Significance
Wild salmon in the North Pacific Ocean, particularly pink salmon, have grown greatly since the mid-1970s apparently due to bottom-up effects of climate change on ocean physics and production processes. Pink salmon spend less than 2 y at sea and most stocks alternate between high and low levels of abundance every other year. In years of high abundance, they now constitute a pelagic consumer front as they return to their spawning rivers, exert top-down control over the open ocean ecosystem by outcompeting other species for shared prey resources, and drive major ecological shifts between years of high and low abundance. Their effect on competing species must be considered in international conservation policies and when developing informed ecosystem-based management strategies.
Keywords: ocean ecology, exploitative competition, consumer front, interaction strength, carrying capacity
Abstract
Climate change in the last century was associated with spectacular growth of many wild Pacific salmon stocks in the North Pacific Ocean and Bering Sea, apparently through bottom-up forcing linking meteorology to ocean physics, water temperature, and plankton production. One species in particular, pink salmon, became so numerous by the 1990s that they began to dominate other species of salmon for prey resources and to exert top-down control in the open ocean ecosystem. Information from long-term monitoring of seabirds in the Aleutian Islands and Bering Sea reveals that the sphere of influence of pink salmon is much larger than previously known. Seabirds, pink salmon, other species of salmon, and by extension other higher-order predators, are tightly linked ecologically and must be included in international management and conservation policies for sustaining all species that compete for common, finite resource pools. These data further emphasize that the unique 2-y cycle in abundance of pink salmon drives interannual shifts between two alternate states of a complex marine ecosystem.
Predator control of community structure and ecosystem function became a tenet of intertidal and nearshore marine ecology following early studies of Paine and others (1–3), yet with few exceptions (4, 5), until more recent times the idea has been less well appreciated for open oceans. Growing attention now is being paid to the overexploitation of pelagic species, particularly those at higher trophic levels currently and in the past, and effects on ocean ecosystems of the loss, or development, of top-down forcing (6–12).
The prevailing view has long held that most biological change in ocean ecosystems, apart from human exploitation, is driven from the bottom up (13–16). One striking example that has been linked to bottom-up processes driven by climate change is the burgeoning abundance of wild Pacific salmon (Oncorhynchus spp.), and in particular pink salmon (Oncorhynchus gorbuscha), in the subarctic North Pacific Ocean and Bering Sea (SNPO/BS). Underpinning the notion initially were studies that found (i) strong coherence between decadal patterns in the Aleutian Low pressure system, which exerts a large influence over climate in the North Pacific Ocean, and patterns in salmon production across a broad region of the SNPO/BS (17, 18); (ii) decadal patterns in primary production that could be explained by the effect of the Aleutian Low pressure system on basin scale wind fields (19); and (iii) decadal patterns in zooplankton, squid, and pelagic fish production that also were correlated with meteorological forcing over the North Pacific Ocean and consistent with patterns in primary production (20). Thus, the general explanation for waxing and waning abundances of salmon over the record in the 20th century was that physical forcing by shifts in the strength and position of the Aleutian Low altered winds, ocean temperatures, and primary and secondary production to the benefit or detriment of salmon. A decadal scale oscillation in the Aleutian Low, now often referred to as the Pacific Decadal Oscillation (PDO) (21), has been linked to numerous physical and biological variability in the SNPO/BS in addition to salmon abundance (21–23).
It was subsequently shown that salmon population responses and their relation to the PDO were out of phase between Alaska and the northwest coast of North America during much of the 20th century (24); that warm anomalies in coastal temperatures were associated with increased survival of salmon in Alaska; and that regional-scale variability in ocean temperature was a better predictor of salmon survival than large, basin-scale variability characterized by the PDO (25). A recent analysis from around the rim of the North Pacific Ocean found regional covariance in abundance of pink salmon, chum salmon (Oncorhynchus keta), and sockeye salmon (Oncorhynchus nerka) associated with the Aleutian Low, and with smaller scale spatially coherent, but regionally distinct, patterns in climate (26).
Water temperature can be important to the early growth and survival of pink salmon fry directly by its effect on physiology and indirectly by its effect on the timing and development of zooplankton prey stocks in nursery areas, which commonly is advanced and greater in warmer years than in cooler years. In cooler springs, fry grow more slowly and a greater number die both from lack of food and from an increased susceptibility to predators (27, 28). For example, a conceptual model for Prince William Sound, Alaska, holds that, in years of abundant spring zooplankton, fry grow faster and remain longer in the shelter of inshore nurseries where they are protected from walleye pollock (Theragra chalcogramma) and Pacific herring (Clupea pallasii), two chief predators that remain offshore feeding primarily on swarms of large calanoid copepods and other macrozooplankton. In cooler years of lower zooplankton biomass inshore, fry grow more slowly, move offshore earlier, and suffer higher predation by pollock and herring due to spatial overlap, smaller size, and less alternative prey for those two predators (28).
Although the relationship between climate and pink salmon survival is likely complex, fluctuations in abundance appear to be modulated in large measure directly and indirectly by the thermal environment in which a stock lives. Such a fundamentally bottom-up explanation is bolstered by observations of high growth and survival rates of pink salmon during the period of warmer ocean temperatures and population increase (29, 30), and at this time provides a more parsimonious explanation for population dynamics than would explanations invoking strictly top-down control across such a broad region. Now, however, several lines of evidence indicate that pink salmon themselves are having a large top-down influence on other salmon species, other upper trophic level pelagic species, plankton standing stocks, and by inference, the functioning of the open-ocean ecosystem in the SNPO/BS.
Pink Salmon in an Ecosystem Context
Pink salmon are the most abundant of the wild Pacific salmon, representing about 70% of all returning fish each year across their range (31). They have several early life history characteristics that seem to explain their relatively great abundance, and a short 2-y life cycle from egg to spawning adult (30). Most stocks have interannually alternating weak and strong runs and strong runs can be in either even years or odd years: shifts between strong runs in odd years to strong runs in even years (and visa versa) have been documented in some stocks, even-year and odd-year brood lines are reproductively isolated, and the abundance of fish in strong runs is commonly far greater than in weak runs (32–36). The cause of this phenomenon is not known.
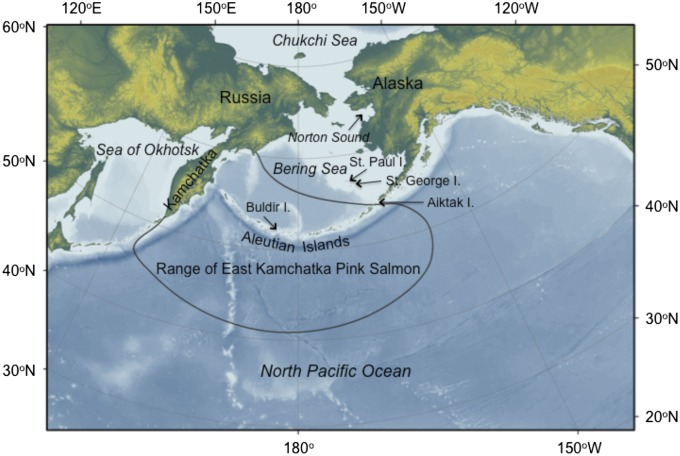
The majority of pink salmon in the northwestern and central SNPO/BS are of Asian origin, especially fish that spawn in rivers of the eastern Kamchatka Peninsula and western Bering Sea (Fig. 1) that are odd-year–dominant stocks (34). Although data are reported as “eastern Kamchatka,” more than 90% of those fish spawn in river tributaries of the western Bering Sea (38). The total run size (catch plus escapement) has been steady in even years for the past several decades, averaging about 17 ± 2 (SEM) × 106 fish per y from 1972 through 2012, whereas runs in odd years rose from an average of about 47 ± 6 × 106 fish per y in 1971–1987, to 83 ± 7 × 106 fish per y in 1989–2007, and to 173 × 106 fish in 2009 and 225 × 106 fish in 2011 (34, 39) (Table S1). The increases were not augmented by hatchery releases (34). A smaller stock of even-year–dominant pink salmon spawns in the eastern Bering Sea, primarily in Norton Sound, with run sizes there averaging 3.3 × 106 fish in even years and 0.56 × 106 fish in odd years between 1997 and 2012 (40).
Fig. 1.
The northern North Pacific Ocean and Bering Sea and locations of places discussed in the text. Salmon distribution is adapted from ref. 37.
Questions concerning the carrying capacity of the North Pacific Ocean in regard to salmon emerged in the early 1990s with increasing overall numbers of fish (41, 42). Differences in diets, growth, condition, distribution, and catch of three competing species—pink salmon, sockeye salmon, and chum salmon—in even years compared with odd years suggested that pink salmon were placing a disproportionately high demand on pelagic production (43–48). It was further suggested that biennial oscillations in standing stocks of phytoplankton and zooplankton in the central SNPO/BS, apparent by 1990 and out of phase with each other, represented a trophic cascade initiated in odd years by prey demand of pink salmon—during odd years, relaxed grazing pressure by depressed numbers of macrozooplankton, among the primary prey of pink salmon, led to an elevated standing stock of phytoplankton in summer (49, 50). This conclusion was reached after considering variability in physical indices and forcing factors, including the Northern Hemisphere Zonal Index, solar radiation flux, surface wind speed, sea surface temperature, salinity, density, and nutrient levels that were associated with interannual and decadal patterns in production at lower trophic levels, but not systematic biennial oscillations.
Such a relationship between zooplankton and phytoplankton abundance would explain a conspicuous biennial alternation in body size of Neocalanus copepods in the central North Pacific Ocean, during a study spanning the 1980s and 1990s, that generally was poorly correlated with climatological and environmental variables as well (integrated mean water column temperature from surface to 150 m, vertical stability index, North Pacific Index, and Southern Oscillation Index) (51). The authors found that individuals were larger in odd years, when competition for phytoplankton would have been less, than in even years when competition would have risen. The one significant correlation they did report was a positive one between body size of Neocalanus cristatus and chlorophyll a concentration, which would be expected in this scenario.
Seabirds in a Pink Salmon Context
Observations in the Bering Sea in odd years of lower body mass and liver mass of short-tailed shearwaters (Puffinus tenuirostris), a Southern Hemisphere seabird that spends the austral winter in the SNPO/BS and Chukchi Sea (52), and two to five times higher strandings of shearwaters on the coast of eastern Kamchatka (53), provided the first evidence (to the authors’ knowledge) of the influence of pink salmon over a competing species besides other salmon (and see ref. 54). Among the important prey of pink salmon (29, 55, 56), copepods (Neocalanus spp.), euphausiids (Thysanoessa spp.), squids (Gonatidae), myctophids (Myctophidae), and Atka mackerel (Pleurogrammus monopterygius) are at times also important prey of shearwaters and of resident seabirds wintering in the subarctic North Pacific Ocean and nesting in the Aleutian Islands and Bering Sea (52, 57–66).
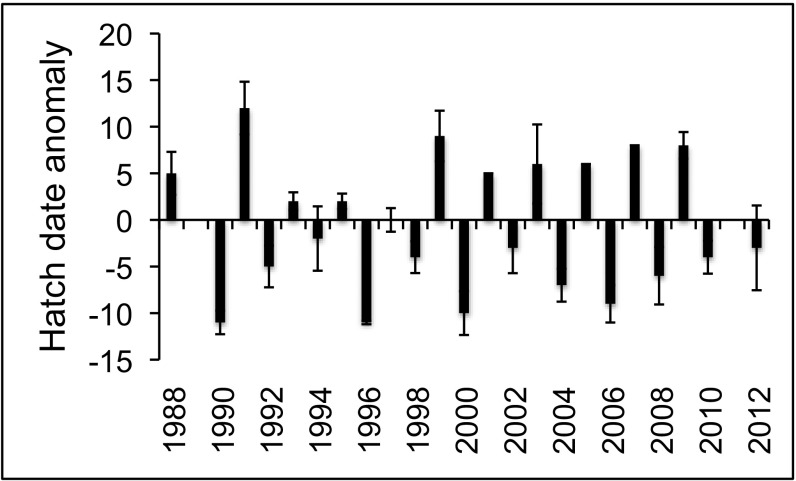
Systematic annual monitoring of nesting seabirds at four major colonies in the southern Bering Sea and Aleutian Islands (Fig. 1) began in 1984 at St. George Island and St. Paul Island (Pribilof Islands), 1988 at Buldir Island (western Aleutian Islands), and 1995 at Aiktak Island (eastern Aleutians) (67–70). One of the most conspicuous patterns over the years, and the one that first alerted us to the possibility of a connection between the birds and pink salmon, is the alternating early (even year)–late (odd year) nesting phenology of tufted puffins (Fratercula cirrhata) at Buldir (Fig. 2). This led us to examine phenology and up to five additional elements of the breeding biology of tufted puffins and as many as 15 other seabird species of two feeding guilds—omnivores (12 species that consume a mixture of fishes, squids, zooplankton, and other invertebrates) and planktivores (4 species that consume primarily zooplankton)—at those islands for similar even-year–odd-year patterns. We further examined the possible connection between seabirds and salmon by comparing seabird nesting parameters to the annual run size of eastern Kamchatka pink salmon, and in particular cases to the annual run size of pink salmon in western Alaska. Using these approaches, we found compelling evidence that pink salmon have a major influence on diets, numbers, phenology, fecundity, and/or productivity of one or more species (in the order of 107 individuals) at one or more of these islands.
Fig. 2.
Nesting phenology (mean hatch date anomaly, days) using the example of tufted puffins at Buldir Island. Positive values are late, and negative values are early: no data for 1989 or 2011. Error bars denote ±1 SEM. Data are from ref. 67. See Tables S2 and S3 for phenology data for all species tested.
Results
We ran 111 tests of the null hypothesis that there was no difference in mean values of six nesting parameters of 16 species at four islands between even and odd years (Table 1 and Tables S2 and S3). Rejection of the null hypothesis for the individual parameters at α = 0.10 ranged from 29% to 84% and was 50% overall. These rates exceeded in all cases the number that would be expected by chance alone and demonstrated strong directionality and the large magnitude of effect that we hypothesize pink salmon have on seabirds, and the ecosystem, of the SNPO/BS.
Table 1.
Comparisons of seabird nesting parameters in even years versus odd years
| Parameter | No. of tests | No. rejections (%) | No. expected |
| Clutch size | 9 | 4 (44) | 1 |
| Hatch date | 25 | 21 (84) | 3 |
| Laying success | 8 | 6 (75) | 1 |
| Hatching success | 27 | 9 (33) | 3 |
| Fledging success | 21 | 6 (29) | 2 |
| Productivity | 21 | 9 (43) | 2 |
| Overall | 111 | 55 (50) | 11 |
Number of tests of the null hypothesis of no difference between mean values of seabird nesting parameters in even years versus odd years, the number (percentage) of hypotheses that were rejected at α = 0.10, and the number that would be expected to be rejected at α = 0.10 due to chance alone.
Omnivorous Seabirds.
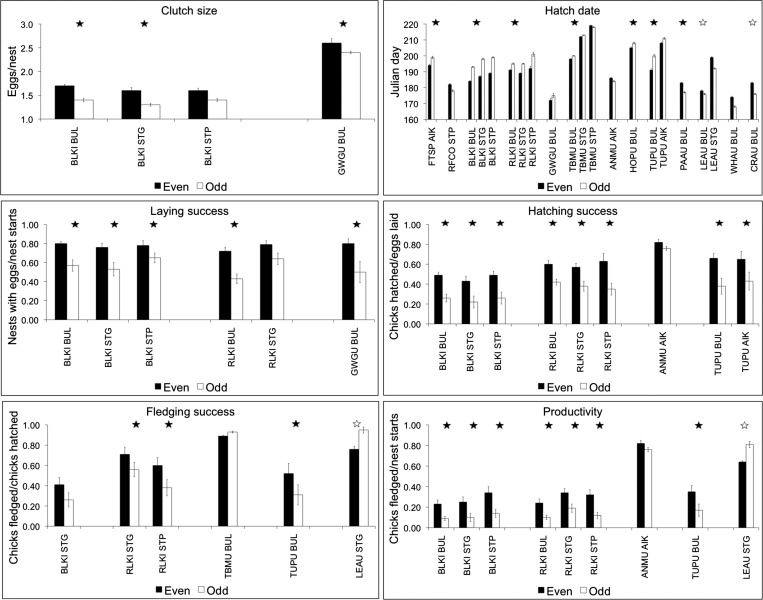
Phenology, as indexed by mean hatch date, was later (at α = 0.10) in odd years than in even years for 13 of the 20 species/island samples and was seen on all islands: the difference was particularly pronounced in black-legged kittiwakes (Rissa tridactyla), which had mean hatch dates that were 9–12 d later in odd years (Fig. 3 and Tables S2 and S3). It was earlier by 4 d for red-faced cormorants (Phalacrocorax urile) at St. Paul, and earlier for ancient murrelets (Synthliboramphus antiquus) at Aiktak and for common murres (Uria aalge) and thick-billed murres (Uria lomvia) at St. Paul by 2, 4, and 1 d, respectively. Clutch size was smaller in odd years than in even years for black-legged kittiwakes at all three islands and for glaucous-winged gulls (Larus glaucescens) at Buldir. Laying success (number of nests with eggs per number of nest starts) was lower in odd years for black-legged kittiwakes at all three islands, for red-legged kittiwakes (Rissa brevirostris) at Buldir and St. George (and by 15% at St. Paul, although it missed the α = 0.10 criterion), and for glaucous-winged gulls at Buldir. Hatching success (number of eggs hatching per number of eggs laid) was lower in odd years for both species of kittiwakes at all three islands, for tufted puffins at Buldir and Aiktak, and for ancient murrelets at Aiktak. Fledging success (number of chicks fledged per number of eggs hatched) was lower in odd years for tufted puffins at Buldir, both species of kittiwakes at St. George, and red-legged kittiwakes at St. Paul (and black-legged kittiwakes by 12%, although it too missed the α = 0.10 criterion), but was higher in odd years for thick-billed murres at Buldir. Productivity (number of chicks fledged per number of nest starts) was lower in odd years for both species of kittiwake at all three islands, for ancient murrelets at Aiktak, and for tufted puffins at Buldir.
Fig. 3.
Mean values of seabird breeding parameters that exhibited differences between even years and odd years at α = 0.10. See Tables S2 and S3 for all parameter tests in all species and sample sizes. Error bars denote ±1 SEM. Filled stars indicate relationships to eastern Kamchatka pink salmon abundance (linear regression, α = 0.10); open stars indicate relationships to western Alaska pink salmon abundance at α = 0.10 (Table S4). See Table S2 for species abbreviations. BUL, Buldir Island; STG, St. George Island; STP, St. Paul Island. Data are from refs. 67–70.
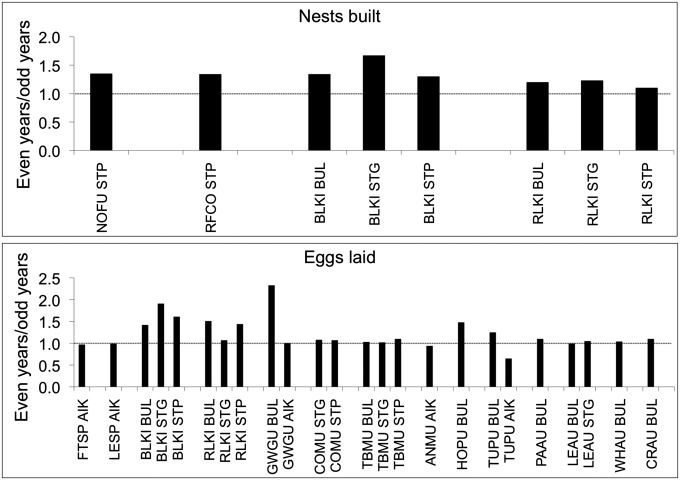
Counts of nests of some species that build nests (not all species of seabirds do) at Buldir and the Pribilofs were made in sufficient numbers of even and odd years to compare differences, and in all cases more nests were built in even years than in odd years (Fig. 4). Although these are small sample sizes and not all differences had high significance levels (Table S5), they strongly support the other even-year–odd-year evidence of an effect of pink salmon on these birds. In addition, finally, black-legged kittiwakes at all three islands, red-legged kittiwakes at Buldir and St. Paul, and glaucous-winged gulls, horned puffins (Fratercula corniculata), and tufted puffins at Buldir laid conspicuously more eggs in even years than in odd years (Fig. 4).
Fig. 4.
Ratios of the numbers of nests built and eggs laid in even years compared with odd years across all years. See Table S2 for species abbreviations. AIK, Aiktak Island; BUL, Buldir Island; STG, St. George Island; STP, St. Paul Island. Data are from refs. 67–70.
Nesting parameters of several of the omnivores also exhibited negative correlations with the run size of eastern Kamchatka pink salmon (Fig. 3 and Table S4). Clutch size of black-legged kittiwakes at Buldir and St. George was well correlated with salmon abundance, and of glaucous-winged gulls at Buldir. The hatch date for five of six species at Buldir was highly correlated with salmon abundance, although not correlated at either of the Pribilof islands despite large differences in mean hatch dates between even and odd years. Laying success of black-legged kittiwakes at all three islands and of red-legged kittiwakes and glaucous-winged gulls at Buldir was correlated with salmon abundance. Hatching success of both species of kittiwakes at all three islands and of tufted puffins at Buldir and Aiktak was also correlated with salmon abundance. Fledging success was generally less well correlated with salmon abundance, reflecting the smaller, or lack of, differences between mean values in even and odd years, but still was correlated for black-legged kittiwakes at St. Paul, for red-legged kittiwakes at St. George and St. Paul, and for tufted puffins at Buldir. Productivity was strongly correlated with pink salmon abundance for both species of kittiwakes at all islands and for horned and tufted puffins at Buldir.
There were no consistent geographic patterns in the magnitude of differences between mean values of parameters in even and odd years for the three species that were sampled at Buldir, St. George, and St. Paul (Table 2), nor were there consistent geographic patterns in the strength of relationships of nesting parameters to eastern Kamchatka pink salmon run size (Table S4).
Table 2.
Values of nesting parameters in even years divided by values in odd years for omnivorous species that were each measured at Buldir (BUL), St. George (STG), and St. Paul (STP)
| Species location | Clutch size | Hatch date | Laying success | Hatching success | Fledging success | Productivity |
| BLKI BUL | 1.21 | 0.95 | 1.40 | 1.88 | 1.00 | 2.56 |
| BLKI STG | 1.23 | 0.94 | 1.43 | 1.95 | 1.58 | 2.50 |
| BLKI STP | 1.14 | 0.94 | 1.20 | 1.88 | 1.34 | 2.43 |
| RLKI BUL | na | 0.98 | 1.67 | 1.43 | 0.96 | 2.60 |
| RLKI STG | na | 0.97 | 1.23 | 1.50 | 1.28 | 1.74 |
| RLKI STP | na | 0.96 | 1.35 | 1.75 | 1.58 | 2.67 |
| TBMU BUL | na | 0.99 | na | 1.03 | 0.96 | 1.00 |
| TBMU STG | na | 1.00 | na | 0.98 | 1.00 | 1.00 |
| TBMU STP | na | 1.00 | na | 0.91 | 0.99 | 0.89 |
Planktivorous Seabirds.
The limited data on breeding parameters of planktivores—four species at Buldir and one species at St. George—revealed either an opposite pattern to that of omnivores, or no pattern at all (Fig. 3 and Tables S2 and S3). Only the hatch date was very different between even years and odd years at Buldir, and in all cases it was earlier (by up to 7 d) in odd years. The hatch date for least auklets (Aethia pusilla) at St. George also was much earlier in odd years, and fledging success and productivity were higher. The hatch dates of least auklets and crested auklets (Aethia cristatella) at Buldir were well correlated with Norton Sound pink salmon abundance, as were fledging success and productivity of least auklets at St. George (Fig. 3 and Table S4).
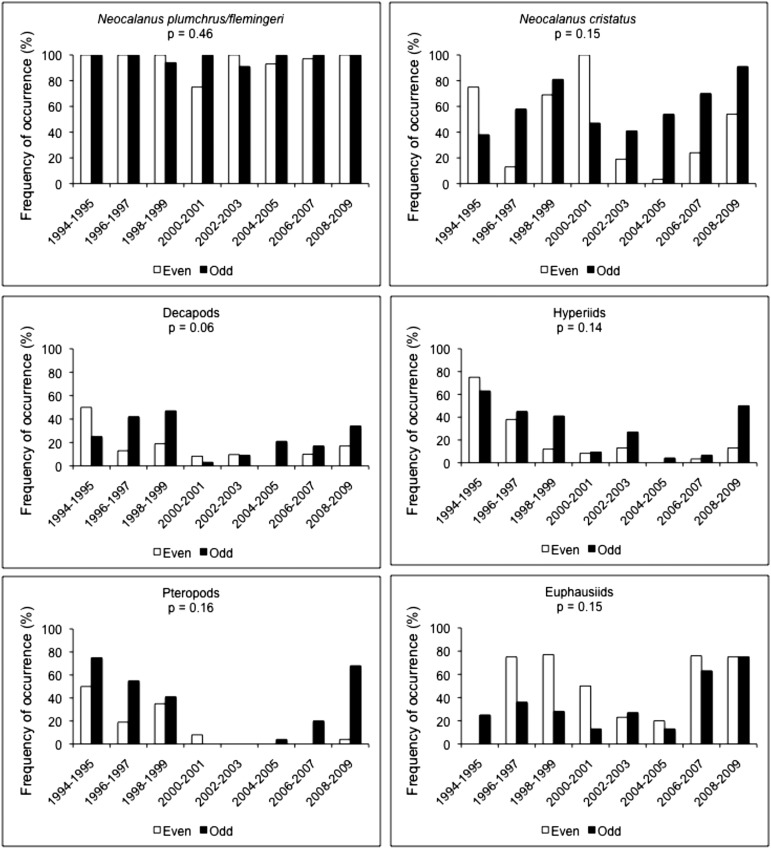
Diets of least auklets at Buldir during the chick period (mid-June to mid-July) differed between even years and odd years (Fig. 5). In 1994–2009, the dominant prey of least auklets, the large calanoid copepod taxon Neocalanus plumchrus/flemingeri (the two species are difficult to differentiate), had a mean frequency of occurrence of 97 ± 2% and there was no difference between even and odd years. In 1994–2006, it made up an estimated 65 ± 7% of the biomass of prey brought to chicks; however, the proportion in even years (excepting 2000 when it was anomalously low at just 12%) was 77 ± 6%, but 62 ± 9% in odd years (P = 0.18). Although the apparent difference did not have high statistical significance, it is consistent with even-year–odd-year differences found in the consumption of principal secondary prey—that is, with the exception of euphausiids, consumption of N. cristatus, decapods, hyperiids, and pteropods was generally higher in odd years than in even years. The lower consumption of euphausiids in odd years may have been proportional to a lower abundance of euphausiids in odd years due to pink salmon predation.
Fig. 5.
Frequency of occurrence (percentage) of principal prey in diets of least auklets at Buldir Island. Significance levels (P, Student t test; n = 16 y) are for differences between mean values in even years and odd years across all years. Data are from ref. 67.
Likewise, whiskered auklets (Aethia pygmaea) at Buldir (67) consumed more Neocalanus cristatus, their dominant prey, during the chick rearing period in even years than in odd years (52 ± 8% versus 32 ± 11%; P = 0.18). Although this difference also was not highly significant, it and the differences in least auklet diets between even and odd years are likely biologically significant. Such a conclusion is supported by continuous plankton recorder data primarily from the southern Bering Sea (173 W × 173 E, 52 N × 54 N; appendix 5 in ref. 66) that show large differences in the abundance of N. cristatus and N. plumchrus/flemingeri across even and odd years—4.8 ± 2.3 g versus 0.06 ± 0.01 g, P = 0.14; and 3.9 ± 0.6 g versus 0.6 ± 0.3 g, P = 0.0071, respectively (Fig. 6).
Fig. 6.
Biomass of Neocalanus copepods in continuous plankton recorder standardized tows in the southern Bering Sea. Significance levels (P, Student t test; n = 7 y) are for differences between mean values in even years and odd years across all years. Data are from ref. 66.
Discussion
The great interaction strength of pink salmon during years of high abundance apparently derives from voracious consumption to fuel exceptionally rapid growth in spring–summer of their second year—the mass of maturing fish increases by some 500%, from about 300 to 1,500 g, in just 4 mo between March and July when they spawn (29). Prominent among their prey are species important to the structure of the plankton community of the SNPO/BS and to other consumers, such as seabirds, both directly and indirectly as trophic links. Exploitative competition is common within many trophic levels in many ecosystems, but there are few cases where it has been identified or suspected among lower and higher trophic levels in the open ocean (e.g., refs. 71–74). Now we show evidence of strong exploitative competition by pink salmon visited upon pelagic species, besides other species of salmon, in the SNPO/BS: in years of high abundance pink salmon consume zooplankton and micronekton in sufficient amounts to compromise a variety of nesting parameters of resident seabirds, as well as the survival of migratory seabirds.
Most of the omnivorous seabirds considered here winter in the northern North Pacific Ocean and southern Bering Sea (59, 60, 75, 76). Thus, most species are exposed to competition with pink salmon during much of the year, and competition would intensify rapidly in late spring and early summer when fish move back into the Bering Sea from the North Pacific Ocean. Any carryover effects of prebreeding food stress on nesting success (77) would exacerbate the effect of continuing competition through the early to mid stages of nesting.
This comports reasonably well with the timing of the migration of eastern Kamchatka pink salmon (78). Most fish are in the northern North Pacific Ocean through May (coincident with prelaying), which would explain the strong relationships of phenology and laying success with salmon abundance. The fish begin moving back into the Bering Sea through the Aleutian Islands and Bering Sea basin in June (coincident with laying and incubation), and by July (coincident with peak hatch) they are moving into the central and western Bering Sea. Thus, by the chick period (July–August) the bulk of the fish are in or approaching their spawning rivers and their influence is apparently diminished. Their distribution and the timing of migration vary between years depending on oceanographic conditions, particularly temperature (79), which would be expected to lead to interannual variability in the strengths of relationships with various elements of seabird breeding biology.
There is the question of why planktivorous seabirds exhibit the opposite pattern in phenology—that it is advanced in odd years and delayed in even years and why least auklets on St. George have higher fledging success and productivity in odd years. However, the consistent differences between mean values in even years and odd years also suggest a connection to pink salmon. One possibility is that the differences are due not to effects of eastern Kamchatka pink salmon, but to effects of pink salmon from western Alaska that spawn in rivers emptying into the eastern Bering Sea and that have strong runs in even years (34). These fish appear to move out of the Bering Sea and into the northeastern North Pacific in winter, and maturing fish return in spring through the central and eastern Aleutian Islands and southeastern Bering Sea (78, 79). Although the western Alaska stock is smaller than the eastern Kamchatka stock, as they return in spring and early summer their numbers are concentrated in a comparatively smaller geographic region, which would concentrate possible effects on resource pools shared with auklets and other species. Thus, least auklets on St. George would compete with them from prelaying through much of the breeding season, which would explain the correlations between their breeding parameters and western Alaska pink salmon abundance. Auklets from elsewhere that winter in the eastern Aleutian Islands, for example, whiskered auklets (80, 81), would be exposed to competition with western Alaska pink salmon in winter–spring, but likely not in summer, which would explain why only phenology differs between even and odd years and why it is delayed in even years. Alternatively, auklets from the western Aleutian Islands, if they winter in the western North Pacific Ocean off Japan and the Kurile Islands (82), might be exposed to competition with Sea of Okhotsk pink salmon, which also are dominant in even years and highly abundant (34).
Neocalanus plumchrus/flemingeri and N. cristatus commonly dominate the biomass of zooplankton in the SNPO/BS and are major conduits of energy between phytoplankton and higher trophic levels, in large measure because they accumulate a high lipid content in summer in preparation for overwinter diapause (83). They are thus high-quality prey for planktivores including least auklets, whiskered auklets, pink salmon, and others. Although some of the secondary prey of least auklets and whiskered auklets also have comparatively high lipid concentrations, e.g., euphausiids, many are of much lower energy density (84). The rise in occurrence of secondary prey in auklet diets in odd years is presumably related to the same phenomenon in chum salmon in the SNPO/BS—in even years chum salmon diets include high lipid copepods, euphausiids, and other crustaceans, but in odd years their diets are dominated by lower lipid prey, primarily gelatinous taxa such as pteropods, appendicularia, and coelenterates, due apparently to the depressing effect on crustacean biomass of pink salmon predation (43). Similarly, diets of pink, chum, and sockeye salmon in the northeastern North Pacific Ocean and Bering Sea contain different levels of important prey in even and odd years (46, 56).
Depression of seabird productivity cannot be tied to long-term trends in the abundance of any of the species, trends that vary among species and islands (67–70, 85). However, the combination of fewer birds attempting to nest in odd years; fewer eggs being laid, and later, by those that do attempt to nest; and poorer reproductive success by some species raises questions for the future. Seabirds are long-lived, K-selected animals, a strategy that dampens effects of interannual variability in productivity on abundance, but increases the sensitivity of populations to adult mortality. However, over the long term they do depend on reproduction, and the combination of depressed productivity every other year, by as much as 62% for both species of kittiwake, coupled with possible deleterious effects of physiological stress on developing chicks and reproductive life spans of adults experiencing biennial physiological stress (86) could lead to declines in the abundance of the more sensitive species if pink salmon numbers remain at high levels and seabird mortality begins to outpace recruitment. That not all seabird species were affected equally likely reflects differing degrees of ecological separation from pink salmon, which could include the extent of dietary overlap and spatial and temporal physical overlap, and the behavioral ability of some (e.g., murres in particular) to buffer effects of variability in prey quantity and quality on breeding success (87).
Ocean temperature has steadily risen in the western Bering Sea since the middle of the last century, with a pronounced increase in the 2000s that corresponded to the most recent increase in pink salmon abundance (39). One would expect that there is an optimum thermal window above and below which salmon populations cease to prosper (88–91), although the unusually large aerobic scope and cardiorespiratory capacity of pink salmon significantly broadens their range of thermal tolerance (92). However, even if pink salmon abundance experienced no further growth, important questions remain concerning their impact on ecosystem function in the SNPO/BS and the capacity of ocean production processes to support the current biomass of many higher trophic level species including salmon, other fishes, seabirds, and recovering populations of great whales. The potential problem may grow over the course of this century if habitat shrinks due to projected ocean warming and competitive dominance of pink salmon increases if they and other species become more tightly crowded (91).
The resource vacuum and altered community composition left behind as pink salmon migrate back through the SNPO/BS in spring–summer are functionally equivalent to effects of consumer fronts described in a variety of terrestrial and marine ecosystems (93). Although consumer (fish) density would not be as high per square meter as in other ecosystem types, at the geographic scale over which the process occurs (about 1.3 × 106 km2 in the Bering Sea basin alone) it bears a resemblance, particularly if pink salmon tend to coalesce as their migration progresses. In this case, the movement of the consumers is not necessarily driven by serial depletion of resources at the “front,” but by the biological imperative to return to their natal streams to reproduce. Consumer fronts in open ocean ecosystems previously have not been described (93), and the indication that the return migration of eastern Kamchatka pink salmon now effectively constitutes one in odd years apparently derives from the role that climate change has played in the growth in their abundance. In aggregate, the direct, indirect, and cascading effects of pink salmon suggest that they have a destabilizing effect on the ecosystem of the SNPO/BS.
Additional pressure in the North Pacific comes from the growing number of hatchery produced pink and chum salmon—e.g., some 3 × 109 chum smolts are released each year in Japan and the annual run size has been in the order of 50–80 × 106 fish since the 1980s, plus there are many more in the ocean given their multiyear life history strategy, and most of them spend the summer–fall feeding period in the Bering Sea (31, 37, 45). A recommendation has been made to increase Russian hatchery production of chum salmon (94), which currently is negligible. In the northeastern North Pacific, hatchery production of pink salmon in Prince William Sound began in the mid-1970s and has grown to annual runs as high as nearly 70 × 106 fish (34). Record-breaking runs of wild pink salmon in summer 2013 from Washington State to the Gulf of Alaska (95–97) highlight the continuing trend.
Interannual switching between alternate ecosystem states of the SNPO/BS driven by pink salmon must be accounted for when attempting to explain patterns of change in populations of species at lower and higher trophic levels and when building ocean ecosystem models. Key forcing from the salmon is additive to, perhaps dominant to in some cases, whatever other drivers are important in the environment. The abundance of pink salmon, owing to their life history strategy, is an uncommon case of too many fish in the sea, and the ecosystem-scale effect they have needs to become part of international resource common-pool policy discussions that include seabirds, and by extension additional competing species (98). The response of wild salmon, and other commercially targeted fishes, to climate change has important management implications (98–102) and conservation implications as revealed by this study. The large and growing number of hatchery-reared salmon raises additional concern about the carrying capacity of the SNPO/BS, although such concern is not universally embraced (103).
Pacific salmon has considerable societal importance, as the commercial fishery is a multibillion dollar industry employing tens of thousands of people (104) and feeding millions of people. There is an obvious strategy in using the oceans as unattended feedlots, but we know that the feed troughs will not be perpetually full, and despite the nutritious protein and fatty acids of free-range salmon, it is time to consider additional issues as well. The need to sustainably accommodate not only salmon but other denizens of the sea could potentially turn salmon fishery management in certain cases from the now common practice of imposing catch limits and raising hatchery production to enhance stocks, to relaxing catch limits and encouraging larger harvests and smaller hatchery releases to help maintain equity among all of the trophically linked consumers—in other words, to devise a broad-scale ecosystem-based management strategy.
Materials and Methods
Values of seabird breeding parameters at the four monitoring colonies are reported annually by the US Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge (67–70). Unless otherwise noted, all seabird data were from these sources—additional data were available in ref. 66 as cited. We used the data as reported for all parameters except number of eggs laid, which we calculated by adjusting the reported number of chicks hatched annually on phenology monitoring plots by the average hatching success of eggs in even and odd years. We compared mean values of nesting parameters in even years to those in odd years using Student t test.
Annual run sizes (catch plus escapement, millions of fish) of wild eastern Kamchatka pink salmon were reported for 1952–2005 in ref. 34, and catch only (tonnes) for 1971–2009 in ref. 39 and 2010–2012 (as shown in Table S1). We estimated the run size in 2006–2012 using the relationship between run size and catch in 1971–2005 (r2 = 0.90, P < 0.0001). We used the annual run size of wild pink salmon in Norton Sound from 1997 to 2012 (40) as an index of run size in western Alaska. There are no hatchery programs in eastern Kamchatka or western Alaska. Run sizes were log normal transformed to compute values of linear regressions against nesting parameters.
We used α = 0.10 to parse the full dataset for discussion and clarity in presentation in figures—it does not imply a firm judgment about the statistical or biological significance of differences between mean values in even and odd years or the slopes of regressions of nesting parameter values against salmon abundance. Significance levels of all tests of null hypotheses are reported in Tables S2–S5.
Supplementary Material
Acknowledgments
We thank J. Estes, K. Myers, B. Agler, S. Iverson, and one anonymous reviewer for constructive comments that improved this manuscript, and G. Khen for providing eastern Kamchatka pink salmon harvest data. We are indebted to V. Byrd for establishing the long-term seabird monitoring program administered by the Alaska Maritime National Wildlife Refuge, US Fish and Wildlife Service, and the many dedicated personnel who continue to maintain and improve it. Partial support for A.M.S. was provided by the Institute of Marine Science, University of Alaska, Fairbanks.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Commentary on page 6534.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1319089111/-/DCSupplemental.
References
- 1.Paine RT. Food web complexity and species diversity. Am Nat. 1966;100(910):65–75. [Google Scholar]
- 2.Paine RT. A note on trophic complexity and community stability. Am Nat. 1969;103(929):91–93. [Google Scholar]
- 3.Estes JA, Palmisano JF. Sea otters: Their role in structuring nearshore communities. Science. 1974;185(4156):1058–1060. doi: 10.1126/science.185.4156.1058. [DOI] [PubMed] [Google Scholar]
- 4.Laws RM. Seals and whales of the Southern Ocean. Philos Trans R Soc Lond B. 1977;279(963):81–96. [Google Scholar]
- 5.May RM, Beddington JR, Clark CW, Holt SJ, Laws RM. Management of multispecies fisheries. Science. 1979;205(4403):267–277. doi: 10.1126/science.205.4403.267. [DOI] [PubMed] [Google Scholar]
- 6.Estes JA, Tinker MT, Williams TM, Doak DF. Killer whale predation on sea otters linking oceanic and nearshore ecosystems. Science. 1998;282(5388):473–476. doi: 10.1126/science.282.5388.473. [DOI] [PubMed] [Google Scholar]
- 7.Springer AM, et al. Sequential megafaunal collapse in the North Pacific Ocean: An ongoing legacy of industrial whaling? Proc Natl Acad Sci USA. 2003;100(21):12223–12228. doi: 10.1073/pnas.1635156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank KT, Petrie B, Choi JS, Leggett WC. Trophic cascades in a formerly cod dominated ecosystem. Science. 2005;308(5728):1621–1623. doi: 10.1126/science.1113075. [DOI] [PubMed] [Google Scholar]
- 9.Pauly D, Watson R, Alder J. Global trends in world fisheries: Impacts on marine ecosystems and food security. Philos Trans R Soc Lond B Biol Sci. 2005;360(1453):5–12. doi: 10.1098/rstb.2004.1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314(5800):787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 11.Baum JK, Worm B. Cascading top-down effects of changing oceanic predator abundances. J Anim Ecol. 2009;78(4):699–714. doi: 10.1111/j.1365-2656.2009.01531.x. [DOI] [PubMed] [Google Scholar]
- 12.Ainley DG, et al. Impacts of cetaceans on the structure of Southern Ocean food webs. Mar Mamm Sci. 2010;26(2):482–498. [Google Scholar]
- 13.Aebischer NJ, Coulson JC, Colebrook JM. Parallel long term trends across four marine trophic levels and weather. Nature. 1990;347:753–755. [Google Scholar]
- 14.Laevastu T. Marine Climate, Weather and Fisheries. New York: Wiley; 1993. [Google Scholar]
- 15.Hazen EL, et al. Predicted habitat shifts of Pacific top predators in a changing climate. Nat Clim. 2013;3:234–238. [Google Scholar]
- 16.Petitgas P, et al. Impacts of climate change on the complex life cycles of fish. Fish Oceanogr. 2013;22(2):121–139. [Google Scholar]
- 17.Beamish RJ, Bouillon DR. Salmon production trends in relation to climate. Can J Fish Aquat Sci. 1993;50(5):1002–1016. [Google Scholar]
- 18.Francis RC, Hare SR. Decadal-scale regime shifts in the large marine ecosystems of the Northeast Pacific: A case for historical science. Fish Oceanogr. 1994;3(4):279–291. [Google Scholar]
- 19.Polovina JJ, Mitchum GT, Evans GT. Decadal and basin-scale variation in mixed layer depth and the impact on biological production in the Central and North Pacific. Deep-Sea Res. 1995;42:1701–1716. [Google Scholar]
- 20.Brodeur RD, Ware DM. Long-term variability in zooplankton biomass in the subarctic Pacific Ocean. Fish Oceanogr. 1992;1(1):32–39. [Google Scholar]
- 21.Mantua NJ, Hare SR, Zhang Y, Wallace JM, Francis RC. A Pacific interdecadal climate oscillation with impacts on salmon production. Bull Am Meteorol Soc. 1997;78(6):1069–1079. [Google Scholar]
- 22. Ebbesmeyer CC, et al. (1991) 1976 step in the Pacific climate: Forty environmental changes between 1968-1975 and 1977-1984. Proceedings of the Seventh Annual Pacific Climate (PACLIM) Workshop, April 1990, eds Betancourt JL, Tharp VL (California Department of Water Resources, Interagency Ecological Studies Program, West Sacramento, CA), Technical Report 26, pp 115–126.
- 23.Hare SR, Mantua NJ. Empirical evidence for North Pacific regime shifts in 1977 and 1989. Prog Oceanogr. 2000;47:103–145. [Google Scholar]
- 24.Hare SR, Mantua NJ, Francis RC. Inverse production regimes: Alaska and West Coast Pacific salmon. Fisheries (Bethesda, Md) 1999;24(1):6–14. [Google Scholar]
- 25.Mueter FJ, Peterman RM, Pyper BJ. Opposite effects of ocean temperature on survival rates of 120 stocks of Pacific salmon (Oncorhynchus spp.) in northern and southern areas. Can J Fish Aquat Sci. 2002;59(3):456–463. [Google Scholar]
- 26.Stachura MM, Mantua NJ, Scheuerell MD. Oceanographic influences on patterns in North Pacific salmon abundance. Can J Fish Aquat Sci. 2013;71(2):226–235. [Google Scholar]
- 27.Karpenko VI. Ocean mortality of northeast Kamchatka pink salmon and influencing factors. N Pac Anadr Fish Comm Bull. 1998;1:251–261. [Google Scholar]
- 28.Cooney T. In: Long-Term Ecological Change in the Northern Gulf of Alaska. Spies RB, editor. Oxford: Elsevier; 2007. pp. 76–81. [Google Scholar]
- 29.Karpenko VI, Koval MV. Feeding strategies and trends of pink and chum salmon growth in the marine waters of Kamchatka. N Pac Anadr Fish Comm Tech Rep. 2012;8:82–86. [Google Scholar]
- 30.Ruggerone GT, Myers KW, Agler BA, Nielsen JL. Evidence for bottom-up effects on pink and chum salmon abundance and the consequences for other salmon species. N Pac Anadr Fish Comm Tech Rep. 2012;8:94–98. [Google Scholar]
- 31.Ruggerone GT, Peterman RM, Dorner B, Myers KW. Magnitude and trends in abundance of hatchery and wild pink salmon, chum salmon, and sockeye salmon in the North Pacific Ocean. Mar Coast Fish. 2010;2(1):306–328. [Google Scholar]
- 32.Neave F. “Even-year” and “odd-year” pink salmon populations. Proc Trans Royal Soc Can Ser. 1952;3(46):55–70. [Google Scholar]
- 33.Krkosek M, Hilborn R, Peterman RM, Quinn TP. Cycles, stochasticity and density dependence in pink salmon population dynamics. Proc Royal Sci B. 2011;278(1714):2060–2068. doi: 10.1098/rspb.2010.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ruggerone GT, Peterman RM, Dorner B, Myers KW, Mantua NJ (2010) Abundance of Adult Hatchery and Wild Salmon by Region of the North Pacific (School of Aquatic and Fisheries Sciences, Univ of Washington, Seattle), Report SAFS-UW-1001.
- 35. Bugayev VF (2002) On Pink Salmon (Oncorhynchus gorbuscha) Abundance Influence on Asian Sockeye Salmon (Oncorhynchus nerka) Abundance, North Pacific Anadromous Fish Commission Document 628 (Kamchatka Research Institute of Fisheries and Oceanography, Petropavlovsk-Kamchatsky, Russia)
- 36.Beacham TD, McIntosh B, MacConnachie C, Spilsted B, White BA. Population structure of pink salmon (Oncorhynchus gorbuscha) in British Columbia and Washington, determined with microsatellites. Fish Bull. 2012;110(2):242–256. [Google Scholar]
- 37.Ruggerone GT, Zimmerman M, Myers KW, Nielsen JL, Roders DE. Competition between Asian pink salmon (Oncorhynchus gorbuscha) and Alaskan sockeye salmon (O. nerka) in the North Pacific Ocean. Fish Oceanogr. 2003;12(3):209–219. [Google Scholar]
- 38.Antonov NP. Commercially Harvested Species of Fish of the Kamchatka Region: Biology, Stocks and Fisheries. Moscow: VNIRO Publishers; 2011. [Google Scholar]
- 39.Khen GV, Basyuk EO, Vanin NS, Matveev VI. Hydrography and biological resources in the western Bering Sea. Deep Sea Res Part II Top Stud Oceanogr. 2013;94:106–120. [Google Scholar]
- 40.Alaska Department of Fish and Game . Norton Sound Salmon Season Summary. Nome, AK: Alaska Department of Fish and Game, Division of Commercial Fisheries; 2012. [Google Scholar]
- 41. Welch DW, Morris JFT (1994) Evidence for Density-Dependent Marine Growth in British Columbia Pink Salmon Populations, North Pacific Anadromous Fish Commission Document 97 (Department of Fisheries and Oceans, Nanaimo, BC, Canada)
- 42.Helle JH, Hoffman MS. Size decline and older age at maturity of two chum salmon (Oncorhynchus keta) stocks in western North America, 1972–92. Can Spec Publ Fish Aquat Sci. 1995;121:245–260. [Google Scholar]
- 43.Tadokoro K, Ishida Y, Davis ND, Ueyanagi S, Sugimoto T. Change in chum salmon (Oncorhynchus keta) stomach contents associated with fluctuation of pink salmon (O. gorbuscha) abundance in the central subarctic Pacific and Bering Sea. Fish Oceanogr. 1996;5(2):89–99. [Google Scholar]
- 44.Azumaya T, Ishida Y. Density interactions between pink salmon (Oncorhynchus gorbuscha) and chum salmon (O. keta) and their possible effects on distribution and growth in North Pacific Ocean and Bering Sea. N Pac Anadr Fish Comm Bull. 2000;2:165–174. [Google Scholar]
- 45.Ishida Y, Azumaya T, Fukuwa M, Davis N. Interannual variability in stock abundance and body size of Pacific salmon in the central Bering Sea. Prog Oceanogr. 2002;55:223–234. [Google Scholar]
- 46.Kaeriyama M, et al. Change in feeding ecology and trophic dynamics of Pacific salmon (Oncorhynchus spp.) in the central Gulf of Alaska in relation to climate events. Fish Oceanogr. 2004;13(3):197–207. [Google Scholar]
- 47.Ruggerone GT, Nielsen JL. Evidence for competitive dominance of pink salmon (Oncorhynchus gorbuscha) over other salmonids in the North Pacific Ocean. Rev Fish Biol Fish. 2004;14(3):371–390. [Google Scholar]
- 48.Kaga T, Sato S, Azumaya T, Davis ND, Fukuwaka M-a. Lipid content of chum salmon Oncorhynchus keta affected by pink salmon O. gorbuscha abundance in the central Bering Sea. Mar Ecol Prog Ser. 2013;478:211–221. [Google Scholar]
- 49.Shiomoto A, Tadokoro K, Nagasawa K, Ishida Y. Trophic relations in the subarctic North Pacific ecosystem: Possible feeding effect from pink salmon. Mar Ecol Prog Ser. 1997;150:75–85. [Google Scholar]
- 50.Sugimoto T, Tadokoro K. Interannual-interdecadal variations in zooplankton biomass, chlorophyll concentration and physical environment in the subarctic Pacific and Bering Sea. Fish Oceanogr. 1997;6(2):74–93. [Google Scholar]
- 51.Kobari T, et al. Interannual variations in abundance and body size in Neocalanus copepods in the central North Pacific. J Plankton Res. 2003;25(5):483–494. [Google Scholar]
- 52.Toge K, et al. The relationship between pink salmon biomass and the body condition of short-tailed shearwaters in the Bering Sea: Can fish compete with seabirds? Proc Royal Sci B. 2011;278(1718):2584–2590. doi: 10.1098/rspb.2010.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lobkov EG (1991) Phenomenon of the cyclic increase of mortality of seabirds in coastal Kamchatka. Proceedings of the 10th All-Union Ornithological Conference, Minsk, Belarus: Navuka i Tekhnika (Navuka i Tekhnika, Minsk, Belarus), pp 99–101.
- 54.Zador S, Hunt GL, Jr, TenBrink T, Aydin K. Combined seabird indices show lagged relationships between environmental conditions and breeding activity. Mar Ecol Prog Ser. 2013;485:245–258. [Google Scholar]
- 55. Nagasawa K, Davis ND, Uwano Y (1997) Japan-U.S. Cooperative High-Seas Salmonid Research Aboard the R/V Wakatake Maru from June 11 to July 25, 1997, North Pacific Anadromous Fish Commission Document 266 (National Research Institute of Far Seas Fisheries, Fisheries Agency of Japan, Shimizu, Japan)
- 56.Davis ND, Fukuwaka M, Armstrong JL, Myers KW. Salmon food habits studies in the Bering Sea, 1960 to present. N Pac Anadr Fish Comm Tech Rep. 2005;6:24–28. [Google Scholar]
- 57.Ogi H, Kubodera T, Nakamura K. The pelagic feeding ecology of the Short-tailed Shearwater Puffinus tenuirostris in the subarctic Pacific region. Rep Yamashina Inst Ornithol. 1980;12:157–182. [Google Scholar]
- 58.Hunt GL, Jr, Baduini C, Jahncke J. Diets of short-tailed shearwaters in the southeastern Bering Sea. Deep Sea Res Part II Top Stud Oceanogr. 2002;49:6147–6156. [Google Scholar]
- 59. Wehle DHS (1976) Summer foods and feeding ecology of Tufted and Horned Puffins on Buldir Island, Alaska—1975. MSc thesis (Univ of Alaska, Fairbanks, AK)
- 60.Ogi H. The pelagic feeding ecology of thick-billed murres in the North Pacific, March-June. Bull Fac Fish Hokkaido Univ. 1980;31:50–72. [Google Scholar]
- 61.Hunt GL, Jr, Burgesson B, Sanger GA. In: The Eastern Bering Sea Shelf: Oceanography and Resources. Hood DW, Calder JA, editors. Juneau, AK: NOAA; 1981. pp. 629–648. [Google Scholar]
- 62.Springer AM, Piatt JF, van Vliet GB. Seabirds as proxies of marine habitats and food webs in the western Aleutian Arc. Fish Oceanogr. 1996;5(1):45–55. [Google Scholar]
- 63.Springer AM, Byrd GV, Iverson SJ. Hot oceanography: Planktivorous seabirds reveal ecosystem responses to heating of the Bering Sea. Mar Ecol Prog Ser. 2007;352:289–297. [Google Scholar]
- 64.Ito M, Takahashi A, Kokubun N, Kitaysky AS, Watanuki Y. Foraging behavior of incubating and chick-rearing thick-billed murres Uria lomvia. Aquat Biol. 2010;8(3):279–287. [Google Scholar]
- 65.Harding A, et al. Does location really matter? An inter-colony comparison of seabirds breeding at varying distances from productive oceanographic features in the Bering Sea. Deep Sea Res Part II Top Stud Oceanogr. 2013;94:178–191. [Google Scholar]
- 66.Bond AL, Jones IL, Williams JC, Byrd GV. Diet of auklet chicks in the Aleutian Islands, Alaska: Similarity among islands, interspecies overlap, and relationships to ocean climate. J Ornithol. 2012;153(1):115–129. [Google Scholar]
- 67. Warzybok JA, Drummond BA, Williams JC (2013) Biological monitoring at Buldir Island, Alaska in 2012. U.S. Fish and Wildlife Service Report, AMNWR 2012/02 (U.S. Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge, Homer, AK). Available at https://absilcc.org/science/amnwr/sitepages/library.aspx. Accessed February 25, 2013.
- 68. Bechaver CA, Gehrig JM (2011) Biological monitoring at Aiktak Island, Alaska in 2011. U.S. Fish and Wildlife Service Report, AMNWR 2011/12 (U.S. Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge, Homer, AK). Available at https://absilcc.org/science/amnwr/sitepages/library.aspx. Accessed October 19, 2011.
- 69. Klostermann MR, Drummond BA (2012) Biological monitoring at St. George Island, Alaska in 2012. U.S. Fish and Wildlife Service Report, AMNWR 2012/08 (U.S. Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge, Homer, AK). Available at https://absilcc.org/science/amnwr/sitepages/library.aspx. Accessed April 26, 2013.
- 70. Thomson G, Drummond BA (2012) Biological monitoring at St. Paul Island, Alaska in 2012. U.S. Fish and Wildlife Service Report, AMNWR 2012/09 (U.S. Fish and Wildlife Service, Alaska Maritime National Wildlife Refuge, Homer, AK). Available at https://absilcc.org/science/amnwr/sitepages/library.aspx. Accessed April 26, 2013.
- 71.Wootton JT. The nature and consequences of indirect effects in ecological communities. Annu Rev Ecol Syst. 1994;25:443–466. [Google Scholar]
- 72.Fogarty MJ, Murawsky SA. Large-scale disturbance and the structure of marine ecosystems: Fishery impacts on Georges Bank. Ecol Appl. 1998;8(Suppl 1):S6–S22. [Google Scholar]
- 73.Myers RA, Worm B. Rapid worldwide depletion of predatory fish communities. Nature. 2003;423(6937):280–283. doi: 10.1038/nature01610. [DOI] [PubMed] [Google Scholar]
- 74.Ainley DG, Ballard G, Dugger KM. Competition among penguins and cetaceans reveals trophic cascades in the western Ross Sea, Antarctica. Ecology. 2006;87(8):2080–2093. doi: 10.1890/0012-9658(2006)87[2080:capacr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 75. Orben RA, Irons DB, Shaffer SA, Roby DD (2012) Winter distribution and ecology of black-legged kittiwakes (Rissa tridactyla) and thick-billed murres (Uria lomvia) breeding at two Bering Sea colonies with differing population trends. North Pacific Research Board Project 911 Final Report (North Pacific Research Board, Anchorage, AK)
- 76. Orben RA, et al. (2012) Winter migration of red-legged and black-legged kittiwakes from the Pribilof Islands, Alaska. 2012 Alaska Marine Science Symposium Book of Abstracts (NOAA Alaska Fisheries Science Center/North Pacific Research Board, Seattle, WA), p 166. Available at www.alaskamarinescience.org/documents/2012_AMSS_AbstractBook_000.pdf.
- 77.Sorensen MC, Hipfner JM, Kyser TK, Norris DR. Carry-over effects in a Pacific seabird: Stable isotope evidence that pre-breeding diet quality influences reproductive success. J Anim Ecol. 2009;78(2):460–467. doi: 10.1111/j.1365-2656.2008.01492.x. [DOI] [PubMed] [Google Scholar]
- 78. Myers KW, Aydin KY, Walker RV, Fowler S, Dahlberg ML (1996) Known ocean ranges of stocks of Pacific salmon and steelhead as shown by tagging experiments, 1956-1995. North Pacific Anadromous Fish Commission Document 192 (Univ of Washington, Fisheries Research Institute, Seattle)
- 79.Myers KW, Klovach NV, Gritsenko OF, Urawa S, Royer TC. Stock-specific distributions of Asian and North American salmon in the open ocean, interannual changes, and oceanographic conditions. N Pac Anadr Fish Comm Bull. 2007;4:159–177. [Google Scholar]
- 80. Troy D (1992) Seabird and Marine Mammal Use of the Unimak Pass Region. OCS Study MMS 92-0046 (Minerals Management Service, Anchorage, AK), pp 183–193. [Google Scholar]
- 81.Renner M, Hunt GL, Jr, Piatt JF, Byrd GV. Seasonal and distributional patterns of seabirds along the Aleutian Archipelago. Mar Ecol Prog Ser. 2008;357:301–311. [Google Scholar]
- 82. Robinson JL, Jones IL (2013) Year-round spatial and temporal distribution of a small, diving seabird, the Crested Auklet (Aethia cristatella), originating from a breeding site at Buldir Island, Aleutian Islands. 2013 Alaska Marine Science Symposium Book of Abstracts (NOAA Alaska Fisheries Science Center/North Pacific Research Board, Seattle, WA), p 68. Available at www.alaskamarinescience.org/past/documents/AMSS_Book_of_Abstracts_2013.pdf.
- 83.Vidal J, Smith SL. Biomass, growth, and development of populations of herbivorous zooplankton in the southeastern Bering Sea during spring. Deep-Sea Res. 1986;33(4):523–556. [Google Scholar]
- 84.Davis ND, Myers KW, Ishida Y. Caloric value of high-seas salmon prey organisms and simulated salmon ocean growth and prey consumption. N Pac Anadr Fish Comm Bull. 1998;1:146–162. [Google Scholar]
- 85.Byrd GV, Schmutz JA, Renner HM. Contrasting population trends of piscivorous seabirds in the Pribilof Islands: A 30-year perspective. Deep Sea Res Part II Top Stud Oceanogr. 2008;55(16-17):1846–1855. [Google Scholar]
- 86.Kitaysky AS, et al. Food availability and population processes: Severity of nutritional stress during reproduction predicts survival of long-lived seabirds. Funct Ecol. 2010;24(3):625–637. [Google Scholar]
- 87.Burger AE, Piatt JF. Flexible time budgets in breeding common murres: Buffers against variable prey abundance. Stud Avian Biol. 1990;14:71–83. [Google Scholar]
- 88.Welch DW, Chigirinsky AI, Ishida Y. Upper thermal limits on the oceanic distribution of Pacific salmon (Oncorhynchus spp.) in the spring. Can J Fish Aquat Sci. 1995;52(3):489–503. [Google Scholar]
- 89.Welch DW, Ishida Y, Nagasawa K. Thermal limits and ocean migrations of sockeye salmon (Oncorhynchus nerka): Long-term consequences of global warming. Can J Fish Aquat Sci. 1998;55(4):937–948. [Google Scholar]
- 90.Azumaya T, Nasagawa T, Temnykh OS, Khen G. Regional and seasonal differences in temperature and salinity limitations of Pacific salmon (Oncorhynchus spp.) N Pac Anadr Fish Comm Bull. 2007;4:179–187. [Google Scholar]
- 91.Abdul-Aziz OI, Mantua NJ, Myers KW. Potential climate change impacts on thermal habitats of Pacific salmon (Oncorhynchus spp.) in the North Pacific Ocean and adjacent seas. Can J Fish Aquat Sci. 2011;68(9):1660–1680. [Google Scholar]
- 92.Clark TD, Jeffries KM, Hinch SG, Farrell AP. Exceptional aerobic scope and cardiovascular performance of pink salmon (Oncorhynchus gorbuscha) may underlie resilience in a warming climate. J Exp Biol. 2011;214(Pt 18):3074–3081. doi: 10.1242/jeb.060517. [DOI] [PubMed] [Google Scholar]
- 93.Silliman BR, et al. Consumer fronts, global change, and runaway collapse in ecosystems. Annu Rev Ecol Evol Syst. 2013;44(1):503–538. [Google Scholar]
- 94.Kaev AM. Wild and hatchery reproduction of pink and chum salmon and their catches in the Sakhalin-Kuril region, Russia. Environ Biol Fishes. 2012;94:207–218. [Google Scholar]
- 95. Hume M (September 12, 2013) Pink salmon reaching Fraser River in massive numbers. The Globe and Mail. Available at www.theglobeandmail.com/news/british-columbia/pink-salmon-reaching-fraser-river-in-massive-numbers/article14298697.
- 96. Martin MC (September 20, 2013) Record fishing year in Southeast. Juneau Empire. Available at http://juneauempire.com/outdoors/2013-09-20/record-fishing-year-southeast#.UyhsjF4vSHk.
- 97. Yuasa M (October 7, 2013) No end in sight just yet for huge pink salmon return. Seattle Times. Available at http://blogs.seattletimes.com/reeltimenorthwest/2013/09/04/no-end-in-sight-just-yet-for-huge-pink-salmon-return.
- 98.Holt CA, Rutherford MB, Peterman RM. International cooperation among nation-states of the North Pacific Ocean on the problem of competition among salmon for a common pool of prey resources. Mar Policy. 2008;32(4):607–617. [Google Scholar]
- 99.Schindler DE, et al. Climate change, ecosystem impacts, and management for Pacific salmon. Fisheries (Bethesda, Md) 2008;33(10):502–506. [Google Scholar]
- 100.Yatsu A, et al. Elucidating dynamic responses of North Pacific fish populations to climatic forcing: Influence of life-history strategy. Prog Oceanogr. 2008;77(2-3):252–268. [Google Scholar]
- 101.Kaeriyama M, Seo H, Kudo H, Nagata M. Perspectives on wild and hatchery salmon interactions at sea, potential climate effects on Japanese chum salmon, and the need for sustainable salmon fishery management reform in Japan. Environ Biol Fishes. 2012;94(1):165–177. [Google Scholar]
- 102.Melnychuk MC, Banobi JA, Hilborn R. The adaptive capacity of fishery management systems for confronting climate change impacts on marine populations. Rev Fish Biol Fish. 2013 doi: 10.1007/s11160-013-9307-9. [DOI] [Google Scholar]
- 103.Shuntov VP, Temnykh OS. North Pacific Ocean carrying capacity—is it really too low for highly abundant salmon stocks? Myths and realities. N Pac Anadr Fish Comm Tech Rep. 2005;6:3–7. [Google Scholar]
- 104. The Research Group (2009) North Pacific Salmon Fisheries Economic Measurement Estimates, Version 1.2 (The Research Group, Corvallis, OR). Available at www.wildsalmoncenter.org/pdf/Salmon_Economic_Valuation.pdf. Accessed December 9, 2013.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.