Abstract
Introduction
An increasing number of basic, translational and clinical studies demonstrate the importance of the protein tyrosine kinase receptor, c-Met, in the progression of prostate cancer. c-Met is overexpressed in primary prostate cancers, further increased in expression in bone metastases and is associated with development of castrate resistant disease. Because of its importance as a target, c-Met inhibitors have reached clinical trial for advanced, castrate resistant prostate cancer.
Areas covered
In this review, altered expression of c-Met and HGF in prostate tumors and the microenvironment and how they contribute to growth and invasion of prostate cancer cells is described. Next, preclinical studies providing the support for use of c-Met inhibitors are discussed. Finally, early promising results from c-Met inhibitors in clinical trial, and future prospects for c-Met inhibitors in the treatment of advanced stage prostate cancer are discussed.
Expert opinion
An emerging theme in treating metastatic prostate cancer is the requirement to target both the epithelial and stromal compartments. Results from clinical trials suggest that inhibitors of c-Met that block stromal-mediated c-Met activation in prostate tumors may be important therapeutic agents in at least a subset of patients with metastatic prostate cancer. However, as many of the inhibitors have multiple targets, the efficacy of targeting c-Met alone remains to be determined.
Keywords: c-Met, HGF, prostate cancer
1. Introduction
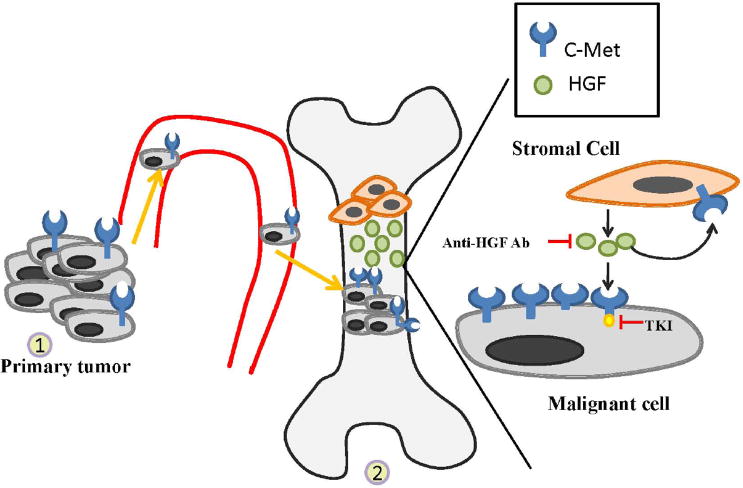
Prostate cancer (PCa) is the most commonly diagnosed non-cutaneous malignancy, the sixth leading cause of cancer related deaths among men worldwide and the second leading cause of cancer deaths in men in the United States [1, 2]. Approximately 90% of patients with metastatic castrate-resistant prostate cancer (CRPC) develop distal secondary bone metastasis, especially within the axonal skeleton [3]. While both chemotherapies (such as docetaxel and cabazitaxel) and androgen-ablative therapies (such as abiraterone acetate) have improved the survival of patients with metastatic castrate-resistant prostate cancer (mCRPC) [4-6], nearly every patient with bone metastasis eventually succumbs to the disease. However, as the relationship between tumor and microenvironment is becoming better understood, clinical trials are more frequently designed to target both the epithelial (tumor) “compartment” and the microenvironment “compartment”, and these trials show considerable promise in prolonging life of patients with prostate cancer bone metastasis. Many studies have demonstrated that several protein tyrosine kinases play important functions in both the tumor and microenvironment, and several inhibitors of tyrosine kinases, including Src, PDGF-R, IGF-R, FGF-R and c-Met are now in clinical trial for advanced prostate cancer. In each case, preclinical and emerging clinical evidence demonstrate that not only is the tumor targeted, but also tumor/microenvironment interactions that affect kinase activation are affected, often measured by decrease in markers of bone turnover. While c-Met is emerging as a target for many solid tumors, an increasing number of studies from the laboratory and the clinic have implicated c-Met as an especially attractive target for late-stage prostate cancer. As detailed below, overexpression of c-Met to very high levels is a very common occurrence in prostate cancer. Further, HGF is abundantly expressed in the tumor microenvironment, leading to c-Met activation and downstream signaling that promotes several properties of tumor progression and metastasis. In addition, c-Met expression and activation appears to be one of the common mechanisms of resistance to other targeted therapies. Given these multiple roles of c-Met in prostate cancer, several c-Met inhibitors have been developed. While their use in clinical trials specifically for prostate cancer has begun only relatively recently, there is considerable excitement in response of patients in some of these early clinical trials. In this review, we will focus on the evidence implicating the HGF/c-Met signaling axis in prostate cancer progression and metastatic growth, and then discuss inhibitors of the pathway currently being studied in clinical trials. Finally, we will assess prospects for c-Met inhibitors in treatment of PCa bone metastases.
Overview of c-Met and HGF
The structure and functions of c-Met and its ligand HGF/SF have been extensively discussed elsewhere [7, 8], and thus will be only briefly summarized here. C-Met, also known as Hepatocyte Growth Factor Receptor (HGFR), is a surface receptor with intrinsic protein tyrosine kinase (PTK) activity [9, 10]. C-Met is primarily expressed in epithelial and endothelial cells. The sole ligand for c-Met, HGF, belongs to the plasminogen subfamily of S1 peptidases, although HGF itself has no protease activity [11]. HGF expression is restricted primarily to cells of mesenchymal origin, and is abundant in the microenvironment of metastatic prostate cancer in the bone. Engagement of HGF with c-Met leads to activation of numerous signaling cascades, especially those related to invasion and properties of epithelial to mesenchymal transition [12, 13]. Among signaling molecules activated are the non-receptors tyrosine kinases, c-Src and c-Fyn, important because Src is involved PCa growth at the metastatic site by affecting tumor invasion [14] and bone turnover [15] and Fyn may be involved in tropism of PCa cells [16]. The c-Met receptor also interacts with CD44, integrin and focal adhesion kinase (FAK) [17-19], molecules aberrantly overexpressed and or activated in PCa. In addition, recent data demonstrate that crosstalk between c-Met and other RTKs leads to non-canonical c-Met phosphorylation (i.e. phosphorylation in the absence of HGF). For example, Epidermal Growth Factor Receptor (EGFR) stimulation utilizes a delayed, c-Src-dependent lateral signaling leading to increased c-Met phosphorylation [20, 21]. Although yet to be fully investigated, these results suggest that c-Met activation may be a mechanism of resistance to some therapies used in prostate cancer. Thus, numerous mechanisms by which c-Met is activated in PCA cells point to its central role in signal transduction and importance as a target in PCa.
3. Alterations in c-Met and HGf during Prostate Cancer Progression
In many solid tumors, c-Met becomes activated by mutation, gene amplification, and constitutive HGF expression [22]. However, in prostate cancer, c-Met mutations have not been reported and c-Met overexpression appears to occur via transcriptional de-repression. Transcriptional regulation of c-Met expression by altered expression of micro RNAs (miRNAs) is also an emerging mechanism for c-Met overexpression in prostate cancer. The miRNAs are 18-22 nucleotides long small non-coding RNAs. Altered expression of several miRNAs that regulate c-Met expression have been demonstrated in numerous solid tumors [23-26]. Specifically, miR-199a*, miR-34b and miR-34c inhibited Met expression and its biological functions in different cancer cell lines [27, 28]. Roles of miRNAs in prostate progression and metastasis are currently under study, with preliminary work demonstrating that miR-34a regulates c-Met expression in prostate tumor cells and is decreased in expression in cells selected for increased metastatic potential (and have higher c-Met expression) versus lower metastatic isogenic counterparts (and have lower c-Met expression) [29].
3. c-Met and expression in prostate cancer and serum of prostate cancer patients
C-Met expression increases throughout the stages of prostate cancer development and metastasis (Figure 1) [30]. Further, very recent work suggests that putative prostate cancer stem cells express c-Met and the c-Met levels are associated with progression of bone metastasis [31]. C-met expression increases in more invasive prostate tumor cell lines relative to less invasive isogenic cell lines [32]. Additionally, in cell lines expressing PSA, the c-Met level was inversely correlated with PSA production [33]. This result could imply that c-Met levels might serve as an early indicator of disease recurrence in absence of PSA increase.
Fig. 1.
In patients with localized prostate cancer undergoing radical prostatectomy, 40% of tumors stained positive for c-Met expression [34]. In this cohort, c-Met expression was directly correlated with the Gleason score [35]. By immunohistochemistry, c-Met overexpression was documented in 54% of lymph node disease and 83% of bone metastases. Additionally, in patients undergoing radical prostatectomy, the preoperative serum level of HGF is a strong predictor of prostate cancer metastasis to lymph nodes and disease recurrence [36, 37]. In accord with this finding, serum HGF and urine c-Met levels are significantly elevated in patients with metastatic disease compared to patients with localized disease [38]. Thus, urinary c-Met excretion might serve as an early indicator of recurrence in the absence of a PSA increase.
3.2 c-Met and Androgen Receptor Regulation
There is increasing evidence from data derived from pre-clinical models and primary human tumors that c-Met is a critical oncogenic pathway that contributes to the evolution of castrate-resistant disease. For example, in vitro studies demonstrated an inverse correlation between the androgen receptor (AR) levels and c-Met expression in prostate cancer cell lines [33, 38, 39]. AR represses c-Met expression via inhibition of the Sp1-induced transcription of the c-Met gene. As expected, AR knock down results in c-Met upregulation [33]. Moreover, c-Met levels were elevated after chemical or surgical castration in animal models of prostate cancer as well as in the human disease, providing in vivo support for the cell line observations [33]. Furthermore, the anti-tumor effect of c-Met inhibitors is greatly enhanced when combined with androgen ablation therapy in pre-clinical models where c-Met is also upregulated by androgen-independent mechanisms. For example, hypoxia has been shown to upregulate c-Met through a HIF-1α site in the c-Met promoter [40, 41].
3.3 c-Met and therapy resistance in prostate cancer
HGF-induced c-Met activation protects prostate cancer cells against cytotoxicity and apoptosis induced by DNA damaging agents (e,g. adriamycin and ionizing radiation) [42]. In the case of targeted agents, activation of the c-Met pathway stimulates angiogenesis in Sunitinib-resistant tumors, suggesting a potential role in resistance to antiangiogenic treatments [43]. In other tumor types, c-Met is known to mediate resistance to Src inhibitors [44] and EGF-R inhibitors [21], especially in patients with EGF-R mutations [45]. Whether c-Met will play a role in resistance to targeted therapies and chemotherapy in prostate cancer remains to be determined, but the cross talk between growth factor receptors suggests this is an area that merits further study. Indeed, we have demonstrated that long-term exposure in mice bearing prostate cancer treated with dasatinib, a Src family kinase inhibitor leads to c-Met activation and accelerated tumor growth (Varkaris and Gallick, unpublished), suggesting that future studies should examine c-Met status in patients resistant to other therapies. As discussed above, measuring levels of HGF in the circulation and microenvironment is also important.
4.0 Development of novel c-Met inhibitors
The recent understanding of the important role of the HGF/c-Met axis in the dynamic tumor-microenvironment interactions that promote metastatic growth of prostate tumor cells in the bone led to the impetus to test HGF/c-Met inhibitors in patients with mCRPC. Several strategies have been devised to interfere with HGF/c-Met signalling. These include development of multi-targeted small molecule tyrosine kinase inhibitors that inhibit c-Met kinase activity and monoclonal antibodies that interfere with HGF/c-Met interaction. Molecules being studied currently will be discussed below. Characteristics of these compounds are presented in Table 1.
Table 1. C-Met inhibitors in Early Phase Clinical Trials.
| Agent | Molecular Target | Mechanism of action | Developmental Status |
|---|---|---|---|
| XL 184 (Cabozatinib) | c-Met, VEGFR2 | Competitive to ATP binding* | Phase II |
| GSK 1363089 (foretinib) | c-Met, Ron, AXL, VEGFR2 and PDGFR | Competitive to ATP binding | Phase I/II |
| BMS-777607 | c-Met | Competitive to ATP binding | Phase I/II |
| PF-2341066 (crizotinib) | c-Met, ALK | Competitive to ATP binding | Phase I |
| ARQ-197 | c-Met | Non competitive to ATP binding | Phase I/II |
| AMG 102 | HGF | Human monoclonal antibody | Phase I/II |
Selectivity among all the inhibitors to c-Met varies.
4.1 Small molecule tyrosine kinase inhibitors
Several small molecule inhibitors that potently affect c-Met signaling are in various stages of preclinical and clinical development. Selectivity of the inhibitors towards c-Met varies, with most having well-identified additional targets. Most of the small molecule tyrosine kinase inhibitors (TKIs) are orally bioavailable with a favorable toxicity profile. These characteristics led to the rapid introduction of some TKIs into clinical trials. This section will discuss TKIs used in both preclinical and clinical trials.
BMS-777607 is a potent, ATP-competitive c-Met inhibitor. Recent in vitro data have shown that BMS-777607 affects several properties associated with prostate cancer metastasis, including inhibition of scattering, migration and invasion of prostate tumor cells in doses less or equal to 1μM. BMS-777607 suppressed HGF induced proliferation increase in doses of 3 and 10μM without apparent cytotoxic effects [46]. Additional preclinical studies support the hypothesis that c-Met inhibition by BMS-777607 suppresses the invasive and metastatic phenotype more effectively than local growth of prostate cancer. This agent is currently under investigation in phase I and II trials in patients with advanced or metastatic solid tumors [47].
PF-2341066 (crizotinib) is another ATP-competitive c-Met inhibitor with additional potent activity against Anaplastic Lymphoma Kinase (ALK) [48]. PF-2341066 showed a moderate anti- proliferative activity against AR negative PC3 prostate cancer cells in doses of 2.5μM. Activity was also observed in AR positive cell lines, (LNCaP and C4-2). Recent in vitro work has demonstrated that drug responsiveness was inversely associated with AR levels and whether the cells are androgen sensitive. In vivo, PF-2341066 suppressed the growth of AR positive androgen-independent prostate cells xenografts (C4-2 cells) [48]. Also, c-Met inhibition showed synergistic activity with androgen ablation in the growth inhibition of prostate cancer cell xenografts after castrate resistant progression [48]. These studies suggest that PF-2341066 (and perhaps other c-Met inhibitors) are likely to have greater anti-tumor effects when combined with anti-androgen therapies. Crizotinib is currently in phase I clinical evaluation [49].
ARQ-197 is a highly selective but less potent non-ATP competitive TKI. In preclinical studies, ARQ-197 has shown antitumor activity against a broad spectrum of cancer cell lines including PC3 cells [50, 51]. Additionally, ARQ-197 demonstrated dose-dependent tumor growth inhibition ranging from 50 to 80% in PC3-derived xenografts grown in immunocompromised mice. These preclinical studies have led to Phase I and II clinical trials in patients with solid tumors who failed first-line treatment. Preliminary evidence of antitumor activity was demonstrated with a favorable safety profile. Only a few prostate cancer patients were included in this trial [52].
Foretinib/ GSK 1363089 is a multi-targeted TKI whose targets include c-Met, Ron, AXL, VEGFR2 and PDGFR [53]. Foretinib has similar pharmacologic effects to XL-184 (see below). Although Foretinib has been tested in several solid tumors in phase I and phase II studies, few patients with prostate cancer were included in these trials [54].
4.2 Monoclonal Antibodies against c-Met and HGF
Anti HGF and c-Met monoclonal antibodies demonstrate a higher specificity against the HGF/c-met axis as compared to TKIs. Furthermore, antibody targeting provides the potential of initiating a host-immune response against tumor cell. Several immunotherapeutic products have recently been tested in later-phase clinical trials and demonstrated evidence of clinical benefit-. AMG 102 is a human monoclonal IgG antibody that binds and neutralizes human HGF [55]. Interestingly, AMG 102 was found to enhance the antitumor activity of conventional chemotherapeutics including docetaxel in U-87 MG (human glioblastoma-direved cell line that previously described to contain a HGF autocrine loop) cells and xenografts [56]. AMG 102 is currently undergoing clinical evaluation in a Phase I/II trials in combination with mitoxandrone and prednisone in patients with docetaxel pretreated castrate-resistant prostate cancer [57].
4.3 XL184 (cabozatinib)
XL-184 (cabozatinib) is a potent, ATP competitive dual inhibitor of c-Met and VEGFR-2. XL-184 is also active at higher IC50s against other RTKs including RET, KIT, FLT3 and TIE2. Preclinical evaluation of cabozatinib showed promising anti-proliferative and anti-angiogenic properties. Cabozatinib is currently been tested as a single agent in a phase I clinical trial in patients with advanced solid tumor malignancies. Preliminary analyses demonstrated dramatic responses in patients with mCRPC [58, 59, 60]. Of 65 patients with documented bone metastasis at baseline, 56 patients (86%) had a complete or partial resolution on bone scans as early as six weeks after treatment initiation. The effect on bone metastases was also reflected in improvement of pain. Pain was improved in 64% and narcotics were decreased or stopped altogether in 46% of patients who were narcotic-dependent at the time of enrollment into the study (n=28). In addition, cabozatinib produced a more than 50% decrease in biochemical bone markers including serum C-telopeptide and alkaline phospatase in more than half of the evaluable patients. Given these encouraging phase I results, a non-randomized expansion cohort for patients with mCRPC is currently underway. Additional studies are also planned to define the optimal dose of XL-184 since toxicities have been reported from the phase I effort, including fatigue, dysgeusia, hypothyrodism, and in rare instances, death.
5. Conclusion
Evidence to date suggests that aberrant activation of the HGF/c-Met axis in prostate cancer epithelial cells appears to be a relatively late event in tumor progression. C-Met expression increases in advanced stages of the disease, with the highest expression observed in bone metastases. HGF, produced from the stromal compartment of the bone, contributes to c-Met activation at this most common metastatic site in prostate cancer. Thus, the HGF/c-Met axis fits well with the emerging paradigm that growth of prostate cancer in the bone depends on tumor/microenvironment interactions and, indeed, is driven by the microenvironment. Current results thus support signaling through c-Met as an important contributor to the “vicious cycle” of prostate cancer bone metastasis. For these reasons, numerous c-Met inhibitors are being tested in clinical trials, with very promising early results obtained with XL-184, a multi-targeted TKI. While trials of c-Met inhibitors in prostate cancer are still in early stages, the large number of studies (both planned and currently underway) will likely lead answers as to whether of not c-Met inhibitors will become part of the treatment algorithm for a least a subset of patients with advanced prostate cancer.
6. Expert Opinion
Over the past two years, the HGF/c-Met signaling axis has emerged as one of the most exciting targets for the treatment of mCRPC. The very high levels of c-Met expression in bone metastases coupled with the presence of its ligand, HGF in the serum and tumor microenvironment fit well with the hypothesis that successful treatment of CRPC involves targeting both the epithelial and the bone microenvironment [61]. Based on early but promising results of tumor regression induced by some multi-targeted c-Met inhibitors, there has been a surge of interest into how c-Met itself contributes to metastatic prostate cancer growth in the bone. For example, results from the phase II trial of XL-184 showing resolution of bone lesions as early as six weeks in a majority of patients is compelling. Such findings have never been reported with any conventional or novel targeted treatment. Nevertheless, some caution is warranted in interpreting these results, since osteoblasts in the bone are known to express high levels of c-Met [33]. Thus the favorable changes on radionuclide bone scan may represent a suppression of osteoblast activity in addition to an anti-tumor effect. Better correlation with other markers of disease response is needed to confirm the current observations. In addition, the use of multi-targeted inhibitors always brings into question whether other targets are as, or more important, than c-Met itself. For example, XL-184 targets not only c-Met, but also VEGFR-2, and at higher doses, other kinases as well, as described above. Thus, the high response rates seen in patients with mCRPC may be due to inhibition of multiple additional kinases that cross-talk with c-Met, including Focal Adhesion Kinase (FAK- regulated by the integrin pathway), EGFR, and IGF-1R. Whether activation of pathways that normally cross-talk with c-Met might contribute to resistance to c-Met inhibitors remains to be studied. Nevertheless, these results suggest that c-Met inhibitors in combination with other signal transduction inhibitors are likely to have the greatest efficacy for mCRPC.
Ideally, before studying whether a novel therapy prolongs survival in a large randomized phase III clinical trial, complementary (pre)clinical studies are warranted to answer some of the specific questions raised above. Further, the current results are seen in bone metastases, and the crosstalk of different pathways in the bone remains to be fully elucidated. However, an emerging theme in therapy development is that novel agents that target both the epithelial and stromal compartments hold the key to advances in treating this disease. Thus, the ability of c-Met inhibition to block bidirectional crosstalk between the epithelial-stromal compartments that promotes tumor growth is worth pursuing. Further studies are needed to elucidate the role of c-Met and RTKs crosstalk in prostate cancer progression and chemoresistance.
highlights.
Tumor-microenvironment interactions are essential for prostate cancer invasive growth, metastasis and drug resistance. The c-Met/ HGF signaling pathway is an important contributor to epithelial –stromal interactions.
In prostate cancer, c-Met activation is mediated through a paracrine mechanism. C-Met is overexpressed in prostate cancer cells and HGF is abundantly excreted by stromal cells.
C-Met expression increases throughout the stages of prostate cancer progression, indicating that the c-Met/ HGF pathway contributes to tumor progression.
In vitro studies have shown an inverse correlation between c-Met expression and prostate cancer cell differentiation, AR expression and PSA production.
The c-Met/ HGF signaling pathway inhibition is a promising therapeutic target for the treatment of metastatic prostate cancer.
To date, several c-Met/HGF inhibitors, including small molecule tyrosine kinase inhibitors and antibodies that bind to HGF or c-Met are currently under clinical evaluation.
Early clinical data demonstrate compelling antitumor effects in patients with metastatic castrate resistant prostate cancer with some multi-targeted c-Met inhibitors.
Acknowledgments
GEG and CJL were supported by SPORE 1P50 CA14038801 and two awards from Prostate Cancer Foundation (“Mechanism of Resistance to Androgen Biosynthesis Inhibition in Castrate-Resistant Prostate Cancer Bone Metastasis” and “Individualized Microenvironment Targeted Therapy for Prostate Cancer Bone Metastases: Simultaneous Evaluation of Multiple Therapeutic Targets”). FD was supported by NIH grant T32 CA009666. SG was supported by UTHealth Innovation for Cancer Prevention Research Pre-doctoral Fellowship, The University of Texas School of Public Health – Cancer Prevention and Research Institute of Texas grant # RP101503.
Abbreviations
- AR
androgen receptor
- ALK
anaplastic Lymphoma kinase
- c-Met
mesenchymal-epithelial transition factor
- CRPC
castrate resistant prostate cancer
- EMT
Epithelial to Mesenchymal transition
- FAK
Focal adhesion kinase
- HGF
Hepatocyte Growth Factor
- HGF-R
Hepatocyte Growth Factor Receptor
- mCRPC
metastatic castrate resistant prostate cancer
- NSCLC
non small cell lung cancer
- PCa
Prostate Cancer
- PTK
Protein Tyrosine Kinase
- RTK
Receptor Tyrosine Kinase
- SF
Scatter Factor
- TKI
tyrosine kinase inhibitor
Footnotes
The authors state no conflict of interest and have received no payment in preparation of this manuscript.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, et al. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10. Available at http://globocan.iarc.fr/factsheets/populations/factsheet.asp?uno=840.
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Bubendorf L, Schöpfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31:578–583. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 4.Tannock Ian F, MD, PhD, de Wit Ronald, MD, Berry William R, MD, et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995–2005. doi: 10.1056/NEJMoa1014618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376:1147–1154. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 7.Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- 8.Liu X, Yao W, Newton RC, Scherle PA. Targeting the c-MET signaling pathway for cancer therapy. Expert Opin Investig Drugs. 2008;17:997–1011. doi: 10.1517/13543784.17.7.997. [DOI] [PubMed] [Google Scholar]
- 9**.Giordano S, Di Renzo MF, Narsimhan RP, et al. Biosynthesis of the protein encoded by the c-met proto-oncogene. Oncogene. 1989;4:1383–8. First description of the c-Met protein. [PubMed] [Google Scholar]
- 10.Birchmeier C, Birchmeier W, Gherardi E, Vande Woude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–25. doi: 10.1038/nrm1261. [DOI] [PubMed] [Google Scholar]
- 11**.Bottaro DP, Rubin JS, Faletto DL, Chan AM, et al. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–4. doi: 10.1126/science.1846706. Identification of the c-Met ligand. [DOI] [PubMed] [Google Scholar]
- 12.Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–42. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- 13.Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park SI, Zhang J, Phillips KA, et al. Targeting SRC family kinases inhibits growth and lymph node metastases of prostate cancer in an orthotopic nude mouse model. Cancer Res. 2008;68:3323–33. doi: 10.1158/0008-5472.CAN-07-2997. [DOI] [PubMed] [Google Scholar]
- 15.Araujo JC, Poblenz A, Corn P, et al. Dasatinib inhibits both osteoclast activation and prostate cancer PC-3-cell-induced osteoclast formation. Cancer Biol Ther. 2009;8:2153–9. doi: 10.4161/cbt.8.22.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen AR, David SY, Liao C, et al. Fyn is downstream of the HGF/MET signaling axis and affects cellular shape and tropism in PC3 cells. Clin Cancer Res. 2011;17:3112–22. doi: 10.1158/1078-0432.CCR-10-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghatak S, Hascall VC, Markwald RR, Misra S. Stromal hyaluronan interaction with epithelial CD44 variants promotes prostate cancer invasiveness by augmenting expression and function of hepatocyte growth factor and androgen receptor. J Biol Chem. 2010;285:19821–32. doi: 10.1074/jbc.M110.104273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trusolino L, Bertotti A, Comoglio PM. A signaling adapter function for alpha6beta4 integrin in the control of HGF-dependent invasive growth. Cell. 2001;107:643–54. doi: 10.1016/s0092-8674(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 19.Chen SY, Chen HC. Direct interaction of focal adhesion kinase (FAK) with Met is required for FAK to promote hepatocyte growth factor-induced cell invasion. Mol Cell Biol. 2006;26:5155–67. doi: 10.1128/MCB.02186-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20*.Xu L, Nilsson MB, Saintigny P, et al. Epidermal growth factor receptor regulates MET levels and invasiveness through hypoxia-inducible factor-1alpha in non-small cell lung cancer cells. Oncogene. 2010;29:2616–27. doi: 10.1038/onc.2010.16. Work in this manuscript demonstrated the correlation between HIF-1α and c-Met levels in non-small cell lung cancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulak AM, Gubish CT, Stabile LP, Henry C, et al. HGF-independent potentiation of EGFR action by c-Met. Oncogene. 2011;30:3625–35. doi: 10.1038/onc.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Newton RC, Scherle PA. Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med. 2010;16:37–45. doi: 10.1016/j.molmed.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Lee UJ, Kim MN, et al. MicroRNA miR-199a* regulates the MET proto-oncogene and the downstream extracellular signal-regulated kinase 2 (ERK2) J Biol Chem. 2008;283:18158–66. doi: 10.1074/jbc.M800186200. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Fu H, Tie Y, et al. miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer Lett. 2009;275:44–53. doi: 10.1016/j.canlet.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 25.Hu G, Chen D, Li X, Yang K, et al. miR-133b regulates the MET proto-oncogene and inhibits the growth of colorectal cancer cells in vitro and in vivo. Cancer Biol Ther. 2010;10:190–7. doi: 10.4161/cbt.10.2.12186. [DOI] [PubMed] [Google Scholar]
- 26.Cai KM, Bao XL, Kong XH, et al. Hsa-miR-34c suppresses growth and invasion of human laryngeal carcinoma cells via targeting c-Met. Int J Mol Med. 2010;25:565–71. doi: 10.3892/ijmm_00000378. [DOI] [PubMed] [Google Scholar]
- 27.Migliore C, Petrelli A, Ghiso E, et al. MicroRNAs impair MET-mediated invasive growth. Cancer Res. 2008;68:10128–36. doi: 10.1158/0008-5472.CAN-08-2148. [DOI] [PubMed] [Google Scholar]
- 28.Salvi A, Sabelli C, Moncini S, et al. MicroRNA-23b mediates urokinase and c-met downmodulation and a decreased migration of human hepatocellular carcinoma cells. FEBS J. 2009;276:2966–82. doi: 10.1111/j.1742-4658.2009.07014.x. [DOI] [PubMed] [Google Scholar]
- 29.Gaur S, Gallick EG. Elucidating the role of c-Met overexpression and its regulation in prostate cancer progression. Second AACR International Conference on Frontiers in Basic Cancer Research Poster Session; Sept 14 – 18, 2011; San Francisco, CA. [Google Scholar]
- 30*.Knudsen BS, Gmyrek GA, Inra J, et al. High expression of the Met receptor in prostate cancer metastasis to bone. Urology. 2002;60:1113–7. doi: 10.1016/s0090-4295(02)01954-4. This study showed the expression of c-Met in prostate cancer metastasis. [DOI] [PubMed] [Google Scholar]
- 31.Colombel M, Eaton CL, Hamdy F, et al. Increased expression of putative cancer stem cell markers in primary prostate cancer is associated with progression of bone metastases. Prostate. 2011 Aug 31; doi: 10.1002/pros.21473. [DOI] [PubMed] [Google Scholar]
- 32.van Leenders GJ, Gage WR, Hicks JL, et al. Intermediate cells in human prostate epithelium are enrichedin proliferative inflammatory atrophy. Am J Pathol. 2003;162:1529–1537. doi: 10.1016/S0002-9440(10)64286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33**.Verras M, Lee J, Xue H, et al. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 2007;67:967–75. doi: 10.1158/0008-5472.CAN-06-3552. This is the first study that shows the interaction of the androgen signaling and c-Met expression in prostate cancer cells. [DOI] [PubMed] [Google Scholar]
- 34**.Humphrey PA, Zhu X, Zarnegar R, et al. Hepatocyte growth factor and its receptor (c-MET) in prostatic carcinoma. Am J Pathol. 1995;147:386–96. This pivotal study shows the expression of c-Met in prostate cancer tissue and cell lines, and the effects of HGF stimulation in prostate cancer cell lines. [PMC free article] [PubMed] [Google Scholar]
- 35.Pisters LL, Troncoso P, Zhau HE, et al. c-met proto-oncogene expression in benign and malignant human prostate tissues. J Urol. 1995;154:293–8. [PubMed] [Google Scholar]
- 36.Yasuda K, Nagakawa O, Akashi T, et al. Serum active hepatocyte growth factor (AHGF) in benign prostatic disease and prostate cancer. Prostate. 2009;69:346–51. doi: 10.1002/pros.20890. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Karakiewicz PI, Roehrborn CG, et al. Predictive value of plasma hepatocyte growth factor/scatter factor levels in patients with clinically localized prostate cancer. Clin Cancer Res. 2008;14:7385–90. doi: 10.1158/1078-0432.CCR-07-5110. [DOI] [PubMed] [Google Scholar]
- 38.Russo AL, Jedlicka K, Wernick M, et al. Urine analysis and protein networking identify met as a marker of metastatic prostate cancer. Clin Cancer Res. 2009;15:4292–8. doi: 10.1158/1078-0432.CCR-09-0599. [DOI] [PubMed] [Google Scholar]
- 39.Maeda A, Nakashiro K, Hara S, et al. Inactivation of AR activates HGF/c-Met system in human prostatic carcinoma cells. Biochem Biophys Res Commun. 2006;347:1158–65. doi: 10.1016/j.bbrc.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 40*.Pennacchietti S, Michieli P, Galluzzo M, et al. Hypoxia promotes invasive growth by transcriptional activation of the met protooncogene. Cancer Cell. 2003;3:347–61. doi: 10.1016/s1535-6108(03)00085-0. This study shows the hypoxia induced c-Met transcriptional activation. [DOI] [PubMed] [Google Scholar]
- 41.Scarpino S, Cancellario d’Alena F, Napoli A, et al. Increased expression of Met protein is associated with up-regulation of hypoxia inducible factor-1 (HIF-1) in tumour cells in papillary carcinoma of the thyroid. J Pathol. 2004;202:352–8. doi: 10.1002/path.1522. [DOI] [PubMed] [Google Scholar]
- 42.Fan S, Ma YX, Wang JA, et al. The cytokine hepatocyte growth factor/scatter factor inhibits apoptosis and enhances DNA repair by a common mechanism involving signaling through phosphatidyl inositol 3’ kinase. Oncogene. 2000;19:2212–23. doi: 10.1038/sj.onc.1203566. [DOI] [PubMed] [Google Scholar]
- 43.Shojaei F, Lee JH, Simmons BH, et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70:10090–100. doi: 10.1158/0008-5472.CAN-10-0489. [DOI] [PubMed] [Google Scholar]
- 44.Sen B, Peng S, Saigal B, et al. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res. 2011;17:514–24. doi: 10.1158/1078-0432.CCR-10-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–43. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 46.Dai Y, Siemann DW. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Mol Cancer Ther. 2010;9:1554–61. doi: 10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 47.Multiple Ascending Dose Study of BMS-777607 in Subjects With Advanced or Metastatic Solid Tumors. http://clinicaltrials.gov/ct2/show/NCT00605618.
- 48.Tu WH, Zhu C, Clark C, et al. Efficacy of c-Met inhibitor for advanced prostate cancer. BMC Cancer. 2010;10:556. doi: 10.1186/1471-2407-10-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.A Phase 1 Absolute Bioavailability Study For Oral crizotinib In Healthy Volunteers. http://clinicaltrials.gov/ct2/results?term=crizotinib.
- 50.Youzhi Li, Dongshu Chen, Wei Zhou, et al. Broad Spectrum anti-cancer activity of ARQ 197, a highly selective oral c-Met Inhibitor, in multiple xenograft models. AACR Annual Meeting; 2007. abst 2216. [Google Scholar]
- 51.Adjei AA, Schwartz B, Garmey E. Early Clinical Development of ARQ 197, a Selective, Non-ATP-Competitive Inhibitor Targeting MET Tyrosine Kinase for the Treatment of Advanced Cancers. Oncologist. 2011;16:788–99. doi: 10.1634/theoncologist.2010-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yap TA, Olmos D, Brunetto AT, et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J Clin Oncol. 2011;29:1271–9. doi: 10.1200/JCO.2010.31.0367. [DOI] [PubMed] [Google Scholar]
- 53.Qian F, Engst S, Yamaguchi K, et al. Inhibition of tumor cell growth, invasion, and metastasis by EXEL-2880 (XL880, GSK1363089), a novel inhibitor of HGF and VEGF receptor tyrosine kinases. Cancer Res. 2009;69:8009–16. doi: 10.1158/0008-5472.CAN-08-4889. [DOI] [PubMed] [Google Scholar]
- 54.Eder JP, Shapiro GI, Appleman LJ, et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin Cancer Res. 2010;16:3507–16. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 55.Jun HT, Sun J, Rex K, et al. AMG 102, a fully human anti-hepatocyte growth factor/scatter factor neutralizing antibody, enhances the efficacy of temozolomide or docetaxel in U-87 MG cells and xenografts. Clin Cancer Res. 2007;13:6735–42. doi: 10.1158/1078-0432.CCR-06-2969. [DOI] [PubMed] [Google Scholar]
- 56*.Burgess TL, Sun J, Meyer S, et al. Biochemical characterization of AMG 102: a neutralizing, fully human monoclonal antibody to human and nonhuman primate hepatocyte growth factor. Mol Cancer Ther. 2010;9:400–9. doi: 10.1158/1535-7163.MCT-09-0824. First characterization of an HGF monoclonal antibody eventually used in clinical trial. [DOI] [PubMed] [Google Scholar]
- 57.AMG 102 in Combination with Mitoxantrone and Prednisone in Subjects With Previously Treated Castrate Resistant Prostate Cancer. http://clinicaltrials.gov/ct2/show/NCT00770848.
- 58.Smith David C, Spira Alexander, De Grève Jacques, et al. Phase 2 Study of XL184 in a Cohort of Patients with Castration Resistant Prostate Cancer (CRPC) and Measurable Soft Tissue Disease. 22nd EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; November 16-19, 2010; Berlin, Germany. [Google Scholar]
- 59.Smith DC, Smith MR, Small EJ, et al. Phase II study of XL184 in a cohort of patients (pts) with castration-resistant prostate cancer (CRPC) and measurable soft tissue disease. J Clin Oncol. 2011;29(suppl 7) abstr 127. [Google Scholar]
- 60*.Hussain, Smith MR, Sweeney C, Corn PG, et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial. J Clin Oncol. 2011;29(suppl) abstr 4516. This is the first phase II clinical trial of a c-Met inhibitor in patients with metastatic prostate cancer with promising results. Most of the patients experienced a complete or partial resolution on bone scan. [Google Scholar]
- 61.Logothetis CJ, Navone NM, Lin SH. Understanding the biology of bone metastases: key to the effective treatment of prostate cancer. Clin Cancer Res. 2008;14:1599–602. doi: 10.1158/1078-0432.CCR-07-4603. [DOI] [PubMed] [Google Scholar]