Abstract
Objective
To report the prevalence of prescription opioid use and evaluate the trends in a large cohort of Medicaid-enrolled pregnant women.
Methods
A cohort of pregnancies was identified using data from the Medicaid Analytical eXtract for the period of 2000-2007. Dispensing of opioids, as a class and separately for individual agents, was evaluated using claims from filled prescriptions. Variations in patterns of prescription opioid fills were examined by demographic characteristics, by geographic region, and over time. Median number of opioid prescriptions dispensed and cumulative days of availability for prescription opioids during pregnancy were reported.
Results
The study population consisted of over 1.1 million women with completed pregnancies, from 46 US states and Washington, DC. One out of five women from our cohort (21.6%) filled a prescription for an opioid during pregnancy; this proportion increased from 18.5% in 2000 to 22.8% in 2007. Substantial regional variation was seen with the proportion of women who filled a prescription during pregnancy, ranging between 9.5% and 41.6% across the states. Codeine and hydrocodone were the most commonly prescribed opioids. Among women filling at least one opioid prescription, the median (IQR) number of prescriptions filled was 1 (1-2) and the median (IQR) cumulative days of opioid availability during pregnancy were 5 (3-13) days.
Conclusion
We observed high and increasing number of filled prescriptions for opioids during pregnancy among Medicaid-enrolled women. These findings call for further safety evaluations of these drugs and their effects on the developing fetus in order to inform clinical practice.
Introduction
Yazdy et al.(1) recently reported in this journal an increased risk of neural tube defects after first trimester maternal opioid use. This follows the study by Broussard et al.(2) from the National Birth Defects Prevention Study that suggested an association between maternal opioid analgesic use and a number of congenital malformations including neural tube defects, cardiac septal defects, and gastroschisis. These findings, coupled with the well-established risk of neonatal withdrawal syndrome following prolonged opioid exposure in late pregnancy (3), raise concerns about the use of opioids in pregnancy.
In light of these emerging data questioning the safety of prescription opioids for the fetus, it is imperative to study the extent of their use during pregnancy to gain insight into the potential public health impact of maternal opioid exposure during pregnancy. We therefore evaluated the use of prescription opioids in a large national cohort of Medicaid-insured pregnant women in the US. This is a highly relevant population to study this question as Medicaid covers the medical expenses for more than 40% of births in the US (4), and the rates of prescription opioid use are reported to be disproportionately high among Medicaid enrollees compared to commercially insured patients in the general population (5).
Methods
The study population consisted of over 1.1 million women with completed pregnancies, and was drawn from the Medicaid Analytical eXtract for 46 U.S. states and Washington, DC, for the period of 2000-2007. Montana and Connecticut were excluded because of difficulty in linking mothers and infants, Michigan was excluded because of incomplete data, and data from Arizona were not available. We identified all completed pregnancies in women aged 12-55 years linked to live-born infants. We estimated the date of last menstrual period (LMP) based on the delivery date combined with a validated algorithm based on diagnosis codes (6). The LMP was assigned to be 245 days before the delivery date for pregnancies that had maternal or infant ICD-9 codes indicative of preterm delivery (644.0, 644.2, and 765.x) and to be 270 days before the delivery date for all other pregnancies. Finally, we required all women to be Medicaid eligible throughout pregnancy. To ensure a complete, longitudinal stream of healthcare claims throughout pregnancy, we excluded women with supplementary private insurance, women with restricted benefits and women in selected capitated managed care plans. Derivation of this cohort has previously been described in detail elsewhere (7).
Filled prescriptions of opioid analgesics were identified using pharmacy-dispensing claims. We then defined three trimesters using the date of LMP; the first trimester extended from the LMP through day 90 of pregnancy, the second trimester was the following 90 days, and the third trimester began 181 days after estimated LMP and continued until delivery. Based on the dispensing date, each prescription was classified as dispensed in the respective trimester. We accumulated days supply for each filled opioid prescription to derive the cumulative days of opioid availability during pregnancy overall and during each trimester. We assumed that opioids were consumed regularly at the minimum specified interval even if prescribed on an as-needed basis. Cumulative days of opioid availability were reported as median (interquartile range (IQR)).In addition to prescriptions at the class level, we also explored prescriptions filled for individual opioid agents during each trimester. The opioids considered in our analysis included hydrocodone, codeine, oxycodone, propoxyphene, tramadol, meperidine, hydromorphone, morphine, fentanyl, buprenorphine, methadone, pentazocine, tapentadol, and oxymorphone.
Patient characteristics, including race, age, geographic region, most frequent pain diagnoses, and caesarean sections, were presented for women who did and did not fill an opioid prescription during pregnancy. Regional and time trends adjusting for demographic characteristics for prescription opioids fills were examined using mixed effects regression analyses. All analyses were conducted using SAS version 9.3 (SAS institute, Cary, NC). The use of this de-identified database for research was approved by the Institutional Review Board at the Brigham and Women’s Hospital.
Results
The study population consisted of over 1.1 million women with completed pregnancies, from 46 US states and Washington, DC. Overall, approximately one in five Medicaid-enrolled pregnant women filled at least one prescription for an opioid at any time during her pregnancy (21.6% or 239,381 of 1,106,757) between 2000 and 2007. Table 1 shows some key demographic characteristics of our cohort. The proportion of women who received an opioid prescription was 29.0% among whites, 19.1% among black, and 13.4% among Hispanic. The mean age (±SD) for women who received opioid prescriptions was 24.3± 5.3 years and for women who did not receive opioid prescriptions during pregnancy it was 23.9 ± 5.9 years. The majority of pregnant women receiving an opioid prescription had a diagnosis of abdominal pain (48.4%), lower back pain (33.0%), headache syndromes (13.3%), joint pain (11.2%), or migraine (7.9%) at some point during their pregnancy.
Table 1.
Demographic Characteristics of Medicaid Enrolled Pregant Women (2000-2007) by Their Opioid Analgesic Prescription Fill Status
| Characteristic | Filled a Prescription for Opioid Analgesic During Pregnancy |
P * | |
|---|---|---|---|
| Yes (n=239,381) | No (n=867,376) | ||
| Age in years (Mean (SD)) | 24.3 (5.3) | 23.9 (5.9) | <0.001 |
| Race | |||
| White | 127,495 (53.2%) | 312,734 (36.1%) | <0.001 |
| Black | 71,506 (29.9%) | 302,020 (34.8%) | <0.001 |
| Hispanic | 23,650 (9.9%) | 152,646 (17.6%) | <0.001 |
| Others † | 16,730 (7.0%) | 99,976 (11.5%) | <0.001 |
| Region ‡ | |||
| Northeast | 22,231 (9.3%) | 140,186 (16.2%) | <0.001 |
| Midwest | 81,333 (34.0%) | 252,419 (29.1%) | <0.001 |
| South | 85,795 (35.8%) | 227,189 (26.2%) | <0.001 |
| West | 50,022 (20.9%) | 247,582 (28.5%) | <0.001 |
| 5 most frequently diagnosed pain conditions during pregnancy | |||
| Abdominal pain | 115,907 (48.4%) | 244,273 (28.2%) | <0.001 |
| Lower back pain | 78,998 (33.0%) | 112,979 (13.0%) | <0.001 |
|
Headache syndromes other
than migraine |
31,950 (13.3%) | 49,787 (5.7%) | <0.001 |
| Joint pain | 26,789 (11.2%) | 33,641 (3.9%) | <0.001 |
| Migraine | 18,932 (7.9%) | 14,951 (1.7%) | <0.001 |
| Cesarean delivery | 71,134 (29.7%) | 221,047 (25.5%) | <0.001 |
For t-tests in age comparison and chi-square test in other comparisons.
Other races include american indian, asian, native hawaiian, mixed and unknown.
Excluding data from Montana, Michigan, Arizona and Connecticut.
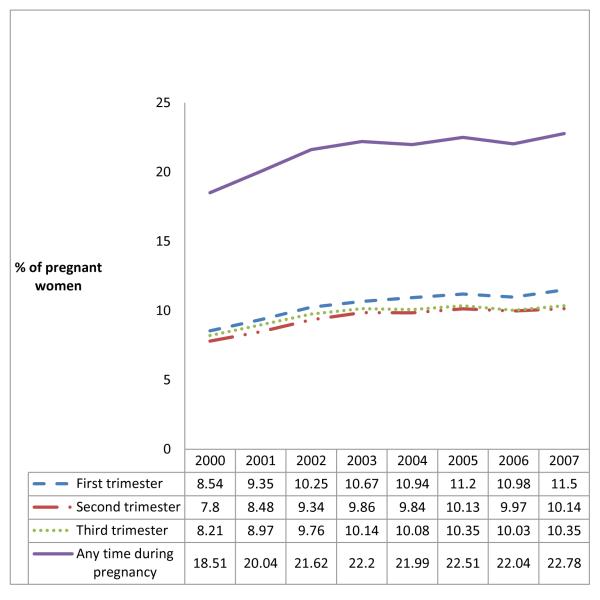
The proportion of women who filled an opioid prescription was 10.5% in the first trimester, 9.6% in the second trimester and 9.8% in the third trimester. Among women filling any opioid prescription, the median (IQR) cumulative days of opioid availability during pregnancy was 5 (3-13) days overall (assuming the medication was taken continuously at the minimum prescribed interval); and 5 (3-12), 5 (3-10) and 5 (3-12) days respectively during the three trimesters. The median (IQR) number of prescriptions filled during pregnancy was 1 (1-2) among women who filled at least one prescription. The proportion of pregnant women potentially exposed to opioids chronically - defined as cumulative days of opioid availability greater than 30 during pregnancy - was 2.5% (28,118 of 1,106,757).
Codeine and hydrocodone accounted for the majority of the opioid prescriptions. Overall, 11.1% women filled prescriptions for codeine, 10.0% for hydrocodone, 2.9% for propoxyphene, and 2.2% for oxycodone at any time during pregnancy. Prescriptions for other opioids including tramadol, meperidine, hydromorphone, morphine, fentanyl, buprenorphine, methadone, pentazocine, tapentadol, and oxymorphone were rarely filled (1.5% combined) at any time during pregnancy within our cohort.
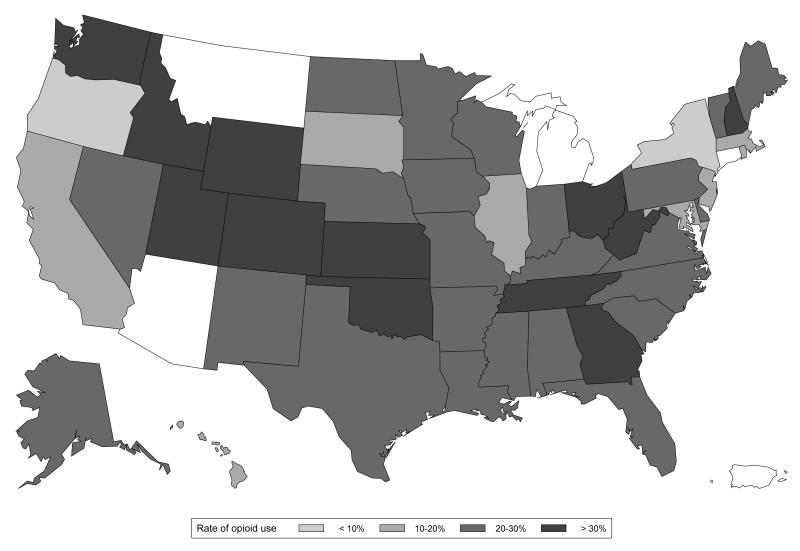
Figure 1 illustrates opioid dispensings at any time during pregnancy by state. Substantial regional variation was seen with the proportion of women who filled a prescription during pregnancy ranging between 9.5% and 41.6% across the states. The five states with the highest rate of opioid prescription were Utah (41.6%), Idaho (35.6%), New Hampshire (34.3%), Wyoming (34.1%), and Tennessee (33.6%). The proportion of women who filled an opioid prescription at any time during pregnancy gradually increased from 18.5% in 2000 to 22.8% in 2007, marking an increase of 23.1% (p-value for the test of linear trend <0.001, Figure 2). This time trend of increasing opioid prescription fills was also seen during each trimester separately (p<0.001for all three time periods). Adjustment for age and race, and accounting for random variability through the use of mixed effects regression did not meaningfully change the estimates of prescription opioid by state or year (data not shown), implying that neither the geographic variation nor the trend over time can be explained by differences in age and/or race distributions.
Figure 1.
Regional variation in the rates of prescription opioid dispensing during pregnancy, Medicaid 2000-2007
*Footnote: Arizona, Michigan, Montana and Connecticut (white) are not represented in the cohort because of incomplete claims information.
Figure 2.
Proportion of pregnant women who filled an opioid prescription, Medicaid 2000-2007
*footnote: p-value for the tests of linear trend <0.001 for all the trimesters and at any time during pregnancy.
Discussion
In a cohort of more than 1 million Medicaid enrolled pregnant women, about 1 in five women was dispensed prescription opioids during pregnancy and we noted a substantial increase in filled prescriptions between 2000 and 2007. Pronounced regional and racial variations were observed in this population, with several states reporting a frequency of opioid dispensing during pregnancy in excess of 30%. Codeine and hydrocodone represented most of the filled prescriptions.
A rampant increase in prescription opioid use in the last two decades has been documented for the US general population (8). This trend is accompanied by a rise in abuse of these agents leading to significant increases in opioid related emergency department visits (9) and deaths (10). The data suggesting higher potential for abuse has prompted the Drug Enforcement Agency to consider re-scheduling of the most widely used prescription opioid, hydrocodone (11). Our data, based on a nationally representative sample of pregnant women, suggest that the epidemic of prescription opioid use in the general population extends to pregnancy. In light of the recent studies suggesting the teratogenic potential of these medications (1,2), and the known risks of neonatal withdrawal following in-utero exposure (3), this is a significant public health concern.
The findings from this study are in agreement with several prior studies conducted in cohorts of pregnant women. In a study of Medicaid beneficiaries from Tennessee, a two-fold increase in the use of opioids during pregnancy was noted between 1995 and 2009, reaching 36.8% (12). We recently studied a cohort of commercially insured women and found 14.4% of pregnant women were exposed to prescription opioids at any time during pregnancy, with approximately 6% of women exposed during each of the trimesters (13). As in the present study, hydrocodone, codeine, propoxyphene, and oxycodone were found to be the most frequently used agents. This study also documented substantial regional variability in the frequency with which opioids were prescribed during pregnancy. The higher use of prescription opioids we observed in women with low socio-economic status (Medicaid) compared with more affluent women (commercially-insured) is consistent with trends observed in the general population (5). An earlier study conducted in a commercially insured population from California and Washington state noted an increasing trend of long term prescription opioid use among women aged 18-44 years (including pregnant and non-pregnant women) (14).
Our study has several limitations that deserve discussion. First, our study does not capture the use of illicitly obtained prescription opioids since studying the prevalence of illicit drug use is not possible using the nationwide healthcare utilization data that are the basis for the present study. Second, our data source only captures prescriptions filled by patients on an outpatient basis. Any illicit or inpatient opioid use is therefore not accounted for, which would result in underestimation of real use. Third, expected duration of use was estimated based on days supply, and is likely to be an underestimate of the real duration for medications that are typically taken on an as-need basis. Fourth, women with pregnancies not resulting in a live birth are not included in our study. We postulate that if opioids are associated with spontaneous or therapeutic terminations of pregnancies, our results may underestimate the total opioid use. On the other hand, diversion or not taking filled prescriptions of opioid analgesic would result in an overestimation of the actual opioid ingestion. Nevertheless, automated pharmacy dispensing information is usually seen as the gold standard of drug exposure compared to self-reported information(15) or prescribing records in outpatient medical records (16).
In conclusion, our findings of high and increasing number of filled prescriptions for opioids during pregnancy among Medicaid-enrolled women call for further safety evaluations of these drugs and their effects on the developing fetus in order to inform clinical practice.
Acknowledgments
Supported by AHRQ Award R01 HSO18533. Brian Bateman received additional funding through a K-award (K08HD075831) from the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the NIH, and Krista Huybrechts received additional funding through a K-award (K01 MH099141) from the National Institute of Mental Health.
The authors thank Helen Mogun, MS (Division of Pharmacoepidemiology, Brigham and Women’s Hospital, Boston, MA) for preparing the analytic dataset.
Sonia Hernandez-Diaz has consulted for Novartis, AstraZeneca, and GSK_biologics for unrelated projects. Brian Bateman is supported through a K-award (K08HD075831 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
Footnotes
Financial Disclosure: The other authors did not report any potential conflicts of interest.
References
- 1.Yazdy MM, Mitchell AA, Tinker SC, Parker SE, Werler MM. Periconceptional Use of Opioids and the Risk of Neural Tube Defects. Obstet Gynecol. 2013;122(4):838–844. doi: 10.1097/AOG.0b013e3182a6643c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Broussard CS, Rasmussen SA, Reefhuis J, Friedman J, Jann MW, Riehle-Colarusso T, et al. Maternal treatment with opioid analgesics and risk for birth defects. Am J Obstet Gynecol. 2011;204(4):314, e311–311. doi: 10.1016/j.ajog.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 3.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307(18):1934–1940. doi: 10.1001/jama.2012.3951. [DOI] [PubMed] [Google Scholar]
- 4.Garcia G. Maternal and Child Health (MCH) Update: States Increase Eligibility for Children’s Health in 2007. National Governors Association; 2008. [Google Scholar]
- 5.Sullivan MD, Edlund MJ, Fan M-Y, DeVries A, Brennan Braden J, Martin BC. Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: the TROUP study. Pain. 2008;138(2):440–449. doi: 10.1016/j.pain.2008.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2013;22:16–24. doi: 10.1002/pds.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palmsten K, Huybrechts KF, Mogun H, Kowal MK, Williams PL, Michels KB, et al. Harnessing the Medicaid Analytic eXtract (MAX) to evaluate medications in pregnancy: design considerations. PloS one. 2013;8(6):e67405. doi: 10.1371/journal.pone.0067405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depen. 2006 Feb 1;81(2):103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 9.The DAWN report . Highlights of the 2010 Drug Abuse Warning Network (DAWN) Findings on Drug-Related Emergency Department Visits. Center for Behavioral Health Statistics and Quality , Substance Abuse and Mental Health Services Administration; 2012. [PubMed] [Google Scholar]
- 10.Paulozzi LJ, Xi Y. Recent changes in drug poisoning mortality in the United States by urban-rural status and by drug type. Pharmacoepidemiol Drug Saf. 2008 Oct;17(10):997–1005. doi: 10.1002/pds.1626. [DOI] [PubMed] [Google Scholar]
- 11.US Food and Drug Administration January 24-25, 2013: Meeting of the Drug Safety and Risk Management Advisory Committee Meeting Announcement; [Retrieved September 20, 2013]. Available at http://www.fda.gov/AdvisoryCommittees/Calendar/ucm332857.htm. [Google Scholar]
- 12.Epstein RA, Bobo WV, Martin PR, Morrow JA, Wang W, Chandrasekhar R, et al. Increasing pregnancy-related use of prescribed opioid analgesics. Ann Epidemiol. Aug. 2013;23(8):498–503. doi: 10.1016/j.annepidem.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bateman B, Hernández-Díaz S, Rathmell J, Seeger J, Doherty M, Fischer M, et al. Patterns of opioid utilization in pregnancy in a large cohort of insurance beneficiaries in the United States. Anesthesiology. 2014 doi: 10.1097/ALN.0000000000000172. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Campbell CI, Weisner C, LeResche L, Ray GT, Saunders K, Sullivan MD, et al. Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health. 2010;100(12):2541–2547. doi: 10.2105/AJPH.2009.180646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West S, Savitz D, Koch G, Strom B, Guess H, Hartzema A. Recall accuracy for prescription medications: self-report compared with database information. Am J Epidemiol. 1995;142:1103–12. doi: 10.1093/oxfordjournals.aje.a117563. [DOI] [PubMed] [Google Scholar]
- 16.West S, Strom B, Freundlich B, Normand E, Koch G, Savitz D. Completeness of prescription recording in outpatient medical records from a health maintenance organization. J Clin Epidemiol. 1994;47:165–7. doi: 10.1016/0895-4356(94)90021-3. [DOI] [PubMed] [Google Scholar]