Significance
The mechanisms through which our brain generates complex cognitive percepts from simple sensory and motor events remain unknown. An important question is whether the basic brain structures controlling movements and perceptions directly participate in higher-order cognitive processes such as language comprehension. Using neurophysiology, we found ultrarapid (starting at ∼80 ms) activations in the human motor cortex in response to unattended action-related verbs and nouns, with words related to different body parts activating corresponding body representations. Accompanying this category-specific activity was activation suppression by words with area-incompatible meaning, demonstrating operation of the neurophysiological principles of lateral/surround inhibition in language processing. These instant activations and deactivations emerging for words of different types in the absence of attention advocate automatic involvement of neural sensorimotor circuits in language comprehension.
Keywords: embodied cognition, lexical semantics, magnetoencephalography, MEG, mismatch negativity
Abstract
To address the hotly debated question of motor system involvement in language comprehension, we recorded neuromagnetic responses elicited in the human brain by unattended action-related spoken verbs and nouns and scrutinized their timecourse and neuroanatomical substrates. We found that already very early on, from ∼80 ms after disambiguation point when the words could be identified from the available acoustic information, both verbs and nouns produced characteristic somatotopic activations in the motor strip, with words related to different body parts activating the corresponding body representations. Strikingly, along with this category-specific activation, we observed suppression of motor-cortex activation by competitor words with incompatible semantics, documenting operation of the neurophysiological principles of lateral/surround inhibition in neural word processing. The extremely early onset of these activations and deactivations, their emergence in the absence of attention, and their similar presence for words of different lexical classes strongly suggest automatic involvement of motor-specific circuits in the perception of action-related language.
The old debate on localization of cognitive functions in the brain was recently reinvigorated with the advent of a concept of mirror neurons and a closely related framework of grounded cognition (1–8). The mirror neuron theory stemmed from a seminal discovery of neurons that activate equally when a specific action is performed by the tested individual or when observing the same action performed by others, giving a strong neurophysiological proof for the concept of comprehension and learning through simulation (for a review, see ref. 1). This is enabled by the presence of perception-action circuits in the brain that can provide motor areas with multimodal sensory information (2). An array of findings in mirror neuron and related research strongly suggest that the motor system is not merely a “slave” or an “output” of any central processing, but that it also takes an active role in perception and comprehension of external events. In cognitive science, which had suggested the emergence of concepts from individual experiences long before these neurophysiological discoveries (3–5), a similar strand of research led to a more general framework of “grounding” (or “embodiment”) of cognitive functions and representations in bodily sensations and actions, which was supported through a range of behavioral and neurophysiological experiments (6–8).
Nowhere these approaches resonated more than in the neuroscience of language. Following breakthrough neurological studies of the 19th century (9, 10), the human language function was for many decades confined to a small set of cortical areas in the left hemisphere. More recent research, however, challenged these views in favor of linguistic representations distributed over a range of brain areas, which span beyond the core language cortices of Broca and Wernicke and form circuits whose configuration depends on the exact sensory and motor reference of a specific representation (11). Based on neurobiological principle of associative learning, coactive neurons become linked in a distributed neuronal circuit that is formed in the process of language acquisition and that may, for example, bind the information of the word’s sensory perception (temporal cortex) with its articulatory program (inferior-frontal cortex) and a sensory reference (e.g., visual cortex for imageable concrete objects) and/or a motor one (e.g., motor cortex for words describing actions). The cortical systems for language and actions are reciprocally connected allowing for language and action-related information to interact in such distributed neuronal assemblies. It has been shown, for example, that words referring to different body parts (e.g., kick, pick, lick) lead to differential activation in the motor strip (12, 13), organized in somatotopic fashion similar to the somatotopy of body representations (14). Further, even perception of individual speech sounds (e.g., labial “p” vs. dental “t”) leads to differential motor strip activity in lip and tongue areas, respectively (15), in line with predictions of the motor theory of speech developed long before the advent of neuroimaging or the discovery of mirror neurons (16).
These views are, however, hotly contested in the literature, the most common argument being that the motor activation for language or action observation is epiphenomenal and does not constitute a part of the comprehension process per se (17, 18). This argument is especially easy to make with respect to hemodynamic neuroimaging data (such as functional magnetic resonance imaging, fMRI) that have very poor time resolution and, hence, delayed covert action simulation or imagery indeed cannot be excluded. It is, however, more difficult to argue against a small but growing body of time-resolved electro- and magneto-encephalogprahic (EEG, MEG) results that show a rapid (∼140–200 ms) activation of motor areas in response to action words (13, 19–21). With different theories of language, diverse as they may be, placing lexically and semantically specific processing at 150–500 ms and most often at 350–400 ms (22), it may be hard to argue that word-specific activations before 200 ms reflect a late postcomprehension process.
However, most recent investigations suggested that the earliest brain reflections of lexical access can be seen much earlier, already at 50–80 ms (23). This earliness, in turn, may indicate that the speed of language processing in the brain is faster than believed previously and that even the 150- to 200-ms activations may therefore be late and possibly even secondary. Importantly, previous research focused on the main peaks of event-related responses, possibly failing to locate a specific activation outside these peak intervals. Further, the earlier focus of research on action-related verbs may have confounded the results because verbs had been suggested to be preferentially represented in the more frontal cortices (24–26). In a typical experiment using English language, it is not possible to fully differentiate a verb from a noun (e.g., “pick” could be both interpreted as the action of picking or the object of choice); although some recent work tried addressing this confound (e.g., refs. 27 and 28), it did not have the temporal resolution to answer the neural timing question. Finally, although fMRI studies controlled the localization of motor cortices through a motor localizer task (12), previous EEG/MEG studies suggesting rapid motor systems involvement in comprehension mainly used a crude localization of brain activity relying on blurry source solutions built on template brain surfaces or even spherical models, usually in the absence of a localizer task.
Thus, to more fully elucidate the role of motor circuits in language perception, it appears essential to (i) scrutinize the entire time course of action word processing in the brain rather than concentrate on response maxima, (ii) use experimental language where verbs and nouns are unambiguously distinguished to investigate perception of action words that are/are not verbs, (iii) remove stimulus-related experimental task and even attention on stimuli to minimize the risk on imagery or simulation, and (iv) use time-resolved electrophysiological imaging techniques in combination with a motor localizer task and individual brain surfaces for source localization precision. These challenges were successfully tackled in the current study. We used high-density magentoencephalography to record auditory mismatch field responses, a neurophysiological index of linguistic memory circuit activation (29), elicited by a set of tightly controlled Russian action-related verbs and nouns, which were related to different body parts (kick, throw, swallow) and which the subjects were instructed to ignore while concentrating on a primary visual task. We then scrutinized motor cortex activity in response to these items by means of calculating focal cortical current sources based on individual magnetic resonance (MR) images, comparing them between different semantic subcategories and benchmarking them against a movement-related cortical activity as such. What we found is somatotopically specific ultrarapid activation of cortical motor structures in response to passively presented spoken words, providing strong evidence for the automatic involvement of motor-specific circuits in spoken language comprehension. Furthermore, these results show that the word comprehension process in the brain is subject to the operation of the neurophysiological mechanism of surround inhibition, whereby activation in competing motor representations could be suppressed by semantically incoherent verbal input.
Results
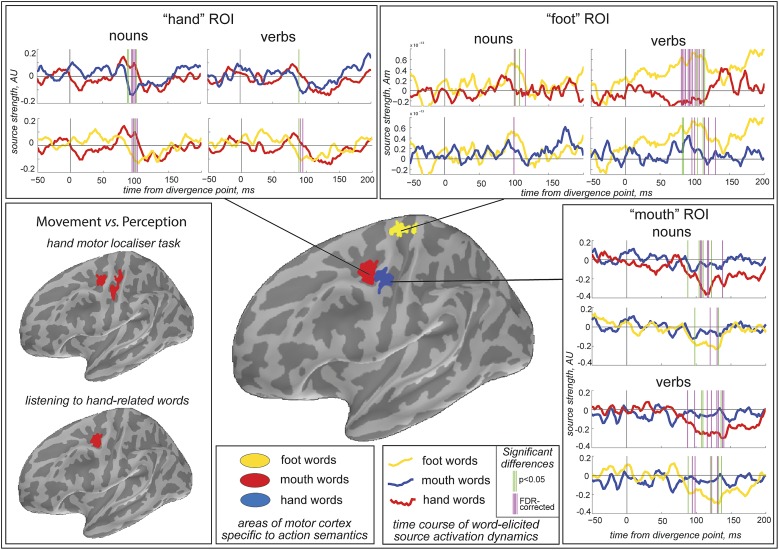
Motor localizer task produced significant activation for finger movements in lateral central and precentral sulci, which could be attributed to hand representations in the primary motor and premotor cortices, respectively, in line with the known functional organization of the motor cortex (Fig. 1, Left). All spoken stimuli elicited clear event-related fields (ERFs), based on which individual cortical source solutions could be successfully calculated. Contrasting the mismatch-response source activations elicited by different action word types in the motor cortex resulted in three significant clusters that were located for the most part anterior to the central sulcus, falling mainly in precentral gyrus and sulcus (Fig. 1, Center). Importantly, spatial distribution of these areas showed a clear divergence between different word types: dorso-medial location for the leg stimuli; more lateral distribution for the hand stimuli; and, posterior-ventrally to the latter, activation specific for the face words. Remarkably, the hand-dominant word area almost precisely corresponded to the activation elicited by the hand localizer task in the premotor cortex but did not include the primary motor component seen in the localizer (Fig. 1, Left).
Fig. 1.
Semantically specific activation of somatotopically organized areas in the left motor cortex in response to different action words. (Lower Center) Motor areas with significantly increased for foot (yellow), hand (red), and mouth (blue) words. (Upper and Right) Dynamics of MNE source amplitudes of neuromagnetic mismatch response (mean over ROI) in foot, hand, and face ROIs suggested a characteristic increase for both nouns and verbs of the three semantic categories that near-instantaneously followed the word disambiguation point, was specific to the referential body semantics of each stimulus and took place early on after word disambiguation; vertical bars indicate significant differences between semantic categories. (Lower Left) Comparison of hand-movement source activation with that elicited by listening to hand-related words. Note almost identical premotor activity but not the motor cortex one.
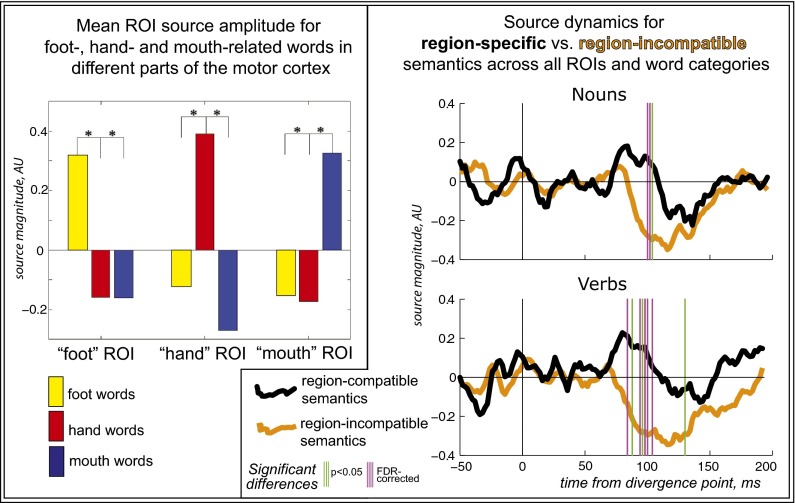
Further statistical investigation of the source activation time course in these regions (Fig. 1) confirmed their specificity for the words’ semantics and revealed temporal windows in which body part-specific areas are first activated somatotopically by action words. For the hand-, leg-, and face-related action words of both lexical categories (verbs and nouns) the selective somatotopic motor cortex response at the respective regions of interest (ROI) occurred approximately at the same time, approximately 80–130 ms after the disambiguation point, with small latency divergence in maximal amplitude of this effect: 90–100 ms (hand), 85–95 ms (leg), and 115–125 ms (face). The strong dependency of this response on action words semantics but not on the lexical category was further confirmed by analyses of variance of the standardized minimum-norm estimate (MNE) values integrated across response intervals. ANOVA showed significant interaction ROI × semantic category (F(4, 80) = 4.8, P < 0.002) and no significant main effect or interaction involving the lexical class factor (verb vs. noun). Thus, overall activation patterns in the three regions appear to diverge between the three semantic categories. Remarkably, although verbs and nouns were acoustically rather distinct (with more auditory similarity within each lexical class than within semantic category), no verb-noun differences could be found in the word-specific motor cortex activity. The latter allowed us to pool verb and noun responses together for further analyses. For these pooled activations, in the “hand” region, the hand-related words had higher maximum response amplitude than both leg-related (F(1,20) = 7.47, P < 0.012) and face-related (F(1,20) = 7.34, P < 0.014) words, whereas in the “leg” region, the leg-related words produced greater response amplitude comparing to both hand- (F(1,20) = 6.92; P < 0.017) and face-related (F(1,20) = 5.90; P < 0.025) stimuli, and in the “face” region, response amplitude for the face-related words was greater than that for both hand- (F(1,20) = 4.35; P = 0.050) and leg-related (F(1,20) = 4.61; P = 0.044) words. Within each ROI, no significant differences were found between responses to words belonging to semantic categories not specific to the ROI (e.g., no face-hand difference in the leg region, etc.; all F values < 0.25; all P values > 0.6). As evident from the ROI activation timecourses, the differential responses for semantic subcategories were underlain not only by increased activation for the “region-specific” words, but also by deactivation for words with “region-incompatible” semantics (e.g., for leg words a maximum found in the “leg area” was accompanied by negative extrema in both hand and face regions). Fig. 2 demonstrates this effect and corresponding statistical effects most clearly by contrasting activations for the region-specific and region-unspecific action words, pooled across all words and regions. Whereas the mean activation peak for the region-specific words was most prominent at ∼80 ms, the deactivation appeared most pronounced somewhat later, at ∼120 ms.
Fig. 2.
Semantically specific activation and deactivation of the motor neocortex by action-related words. (Left) ROI-mean peak source activity (z-score normalized for optimal comparison between areas) shows clearly enhanced amplitude for the three word types in each motor ROI. (Right) Pooled source dynamics for activity generated by verbs and nouns in their semantically-specific ROIs as opposed to semantically incongruous ones; vertical bars indicate significant differences. Note not only the early increase of semantic activation for region-specific words starts ∼80 ms after word disambiguation point but also a suppression of source activity for region-incompatible semantics that is maximal slightly later (∼120 ms).
Discussion
To explore the nature of motor cortex involvement in action word comprehension, we recorded automatic neuromagnetic brain responses to psycholinguistically and acoustically controlled verbs and nouns related to different body parts, which were presented in a nonattend passive auditory oddball paradigm, and scrutinized sources of motor cortex activation for these items by using individual neuroanatomical constraints and an unbiased distributed current estimates approach to source reconstruction. We found distinct somatopically organized precentral activations that took place early, were accompanied by deactivations in semantically incongruous motor regions, and demonstrated the same patterns for verb and noun stimuli. We will briefly consider these findings below.
Specificity of motor cortex activation to words of different types, namely the dorso-lateral distribution of activity for leg-, hand-, and face-related words closely matching the well-known motor cortex somatotopy (14), is in line with the theoretical premises of grounding of cognitive functions in modality-specific experiences and neural structures (6–8, 11). This view was supported by temporally imprecise fMRI data (12) that could not rule out postcomprehension origins of these phenomena and by MEG and EEG activations at 150–200 ms in studies using limited spatial resolution and imperfect source reconstruction techniques (13, 19, 21). What we report here, however, is an MEG investigation of spoken action words using individual MR-based neuroanatomical boundary-element models that subjected motor-cortex current source space to previously unattained scrutiny, further improved by using activation in a motor localizer task as a functional-anatomical landmark. The result, obtained without any stimulus-related task with attention withdrawn from the spoken input to an unrelated primary visual task, is an early (starting from ∼80 ms after word disambiguation point) somatotopically specific activation of premotor cortex for different subcategories of action words. This activation was seen here in the form of the mismatch field response, an established index of lexical memory-trace activation in the brain (29–31), indicating an ultra-rapid ignition of word-specific memory circuits which include category-specific motor-cortex neurons. The earliness of this semantic response makes it near-parallel to the earliest neural signature of lexical access available to date (∼50–80 ms; ref. 23) and, along with the automatic fashion of its generation, largely excludes a possibility of it being a sign of any secondary, postcomprehension phenomena such as imagery or action simulation. Instead, this result clearly supports the view that memory traces for individual words may encompass a variety of structures, including modality-specific ones outside the core language areas. These circuits are likely formed through associative learning, involving auditory-motor speech experience in conjunction with actions, objects, or concepts defined by particular words, and take shape of robustly interconnected distributed neuronal assemblies capable of ultrarapid automatic activation whenever the respective stimulus is present at the input (32–34).
A further advance of this study, in contrast with the majority of previous investigations, is a simultaneous use of lexically unambiguous verbs and nouns. Despite clear acoustic differences between these stimuli, nouns and verbs did not differ in the motor-cortex activation pattern they elicited. This rules out the possibility that the previously reported specificity of motor cortex activation for action words is an epiphenomenon of the earlier suggested frontal cortex specialization for verbs (24–26). With most action-word vocabulary being verbs, this criticism could be made in relation to studies using e.g., single words of the English language (where lexical class cannot be unambiguously established). The current study, however, is immune to such critique, given the morphologically and acoustically distinct forms taken on by words of different lexical classes in Slavonic languages such as Russian. Despite these surface form differences, MEG activations did not demonstrate any statistically significant divergence between verbs and nouns in the motor strip while showing clear semantic-category distinctions. This implies similar modality-grounded mechanisms of lexico-semantic representation formation operating in the human brain for different lexical classes. This result is in line with a recent fMRI study comparing English action nouns and verbs, which used additional words for lexical disambiguation (28), as well as with recent behavioral and TMS results indicating motor cortex role in noun processing (27, 35). Importantly, while fMRI and behavioral approaches lack the temporal resolution to address the timing of motor cortex involvement, the current MEG study shows its near-instant character. This study is also the first neuroimaging demonstration of motor-cortex involvements in language comprehension using a Slavonic language, which adds to the already available evidence from other languages, further supporting the universal nature of this phenomenon.
Given the notable differences between the verb and noun stimuli in terms of their acoustic structures and the similarity of the motor-cortex activation, it is highly unlikely that the elicited motor somatotopy here can be a by-product of purely acoustic differences between the stimuli. This is made even less likely by the rigorous control over the stimulus acoustic features within each set (see Methods). The word stimuli were all frequent words of Russian well-known to all volunteers. Still, even if there were any lexical frequency-driven effects, they have been shown to influence the response amplitude in core language areas in similar paradigms (36, 37), but are not known and not predicted to elicit somatotopic distinctions in the motor strip.
Anatomically, our findings predominantly originated from left precentral cortical structures. This was most strikingly evident in a comparison between word-elicited activity and the motor localizer task. While the latter clearly identified both central and precentral sources, only the more anterior sources were found in the word condition, testifying to the involvement of premotor - but not primary motor - cortex in language comprehension. While primary cortex activation in itself cannot be ruled out, given the limited spatial resolution of MEG and the relatively small word-elicited activations in comparison with strong movement-related neural firing, we note that this is compatible with previous fMRI data that showed predominantly premotor word-elicited BOLD activation (12), as well as with data on multisensory inputs to the motor cortex being mainly concentrated in the premotor areas (e.g., 2). Further neurophysiological investigations, ideally using direct recordings from the human neocortex, are necessary to resolve the question of rapid automatic involvement of primary motor areas in action language comprehension.
Naturally, the grounded/embodied perspective on language processing is not limited to motor involvement. Depending on the exact referential semantics, other cortical areas have been shown to be involved in language comprehension, e.g., olfactory areas for smell-related words (38), auditory cortices for sound-related ones (39), or occipito-temporal areas for visual object words (40), albeit not nearly as early and automatically as we suggest here. This category-specific activation accompanies (and can sometimes be seen as following) activity in temporo-frontal core language areas, the latter potentially reflecting lexico-semantic processing common to different word types (41). Future studies could investigate whether other modality-specific areas are recruited as rapidly as what we show here for the motor strip and address the exact time course of both generic and specific lexico-semantic activations by combining different word types in a single study and scrutinizing neural activations in the same way as we have done here.
A remarkable finding in the current study is that of concurrent activation suppression in those motor regions whose somatotopic specificity is incompatible with the presented word semantics. Inhibition effects related to action words with incongruous semantics are well known from behavioral experiments, including detailed investigations of interference and facilitation between effector movement dynamics and action word processing (42–45). These studies showed specific effects of, for example, hand movements by study participants on memory for hand-related words or on processing of action-related sentences, implying causal relationship between activity of the sensorimotor system and semantic processing of action-related language (46).
However, the current study is, to our knowledge, the first demonstration of the operation of inhibitory mechanisms in early neurophysiological motor-system activity underlying word comprehension per se. Suppression of excitability in an area interfering with an activated neural network is a physiological mechanism that focuses neuronal activity and helps to select only the appropriate neuronal response. This principle is known as the so-called lateral inhibition for interactions between neighboring neurons or as surround inhibition at the mass neuron-population level. Such an inhibition of competing somatotopic neural representations of body parts is an essential mechanism in the motor system, where it can aid the selective execution of desired movements (47–49). It may also serve for sharpening neuronal representations of external events and improving their perceptual discrimination (50), something that may be necessary for optimal semantic discrimination of different action word types. The time course of these deactivation processes appears to closely follow the category-specific activation dynamics, with an onset at approximately 80 ms and a peak at ∼120 ms, similarly testifying to their rapid automatic character. Future studies could address the exact mechanisms involved in neural inhibition between different linguistic representations in more detail.
In sum, we find rapid automatic neural activation and suppression occurring in the brain’s motor cortex in response to spoken action words of different semantic subcategories. The extremely early onset of these activations and deactivations, their emergence in the absence of attention to stimuli, and their similar presence for words of different lexical classes testify, in our view, to automatic involvement of motor-specific circuits in the perception of action-related language. Finally, future studies appear necessary to further elucidate the role of primary vs. secondary motor cortices in these processes, to confirm the generalizability of the reported phenomena to other, ecologically more valid paradigms and other semantic categories, as well as to investigate in more detail possible interactions between automaticity and top-down attentional control in generating these earliest neurophysiological reflections of semantic word processing.
Methods
We chose Russian as a testing language as it has unambiguous distinctions between different lexical classes. In three experimental blocks, we presented 21 healthy native Russian-speaking volunteers (right-handed, mean age 24.5, 8 females) with disyllabic action-related verbs and nouns: (i) hand-related  , v., “throw/toss”) and
, v., “throw/toss”) and  , n., “a throw,” i.e., act of throwing), (ii) leg/foot-related
, n., “a throw,” i.e., act of throwing), (ii) leg/foot-related 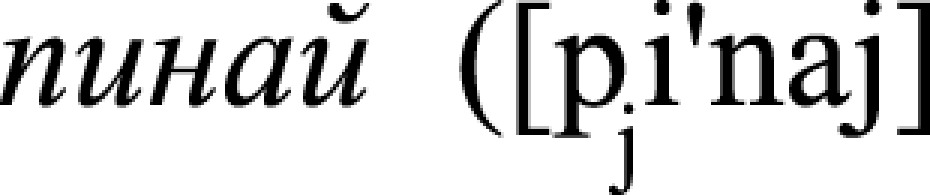 , v., “kick”) and
, v., “kick”) and 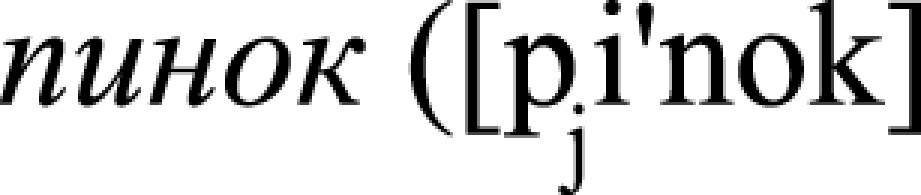 , n., “a kick”), and (iii) face-related
, n., “a kick”), and (iii) face-related 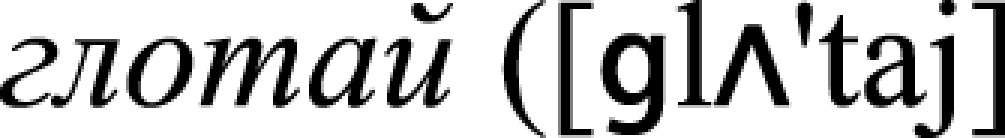 , v., “swallow”) and
, v., “swallow”) and  , n., “a sip”). Previous neurophysiological research showed that activation of individual word memory traces may be recorded in the form of a so-called mismatch negativity (MMN) response when linguistic materials (typically a small group of acoustically controlled words, as also done here) is presented passively in pseudorandom oddball sequences (29). Notably, such representation-specific potentials are generated automatically, in the absence of attention on the stimuli or stimulus-related tasks (51). Therefore, the three types of stimuli were passively presented in oddball nonattend conditions as rare unexpected (so-called “deviant”) trials among acoustically similar but senseless frequent (“standard”) pseudoword stimuli: *
, n., “a sip”). Previous neurophysiological research showed that activation of individual word memory traces may be recorded in the form of a so-called mismatch negativity (MMN) response when linguistic materials (typically a small group of acoustically controlled words, as also done here) is presented passively in pseudorandom oddball sequences (29). Notably, such representation-specific potentials are generated automatically, in the absence of attention on the stimuli or stimulus-related tasks (51). Therefore, the three types of stimuli were passively presented in oddball nonattend conditions as rare unexpected (so-called “deviant”) trials among acoustically similar but senseless frequent (“standard”) pseudoword stimuli: *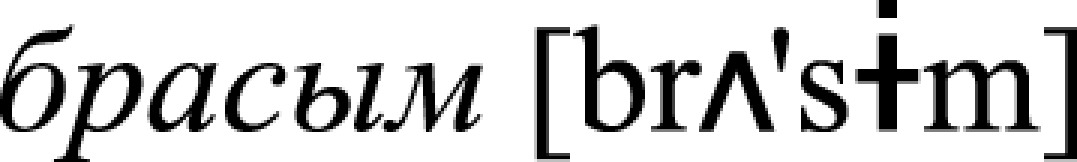 ,
, 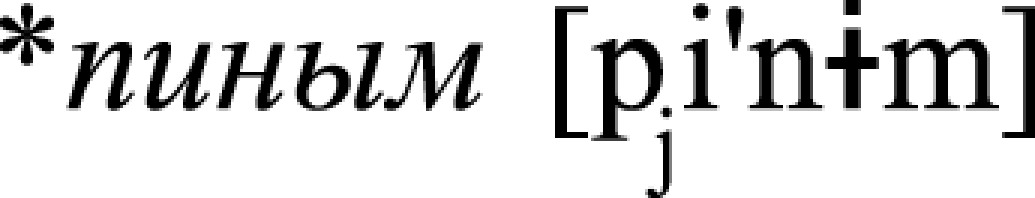 , and
, and 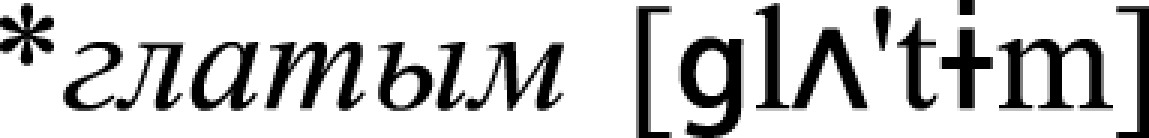 correspondingly. Each of the three blocks included 1,200 stimuli (80% standards, 10% verbs, and 10% nouns with identical onsets), pseudorandomized and presented at a stimulus onset asynchrony jittered between 1,000 and 1,500 ms. The acoustic contrasts between the deviant and standard stimuli were identical across all verbs ([aj] vs. [ɨm] in the stimulus end) and nouns ([ok] vs. [ɨm]), which was achieved by cross-splicing the same endings and stems in different combinations, thus ruling out acoustic confounds within each stimulus type. All stimuli were matched for their length (452 ms), fundamental frequency, and loudness, the critical physical/acoustic features determining auditory evoked responses. Disambiguation point between pseudoword/verb/noun occurred at 261 ms after onset in each condition. This point was identical in all three stimulus groups, which was achieved by cross-splicing stems and offsets, recorded separately to avoid coarticulation effects. Word-recognition points were established in a separate behavioral gating study as being 13–20 ms after the disambiguation point. The subjects were also asked to rate the stimuli for meaningfulness, frequency of use, and relation to the specific body part movements; these ratings confirmed the intended semantic specificity of the stimuli. All word stimuli had above-zero lexical frequency (determined by using Russian National Corpus; www.ruscorpora.ru) and were well-known to all experimental subjects who reported frequent use of all items.
correspondingly. Each of the three blocks included 1,200 stimuli (80% standards, 10% verbs, and 10% nouns with identical onsets), pseudorandomized and presented at a stimulus onset asynchrony jittered between 1,000 and 1,500 ms. The acoustic contrasts between the deviant and standard stimuli were identical across all verbs ([aj] vs. [ɨm] in the stimulus end) and nouns ([ok] vs. [ɨm]), which was achieved by cross-splicing the same endings and stems in different combinations, thus ruling out acoustic confounds within each stimulus type. All stimuli were matched for their length (452 ms), fundamental frequency, and loudness, the critical physical/acoustic features determining auditory evoked responses. Disambiguation point between pseudoword/verb/noun occurred at 261 ms after onset in each condition. This point was identical in all three stimulus groups, which was achieved by cross-splicing stems and offsets, recorded separately to avoid coarticulation effects. Word-recognition points were established in a separate behavioral gating study as being 13–20 ms after the disambiguation point. The subjects were also asked to rate the stimuli for meaningfulness, frequency of use, and relation to the specific body part movements; these ratings confirmed the intended semantic specificity of the stimuli. All word stimuli had above-zero lexical frequency (determined by using Russian National Corpus; www.ruscorpora.ru) and were well-known to all experimental subjects who reported frequent use of all items.
Whereas the stimuli were presented (Presentation 14.4, Neurobehavioral Systems) via plastic ear tubes at 50 dB above individual hearing thresholds, the participants, placed in an electromagnetically and acoustically insulated booth, were asked to concentrate on watching a self-selected videofilm and ignore the sounds. Their brain’s neuromagnetic activity was recorded (passband 0.03–330 Hz, sampling rate 1 kHz) continuously by using 306-channel MEG setup (Elekta Neuromag). To control for eye movements, vertical and horizontal bipolar electrooculograms (EOG) were recorded. To track head position in the MEG helmet dewar, 4 head position identification (HPI) coils were digitized together with fiducial points (using Fastrak 3D digitizer; Polhemus) before recording and their position was continuously recorded throughout the experiment.
Following the auditory stimulation session, motor localizer task was performed: Subjects were asked to slightly move their right index finger while their MEG was recorded. Approximately 120 self-paced movements (which did not include touching a button or self-touch) were recorded through an optical response box by using a laser light beam to register the motion onset and record it synchronously with the MEG data. Because the hand motor area is located laterally to the leg/foot one and somewhat dorsally of the head-related cortex, it is best placed to serve as an anchor for determining the motor cortex location. For this reason, and also because of time restrictions and due to extensive MEG artifacts associated with head and foot movements, face/head and foot localizers were omitted.
To minimize the contribution of magnetic sources from outside the head and to reduce any artifacts, the data from the 306 sensors were processed using the temporal extension of the Signal Space Separation method (SSS, ref. 52) as implemented in MaxFilter 2.0 software (Elekta Neuromag): Static bad channels were detected and excluded from subsequent processing steps, and compensation was made for within-block head movements (as measured by HPI coils). For compatibility between different experimental blocks, the data were converted to standard head position (x = 0 mm; y = 0 mm; z = 45 mm) across all blocks. Epochs from 50 ms before to 950 ms after the onset of each stimulus were used for calculating ERFs for the different stimulus types by using MNE Suite 2.6.0 software (Martinos Center for Biomedical Imaging). Epochs with amplitude exceeding 3 × 10−10 T/m (gradiometers), 12 × 10−10 T (magnetometers), or 150 µV (EOG) were discarded, which resulted in at least 110 artifact-free deviant epochs and 950 standard ones per block, that were used for to calculate average event-related fields for each subject, condition, and stimulus type.
High-resolution structural T1-weighted MRIs were acquired for each participant by using a 1.5 T Toshiba ExcelArt Vantage scanner [repetition time (TR) = 12 ms, echo time (TE) = 5 ms, flip angle = 20°, 160 sagittal slices, slice thickness = 1.0 mm, voxel size = 1.0 × 1.0 × 1.0 mm3]. Cortical matter was segmented in the individual structural MRIs, and the estimated border between gray and white matter was tessellated. Individual single-layer boundary-element models were created for each participant by using watershed segmentation algorithms (FreeSurfer 4.3 software; Martinos Center for Biomedical Imaging) to reconstruct the brain’s cortical gray matter surface as a high-resolution triangularized mesh with 10,424 vertices in each hemisphere. The surface was further “inflated” to unfold cortical sulci to provide their optimal view. Further processing was performed by using the MNE Suite 2.6.0 software. Cortical sources of the observed neuromagnetic activity were estimated by using signals from all 306 sensors and L2 MNE approach, which models the recorded magnetic field distribution with the smallest amount of overall source activity (53, 54).
Magnetic MMN (MMNm, or mismatch field, MMF) response, a neural index of experience-dependent memory traces, was calculated, separately for each subject and deviant type, in the source space by subtracting the source strength of standard response from the deviant one at each vertex. For the comparison of MMNm source strength in the auditory and motor cortex, three cortical regions were used based on the Desikan–Killiany parcellation of the cortical surface as implemented in the FreeSurfer package (55). The left auditory region was delineated by anatomical labels of transverse gyrus and sulcus. The left lateral motor and premotor cortex regions broadly covering motor representations of hand and face muscles comprised standard anatomical labels of central sulcus and precentral gyrus and sulcus. The left dorso-medial motor and premotor cortex regions encompassing motor representations of leg/foot area comprised the anterior part of paracentral lobule on the medial surface of the left hemisphere and the uppermost portion of the central sulcus and precentralgyrus and sulcus. Because the study focused on testing word-specific motor activations, other ROIs or whole brain analysis remained outside the scope of the current analysis.
Following the disambiguation point (marked as zero in all plots of source activation dynamics), the strongest activity was elicited in the auditory cortex which, because of activation leakage characteristic of the MNE method, somewhat spilled into nearby areas including lateral motor cortex. To control for this leakage and remove the resulting contamination of MNE activations in each subject and each experimental condition, source amplitude at every vertex within the lateral motor cortex region was normalized by the mean MNE value across all lateral motor cortex vertices at each time point of the magnetic mismatch response. Although this manipulation eliminates the common trend in the MNE values of affected vertices, it leaves unchanged the putative topographical differences in activation between word categories. Dorsal ROI activity remained unaffected by the auditory cortex contamination.
To define motor cortex areas selectively activated by each of the cognate action word pairs (hand-, leg-, face-related), we used an unbiased data-driven approach relying on quantification of the preponderance in MMNm vertices source strength for any particular word. This quantification was achieved by comparing the MNE amplitude to a given action word and two other words from the same lexical but different semantic categories with a requirement of a minimum of five adjacent voxels in two or more consecutive 10-ms sliding windows showing significant directional differences between stimuli at P < 0.05 one-tailed signed-rank test. The resulting three motor cortex spatial clusters were considered as word-specific motor ROIs. The time course of the mean unsigned MNE values was calculated across all vertices in each of these ROIs and subjected to further statistical analysis.
Wilcoxon signed-rank test was applied to assess differences produced by nouns and verbs belonging to different semantic categories in MNE timecourses in ROIs in every 2-ms time window from 40 ms to 140 ms in relation of disambiguation point. False discovery rate (FDR) correction for multiple comparisons was applied, and both FDR-corrected and uncorrected differences between source activations caused by different stimuli are indicated in MNE timecourses in Fig. 1. Repeated-measures ANOVAs with factors ROI (three levels: motor areas with hand-, leg-, and face-word specificity), semantic category (three levels: hand-, leg-, and face-related action words) and lexical class (nouns/verbs) were performed for the standardized mean MNE amplitudes at the maximum of action word-specific differential activation. No violation of sphericity assumption was revealed by Mauchley sphericity test for any of two-way or three-way ANOVA interactions (all P values > 0.5). Significant interactions were followed up by using planned comparisons.
Acknowledgments
This research was supported by the Medical Research Council, United Kingdom (Core Project MC-A060-5PQ90 and Partnership Grant MR/K005464/1), Higher School of Economics, Moscow (Program of Fundamental Studies TZ 76), and by the Lundbeck Foundation, Denmark.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Rizzolatti G, Craighero L. The mirror-neuron system. Annu Rev Neurosci. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- 2.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 3.Lakoff G, Johnson M. The metaphorical structure of the human conceptual system. Cogn Sci. 1980;4(2):195–208. [Google Scholar]
- 4.Kosslyn SM, Pomerantz JR. Imagery, propositions, and form of internal representations. Cognit Psychol. 1977;9(1):52–76. [Google Scholar]
- 5.Gibson JJ. The Senses Considered as Perceptual Systems. Oxford: Houghton Mifflin; 1966. [Google Scholar]
- 6.Barsalou LW. Grounded cognition. Annu Rev Psychol. 2008;59:617–645. doi: 10.1146/annurev.psych.59.103006.093639. [DOI] [PubMed] [Google Scholar]
- 7.Pulvermüller F. Words in the brain’s language. Behav Brain Sci. 1999;22(2):253–279. discussion 280–336. [PubMed] [Google Scholar]
- 8.Glenberg AM, Gallese V. Action-based language: A theory of language acquisition, comprehension, and production. Cortex. 2012;48(7):905–922. doi: 10.1016/j.cortex.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Broca P. Remarques sur la siège du langage articulé, suivies d'une observation d'aphémie (perte de parole) Bulletins de la Société Anatomique de Paris. 1861;36(2, 6):330–357. [Google Scholar]
- 10.Wernicke C. Der Aphasische Symptomencomplex. Eine Psychologische Studie auf anatomischer Basis. Breslau: Kohn und Weigert; 1874. [Google Scholar]
- 11.Pulvermüller F, Fadiga L. Active perception: Sensorimotor circuits as a cortical basis for language. Nat Rev Neurosci. 2010;11(5):351–360. doi: 10.1038/nrn2811. [DOI] [PubMed] [Google Scholar]
- 12.Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41(2):301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- 13.Shtyrov Y, Hauk O, Pulvermüller F. Distributed neuronal networks for encoding category-specific semantic information: The mismatch negativity to action words. Eur J Neurosci. 2004;19(4):1083–1092. doi: 10.1111/j.0953-816x.2004.03126.x. [DOI] [PubMed] [Google Scholar]
- 14.Penfield W, Rasmussen T. The Cerebral Cortex of Man. New York: Macmillan; 1950. [Google Scholar]
- 15.Pulvermüller F, et al. Motor cortex maps articulatory features of speech sounds. Proc Natl Acad Sci USA. 2006;103(20):7865–7870. doi: 10.1073/pnas.0509989103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberman AM, Mattingly IG. The motor theory of speech perception revised. Cognition. 1985;21(1):1–36. doi: 10.1016/0010-0277(85)90021-6. [DOI] [PubMed] [Google Scholar]
- 17.Lotto AJ, Hickok GS, Holt LL. Reflections on mirror neurons and speech perception. Trends Cogn Sci. 2009;13(3):110–114. doi: 10.1016/j.tics.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hickok G, Hauser M. (Mis)understanding mirror neurons. Curr Biol. 2010;20(14):R593–R594. doi: 10.1016/j.cub.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hauk O, Pulvermüller F. Neurophysiological distinction of action words in the fronto-central cortex. Hum Brain Mapp. 2004;21(3):191–201. doi: 10.1002/hbm.10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boulenger V, Shtyrov Y, Pulvermüller F. When do you grasp the idea? MEG evidence for instantaneous idiom understanding. Neuroimage. 2012;59(4):3502–3513. doi: 10.1016/j.neuroimage.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Pulvermüller F, Shtyrov Y, Ilmoniemi R. Brain signatures of meaning access in action word recognition. J Cogn Neurosci. 2005;17(6):884–892. doi: 10.1162/0898929054021111. [DOI] [PubMed] [Google Scholar]
- 22.Friederici AD. Towards a neural basis of auditory sentence processing. Trends Cogn Sci. 2002;6(2):78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- 23.MacGregor LJ, Pulvermüller F, van Casteren M, Shtyrov Y. Ultra-rapid access to words in the brain. Nat Commun. 2012;3:711. doi: 10.1038/ncomms1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shapiro KA, Pascual-Leone A, Mottaghy FM, Gangitano M, Caramazza A. Grammatical distinctions in the left frontal cortex. J Cogn Neurosci. 2001;13(6):713–720. doi: 10.1162/08989290152541386. [DOI] [PubMed] [Google Scholar]
- 25.Damasio AR, Tranel D. Nouns and verbs are retrieved with differently distributed neural systems. Proc Natl Acad Sci USA. 1993;90(11):4957–4960. doi: 10.1073/pnas.90.11.4957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perani D, et al. The neural correlates of verb and noun processing. A PET study. Brain. 1999;122(Pt 12):2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- 27.Marino BF, Gough PM, Gallese V, Riggio L, Buccino G. How the motor system handles nouns: A behavioral study. Psychol Res. 2013;77(1):64–73. doi: 10.1007/s00426-011-0371-2. [DOI] [PubMed] [Google Scholar]
- 28.Pulvermüller F, Cook C, Hauk O. Inflection in action: Semantic motor system activation to noun- and verb-containing phrases is modulated by the presence of overt grammatical markers. Neuroimage. 2012;60(2):1367–1379. doi: 10.1016/j.neuroimage.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 29.Pulvermüller F, Shtyrov Y. Language outside the focus of attention: the mismatch negativity as a tool for studying higher cognitive processes. Prog Neurobiol. 2006;79(1):49–71. doi: 10.1016/j.pneurobio.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Shtyrov Y, Pulvermüller F. Language in the mismatch negativity design: motivations, benefits and prospects. J Psychophysiol. 2007;21(3-4):176–187. [Google Scholar]
- 31.Näätänen R, Paavilainen P, Rinne T, Alho K. The mismatch negativity (MMN) in basic research of central auditory processing: a review. Clin Neurophysiol. 2007;118(12):2544–2590. doi: 10.1016/j.clinph.2007.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Shtyrov Y. Automaticity and attentional control in spoken language processing: Neurophysiological evidence. Mental Lexicon. 2010;5(2):255–276. [Google Scholar]
- 33.Shtyrov Y, Kujala T, Pulvermüller F. Interactions between language and attention systems: Early automatic lexical processing? J Cogn Neurosci. 2010;22(7):1465–1478. doi: 10.1162/jocn.2009.21292. [DOI] [PubMed] [Google Scholar]
- 34.Garagnani M, Shtyrov Y, Pulvermüller F. Effects of attention on what is known and what is not: MEG evidence for functionally discrete memory circuits. Front Hum Neurosci. 2009;3:10. doi: 10.3389/neuro.09.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gough PM, et al. Nouns referring to tools and natural objects differentially modulate the motor system. Neuropsychologia. 2012;50(1):19–25. doi: 10.1016/j.neuropsychologia.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Alexandrov AA, Boricheva DO, Pulvermüller F, Shtyrov Y. Strength of word-specific neural memory traces assessed electrophysiologically. PLoS ONE. 2011;6(8):e22999. doi: 10.1371/journal.pone.0022999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shtyrov Y, Kimppa L, Pulvermüller F, Kujala T. Event-related potentials reflecting the frequency of unattended spoken words: A neuronal index of connection strength in lexical memory circuits? Neuroimage. 2011;55(2):658–668. doi: 10.1016/j.neuroimage.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 38.González J, et al. Reading cinnamon activates olfactory brain regions. Neuroimage. 2006;32(2):906–912. doi: 10.1016/j.neuroimage.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 39.Trumpp NM, Kliese D, Hoenig K, Haarmeier T, Kiefer M. Losing the sound of concepts: Damage to auditory association cortex impairs the processing of sound-related concepts. Cortex. 2013;49(2):474–486. doi: 10.1016/j.cortex.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Moseley RL, Pulvermuller F, Shtyrov Y. Sensorimotor semantics on the spot: brain activity dissociates between conceptual categories within 150 ms. Sci Rep. 2013;3:1928. doi: 10.1038/srep01928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pulvermüller F, Shtyrov Y. Spatiotemporal signatures of large-scale synfire chains for speech processing as revealed by MEG. Cereb Cortex. 2009;19(1):79–88. doi: 10.1093/cercor/bhn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Glenberg AM, Kaschak MP. Grounding language in action. Psychon Bull Rev. 2002;9(3):558–565. doi: 10.3758/bf03196313. [DOI] [PubMed] [Google Scholar]
- 43.Boulenger V, et al. Subliminal display of action words interferes with motor planning: a combined EEG and kinematic study. J Physiol Paris. 2008;102(1-3):130–136. doi: 10.1016/j.jphysparis.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Nazir TA, et al. Language-induced motor perturbations during the execution of a reaching movement. Q J Exp Psychol (Hove) 2008;61(6):933–943. doi: 10.1080/17470210701625667. [DOI] [PubMed] [Google Scholar]
- 45.Shebani Z, Pulvermüller F. Moving the hands and feet specifically impairs working memory for arm- and leg-related action words. Cortex. 2013;49(1):222–231. doi: 10.1016/j.cortex.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 46.Scorolli C, Borghi AM. Sentence comprehension and action: Effector specific modulation of the motor system. Brain Res. 2007;1130(1):119–124. doi: 10.1016/j.brainres.2006.10.033. [DOI] [PubMed] [Google Scholar]
- 47.Sanes JN, Donoghue JP. Plasticity and primary motor cortex. Annu Rev Neurosci. 2000;23:393–415. doi: 10.1146/annurev.neuro.23.1.393. [DOI] [PubMed] [Google Scholar]
- 48.Sohn YH, Hallett M. Surround inhibition in human motor system. Exp Brain Res. 2004;158(4):397–404. doi: 10.1007/s00221-004-1909-y. [DOI] [PubMed] [Google Scholar]
- 49.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50(4):381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 50.von Békésy G. Sensory Inhibition. Princeton, NJ: Princeton Univ Press; 1967. [Google Scholar]
- 51.Näätänen R, et al. Language-specific phoneme representations revealed by electric and magnetic brain responses. Nature. 1997;385(6615):432–434. doi: 10.1038/385432a0. [DOI] [PubMed] [Google Scholar]
- 52.Taulu S, Kajola M, Simola J. Suppression of interference and artifacts by the Signal Space Separation Method. Brain Topogr. 2004;16(4):269–275. doi: 10.1023/b:brat.0000032864.93890.f9. [DOI] [PubMed] [Google Scholar]
- 53.Ilmoniemi RJ. Models of source currents in the brain. Brain Topogr. 1993;5(4):331–336. doi: 10.1007/BF01128686. [DOI] [PubMed] [Google Scholar]
- 54.Hämäläinen MS, Ilmoniemi RJ. Interpreting magnetic fields of the brain: minimum norm estimates. Med Biol Eng Comput. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- 55.Desikan RS, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]