Significance
Although amyloid fibrils are associated with numerous pathologies, their conformational stability remains largely unknown. In particular, calorimetry, one of the most powerful methods used to study the thermodynamic properties of globular proteins, has not played a significant role in understanding protein aggregation. Here, with β2-microglobulin, we established direct heat measurements of supersaturation-limited amyloid fibrillation using an isothermal titration calorimeter. We also revealed the thermodynamics of amorphous aggregation. By creating a totally new field of calorimetric study of protein misfolding, we can now comprehensively address the thermodynamics of protein folding and misfolding.
Keywords: enthalpy change, metastability, solubility, thermodynamic stability, thioflavin T
Abstract
Amyloid fibrils form in supersaturated solutions via a nucleation and growth mechanism. Although the structural features of amyloid fibrils have become increasingly clearer, knowledge on the thermodynamics of fibrillation is limited. Furthermore, protein aggregation is not a target of calorimetry, one of the most powerful approaches used to study proteins. Here, with β2-microglobulin, a protein responsible for dialysis-related amyloidosis, we show direct heat measurements of the formation of amyloid fibrils using isothermal titration calorimetry (ITC). The spontaneous fibrillation after a lag phase was accompanied by exothermic heat. The thermodynamic parameters of fibrillation obtained under various protein concentrations and temperatures were consistent with the main-chain dominated structural model of fibrils, in which overall packing was less than that of the native structures. We also characterized the thermodynamics of amorphous aggregation, enabling the comparison of protein folding, amyloid fibrillation, and amorphous aggregation. These results indicate that ITC will become a promising approach for clarifying comprehensively the thermodynamics of protein folding and misfolding.
Aggregation has often been an obstacle to studying the structure, function, and physical properties of proteins. However, a large number of aggregates associated with serious diseases, including Alzheimer’s, Parkinson, and prion diseases (1, 2) promoted the challenge of studying protein misfolding and aggregation. Researchers succeeded in distinguishing amyloid fibrils and oligomers from other amorphous aggregates and characterized the ordered structures present in amyloid fibrils or oligomers, which led to the development of the field of amyloid structural biology (3–8). These advances have been attributed to various methodologies that are also useful for studying the structural properties of globular proteins. Even X-ray crystallography has become a powerful approach for studying amyloid microcrystals (5) or oligomers (9). The atomic details of amyloid fibrils are becoming increasingly clearer, and a cross-β structure was shown to be the main structural component of fibrils (5, 6, 8). Although tightly packed core regions of amyloid fibrils have been reported, the overall structures were shown to be dominated by common cross-β structures, which supported the argument for the main-chain dominated architecture in contrast to the side-chain dominated architecture of globular native states (10–12).
These structural studies have been complemented by a series of efforts to clarify the mechanism for the formation of amyloid fibrils (i.e., amyloid fibrillation). The presence of a long lag time in spontaneous fibrillation and rapid fibrillation by the addition of preformed fibrils represent a similarity with the supersaturation-limited crystallization of substances (13–18). We have revisited “supersaturation” and argued its critical role for amyloid fibrillation (17–19). The role of supersaturation in neurodegenerative diseases at the proteome level has been reported recently (20).
However, calorimetry, one of the most powerful methods used to study the thermodynamic properties of globular proteins (21–24), has not played a significant role in understanding protein aggregation. The aggregation of proteins following heat denaturation as monitored by differential scanning calorimetry is an infamous example demonstrating how aggregation can prevent exact analyses (25, 26). To date, few studies have investigated protein aggregation including amyloid fibrils with calorimetry (27–32). Our previous study on the exothermic heat effects accompanying fibril growth was achieved by monitoring the seed-dependent elongation of fibrils formed by β2-microglobulin (β2m), a protein responsible for dialysis-related amyloidosis, using isothermal titration calorimetry (ITC) (28).
In the present study using β2m, we succeeded in characterizing the total heat of spontaneous fibrillation and amorphous aggregation. An analysis of the heat burst associated with fibrillation or amorphous aggregation under various temperatures clarified their thermodynamic properties. The results obtained enabled the calorimetric characterization of amyloid fibrils and amorphous aggregates relative to that of the native globular structures, which opens a new field for the calorimetric study of protein aggregates.
Results
Heat for the Formation of Amyloid Fibrils Monitored by ITC.
At pH 2.5, acid-denatured β2m formed amyloid fibrils in the presence of moderate concentrations of NaCl. As defined by the conformation phase diagram, fibril formation is dependent on protein and NaCl concentrations (Fig. S1) (19, 33). Spontaneous fibrillation was previously shown to be facilitated by various kinds of agitations such as stirring with a magnetic bar (34, 35) or ultrasonication (19, 36–39), leading to a burst phase of fibrillation after a lag phase. Under the conditions of persistent metastability of supersaturation, it is likely that these agitations may create seed-competent conformations. For instance, during air–water interface-dependent protein aggregation, a template-competent conformation is formed (39).
In our studies, we used the ITC instrument to agitate the β2m solution and monitor the heat response of fibrillation. To establish supersaturation in the presence of various concentrations of β2m at pH 2.5 in the cell at 37 °C, the NaCl concentration was increased to a final value of 0.1 M by stepwise injections of a small volume of 1.0 M NaCl (Fig. 1A; Materials and Methods). After each injection, a sharp endothermic or exothermic spike, which represented the heat (q) of salt dilution, occurred and the heat flow (=∂q/∂t) returned back to the original reference power level. Notably, a marked exothermic peak with a half-width of ∼2 h occurred at 0.3 mg⋅mL−1 of β2m at ∼11 h (Fig. 1A). Similar exothermic peaks were observed at other concentrations of β2m. The lag time for the major exothermic peak (Materials and Methods) shortened (Fig. S2A) and the exothermic peak became larger with an increase in the protein concentration. When 0.5 mg⋅mL−1 of the β2m solution at 0.1 M NaCl was prepared in a test tube, set in the ITC cell, and followed by stirring, a similar exothermic burst with a lag time of 3.7 h was observed (Fig. S3). When we consider the time for titration of salt (3.5 h; Fig. 3A), the observed lag time was independent of the methods, although the titration in the ITC cell was much simpler. The results suggested that the titration inside the ITC cell did not bring any additional effects.
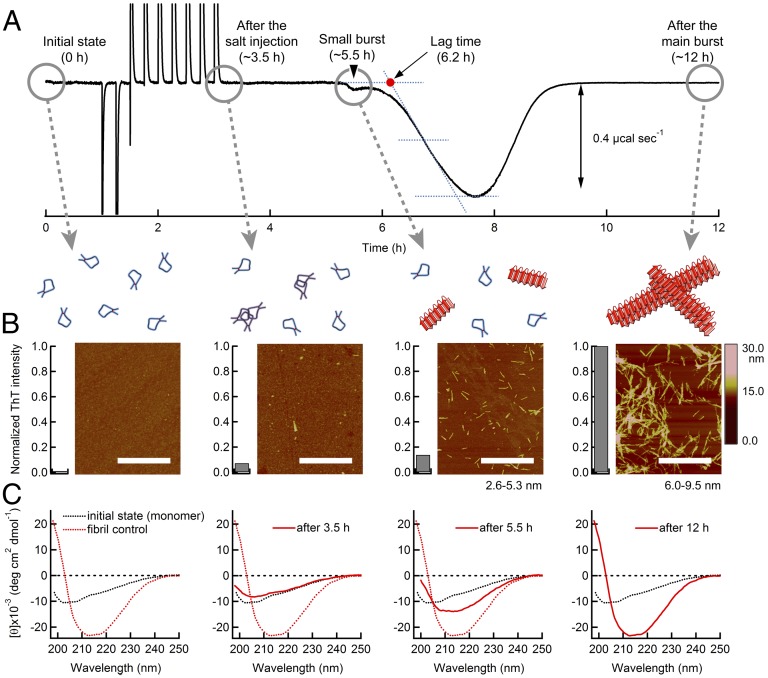
Fig. 1.
Calorimetric observation of the amyloid burst of β2m at various protein concentrations at 37 °C. (A) Thermograms of the fibril formation of β2m at 0.3–6.7 mg⋅mL−1 and pH 2.5 obtained using ITC. Inset shows a close-up view of exothermic heat at 0.3 mg⋅mL−1 β2m. The arrowheads indicate the locations of “small burst.” These also apply to the thermograms of Figs. 2, 3, and 5. (B–D) Characterization of β2m solutions after incubation in ITC cells by AFM images (B), far-UV CD spectra (C), and ThT fluorescence intensities (D). The scale bars on the AFM images indicate 1 μm, and the numbers under images are fibril height. The scale bar on the Right represents the height. These also apply to AFM images in Figs. 3–5. (E) Dependences of the observed heat of the small peak (black inverted triangle), main peak (red circles), total heat including rapid heat effect (green circle), and amorphous aggregation (black circles) on the protein concentration. Inset in C shows the expansion of the heat of amorphous aggregation. The observed heats were normalized by the β2m concentration to give the ΔH values.
Fig. 3.
Monitoring the kinetics of the amyloid burst of β2m using various approaches. (A) ITC profile of 1.1 mg⋅mL−1 β2m at pH 2.5 and 37 °C. The lag time (red dot) is determined by a baseline and a tangent line at the middle of the major peak. (B and C) Conformational changes in β2m during incubation in an ITC cell characterized using AFM images and ThT fluorescence intensities (B) and the far-UV CD spectra (C) at the four time points: “Initial state (0 h),” “After salt injection (∼3.5 h),” “Small burst (∼5.5 h),” and “After main burst (∼12 h).” Conformations of β2m based on AFM, ThT fluorescence, and CD are illustrated above the AFM images: monomers (blue curves), oligomers (magenta curves), and fibrils (red rectangles). The CD spectra at the respective time points are shown by red solid curves. The spectra of monomers (black dotted curves) and mature fibrils (red dotted curves) are shown for comparison.
The total heat calculated based on the peak area was normalized by the protein concentration. The normalized heat did not depend significantly on the protein concentration (Fig. 1E). Moreover, when the stirring speed was varied in the range of 200–1,000 rpm with a fixed protein concentration of 0.5 mg⋅mL−1, the lag time shortened with an increase in the speed (Fig. 2A and Fig. S2B). However, the total heat was independent of the stirring speed (Fig. 2B). These results suggested that the observed heat represented the enthalpy change (ΔH) of the reaction triggered by stirring. Assuming that the observed total heat was ΔH, the ΔH value at 37 °C was estimated to be −77 kJ⋅mol−1 from the dependence on stirring speed or −74 kJ⋅mol−1 from the dependence on protein concentration. The decrease in magnitude of ΔH at high protein concentrations may have been linked with the partial and transient formation of amorphous aggregates with a smaller ΔH value (see below).
Fig. 2.
Dependencies of the observed heat of aggregation on the stirring speed at pH 2.5 and 37 °C. (A) Thermograms of the fibril formation of β2m at 0.5 mg⋅mL−1 at various stirring speeds. The arrowheads indicate the locations of “small burst.” (B) Dependence on the stirring speed of the observed heat normalized by the protein concentration. (C and D) Characterization of β2m solutions after incubation in ITC cells by far-UV CD spectra (C) and ThT fluorescence intensities (D).
After the exothermic peaks, all β2m solutions exhibited a far-UV CD spectrum with a minimum at ∼218 nm, an atomic force microscopy (AFM) image of fibrils with a height of 4.5–9.0 nm and various lengths up to 1 μm, and strong thioflavin T (ThT) fluorescence (Fig. 1 B–D). These results indicated that β2m solutions above 0.3 mg⋅mL−1 in 0.1 M NaCl at pH 2.5 were supersaturated (or metastable) and that agitation by stirring broke this supersaturation, resulting in amyloid fibrillation. We consider that the exothermic peak represents the formation of amyloid fibrils (“amyloid burst”) and the observed heat gives its ΔH value. Similar effects were expected for other salts, the effectiveness of which follows the electroselectivity series (19, 33). One experiment with ammonium sulfate was shown in Fig. S4.
Small Amyloid Burst and Excess Heat Immediately After Salt Titration.
Careful inspection of the ITC thermograms indicated that, in all of the ITC profiles, a small exothermic peak, which we designated “small amyloid burst,” appeared before the main amyloid burst (Figs. 1A, 2A, and 5A). To clarify the significance of these small peaks, we performed CD and AFM measurements and a ThT assay at several time points during the reaction at 1.0 mg⋅mL−1 β2m (Fig. 3 B and C). Neither the CD spectrum nor the AFM image showed significant changes before and immediately after the salt injection spikes, which indicated that the dominant molecular species were still monomers. When the small exothermic peak appeared at the ∼5.5-h time point, the AFM image revealed the presence of short and thin fibrils with a height of 2.6–5.3 nm. A slight change in the CD spectrum and small increase in ThT fluorescence were also observed. These results indicated that some fibrillation, possibly the formation of protofibrils, started at the point of the small burst, and subsequent elongation coupled with the breakage of fibrils to make new growing ends (i.e., secondary nucleation) caused the explosive amyloid burst (Fig. 3 B and C). The exact position and size of the minor peaks were less dependent on the experimental conditions than those of the major peaks (Figs. 1A, 2A, and 5A, and Fig. S2). The total heat accompanying the small exothermic peak was constant (−1.5 kJ⋅mol−1) and independent of the protein concentration (Fig. 1E). Although the observed heat contained information on the ΔH value of protofibril formation, its small fraction precludes further analysis.
Fig. 5.
Temperature dependence of the amyloid burst of β2m monitored by calorimetry. (A) Thermograms at various temperatures between 31 and 43 °C. The arrowheads indicate the locations of “small burst.” (B–D) Characterization of the β2m solution at 1.1 mg⋅mL−1 and 0.1 M NaCl after the heat burst by the far-UV CD (B), ThT fluorescence (C), and AFM (D).
We also recognized a small excess heat effect immediately after each of the stepwise addition of 1.0 M NaCl (Fig. S5). This small but notable heat effect increased with an increase in the concentration of NaCl and β2m, suggesting that it represents the formation of amorphous aggregates. However, after the completion of major amyloid burst, the formation of amorphous aggregates was evident neither from the CD spectra, ThT intensities, nor AFM images (Fig. 1 B–D). Thus, it is possible that a small amount of amorphous aggregates formed after the salt injection finally transformed to the fibrils, although the exact kinetics is unknown. If this is a case, a total heat including those of rapid heat effect, small amyloid burst, and major amyloid burst should represent the ΔH value for amyloid fibrillation. Indeed, the sum of these heat effects was constant (−78 kJ⋅mol−1) over a wide range of concentration, suggesting the validity of assumption (Fig. 1E).
Heat of Amorphous Aggregation.
β2m formed amorphous aggregates at very high NaCl concentrations above 0.8 M at pH 2.5 (Fig. S1) (19). In analogy with the crystallization of substances, amyloid fibrils and amorphous aggregates were shown to be similar to crystals and glasses, respectively (19). In Yoshimura et al. (19), we showed that, whereas crystalline amyloid fibrils formed after a lag phase, glassy amorphous aggregates formed without a lag phase. The rapid and partial formation of amorphous aggregates after the salt titration was consistent with this view (Fig. S5).
It was difficult to increase the NaCl concentration in the cell up to ∼1.0 M by injecting the NaCl solution at a high concentration in the syringe. Thus, we performed an inverse titration: the β2m solution at a high concentration in the syringe was injected into the cell containing 1.0 M NaCl (Fig. 4). On the bases of the low CD signal, amorphous aggregates revealed by the AFM image, and low ThT fluorescence, we confirmed that β2m formed amorphous aggregates. There was no lag phase in amorphous aggregation, which was consistent with our previous results (19). Careful subtraction of the heat for the control experiment revealed the heat of amorphous aggregation. At 37 °C, the control heat effect without β2m was ∼1,250 μcal, whereas that of β2 was around ∼1,190 μcal, with the excess heat of aggregation around ∼5% of the basal heat effects. Again, there was no protein concentration dependence in the range of 0.1–0.7 mg⋅mL−1, which suggested that the heats represented the ΔH of amorphous aggregation (Fig. 4B). The ΔH value for amorphous aggregation was estimated to be −43 kJ⋅mol−1 at 37 °C and assumed to be independent of the protein concentration as indicated by the dotted line in Fig. 1E.
Fig. 4.
Calorimetric observation of amorphous aggregation of β2m at 37 °C. (A) Thermogram of amorphous aggregation revealed by titrating 1.0 M NaCl in the ITC cell with 3.6 mg⋅mL−1 β2m (red) or solvent (black) in the syringe. Titration was repeated 13 times to increase the β2m concentration. The expanded thermogram shows the second titration peak. (B) After subtracting the control, the excess heat was plotted against the final β2m concentration. The results of measurements at various temperatures are shown. (C and D) CD spectrum, ThT fluorescence intensity, and AFM image of the amorphous aggregates formed in the ITC cell at 1.0 M NaCl. CD spectra of fibrils and monomers are also shown.
Temperature Dependency of Aggregation Heat.
ITC measurements of the amyloid burst at 1.0 mg⋅mL−1 were performed at various temperatures between 31 and 43 °C (Fig. 5A). The lag time shortened (Fig. S2C) and the exothermic peak became larger with an increase in temperature. The ΔH value increased in magnitude from −41.3 to −101.1 kJ⋅mol−1 (Fig. 6A and Table S1). We confirmed using far-UV CD, AFM, and ThT assays that the products observed after heat burst at all of the temperatures were amyloid fibrils (Fig. 5 B–D).
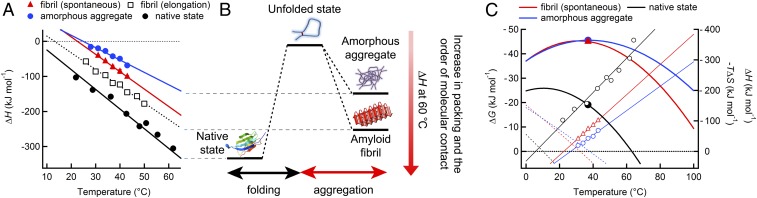
Fig. 6.
Thermodynamic characterization of the folding and misfolding of β2m. (A) Temperature dependencies of ΔH for amorphous aggregation (blue circles), spontaneous (red triangles) and seed-dependent fibrillations (black squares), and folding (black circles). (B) The difference in ΔH at 60 °C among the different conformational states is illustrated. (C) Temperature dependencies of ΔG (curves), ΔH (solid straight lines), and −TΔS (dotted straight lines) for folding to the native state (black lines and circles), spontaneous amyloid fibrillation (red lines and triangles), and amorphous aggregation (blue lines and circles). The ΔG values at 37 °C are shown with the closed symbols. The sign of the ordinate is opposite to that in A to compare the profiles with the standard profile of protein unfolding.
Assuming that the observed heat effect represented ΔH, temperature dependence provided a heat capacity change (ΔCp) of fibrillation based on the relationship of ΔCp = ∂ΔH/∂T. The plot of ΔH against temperature was linear, providing a ΔCp value of −5.0 kJ⋅mol−1⋅K−1 (Fig. 6A and Table S1). We previously obtained the ΔH value and temperature dependence for the seed-dependent elongation of amyloid fibrils of β2m monitored by ITC (28) (Fig. 6A). Although the ΔH values for the spontaneous fibrillation obtained here were slightly smaller than those of seed-dependent elongation, the current ΔCp value was similar to that (−4.8 kJ⋅mol−1⋅K−1) of seed-dependent elongation (28).
We also measured temperature dependence of the heat effects of amorphous aggregation (Figs. 4B and 6A, and Table S1). Although the ΔH values for amorphous aggregates at 1.0 M and various temperatures were smaller in intensity than those of mature fibrils, ΔH changed linearly against temperature, providing a ΔCp value (−3.5 kJ⋅mol−1⋅K−1) that was slightly smaller than that of amyloid fibrils.
Evaluation of Thermodynamic Parameters.
To comprehensively understand the thermodynamics of aggregation, we have to know the changes in free energy (ΔG) and entropy (ΔS) in addition to the ΔH and ΔCp terms, which are directly determined by calorimetry (24). Although the detailed mechanical models of fibril formation remain elusive (16), a simplified model will still be valid for describing the equilibrium between monomers (M) and fibrils (P) (13–15, 28):
 |
where k1 and k−1 are the apparent rate constants for polymerization and depolymerization, respectively. The elongation of fibrils is defined by the equilibrium association constant (K) as follows:
 |
where [P] is the concentration of fibrils and [M] is the concentration of monomers. The equilibrium is clearly independent of [P]. Hence, we obtain the equilibrium monomer concentration [M]e as follows:
[M]e is referred to as the “critical concentration” (13, 15) because fibrils form when the concentration of monomers exceeds [M]e. By determining [M]e, we can calculate the apparent free energy change of fibrillation (ΔGapp) by the following: ΔGapp = −RTlnK = RTln[M]e, where R and T are the gas constant and temperature, respectively. Combined with the ΔH value directly obtained from the ITC measurements, we can obtain the ΔS value by ΔGapp = ΔH – TΔS. Although mechanism 1 might not be exactly true for amorphous aggregation, we assumed that it is also a reversible process determined by solubility and thus is approximated by mechanism 1.
We used an ELISA (SI Text) to determine the [M]e value under various conditions (Table S2). We then estimated the ΔGapp and TΔS for fibrillation and amorphous aggregation. We also estimated the temperature dependencies of these parameters as well as those of ΔH, in which we used ΔGapp values at 37 °C to link the ΔH and TΔS functions. These functions were compared with those for folding to the native state (Fig. 6).
The ΔGapp value of fibrillation (−45.0 kJ⋅mol−1) at 37 °C and pH 2.5 was the same as that of amorphous aggregation (−45.4 kJ⋅mol−1) under the same conditions (Fig. 6C). These values were significantly larger in intensity than that (−21.0 kJ⋅mol−1) of the native state at pH 7.0 (28), although distinct pH values preclude a direct comparison. Although a small range of temperatures used for the experiments makes the extrapolation less accurate at this stage, separation of ΔGapp into the enthalpy and entropy terms indicated that both amyloid fibrils and amorphous aggregates are stabilized enthalpically above 40 °C, whereas they are stabilized entropically below 20 °C.
Discussion
Amyloid formation occurs in supersaturated solutions via a nucleation-dependent manner (18, 40, 41), analogous to crystallization of substances (17, 42). Under the conditions of persistent metastability, nucleation does not occur in practice (14, 18). However, various kinds of agitations can break supersaturation, leading to the formation of fibrils. We used ITC for stirring the solution and for monitoring the accompanying heat effects. The results showed that we can perform calorimetric measurements of amyloid fibrillation of β2m as well as amorphous aggregation revealing the ΔH and ΔCp values. By combining these values with ΔG obtained from the solubility of β2m monomers, we can address the thermodynamics of protein aggregation (Fig. 6 and Tables S1 and S2). The methodology is straightforward and can be applied to study various amyloid fibrils as well as amorphous aggregates.
The heat capacity change upon protein unfolding has been primarily determined by the hydration of polar and apolar groups and to a much lesser extent by the disruption of internal noncovalent interactions such as van der Waals interactions, H bonds, and ionic interactions (21, 24). Considering the morphological difference between the intramolecularly folded native state and intermolecularly associated amyloid fibrils and assuming the same packing densities, the extent of burial should be higher for fibrils assuming the same packing densities. The ΔCp values for the native, amyloid, and amorphous conformations were −5.6, −5.0, and −3.5 kJ⋅mol−1⋅K−1, respectively (Fig. 6A and Table S1). The similar values of ΔCp upon protein folding and amyloid formation suggest a similar overall burial of surfaces in the two forms. We consider that the tightly packed core regions, as observed in amyloid microcrystals (5, 6), coexist with the less densely packed noncore regions with cavities accessible to bulk water (3), leading to an overall similar extent of burial of surfaces (12, 28). In contrast, the smaller ΔCp value of amorphous aggregation suggests looser packing, which is consistent with the absence of notable ordered structures.
Two main effects have been shown to be responsible for the ΔH of protein unfolding: the hydration of the buried hydrophobic and polar groups that become exposed in the unfolded state, and the disruption of internal interactions such as van der Waals interactions, and H bonds (21, 24). The magnitude of the ΔH of amyloid fibrils (normalized by protein concentration) was significantly less than that of the folding of native β2m (Fig. 6 and Table S1). The ΔH values for amorphous aggregation were even smaller in intensity. From the observed similarity of the ΔCp values, we assumed a similar contribution of the hydration of the buried groups between native and fibril conformations. Therefore, the observed decrease in ΔH appeared to be the result of different internal interactions (28). It is generally accepted that there is a stronger and more persistent backbone H-bond network in the amyloid structure than there is in the globular fold of proteins, leading to an increase in the β-sheet content (3, 12, 43). However, H bonds should increase the magnitude of the ΔH value, which is inconsistent with the results.
Thus, a reasonable explanation for the ΔH order in magnitude of “native structure > amyloid fibril > amorphous aggregate” is that it dominantly represents side-chain packing in folded or misfolded structures (Fig. 6B). The overall side-chain packing in the amyloid form cannot be as optimal as that in the native state because the structure is determined by extensively H-bonded β-structured backbones (11, 12). The loss of tight packing may be more serious for amorphous aggregation.
The separation of overall stability of amyloid fibrils (ΔG) into the ΔH and TΔS terms illustrates that the contributions of the two terms vary depending on temperature. Fibrillation is determined by the favorable entropic term at ∼20 °C at which ΔH is close to zero. ΔG is minimal at ∼35 °C, at which the fibrils exhibit maximal stability and, thus, ΔS is zero because ΔS = ∂ΔG/∂T. Fibrillation is then determined by the favorable enthalpy term at 35 °C. Thus, the temperature-dependent enthalpy–entropy interplay determines the stability of amyloid fibrils. To understand this interplay, we have to estimate amyloid-specific factors such as the entropy loss resulting from a rigid H bonding of backbones and a reduction in the number of monomers as well as the enthalpy gain obtained from numerous molecular contacts.
In conclusion, we showed that quantitative calorimetric analysis with ITC was indeed possible for the supersaturation-limited amyloid fibrillations. Stirring inside the ITC cell can break persistent supersaturation, which triggers fibrillation. Compared with the single crystals of substances, amyloid fibrils retain a thin and linear morphology. Moreover, the shear forces of stirring keep fibrils dispersed in solution and fragment fibrils, which accelerate seed-dependent propagation. These enabled accurate calorimetric measurements of the amyloid burst, making the thermodynamic characterization of fibrillation possible. By carefully adjusting these conditions, we can also monitor the heat of amorphous aggregation. Accordingly, ITC will become a promising approach for clarifying the thermodynamic properties of protein aggregates.
Materials and Methods
Assays of Amyloid Fibrils.
Expression and purification of human β2m are described in SI Text. The formation of fibrils and amorphous aggregates was characterized by various methods including ThT fluorescence, AFM, CD, and ELISAs. The details are described in SI Text.
ITC Measurements.
ITC measurements for the spontaneous fibrillization of β2m at 0.3–6.7 mg⋅mL−1 dissolved in 10 mM HCl solution (pH 2.5) were performed with a VP-ITC instrument (GE Healthcare) at the desired temperatures (31–43 °C). The consecutive injections of 20 μL of the 10 mM HCl solution containing 1 M NaCl in the syringe into the β2m solution in the cell were conducted following a 60-min initial delay for complete equilibration. To minimize the heat effects caused by the difference in temperature, the consecutive injections were required because the temperature of the solution inside injection syringe was not controlled except 20 μL in the needle. The first titration of 2 μL was adopted to minimize the influence of residual bubbles and imperfect solution filling the syringe. Nine salt titrations in total, spaced at intervals of 900 s, were performed with a duration of 4 s for the first titration and 40 s for the others to reach the final NaCl concentration of 100 mM. Changes in the heat flow in microcalories per second were monitored in real time with 10 μcal⋅s−1 of reference power. The reaction cell was continuously stirred at 600 rpm. Lag time was defined by a period between the time starting the measurement under stirring and the time of major heat effect occurred as shown in Fig. 3A. To examine the effects of the stirring speed on fibrillation, the stirring speed was changed from 200 to 1,000 rpm. To monitor amorphous aggregation, 3.5 mg⋅mL−1 β2m in 10 mM HCl solution without salt was inversely titrated into 10 mM HCl solution containing 1.0 M NaCl at the desired temperatures (31–43 °C). The parameters for ITC measurements except for shortening the initial delay to 30 min were identical to those used for fibril formation. The total heat effects, which were shown to be equal to the ΔH values, were calculated using peak areas after subtracting the heat of dilution and baseline corrections.
Supplementary Material
Acknowledgments
We thank Ms. Kyoko Kigawa for the expression and purification of β2m. This work was supported by the Japanese Ministry of Education, Culture, Sports, Science and Technology. J.K. is supported by Bolyai János fellowship of the Hungarian Academy of Sciences and Hungarian Scientific Research Fund (OTKA; Grant 81950).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322602111/-/DCSupplemental.
References
- 1.Sipe JD, et al. Amyloid fibril protein nomenclature: 2012 recommendations from the Nomenclature Committee of the International Society of Amyloidosis. Amyloid. 2012;19(4):167–170. doi: 10.3109/13506129.2012.734345. [DOI] [PubMed] [Google Scholar]
- 2.Jucker M, Walker LC. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501(7465):45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoshino M, et al. Mapping the core of the β2-microglobulin amyloid fibril by H/D exchange. Nat Struct Biol. 2002;9(5):332–336. doi: 10.1038/nsb792. [DOI] [PubMed] [Google Scholar]
- 4.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 5.Sawaya MR, et al. Atomic structures of amyloid cross-β spines reveal varied steric zippers. Nature. 2007;447(7143):453–457. doi: 10.1038/nature05695. [DOI] [PubMed] [Google Scholar]
- 6.Eisenberg D, Jucker M. The amyloid state of proteins in human diseases. Cell. 2012;148(6):1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bemporad F, Chiti F. Protein misfolded oligomers: Experimental approaches, mechanism of formation, and structure-toxicity relationships. Chem Biol. 2012;19(3):315–327. doi: 10.1016/j.chembiol.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Tycko R, Wickner RB. Molecular structures of amyloid and prion fibrils: Consensus versus controversy. Acc Chem Res. 2013;46(7):1487–1496. doi: 10.1021/ar300282r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laganowsky A, et al. Atomic view of a toxic amyloid small oligomer. Science. 2012;335(6073):1228–1231. doi: 10.1126/science.1213151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fändrich M, Dobson CM. The behaviour of polyamino acids reveals an inverse side chain effect in amyloid structure formation. EMBO J. 2002;21(21):5682–5690. doi: 10.1093/emboj/cdf573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chatani E, Kato M, Kawai T, Naiki H, Goto Y. Main-chain dominated amyloid structures demonstrated by the effect of high pressure. J Mol Biol. 2005;352(4):941–951. doi: 10.1016/j.jmb.2005.07.043. [DOI] [PubMed] [Google Scholar]
- 12.Lee YH, Chatani E, Sasahara K, Naiki H, Goto Y. A comprehensive model for packing and hydration for amyloid fibrils of β2-microglobulin. J Biol Chem. 2009;284(4):2169–2175. doi: 10.1074/jbc.M806939200. [DOI] [PubMed] [Google Scholar]
- 13.Jarrett JT, Lansbury PT., Jr Seeding “one-dimensional crystallization” of amyloid: A pathogenic mechanism in Alzheimer’s disease and scrapie? Cell. 1993;73(6):1055–1058. doi: 10.1016/0092-8674(93)90635-4. [DOI] [PubMed] [Google Scholar]
- 14.Naiki H, et al. Establishment of a kinetic model of dialysis-related amyloid fibril extension in vitro. Amyloid. 1997;4:223–232. [Google Scholar]
- 15.Wetzel R. Kinetics and thermodynamics of amyloid fibril assembly. Acc Chem Res. 2006;39(9):671–679. doi: 10.1021/ar050069h. [DOI] [PubMed] [Google Scholar]
- 16.Morris AM, Watzky MA, Finke RG. Protein aggregation kinetics, mechanism, and curve-fitting: A review of the literature. Biochim Biophys Acta. 2009;1794(3):375–397. doi: 10.1016/j.bbapap.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Kitayama H, et al. A common mechanism underlying amyloid fibrillation and protein crystallization revealed by the effects of ultrasonication. Biochim Biophys Acta. 2013;1834(12):2640–2646. doi: 10.1016/j.bbapap.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Lin Y, Lee YH, Yoshimura Y, Yagi H, Goto Y. Solubility and supersaturation-dependent protein misfolding revealed by ultrasonication. Langmuir. 2014;30(7):1845–1854. doi: 10.1021/la403100h. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura Y, et al. Distinguishing crystal-like amyloid fibrils and glass-like amorphous aggregates from their kinetics of formation. Proc Natl Acad Sci USA. 2012;109(36):14446–14451. doi: 10.1073/pnas.1208228109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciryam P, Tartaglia GG, Morimoto RI, Dobson CM, Vendruscolo M. Widespread aggregation and neurodegenerative diseases are associated with supersaturated proteins. Cell Rep. 2013;5(3):781–790. doi: 10.1016/j.celrep.2013.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Makhatadze GI, Privalov PL. Energetics of protein structure. Adv Protein Chem. 1995;47:307–425. doi: 10.1016/s0065-3233(08)60548-3. [DOI] [PubMed] [Google Scholar]
- 22.Leavitt S, Freire E. Direct measurement of protein binding energetics by isothermal titration calorimetry. Curr Opin Struct Biol. 2001;11(5):560–566. doi: 10.1016/s0959-440x(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 23.Ladbury JE, Williams MA. The extended interface: Measuring non-local effects in biomolecular interactions. Curr Opin Struct Biol. 2004;14(5):562–569. doi: 10.1016/j.sbi.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Privalov PL. Thermodynamic problems in structural molecular biology. Pure Appl Chem. 2007;79(8):1445–1462. [Google Scholar]
- 25.Vermeer AW, Norde W. The thermal stability of immunoglobulin: Unfolding and aggregation of a multi-domain protein. Biophys J. 2000;78(1):394–404. doi: 10.1016/S0006-3495(00)76602-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benjwal S, Verma S, Röhm KH, Gursky O. Monitoring protein aggregation during thermal unfolding in circular dichroism experiments. Protein Sci. 2006;15(3):635–639. doi: 10.1110/ps.051917406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dzwolak W, Ravindra R, Lendermann J, Winter R. Aggregation of bovine insulin probed by DSC/PPC calorimetry and FTIR spectroscopy. Biochemistry. 2003;42(38):11347–11355. doi: 10.1021/bi034879h. [DOI] [PubMed] [Google Scholar]
- 28.Kardos J, Yamamoto K, Hasegawa K, Naiki H, Goto Y. Direct measurement of the thermodynamic parameters of amyloid formation by isothermal titration calorimetry. J Biol Chem. 2004;279(53):55308–55314. doi: 10.1074/jbc.M409677200. [DOI] [PubMed] [Google Scholar]
- 29.Sasahara K, Naiki H, Goto Y. Kinetically controlled thermal response of β2-microglobulin amyloid fibrils. J Mol Biol. 2005;352(3):700–711. doi: 10.1016/j.jmb.2005.07.033. [DOI] [PubMed] [Google Scholar]
- 30.Sasahara K, Yagi H, Naiki H, Goto Y. Thermal response with exothermic effects of β2-microglobulin amyloid fibrils and fibrillation. J Mol Biol. 2009;389(3):584–594. doi: 10.1016/j.jmb.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 31.Jeppesen MD, Hein K, Nissen P, Westh P, Otzen DE. A thermodynamic analysis of fibrillar polymorphism. Biophys Chem. 2010;149(1-2):40–46. doi: 10.1016/j.bpc.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 32.Morel B, Varela L, Conejero-Lara F. The thermodynamic stability of amyloid fibrils studied by differential scanning calorimetry. J Phys Chem B. 2010;114(11):4010–4019. doi: 10.1021/jp9102993. [DOI] [PubMed] [Google Scholar]
- 33.Raman B, et al. Critical balance of electrostatic and hydrophobic interactions is required for β2-microglobulin amyloid fibril growth and stability. Biochemistry. 2005;44(4):1288–1299. doi: 10.1021/bi048029t. [DOI] [PubMed] [Google Scholar]
- 34.Kad NM, et al. Hierarchical assembly of β2-microglobulin amyloid in vitro revealed by atomic force microscopy. J Mol Biol. 2003;330(4):785–797. doi: 10.1016/s0022-2836(03)00583-7. [DOI] [PubMed] [Google Scholar]
- 35.Giehm L, Otzen DE. Strategies to increase the reproducibility of protein fibrillization in plate reader assays. Anal Biochem. 2010;400(2):270–281. doi: 10.1016/j.ab.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Ohhashi Y, Kihara M, Naiki H, Goto Y. Ultrasonication-induced amyloid fibril formation of β2-microglobulin. J Biol Chem. 2005;280(38):32843–32848. doi: 10.1074/jbc.M506501200. [DOI] [PubMed] [Google Scholar]
- 37.Chatani E, et al. Ultrasonication-dependent production and breakdown lead to minimum-sized amyloid fibrils. Proc Natl Acad Sci USA. 2009;106(27):11119–11124. doi: 10.1073/pnas.0901422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.So M, et al. Ultrasonication-dependent acceleration of amyloid fibril formation. J Mol Biol. 2011;412(4):568–577. doi: 10.1016/j.jmb.2011.07.069. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimura Y, So M, Yagi H, Goto Y. Ultrasonication: An efficient agitation for accelerating the supersaturation-limited amyloid fibrillation of proteins. Jpn J Appl Phys. 2013;52:07HA01-01–07HA01-08. [Google Scholar]
- 40.Come JH, Fraser PE, Lansbury PT., Jr A kinetic model for amyloid formation in the prion diseases: Importance of seeding. Proc Natl Acad Sci USA. 1993;90(13):5959–5963. doi: 10.1073/pnas.90.13.5959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cabriolu R, Kashchiev D, Auer S. Atomistic theory of amyloid fibril nucleation. J Chem Phys. 2010;133(22):225101. doi: 10.1063/1.3512642. [DOI] [PubMed] [Google Scholar]
- 42.Durbin SD, Feher G. Protein crystallization. Annu Rev Phys Chem. 1996;47:171–204. doi: 10.1146/annurev.physchem.47.1.171. [DOI] [PubMed] [Google Scholar]
- 43.Lee YH, Goto Y. Kinetic intermediates of amyloid fibrillation studied by hydrogen exchange methods with nuclear magnetic resonance. Biochim Biophys Acta. 2012;1824(12):1307–1323. doi: 10.1016/j.bbapap.2012.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.