Microbial rhodopsins function as light-driven (retinal-based) ion pumps, cation channels, or light sensors in various microorganisms [see Heberle et al.’s Retinal Proteins—You can teach an old dog new tricks (1)], are found in all three domains of life (2–4), and were recently reported in viruses as well (5, 6). The first discovered microbial rhodopsin, the light-driven proton pump bacteriorhodopsin (BR), was identified in halophilic (high-salt loving) archaea more than 40 y ago (in 1971). Later on, the light-driven chloride pump halorhodopsin (HR) was discovered in the same archaeal microorganisms (for a historical perspective, see ref. 2). Both pumps were discovered using biophysical and physiological techniques. The widespread bacterial light-driven proton pump proteorhodopsin (PR), however, was discovered using metagenomics (7), and was initially detected based on a weak homology to BR (less than 30% identity on the protein level). Although diverse PRs are found in most marine microorganisms in the photic zone (8–11), BRs and HRs are restricted to halophilic archaea and are found only in hypersaline environments. The work of Yoshizawa et al. (12) in PNAS is now reporting on a new light-driven chloride pump, dubbed ClR (for Cl− rhodopsin), which differs from HRs and is found in a marine bacterium (the flavobacterium Nonlabens marinus). Interestingly, this bacterium also possesses a PR protein and a light-driven sodium pump (NaR) [recently found in other flavobacteria (13–16)], which makes it, according to the authors, the first reported microbe to possess three different types of rhodopsin pumps.
The newly reported bacterial ClR has less than 25% identity to archaeal HR or BR proteins. In addition, none of the known amino acids implicated in chloride transport in HR are conserved in ClR. HR shares less than 35% identity to BR on the protein level, and in HR the corresponding amino acid to aspartate at position 85 of BR (the proton acceptor site) is threonine (yielding a protein with the motif TSA; see Fig. 1 for details). A single amino acid change in BR in which aspartate 85 is replaced by threonine (changing the DTD motif of BR to TTD), turns BR from a proton pump into a chloride pump (17). Interestingly, in the new ClR, position 85 is occupied by asparagine (protein motif NTQ). Mutation of BR in which aspartate 85 is changed to asparagine (yielding a BR mutant protein with the motif NTD) does not turn into a chloride pump (17, 18), and it would therefore be extremely interesting to understand what residues participate in chloride pumping in the new ClR.
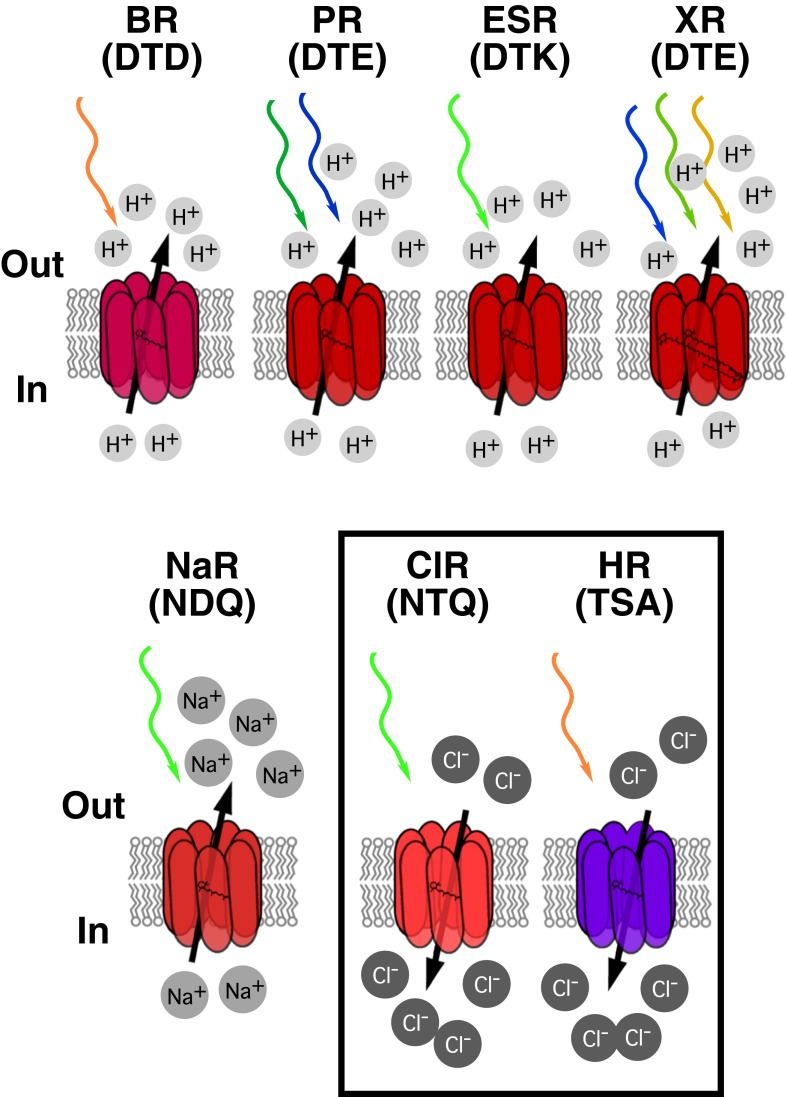
Fig. 1.
Schematic representation of different microbial rhodopsin ion pumps. Amino acids shown in brackets are amino acids found at positions 85, 89, and 96 in each of these rhodopsins (BR numbering). Colored arrows represent different wavelengths at which each rhodopsin absorbs. In the case of PR, the arrows represent the absorption of blue and green rhodopsins (29); XR absorbs at different wavelengths due to its additional carotenoid molecule. ESR, E. sibiricum rhodopsin.
Cultured members of the flavobacteria group have been instrumental in the study of microbial rhodopsins, from the first cultured bacteria showing PR phototrophic activity [Dokdonia sp. strain MED134 (19)] through the identification of an unusual PR from nonmarine permafrost bacteria [Exiguobacterium sibiricum (20)], to the identification of NaR [Nonlabens dokdonensis (basonym: Donghaeana dokdonensis) (13, 15); Dokdonia eikasta (basonym: Krokinobacter eikastus) (16); N. marinus (12)], and now the new ClR [N. marinus (12)]. Perhaps the next challenge is to find functional xanthorhodopsin (XR) homologs in these abundant marine bacteria.
XR is a PR-like proton pump that uses a light-harvesting carotenoid antenna in addition to the retinal chromophore (21). Since its discovery in Salinibacter ruber, a halophilic bacterium, XR was discovered and shown to be functional also in Gloeobacter violaceus, a thylakoid-less cyanobacterium (22). Genes for several XR-like proteins have been reported in the literature lately (14, 23–27), some even in flavobacteria; however, none have yet been shown to behave as XR, namely to have a light-harvesting carotenoid molecule that can transfer energy to retinal.
Current explorations for new microbial rhodopsin ion pumps are restricted to homology searches to known rhodopsins [with only three functional detections reported to date (21, 28, 29)]. Additional creative functional screens should therefore be developed to find novel rhodopsin features.
Footnotes
The authors declare no conflict of interest.
See companion article on page 6732.
References
- 1.Heberle J, Deupi X, Schertler G. Retinal proteins—You can teach an old dog new tricks. Biochim Biophys Acta. 2014;1837(5):531–532. doi: 10.1016/j.bbabio.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 2.Grote M, O’Malley MA. Enlightening the life sciences: The history of halobacterial and microbial rhodopsin research. FEMS Microbiol Rev. 2011;35(6):1082–1099. doi: 10.1111/j.1574-6976.2011.00281.x. [DOI] [PubMed] [Google Scholar]
- 3.Béjà O, Pinhassi J, Spudich JL. Proteorhodopsins: Widespread Microbial Light-Driven Proton Pumps. In: Levin SA, editor. Encyclopedia of Biodiversity. 2 Ed. New York: Elsevier; 2013. pp. 280–285. [Google Scholar]
- 4.Ernst OP, et al. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem Rev. 2014;114(1):126–163. doi: 10.1021/cr4003769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yutin N, Koonin EV. Proteorhodopsin genes in giant viruses. Biol Direct. 2012;7:34. doi: 10.1186/1745-6150-7-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philosof A, Béjà O. Bacterial, archaeal and viral-like rhodopsins from the Red Sea. Environ Microbiol Rep. 2013;5(3):475–482. doi: 10.1111/1758-2229.12037. [DOI] [PubMed] [Google Scholar]
- 7.Béjà O, et al. Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science. 2000;289(5486):1902–1906. doi: 10.1126/science.289.5486.1902. [DOI] [PubMed] [Google Scholar]
- 8.Venter JC, et al. Environmental genome shotgun sequencing of the Sargasso Sea. Science. 2004;304(5667):66–74. doi: 10.1126/science.1093857. [DOI] [PubMed] [Google Scholar]
- 9.Rusch DB, et al. The Sorcerer II Global Ocean Sampling expedition: Northwest Atlantic through eastern tropical Pacific. PLoS Biol. 2007;5(3):e77. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuhrman JA, Schwalbach MS, Stingl U. Proteorhodopsins: An array of physiological roles? Nat Rev Microbiol. 2008;6(6):488–494. doi: 10.1038/nrmicro1893. [DOI] [PubMed] [Google Scholar]
- 11.Finkel OM, Béjà O, Belkin S. Global abundance of microbial rhodopsins. ISME J. 2013;7(2):448–451. doi: 10.1038/ismej.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoshizawa S, et al. Functional characterization of flavobacteria rhodopsins reveals a unique class of light-driven chloride pump in bacteria. Proc Natl Acad Sci USA. 2014;111:6732–6737. doi: 10.1073/pnas.1403051111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung KH. 2012. New type of cation pumping microbial rhodopsins in marine bacteria. 244th ACS National Meeting & Exposition, August 22, 2012 (Philadelphia, PA)
- 14.Riedel T, et al. Genomics and physiology of a marine flavobacterium encoding a proteorhodopsin and a xanthorhodopsin-like protein. PLoS ONE. 2013;8(3):e57487. doi: 10.1371/journal.pone.0057487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon SK, et al. Genomic makeup of the marine flavobacterium Nonlabens (Donghaeana) dokdonensis and identification of a novel class of rhodopsins. Genome Biol Evol. 2013;5(1):187–199. doi: 10.1093/gbe/evs134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue K, et al. A light-driven sodium ion pump in marine bacteria. Nat Commun. 2013;4:1678. doi: 10.1038/ncomms2689. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki J, et al. Conversion of bacteriorhodopsin into a chloride ion pump. Science. 1995;269(5220):73–75. doi: 10.1126/science.7604281. [DOI] [PubMed] [Google Scholar]
- 18.Tittor J, et al. Chloride and proton transport in bacteriorhodopsin mutant D85T: Different modes of ion translocation in a retinal protein. J Mol Biol. 1997;271(3):405–416. doi: 10.1006/jmbi.1997.1204. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-Consarnau L, et al. Light stimulates growth of proteorhodopsin-containing marine Flavobacteria. Nature. 2007;445(7124):210–213. doi: 10.1038/nature05381. [DOI] [PubMed] [Google Scholar]
- 20.Balashov SP, et al. Breaking the carboxyl rule: Lysine 96 facilitates reprotonation of the Schiff base in the photocycle of a retinal protein from Exiguobacterium sibiricum. J Biol Chem. 2013;288(29):21254–21265. doi: 10.1074/jbc.M113.465138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balashov SP, et al. Xanthorhodopsin: A proton pump with a light-harvesting carotenoid antenna. Science. 2005;309(5743):2061–2064. doi: 10.1126/science.1118046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imasheva ES, Balashov SP, Choi AR, Jung KH, Lanyi JK. Reconstitution of Gloeobacter violaceus rhodopsin with a light-harvesting carotenoid antenna. Biochemistry. 2009;48(46):10948–10955. doi: 10.1021/bi901552x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giovannoni SJ, et al. The small genome of an abundant coastal ocean methylotroph. Environ Microbiol. 2008;10(7):1771–1782. doi: 10.1111/j.1462-2920.2008.01598.x. [DOI] [PubMed] [Google Scholar]
- 24.Kang I, et al. Genome sequence of Fulvimarina pelagi HTCC2506T, a Mn(II)-oxidizing alphaproteobacterium possessing an aerobic anoxygenic photosynthetic gene cluster and xanthorhodopsin. J Bacteriol. 2010;192(18):4798–4799. doi: 10.1128/JB.00761-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh HM, et al. Genome sequence of strain IMCC9480, a xanthorhodopsin-bearing betaproteobacterium isolated from the Arctic Ocean. J Bacteriol. 2011;193(13):3421. doi: 10.1128/JB.05088-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vollmers J, et al. Poles apart: Arctic and Antarctic Octadecabacter strains share high genome plasticity and a new type of xanthorhodopsin. PLoS ONE. 2013;8(5):e63422. doi: 10.1371/journal.pone.0063422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Pérez M, et al. Genomes of “Spiribacter”, a streamlined, successful halophilic bacterium. BMC Genomics. 2013;14:787. doi: 10.1186/1471-2164-14-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez A, Bradley AS, Waldbauer JR, Summons RE, DeLong EF. Proteorhodopsin photosystem gene expression enables photophosphorylation in a heterologous host. Proc Natl Acad Sci USA. 2007;104(13):5590–5595. doi: 10.1073/pnas.0611470104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Béjà O, Spudich EN, Spudich JL, Leclerc M, DeLong EF. Proteorhodopsin phototrophy in the ocean. Nature. 2001;411(6839):786–789. doi: 10.1038/35081051. [DOI] [PubMed] [Google Scholar]