared oral gavage of either pure artemisinin or dried A. annua leaves (pACT) in healthy mice (Weathers et al., 2011), we fed pACT to healthy or P. chabaudi-infected mice and measured artemisinin and deoxyartemisinin in the serum for two hours post gavage (Fig. 2). The same data are replotted in Figure 3 to depict the independent kinetics of artemisinin and deoxyartemisinin in healthy vs. infected mice. The first order elimination rate constant for artemisinin in pACT-treated healthy mice was estimated to be 0.80 hr−1, which corresponds to an elimination half-life (T½) of 51.6 min as determined by the method of residuals (45 ± 5.75 via the PK package of Jaki and Wolfsegger, 2011), similar to that observed in rats by Niu et al. (1985). The first order absorption rate constant was estimated at 1.39 hr−1. Cmax and Tmax were 4.33 mg L−1 and 60 min, respectively (Table 1). The area under the curve (AUC) for the length of this study was 299.5 µg·min mL−1. In contrast, the AUC for pACT-treated infected mice was significantly greater at 435.6 mg·min L−1 (Table 1). Serum levels of artemisinin in the infected mice continued to rise over the 120 min of the study period (Fig. 2), and as a result, the T½ was not determined; Cmax and Tmax were estimated at ≥ 6.64 mg L−1 and ≥ 120 min, respectively. The key pharmacokinetic descriptors are shown in Table 1 and are consistent with those of an earlier preliminary study using only healthy mice (Weathers et al., 2011) and with other studies (Niu et al., 1985; Du et al., 2012) using rats fed pure artemisinin.
Figure 2.
Pharmacokinetics of artemisinin and one of it metabolites, deoxyartemisinin in serum of (left) healthy and (right) P. chabaudi-infected mice. Mice (n = 4 – 6 per time point per treatment group) were orally gavaged with dried leaves of A.annua to deliver 100 mg kg−1 body weight dose of artemisinin; bars show SE and * p < 0.05, *** p < 0.001 via Student’s t-test.
Figure 3.
Pharmacokinetics of artemisinin (left) and one of it metabolites, deoxyartemisinin (right) in serum of healthy and P. chabaudi-infected mice. Mice (n = 4 – 6 per time point per treatment group) were orally gavaged with dried leaves of A. annua to deliver 100 mg kg−1 body weight dose of artemisinin; bars show SE and * p < 0.05, *** p < 0.001 via Student’s t- test.
Table 1.
Pharmacokinetic parameters for artemisinin after oral gavage of healthy or P. chabaudi-infected mice with pACT.
| Dose per animal (mg AN kg−1) |
Disease status |
# of subj. | Artemisinin | |||
|---|---|---|---|---|---|---|
| Cmax (mg L−1) |
Tmax (min) |
T½ (min) |
AUC (mg·min L−1) |
|||
| 100 | healthy | 4–6 | 4.33 ± 0.40 | 60 | 51.6 ± | 299.5a |
| 100 | infected | 5 | ≥ 6.64 | ≥ 120 | nd | 435.6b |
Animals were fed A. annua dried leaves (pACT) to deliver 100 mg AN kg−1; average body weight was 23 g; nd, not determined.
Letters a, b after AUC values indicate statistical difference at p≤0.05; values are ± SE.
Batty et al. (2008) compared the pharmacokinetics of dihydroartemisinic acid (DHA) in healthy and Plasmodium-infected mice that were administered a single intraperitoneal dose of DHA (100 mg kg−1) and found no significant difference in elimination half-lives. Since we were unable to determine T½ for infected mice in our experiment, we cannot compare it to healthy mice. Although a direct comparison cannot be made between the pharmacokinetics of DHA and artemisinin, in contrast to Batty et al. (2008), healthy mice in our study had a significantly lower AUC than infected mice.
4.2 Deoxyartemisinin metabolism differs between healthy and infected mice
Because it is one of several liver metabolic products of artemisinin, we also measured deoxyartemisinin (Svensson and Ashton, 1999). Like many other drugs, artemisinin is metabolized in the liver by cytochrome P450s including CYP2B6 and CYP3A4 (Svensson and Ashton, 1999), yielding a number of compounds including deoxyartemisinin, deoxydihydroartemisinin, 9,10-dihydrodeoxyartemisinin, and a metabolite named crystal 7 that are found in human urine (Lee and Hufford, 1990). Each of these degradation products lacks the endoperoxide moiety present in artemisinin, and is therefore therapeutically inactive (Lee and Hufford, 1990). In contrast to its metabolism in healthy mice (Fig. 2), metabolism of artemisinin to deoxyartemisinin appeared to be suppressed in the infected mice (Fig. 2); infection retarded the capacity of the mice to form deoxyartemisinin from artemisinin over the two-hour study period. Overall, artemisinin concentrations decreased with a concomitant rise in deoxyartemisinin levels, but only in healthy subjects. In contrast, in infected mice artemisinin levels continued to rise over the study period whilst deoxyartemisinin levels fell and then remained constant. A. annua contains a diverse group of compounds (Bhakuni et al., 2001) including flavonoids and monoterpenes that have been shown to inhibit P. falciparum (Liu et al., 1992; Elford et al., 1987; Lehane and Saliba, 2008; van Zyl et al., 2006); many also have been shown to inhibit CYP3A4 (Sergent et al., 2009; Choi et al., 2011; de Magalhães et al., 2012).
Results showed an initial dip in artemisinin levels at 15 to 30 minutes in healthy subjects, which we attributed at least partially to conversion of artemisinin to deoxyartemisinin (Fig. 2). The metabolism of artemisinin to deoxyartemisinin appeared to be biphasic, declining slightly between 30 and 60 minutes, and then rising thereafter. The rising arm of the artemisinin pharmacokinetic profile in healthy mice occurred during this period of decreased metabolism to deoxyartemisinin; here, absorption exceeded elimination. When Du et al. (2012) measured deoxyartemisinin in healthy rats given a single oral dose of pure artemisinin (40 mg kg−1), the Tmax of deoxyartemisinin was 30–60 min longer than that of artemisinin (60 min). Their artemisinin metabolism kinetics were the opposite of our results for a single oral dose of pACT where the peak of artemisinin was later than the first peak for deoxyartemisinin, but earlier than the second deoxyartemisinin peak (Fig. 2). The pharmacokinetics in rats is unlikely the same as in mice, so this may account for the differential response between our two sets of results.
4.3 The presence of a plant matrix enhances artemisinin serum levels
We also examined the effects of mouse chow on pure artemisinin entry into the serum. Mouse chow contains a variety of plant materials including soy, oats, wheat, alfalfa, beet pulp, corn, etc. No artemisinin was detected in the serum of mice fed 100 mg AN kg−1 body weight (Table 2), a result consistent with the reported poor availability of the pure drug (Meshnick et al., 1996). When plant material was present either as mouse chow or A. annua pACT, the level of artemisinin in the serum rose to 2.44 and 4.32 mg L−1, respectively, indicating that the presence of plant material, even that in mouse chow, had a positive impact on the appearance of artemisinin in the blood (Table 2). These results were consistent with our preliminary study (Weathers et al., 2011). A. annua leaves can contain as much as 1.4% (w/w) essential oils (Bhakuni et al., 2001), which may enhance solubility and thus bioavailability of the nonpolar artemisinin molecule.
Table 2.
Artemisinin in serum 60 min post pACT or pure artemisinin gavage.
| Mouse treatment | Artemisinin (mg L−1) |
|---|---|
| Healthy untreated (3 pooled mice) a | 0 |
| Healthy + 100 mg AN kg−1 (3 pooled mice) a | 0 |
| Healthy + chow + 100 mg AN kg−1 (3 pooled mice) a | 2.44 |
| Healthy + pACT at 100 mg AN kg−1 (n = 5) | 4.33 ± 0.40b |
| Infected + pACT at 100 mg AN kg−1 (n = 5) | 3.78 ± 0.50b |
These mice were treated in groups of three; blood was harvested and pooled prior to extraction and measurement of artemisinin, so SE was not calculated.
Values are ± SE.
4.4 Dosage considerations
For a human comparison, Giao et al. (2001) showed that when humans were given 500 mg oral doses of pure artemisinin (twice on day 1, then once thereafter for days 2–7), the level of recrudescence was 25%. In a summary of early human trials, oral delivery of at least 600 mg per patient for 5 days was required to achieve ≤ 10% recrudescence (Meshnick et al., 1996). More recently a Kenyan clinical trial with four cohorts (4 × 12 subjects) of malaria patients (ICIPE, 2005) showed that patients receiving dried A. annua leaf tablets containing 11.1 mg artemisinin (twice on day 1, so 22.1 mg d−1) followed by 7.4 mg artemisinin (twice a day for days 2–6, so 14.8 mg d−1) had 9.1% recrudescence (see review by Weathers et al., 2014b).
Furthermore, increasing the amount of dried leaf tablets did not improve therapeutic response, suggesting that maximum effective dose had been achieved. Thus, although the patients treated with dried leaf tablets received about 3–4% the equivalent amount of pure artemisinin used in earlier studies (Meshnick et al., 1996; Giao et al., 2001), they had an equal or better therapeutic outcome than patients treated with pure artemisinin. Those results are consistent with the results of this study.
5.0 CONCLUSIONS
To our knowledge this is the first comparison between healthy and diseased animals of the pharmacokinetics of artemisinin and one of its liver metabolites, deoxyartemisinin, from orally delivered dried leaves of A. annua. When accompanied by plant material, more artemisinin enters the blood stream, demonstrating a beneficial effect of the plant matrix on the bioavailability of artemisinin. These results have implications for possible therapeutic use of pACT in treating malaria and other artemisinin-susceptible diseases.
ACKNOWLEDGMENTS
Authors thank Worcester Polytechnic Institute and University of Massachusetts Center for Clinical and Translational Science (CCTS) for funding this project. The project was also partially supported by Award Number NIH-2R15GM069562-03 from the National Institute of General Medical Sciences. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of General Medical Sciences or the National Institutes of Health.
Glossary
- ACT
artemisinin combination therapy
- AN
artemisinin
- deoxyAN
deoxyartemisinin
- AUC
area under the curve
- pACT
plant-based artemisinin combination therapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: M.A.E., P.J.W., and S.M.R. designed research; M.A.E. performed the mouse-parasite experiments; M.J.T. and P.J.W. grew plants and conducted all biochemical analyses; P.J.W. and G.A-M. analyzed data; M.A.E., P.J.W., M.J.T., G.A-M. and S.M.R. wrote the paper.
The authors declare no conflict of interest.
REFERENCES
- Alin MH, Bjorkman A. Concentration and time dependency of artemisinin efficacy against Plasmodium falciparum in vitro. American Journal of Tropical Medicine and Hygiene. 1994;50:771–776. doi: 10.4269/ajtmh.1994.50.771. [DOI] [PubMed] [Google Scholar]
- Batty KT, Gibbons PL, Davis TME, Ilett KF. Short report: Pharmacokinetics of dihydroartemisinin in a murine malaria model. American Journal of Tropical Medicine and Hygiene. 2008;78:641–642. [PubMed] [Google Scholar]
- Bhakuni RS, Jain DC, Sharma RP, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Current Science. 2001;80:35–48. [Google Scholar]
- Cleveland WS. LOWESS: A program for smoothing scatterplots by robust locally weighted regression. The American Statistician. 1981;35:54. [Google Scholar]
- Choi J-S, Piao Y-J, Kang KW. Effects of quercetin on the bioavailability of doxorubicin in rats: role of CYP3A4 and P-gp inhibition by quercetin. Archives of Pharmaceutical Research. 2011;34:607–613. doi: 10.1007/s12272-011-0411-x. [DOI] [PubMed] [Google Scholar]
- de Magalhães PM, Dupont I, Hendrickx A, Joly A, Raas T, Dessy S, Sergent T, Schneider Y-J. Anti-inflammatory effect and modulation of cytochrome P450 activities by Artemisia annua tea infusions in human intestinal Caco-2 cells. Food Chemistry. 2012;134:864–871. doi: 10.1016/j.foodchem.2012.02.195. [DOI] [PubMed] [Google Scholar]
- Du F, Liu T, Shen T, Zhu F, Xing J. Qualitative-(semi)quantitative data acquisition of artemisinin and its metabolites in rat plasma using an LTQ/Orbitrap mass spectrometer. Journal of Mass Spectrometry. 2012;47:246–252. doi: 10.1002/jms.2958. [DOI] [PubMed] [Google Scholar]
- Duke JA. Handbook of phytochemical constituents of GRAS herbs and other economic plants. Boca Raton, FL: CRC Press LLC; 2001. [Google Scholar]
- Efferth T. Chapter 11 Artemisinin: A Versatile Weapon from Traditional Chinese Medicine. In: Ramawat KG, editor. Herbal Drugs: Ethnomedicine to Modern Medicine. Springer Berlin Heidelberg: DGR; 2009. pp. 173–189. [Google Scholar]
- Elfawal MA, Towler MJ, Reich NG, Golenbock D, Weathers PJ, Rich SM. Dried whole plant Artemisia annua as an antimalarial therapy. PLos ONE. 2012;7:e52746. doi: 10.1371/journal.pone.0052746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elford BC, Roberts MF, Phillipson D, Wilson RJM. Potentiation of the antimalarial activity of qinghaosu by methoxylated flavones. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1987;81:434–436. doi: 10.1016/0035-9203(87)90161-1. [DOI] [PubMed] [Google Scholar]
- Ferreira J, Peaden P, Keiser J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba, and Fumaria officinalis. Parasitology Research. 2011;109:1585–1592. doi: 10.1007/s00436-011-2418-0. [DOI] [PubMed] [Google Scholar]
- Giao PT, Binh TQ, Kager PA, Long HP, Thang NV, van Nam N, de Vries PJ. Artemisinin for treatment of uncomplicated falciparum malaria: Is there a place for monotherapy? American Journal of Tropical Medicine and Hygiene. 2001;65:690–695. doi: 10.4269/ajtmh.2001.65.690. [DOI] [PubMed] [Google Scholar]
- ICIPE. Whole-leaf Artemisia annua-based antimalarial drug: report on proof-of-concept studies, unpublished report. [Accessed on January 17, 2014];2005 http://www.google.com/url?sa=t&rct=j&q=&esrc=s&frm=1&source=web&cd=2&ved=0CDgQFjAB&url=http%3A%2F%2Fwww.iwerliewen.org%2Findex.php%2Fcomponent%2Fedocman%2F%3Ftask%3Ddocument.download%26id%3D96%26Itemid%3D181&ei=J2miUbnFNo-80QGoi4GACw&usg=AFQjCNHoLJmPt4n0AkKyBlXPSyl5W7rc6w&sig2=ppM08X1tZglQLLiaojZx1w&bvm=bv.47008514,d.dmQ.
- Jaki T, Wolfsegger MJ. Estimation of pharmacokinetic parameters with the R package PK. Pharmaceutical Statistics. 2011;10:294–288. [Google Scholar]
- Lee IS, Hufford CD. Metabolism of antimalarial sesquiterpene lactones. Pharmacology and Therapeutics. 1990;48:345–355. doi: 10.1016/0163-7258(90)90053-5. [DOI] [PubMed] [Google Scholar]
- Lehane AM, Saliba KJ. Common dietary flavonoids inhibit the growth of the intraerythrocytic malaria parasite. BMC Research Notes. 2008;1:26. doi: 10.1186/1756-0500-1-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KC-S, Yang SL, Roberts ME, Elford BC, Phillipson JD. Antimalarial activity of Artemisia annua flavonoids from whole plants and cell cultures. Plant Cell Reports. 1992;11:637–640. doi: 10.1007/BF00236389. [DOI] [PubMed] [Google Scholar]
- Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiology Reviews. 1996;60:301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu X, Liyi H, Zhihong R, Zhenyu S. Metabolic fate of qinghaosu in rats; a new TLC densitometric method for its determination in biological material. European Journal of Drug Metabolism and Pharmacokinetics. 1985;10:55–59. doi: 10.1007/BF03189697. [DOI] [PubMed] [Google Scholar]
- O’Connell KA, Gatakaa H, Poyer S, Njogu J, Evance I, Munroe E, Solomon T, Goodman C, Hanson K, Zinsou C, Akulayi L, Raharinjatovo J, Arogundade E, Buyungo P, Mpasela F, Adjibabi CB, Agbango JA, Ramarosandratana BF, Coker B, Rubahika D, Hamainza B, Chapman S, Shewchuk T, Chavasse D. Got ACTs? Availability, price, market share and provider knowledge of anti-malarial medicines in public and private sector outlets in six malaria-endemic countries. Malaria Journal. 2011;10:326. doi: 10.1186/1475-2875-10-326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimus M, Carteron S, Lutgen P. The surprising efficiency of Artemisia annua powder capsules. Medicinal and Aromatic Plants. 2013;2:3. [Google Scholar]
- Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, Polichuk DR, Teoh KH, Reed DW, Treynor T, Lenihan J, Fleck M, Bajad S, Dang G, Diola D, Dorin G, Ellens KW, Fickes S, Galazzo J, Gaucher SP, Geistlinger T, Henry R, Hepp M, Horning T, Iqbal T, Jiang H, Kizer L, Lieu B, Melis D, Moss N, Regentin R, Secrest S, Tsuruta H, Vazquez R, Westblade LF, Xu L, Yu M, Zhang Y, Zhao L, Lievense J, Covello PS, Keasling JD, Reiling KK, Renninger NS, Newman JD. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- Sergent T, Dupont I, van der Heiden E, Scippo M-L, Pussemier L, Larondelle Y, Schneider Y-J. CYP1A1 and CYP3A4 modulation by dietary flavonoids in human intestinal Caco-2 cells. Toxicology Letters. 2009;191:216–222. doi: 10.1016/j.toxlet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Svensson USH, Ashton M. Identification of the human cytochrome P450 enzymes involved in the in vitro metabolism of artemisinin. Journal of Clinical Pharmacology. 1999;48:528–535. doi: 10.1046/j.1365-2125.1999.00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zyl RL, Seatlholo ST, van Vuuren SF, Viljoen AM. The biological activities of 20 nature identical essential oil constituents. Journal of Essential Oil Research. 2006;18:129–133. [Google Scholar]
- Weathers PJ, Arsenault PR, Covello PS, McMickle A, Teoh KH, Reed DW. Artemisinin Production in Artemisia annua: Studies in planta and results of a novel delivery method for treating malaria and other neglected diseases. Phytochemistry Reviews. 2011;10:173–183. doi: 10.1007/s11101-010-9166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers P, Jordan NJ, Lasin P, Towler MJ. Simulated digestion of dried leaves of Artemisia annua consumed as a treatment (pACT) for malaria. Journal of Ethnopharmacology. 2014a;151:858–863. doi: 10.1016/j.jep.2013.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers P, Reed K, Hassanali A, Lutgen P, Engeu PO. Chapter 4. Whole plant approaches to therapeutic use of Artemisia annua, L. (Asteraceae) In: Aftab T, Feirrera JFS, editors. Artemisia annua - Pharmacology and Biotechnology. Springer Berlin: GDR; 2014b. pp. 51–74. [Google Scholar]
- WHO. [Accessed on January 17, 2014];10 Facts on Malaria. 2012 http://www.who.int/features/factfiles/malaria/en/index.html.
- Zhu C, Cook SP. A concise synthesis of (+)-artemisinin. Journal of the American Chemical Society. 2012;134:13577–13579. doi: 10.1021/ja3061479. [DOI] [PubMed] [Google Scholar]