Abstract
Objectives
To compare objective and subjective measurements of napping, and to examine the relationship between evening napping and nocturnal sleep in older adults.
Design
For twelve days, participants wore actigraphs and completed sleep diaries.
Setting
Community
Participants
100 individuals who napped, 60–89 years (including good and poor sleepers with typical age-related medical comorbidities).
Measurements
Twelve days of sleep diary and actigraphy provided subjective and objective napping and sleep data.
Results
Evening naps (within 2 hours of bedtime) were characteristic of the sample with peak nap time occurring between 20:30–21:00 (average nap time occurred between 14:30–15:00). Two categories of nappers were identified: 1) day/evening – those who took both daytime and evening naps, and 2) daytime-only. Interestingly, no participants napped during the evening only. Day/evening nappers significantly underreported evening napping and demonstrated lower objectively measured sleep onset latencies (20 vs 26.5 minutes), less wake after sleep onset (51.4 vs 72.8 minutes), and higher sleep efficiencies (76.8 vs 82%) than daytime-only nappers.
Conclusion
Day/evening napping was prevalent amongst this sample of community-dwelling good/poor sleepers, but was not associated with impaired nocturnal sleep. Although the elimination or restriction of napping is a common element of cognitive-behavioral therapy for insomnia (CBTi), these results suggest that a uniform recommendation to restrict/eliminate napping (particularly evening napping) may not meet the needs of all older individuals with insomnia.
Keywords: napping, older adults, aging, evening, sleep
INTRODUCTION
A significant challenge facing 57% of older adults is difficulty sleeping1. The impact of insomnia on older individuals ranges from a minimum impact of daytime fatigue to impaired cognitive functioning2. Late-life insomnia is attributable, in part, to age-related changes in sleep architecture resulting in lighter sleep, more awakenings, less total sleep time, and longer sleep onset3. Despite these structural changes, sleep complaints are not universal and are minimal in healthy older adults3. For many older individuals, health, situational, and psychological factors combine and add to ontogenetic changes affecting sleep.
Napping, a situational factor associated with late-life insomnia4, is prevalent5 with 1 in 4 older adults napping daily2, 6. Average nap frequency in older adults is one/week7, and average duration is 23.37–458 minutes/day. Clinically, napping is often considered to negatively impact sleep. For example, the sleep hygiene component of cognitive-behavioral treatments for insomnia (CBTi) typically recommends eliminating/reducing napping. However, empirical evidence on the impact of napping on nighttime sleep is mixed – calling this recommendation into question. Although a large portion of the literature failed to find an association between napping and sleep, other studies found negative associations6, 9–11,5 and still others found positive associations2,10–12.
The present study employs several innovations in its examination of the relationship between napping and sleep in older adults. First, methodological factors, such as measurement type (objective/subjective) and brief measurement periods, may have contributed to previous inconsistent findings. Past research relied heavily on surveys or diaries/logs, and evidence suggests older individuals may underreport their napping8, 13. Additionally, previous research relied on limited data collection periods which may not accurately capture the variability in older adults’ napping. Approximately 50% nap only 1–2 days/week6 with considerable variability from weekdays to weekends12. The study’s first aim is to further explore the relationship between objective/subjective napping and to more accurately capture the variability in older adults’ napping habits. This is accomplished by examining both self-reported and actigraphically-measured napping over an extended period of time (12 consecutive days–twice as long as the previously reported maximum of 6 days7, 13) in community-dwelling older adults.
A second innovation involves the examination of multiple facets of napping (frequency, duration, and timing) within a single study. In previous research, the average time of day of napping in older adults ranged from 13:3414–22:007. Interestingly, a number of researchers identified a subset of napping-the evening nap13. Frequency of evening napping increases with age7, 8, 10, and prevalence rates in the elderly range from 45–52%15, 16. Yoon and colleagues13 found that the evening nap timeframe corresponded to clock-times of 20:38–22:38 while the average evening nap duration ranged from 515–31.3 minutes7.
Clinically, the sleep hygiene recommendation to reduce/eliminate napping is commonly modified to permit a brief nap earlier in the day17. The rationale behind this is that early naps are less likely to interfere with nighttime sleep. However, empirical support for this is mixed 13,8,18. The study’s second aim is to examine the impact of evening naps on sleep in older adults. This is accomplished by comparing nighttime sleep variables (objective and subjective total sleep time, sleep onset latency, wake after sleep onset, and sleep efficiency) in two groups–daytime-only nappers and day/evening nappers.
In comparison to previous research which examined napping in an unusually healthy sample13, the current sample contained older individuals with both health complaints and insomnia complaints (good/poor sleepers). Additionally, by conducting general community-based recruitment and sampling participant behavior within the home environment, the present study’s results have greater ecological validity and enhanced generalizability.
METHODS
Procedure
A secondary data analysis was performed using data previously collected19. Participants were recruited from North Florida using media advertisements, community groups, and flyers. Recruitment materials advertised a study of normal sleep patterns and encouraged all types of older sleepers (good, poor, in-between) to participate. Participants received $30 USD. Screening consisted of a brief telephone interview (15–20 minutes) followed by a 1–1 ½ hour interview either at home (76%) or a local continuing care retirement center (24%).
Exclusionary criteria: 1)<60 years; 2)self-reported sleep disorders other than insomnia (apnea, narcolepsy); 3)self-reported symptoms of sleep diagnoses other than insomnia (e.g., heavy snoring, gasping for breath); 4)severe psychiatric disorder (thought disorder, depression); 5)cognitive impairment (impaired score on 3+ Cognistat subtests); 6)use of psychotropic/other sleep-altering medications (beta-blockers); 7)medical conditions that impaired independent daily functioning19.
Following consent, the Cognistat, and demographics/health survey were administered. Next, participants were asked to complete sleep diaries and wear the Actiwatch-L® (data download at end of each week) continuously for 14 days.
Participants
Of the 116 individuals recruited, 103 were enrolled. Thirteen individuals were ineligible due to age, dementia, medication, and sleep apnea. See Table 1 for demographic characteristics. All participants lived in their own homes. The sample included good and poor sleepers. Participants were categorized as poor sleepers if they reported ≥31 minutes of unwanted awake time during the night (sleep onset latency or wake after sleep onset) ≥3 nights/week.
Table 1.
Participant Demographics and Sleep and Napping-Related Characteristics for Overall Sample and by Nap Groups
| Overall Sample | Day/evening nappers | Daytime-only nappers | |
|---|---|---|---|
| Age (M ± SD) | 72.95 (6.93) | 73.33 (7.33) | 72.86 (6.76) |
| Education (years, M ± SD) | 16.16 (3.05) | 16.31 (2.96) | 15.73 (2.86) |
| Gender (% Female) | 64.00 | 61.70 | 72.70 |
| Marital Status (%) | |||
| Single | 6.00 | 5.70 | 11.80 |
| Married | 71.10 | 69.80 | 70.60 |
| Widowed | 14.50 | 18.9 | 5.90 |
| Divorced | 8.40 | 5.70 | 11.80 |
| Ethnicity (%) | |||
| Caucasian | 97.00 | 98.30 | 90.90 |
| African American | 2.00 | 0 | 9.10 |
| Other | 1.00 | 1.70 | 0 |
| Medications1 (M ± SD) | 2.92 (2.19) | 3.133 (2.21) | 2.59 (1.98) |
| Conditions2 (M ± SD) | 2.03 (1.60) | 2.28 (1.77) | 1.32 (1.13) |
| Sleep Classification (%) | |||
| Good Sleepers | 59.00 | 65.00 | 45.40 |
| Poor Sleepers | 41.00 | 35.00 | 54.60 |
| Bedtime (M ± SD) | 22:22 (79 mins) | 22:17 (81 mins) | 22:58 (42 mins) |
| Wake time (M ± SD) | 6:48 (49 mins) | 6:45 (48 mins) | 7:25 (50 mins) |
| Napping Behavior (M ± SD) | |||
| Nap frequency-days nappedo3 | 6.16 (2.87) | 7.13a (2.68) | 3.67a (2.06) |
| Nap frequency-days nappeds3 | 4.26 (3.31) | 3.88 (3.23) | 5.32 (3.75) |
| Nap frequency-# of napso4 | 1.72 (0.65) | 1.20b (0.83) | 0.43b (0.30) |
| Nap duration-overallo5 | 13.15 (2.82) | 13.52c (3.35) | 12.57c (1.89) |
| Nap duration-overalls5 | 18.11 (21.91) | 18.93 (25.76) | 19.82 (15.25) |
| Nap duration-per napo6 | 8.52 (2.97) | 7.88 (2.81) | 10.19 (3.29) |
| Time of dayo7 | 14:52 (142 mins) | 16:48d (100 mins) | 15:21d (187 mins) |
Note. The subscript ‘o’ denotes objectively (actigraphically) measured variables while the subscript ‘s’ denotes variables measured subjectively (sleep diaries).
Total number of medications participants listed.
Total number of medical conditions from the following list: heart problems, cancer, hypertension, neurological disorder, breathing disorder, urinary problems, diabetes, pain, gastrointestinal disorders, mental health disorder, and other.
Nap frequency-days napped refers to the average number of days out of the 12 assessment days when the participant napped;
Nap frequency-# of naps refers to the average number of naps that occurred on a given day;
Nap duration-overall refers to the average total time in minutes spent napping on the days the participants napped;
Nap duration-per nap refers to the average time in minutes spend napping during each nap;
Time of day of naps is calculated as a serial number equivalent to the 24hr clock (0=12am, 0.5=12noon, and 1.0=12 midnight)
Means with different subscripts differ significantly at p < .001.
Means with different subscripts differ significantly at p < .01.
Measures
Objective sleep
Participants wore an Actiwatch-L®20 on their nondominant wrist for 14 consecutive 24-hr periods concurrent with the sleep diary period. Within the Actiwatch-L®, an omnidirectional, piezoelectric accelerometer with a sensitivity of ≥ 0.01 g-force samples data 32 times/second over a 30 second epoch. Data is then analyzed by Actiware-Sleep vol. 3.320. A high sensitivity setting was used since it provides high correlations with PSG-measured total sleep time (.95) for healthy older adults21 and for total sleep time (.73) and sleep onset latency (.93) for individuals with insomnia22. Additionally, actigraphy has high criterion-validity compared to PSG (.80) and high test-retest reliability23 (0.92).
Four objective sleep variables were obtained: sleep onset latencyo-interval between bedtime and sleep start; total sleep timeo-sum of all sleep epochs within a sleep period; sleep efficiencyo-ratio of total sleep time to total time-in-bedx100%; and wake after sleep onseto-time spent awake after initial sleep onset until last awakening. Note: Throughout this report, the subscripts “s” and “o” are used to distinguish between the subjective and objective variables.
Subjective sleep
Participants completed sleep diaries24 each morning for 14 days. However, the first and last days were not analyzed as the actigraphy data for those days was incomplete. Four subjective sleep variables were obtained: sleep onset latencys-initial time from lights out until sleep onset; wake after sleep onsets-time spent awake after initial sleep onset until last awakening; total sleep times-computed by subtracting total wake time from time-in-bed; and sleep efficiencys-ratio of total sleep time to total time-in-bedx100%.
Objective napping
Napping was measured objectively using actigraphy. Methodological questions have arisen regarding the sensitivity of actigraphy to distinguish inactivity due to napping versus resting or watch removal. Interestingly, the sensitivity setting (high) validated for determining sleep bouts in older adults is not applicable for identifying napping. A threshold of 20 activity counts per 30 second epoch resulted in an overestimation of napping, due in part to the misidentification of inactivity (resting/watch removal) as sleep bouts. Therefore, an even higher sensitivity setting was required in order to differentiate mere inactivity from napping. A sensitivity setting of 12 enabled the detection of naps identified by participants in their sleep diaries. Convergent validity for the sensitivity setting was established by comparing objective/subjective naps of participants who kept detailed sleep diaries (e.g. nap 2:30–2:45).
Additionally, concerns about differentiating resting/watch removal from napping were addressed by examining narratives in the participants’ sleep diaries (“watch removed from 6–8 p.m.”) and applying Webster’s rules25. To further ensure the differentiation of ‘watch off’ periods from napping, the sensitivity level was increased to the highest setting (0) and periods which were still identified as naps were noted. If a period of inactivity was labeled as a nap at this setting, it was eliminated from analysis. An activity count of ‘0’ contains less activity then required for sleep and actually represents ‘watch off’ intervals.
Finally, to encompass the nap durations of 515 to 458 minutes found in previous research, naps ranging from 5–180 minutes were included in the analysis. By including naps of >5 minutes, the ability to detect ‘watch off’ periods was increased as the detection of ‘0’ activity for >5 minutes could only be attributed to watch removal.
Five objective napping variables were obtained: frequency-days nappedo-average number of days out of 12 on which a nap occurred; frequency-# of napso-average number of naps that occurred on a given day when the participants napped; duration-overallo-average total time spent napping on the days that participants napped; duration-per napo-average time spent napping during each nap; and time of dayo-sum of the time of day of all naps for each participant divided by the number of naps taken.
Time of day of nap categories
The actigraphic data were used to categorize participants into 2 groups: day/evening nappers (napped during both the day and evening) and daytime-only nappers (solely napped during the day). A nap was labeled an evening nap if it occurred within two hours of the individual’s bedtime for the day being considered13.
Subjective napping
Participants recorded the total number of minutes spent napping each day on their sleep diaries. Two subjective napping variables were obtained: frequency-days nappeds-average number of days during which participants self-reported napping; duration-overalls-average total time spent napping on days napping was reported.
Demographics/Health survey
This two-page, 13-item questionnaire collects information on demographics, sleep disorder symptoms, physical health, and mental health26. The sleep disorders items asked whether the participant had a sleep problem and if they or a bed partner noticed heavy snoring, difficulty breathing or gasping for breath, frequent leg jerks, restlessness before sleep onset, sleep attacks during the day, or paralysis at sleep onset.
Cognitive impairment
Participants were screened for cognitive impairment using the Cognistat27. Individuals who scored in the impaired range on three or more of the ten subscales were excluded. The Cognistat has been found to more sensitively detect cognitive-impairment than the MMSE28 and to differentiate between impairment due to psychiatric illness versus impairment due to organic cognitive impairment29. Interrater reliability ranges from 0.997–1.0030.
Statistical Analysis
Paired samples t tests were conducted to examine differences in objectively and subjectively measured napping variables. Significance was defined at a Bonferroni-adjusted alpha level of 0.025 (t tests) and 0.013 (chi-square tests). Two MANOVAs were conducted to examine differences between the 2 nap groups (daytime-only versus day/evening) in objectively and subjectively measured sleep variables. Additionally, two MANOVAs were performed to examine group differences in sample and nap characteristics. Finally, chi-square analyses were performed to examine group differences in the categorical sample variables (gender, marital status, ethnicity, sleep classification).
RESULTS
Sample and Nap Characteristics
There were no significant group differences [Wilk’s Λ=.86, F(8, 71)=1.41, p=.21, η2=0.14] in terms of age, education, number of medications, health conditions, or average bed/waketimes. The nap groups also did not differ in terms of gender (p=0.35), marital status (p=0.44), race (p=0.05), or sleep classification (good/poor sleepers; p=0.11). See Table 1.
Three individuals were excluded from the analysis as they did not engage in napping throughout the study. Nap frequency-days nappedo was significantly higher than nap frequency-days nappeds (t[98]=−4.74, p=.000, η2=0.19). Conversely, nap duration-overalls was significantly longer than nap duration-overallo (t[96]=2.174, p<.002, η2=0.05).
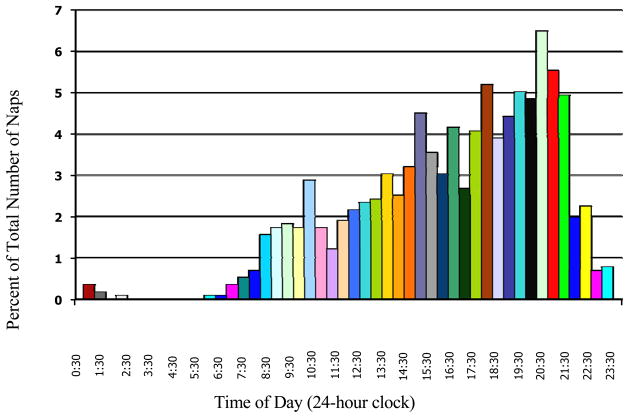
Peak time of day of naps recorded by actigraphy occurred between 20:30–21:00 p.m. (see Figure). Average time of day of naps was between 14:30–15:00. Forty-seven percent of all naps occurred after 6 p.m. Fifty-eight percent of the sample engaged in an evening nap and 85% engaged in a daytime nap. There were no significant gender differences in nap frequency, duration, or time of day.
Figure 1.
Percentage of total actigraphically-measured naps taken by all participants that occurred during each half-hour interval of the 24-hour clock.
There were significant nap group differences in napping [Wilk’s Λ=.62, F(7, 71)=6.24, p=.000, η2=0.38]. Univariate follow-up tests indicated the groups differed in frequency-days napped o [F (1, 77)=26.70, p=.000, η2=0.26], nap frequency-# of napso [F (1, 77)=14.85, p=.000, η2=0.16], nap duration-overall o [F (1, 77)=8.66, p=.004, η2=0.10], and time of day o [F (1, 77)=8.29, p=.005, η2=0.10]. Day/evening nappers had a higher nap frequency both per day and over the 12 day period compared to daytime-only nappers. On average, daytime-only nappers had naps of significantly longer duration while day/evening nappers engaged in naps later in the day.
Nap Categories and Objective Sleep Quantity
Fifty-eight percent of participants were categorized as day/evening nappers, and 27% were daytime-only nappers. The remaining 15% of the sample exhibited very low frequency napping behavior (0–2 naps over the 12 day period). 000Overall, there was a significant main effect of group [Wilk’s Λ=.81, F(4, 77)=4.82, p=.003, η2=0.19]. Univariate followup tests revealed significant differences for sleep onset latencyo [F (1, 80)=4.37, p=.04, η2=0.05], wake after sleep onseto [F (1, 80)=14.27, p=.000, η2=0.15], and sleep efficiencyo [F(1, 80)=12.01, p=.001, η2=0.13]. Day/evening nappers had significantly lower sleep onset latencies (7 minutes), less wake after sleep onset (21 minutes), and greater sleep efficiencies (5%) as measured by actigraphy compared to daytime-only nappers. See Table 2.
Table 2.
Means and Standard Deviations for the Time of Day of Nap Groups for Objective and Subjective Sleep
| Actigraphy sleep and nap variables
| ||||||||
|---|---|---|---|---|---|---|---|---|
| SOL (mins) | WASO (mins) | SE (%) | TST (mins) | |||||
| Nap Group | M | SD | M | SD | M | SD | M | SD |
| Daytime-only | 26.50* | 13.01 | 72.82┼ | 24.65 | 76.80§ | 6.41 | 382 | 43.04 |
| Day/evening | 20.00* | 12.28 | 51.43┼ | 21.99 | 81.95§ | 5.80 | 393 | 58.71 |
| Sleep Diary sleep and nap variables
| ||||||||
|---|---|---|---|---|---|---|---|---|
| SOL (mins) | WASO (mins) | SE (%) | TST (mins) | |||||
| Nap Group | M | SD | M | SD | M | SD | M | SD |
| Daytime-only | 25.61 | 16.74 | 25.19 | 23.50 | 86.05 | 6.65 | 430 | 46.89 |
| Day/evening | 23.92 | 19.73 | 28.72 | 26.28 | 84.97 | 9.43 | 408 | 69.02 |
Note: SOL (sleep onset latency) refers to the number of minutes spent awake from bedtime to the onset of sleep; WASO (wake after sleep onset) refers to the total number of minutes spent awake during the night excluding SOL; SE (sleep efficiency) is a ratio of the time spent sleeping to the time spent in bed; TST (total sleep time) refers to the time spent asleep during the night excluding SOL and WASO.
Group means were significantly different, p<.05.
Group means were significantly different, p<.001.
Group means were significantly different, p<.01.
Nap Categories and Subjective Sleep Quantity
There was no significant main effect of group [Wilk’s Λ=.93, F(12, 222.535)=0.50, p=.95, η2=0.25]. There was a significant correlation between self-reported versus actigraphically-measured nap duration for daytime-only (r = .39, p=.0103), but not for day/evening nappers (r = .16, p=.36). See Table 2.
DISCUSSION
The present study examined the relationship between napping and sleep in community-dwelling older adults who napped. The first aim examined differences between subjectively and objectively measured napping, and the results revealed interesting differences for both frequency and duration. Specifically, subjective nap frequency (4 naps over 12 days) was consistent with previous research12, while objective nap frequency (6 over a 12 day period) was considerably higher12,7. A possible explanation is that actigraphy may be capturing a greater number of evening naps than the sleep diary. Yoon and colleagues13 found only 22.6% of evening nappers self-reported their evening naps. The present study supports and extends these findings as there was a significant underreporting of nap durations by day/evening nappers (more than two and a half times) compared to daytime-only nappers. Regarding nap duration, previous studies reported ranges from 23.3–45 minutes/day7, 8, 13, 14, 16. Average objective (13 minutes) and subjective (18) nap durations in this study were comparatively lower. One explanation for this is that the inclusion of evening naps (typically of shorter duration than daytime naps) would decrease the mean duration.
Time of day of napping was also examined with peak nap time occurring between 8:30–9:00 p.m. This is consistent with previous studies that found the evening to be the most characteristic time for napping in older samples8, 13, 15, 16. One theoretical explanation for the large number of evening naps in the present sample is that evening napping may represent an advance in circadian rhythms. Some older adults experience a ‘phase advance’ resulting in earlier bed- and waketimes13. Therefore, out-of-bed sleep in the evening could represent the start of the nocturnal sleep period. In the present sample, however, there was not a significant difference in bedtimes between the day/evening and daytime-only nappers. Alternatively, evening napping may represent a weakening of the alerting signal from the suprachiasmatic nucleus (SCN),16 which regulates the sleep/wake cycle. Action potentials within the SCN fire in a 24-hour rhythm and reach a maximum firing at mid-day and then fall again at night. It is possible that evening naps could result from a premature drop in the firing of SCN action potentials reducing the alertness of the individual. While there was not a statistically significant difference between the nap groups in terms of medication or conditions, the day/evening nap group reported almost double the number of medical conditions of the daytime-only nap group. It is possible that with greater power, a significant difference in medical conditions would have been detected which could also explain the large number of evening naps in the day/evening nap group. Finally, the large number of evening naps could simply reflect greater sleep needs or trait sleepiness in the present sample.
The second aim examined the relationship between evening naps and sleep. Day/evening and daytime-only nappers differed significantly in terms of objective sleep. Essentially, day/evening nappers had significantly less wake time during the night compared daytime-only nappers. This result conflicts with conventional sleep hygiene recommendations to avoid napping altogether or restrict naps to before noon17, but is consistent with recent recommendations to evaluate the cost/benefits of napping on an individual basis18.
In addition to being statistically significant, the results are clinically significant. An average wake after sleep onset of ≥31 minutes can result in an insomnia diagnosis. Therefore, a 21 minute decrease in wake after sleep onset and a 5% increase in sleep efficiency can have significant clinical impact for elderly sleepers.
Comparisons of the two nap groups revealed interesting differences. Day/evening nappers had a higher nap frequency (both daily and over the 12 days), shorter average duration, and a later average nap time compared to daytime-only nappers. It is intuitive that by engaging in naps during the day/evening, this group likely had a higher frequency of napping. Conversely, napping for longer durations and earlier in the day (daytime-only group) was not associated with better objective sleep.
One limitation of the study is the use of a convenience sample, which restricted generalization of the results to a more diverse sample. Interestingly, comparisons between nappers and non-nappers were prevented, because only 3 out of 103 individuals enrolled did not nap. Because the sample was highly educated and relatively healthy, the napping behavior reported herein may be an underestimation of napping in less healthy, less educated older adults. A second limitation is that the study’s cross-sectional design makes it impossible to draw causal conclusions. Finally, the two measures of napping (objective and subjective) employed have strengths and weaknesses. While actigraphy provides a sensitive measure of an individual’s napping, it is a novel approach that has not typically been applied to the study of napping. Sleep diaries have been used before, but have limited utility for assessing nap duration and time of day of napping.
Future research directions include the establishment of reliability data for both objective and subjective measures of napping. Additionally, the relationship between napping and sleep could be further defined by examining the intraindividual (within-person) variability of napping. This study examined the relationship between napping and sleeping behaviors in community-dwelling older adults who engaged in napping. Significant differences were found between objective and subjective measures of napping. Evening was the most characteristic time for napping. The combination of day/evening naps was not associated with impaired nocturnal sleep. As these results run counter to prevailing treatment recommendations, further research is needed to replicate them, and to subsequently arrive at a consensus regarding treatment recommendations for napping in older adults with insomnia.
Footnotes
Institutions at which the work was performed: University of Florida
Authors’ Contributions: All three authors contributed significantly to the conception and design of the study. Data acquisition was conducted by Drs. McCrae and Rowe. Analysis and interpretation of the data was done by Natalie Dautovich. Drafting of the article was conducted by Dr. McCrae and Natalie Dautovich while all three authors assisted with revising the article. Final approval of the version to be published was given by all three authors. Everyone who contributed significantly to the paper has been acknowledged as authors.
Conflict of Interest Disclosures:
| Elements of Financial/Personal Conflicts |
* Author 1 Natalie Dautovich |
Author 2 Christina McCrae |
Author 3 Meredeth Rowe |
Etc. | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | |||||
| Grants/Funds | X | X | X | |||||
| Honoraria | X | X | X | |||||
| Speaker Forum | X | X | X | |||||
| Consultant | X | X | X | |||||
| Stocks | X | X | X | |||||
| Royalties | X | X | X | |||||
| Expert Testimony | X | X | X | |||||
| Board Member | X | X | X | |||||
| Patents | X | X | X | |||||
| Personal Relationship | X | X | X | |||||
Disclosure statement: This was not an industry supported study. Natalie Dautovich and Drs. McCrae and Rowe have indicated no financial conflicts of interest.
References
- 1.Kryger M, Monjan A, Bliwise D, Ancoli-Israel S. Sleep, health, and aging. Bridging the gap between science and clinical practice. Geriatrics. 2004 Jan;59(1):24–26. 29–30. [PubMed] [Google Scholar]
- 2.Foley DJ, Monjan AA, Brown LS, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: An epidemiologic study of three communities. Sleep. 1995;18(6):425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 3.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-Analysis of Quantitative Sleep Parameters From Childhood to Old Age in Healthy Individuals: Developing Normative Sleep Values Across the Human Lifespan. Sleep: Journal of Sleep and Sleep Disorders Research. 2004;27(7):1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 4.Ancoli-Israel S, Martin JL. Insomnia and daytime napping in older adults. Journal of Clinical Sleep Medicine. 2006;2(3):9. [PubMed] [Google Scholar]
- 5.Foley DJ, Vitiello MV, Bliwise DL, Ancoli-Israel S, Monjan AA, Walsh JK. Frequent napping is associated with excessive daytime sleepiness, depression, pain, and nocturia in older adults: Findings from the National Sleep Foundation ‘2003 Sleep in America’ Poll. American Journal of Geriatric Psychiatry. 2007;15(4):344–350. doi: 10.1097/01.JGP.0000249385.50101.67. [DOI] [PubMed] [Google Scholar]
- 6.Beh H. A survey of daytime napping in an elderly Australian population. Australian Journal of Psychology. 1994;46:100–106. [Google Scholar]
- 7.Yoon I-Y, Kripke DF, Youngstedt SD, Elliott JA. Actigraphy suggests age-related differences in napping and nocturnal sleep. Journal of Sleep Research. 2003;12(2):87–93. doi: 10.1046/j.1365-2869.2003.00345.x. [DOI] [PubMed] [Google Scholar]
- 8.Jean-Louis G, Kripke DF, Assmus JD, Langer RD. Sleep-wake patterns among postmenopausal women: A 24-hour unattended polysomnographic study. Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2000;55(3):M120–m123. doi: 10.1093/gerona/55.3.m120. [DOI] [PubMed] [Google Scholar]
- 9.Hays JC, Blazer DG, Foley DJ. Risk of napping: excessive daytime sleepiness and mortality in an older community population. J Am Geriatr Soc. 1996 Jun;44(6):693–698. doi: 10.1111/j.1532-5415.1996.tb01834.x. [DOI] [PubMed] [Google Scholar]
- 10.Monk TH, Buysse DJ, Carrier J, Billy BD, Rose LR. Effects of afternoon “siesta” naps on sleep, alertness, performance, and circadian rhythms in the elderly. Sleep. 2001 Sep 15;24(6):680–687. doi: 10.1093/sleep/24.6.680. [DOI] [PubMed] [Google Scholar]
- 11.Campbell SS, Murphy PJ, Stauble TN. Effects of nap on nighttime sleep and waking function in older subjects. Journal of the American Geriatrics Society. 2005;53:48–53. doi: 10.1111/j.1532-5415.2005.53009.x. [DOI] [PubMed] [Google Scholar]
- 12.Buysse DJ, Browman KE, Monk TH, Reynolds CF, 3rd, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992 Aug;40(8):779–786. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 13.Yoon I-Y, Kripke DF, Elliott JA, Youngstedt SD, Rex KM, Hauger RL. Age-Related Changes of Circadian Rhythms and Sleep-Wake Cycles. Journal of the American Geriatrics Society. 2003;51(8):1085–1091. doi: 10.1046/j.1532-5415.2003.51356.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirota A, Tanaka H, Nittono H, Hayashi M, Shirakawa S, Hori T. Volitional lifestyle in healthy elderly: its relevance to rest-activity cycle, nocturnal sleep, and daytime napping. Percept Mot Skills. 2002 Aug;95(1):101–108. doi: 10.2466/pms.2002.95.1.101. [DOI] [PubMed] [Google Scholar]
- 15.Ancoli-Israel S, Kripke DF, Mason W, Kaplan OJ. Sleep apnea and periodic movements in an aging sample. J Gerontol. 1985 Jul;40(4):419–425. doi: 10.1093/geronj/40.4.419. [DOI] [PubMed] [Google Scholar]
- 16.Yoon IY, Kripke DF, Elliott JA, Langer RD. Naps and circadian rhythms in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2004 Aug;59(8):844–848. doi: 10.1093/gerona/59.8.m844. [DOI] [PubMed] [Google Scholar]
- 17.Lichstein KL, Morin CM. Treatment of late-life insomnia. Thousand Oaks, CA: Sage Publications, Inc; 2000. [Google Scholar]
- 18.Vitiello M. Effective treatment of sleep disturbances in older adults. Clinical Cornerstone. 2000;2(5):12. doi: 10.1016/s1098-3597(00)90037-1. [DOI] [PubMed] [Google Scholar]
- 19.McCrae CS, Rowe MA, Dautovich ND, Lichstein KL, Durrence HH, Riedel BWTDJ, Bush AJ. Sleep hygiene practices in two community dwelling samples of older adults. Sleep. 2006;29(12):9. doi: 10.1093/sleep/29.12.1551. [DOI] [PubMed] [Google Scholar]
- 20.Mini Mitter Co. I. Actiwatch 16/Actiwatch 64/Actiwatch-L/Actiwatch-Score instruction manual. Mini Mitter Co., Inc; 2001. [Google Scholar]
- 21.Colling E, Wright M, Lahr S, Schmedlen L, DeJongh L, Singer CA. A comparison of wrist actigraphy with polysomnography as an instrument of sleep detection in elderly persons. Sleep. 2000;23:A378. [Google Scholar]
- 22.Cook KG, Lichstein KL, Donaldson J, Nau SD, Lester KW, Aguillard RN. An exploratory validation of actigraphic measures of insomnia. Sleep. 2004;27:A270. [Google Scholar]
- 23.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003 May 1;26(3):342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 24.Lichstein KL, Riedel BW, Means MK. Psychological treatment of late-life insomnia. In: Schulz R, Maddox G, Lawton P, editors. Annual review of gerontology and geriatrics: Vol. 18. Focus on interventions research with older adults. New York: Springer; 1999. pp. 74–110. [Google Scholar]
- 25.Webster JB. An activity-based sleep monitor system for ambulatory use. Sleep: Journal of Sleep Research & Sleep Medicine. 1982;5(4):389–399. doi: 10.1093/sleep/5.4.389. [DOI] [PubMed] [Google Scholar]
- 26.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behaviour Research and Therapy. 2003;41(4):427–445. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 27.Mueller J, Kiernan R, Langston JW. Manual for Cognistat (The Neurobehavioral cognitive status exam) Fairfax, CA: The Northern California Neurobehavioral Group, Inc; 2001. [Google Scholar]
- 28.Fields SD, Fulop G, Sachs CJ, Strain J, Fillit H. Usefulness of the Neurobehavioral Cognitive Status Examination in the hospitalized elderly. Int Psychogeriatr. 1992 Summer;4(1):93–102. doi: 10.1017/s1041610292000929. [DOI] [PubMed] [Google Scholar]
- 29.Wiederman MWMCD. The Neurobehavioral Cognitive Status Exam (NCSE) with geriatric inpatients. Clinical Gerontologist. 1995;15(4):35–47. [Google Scholar]
- 30.Cunic TLD, RL Use of videotaped administrations as a method of establishing interrater reliability for the NCSE. Archives of Clinical Neuropsychology. 2001;16:843–843. [Google Scholar]