SUMMARY
Pneumonic plague is a deadly respiratory disease caused by Yersinia pestis. The bacterial protease Pla contributes to disease progression and manipulation of host immunity, but the mechanisms by which this occurs are largely unknown. Here we show that Pla degrades the apoptotic signaling molecule Fas ligand (FasL) to prevent host cell apoptosis and inflammation. Wild-type Y. pestis, but not a Pla mutant (Δpla), degrades FasL, which results in decreased downstream caspase-3/7 activation and reduced apoptosis. Similarly, lungs of mice challenged with wild-type Y. pestis show reduced levels of FasL and activated caspase-3/7 compared to Δpla infection. Consistent with a role for FasL in regulating immune responses, Δpla infection results in aberrant pro-inflammatory cytokine levels. The loss of FasL or inhibition of caspase activity alters host inflammatory responses and enables enhanced Y. pestis outgrowth in the lungs. Thus, by degrading FasL, Y. pestis manipulates host cell death pathways to facilitate infection.
Keywords: Yersinia pestis, plague, Pla, Fas ligand, inflammation, pneumonia
INTRODUCTION
Infectious pneumonias are a leading cause of worldwide morbidity and mortality, with a greater annual burden of disease than HIV, malaria, and tuberculosis (Mizgerd, 2006). The bacterium Yersinia pestis is an infamous example of an easily transmitted pathogen that causes respiratory infections. As the deadliest form of disease caused by Y. pestis, primary pneumonic plague results in a rapidly progressing, purulent, multifocal severe exudative bronchopneumonia, and is 100% fatal if untreated (Lathem et al., 2005; Perry and Fetherston, 1997). A hallmark of pneumonic plague is the strikingly biphasic pulmonary host inflammatory response. The first 24 h following infection are characterized by rapid bacterial replication in the absence of inflammation. By 36 h, however, the infection rapidly transitions to a highly pro-inflammatory phase that is marked by extensive immune cell influx, inflammatory cytokine production, and tissue damage (Lathem et al., 2005; Price et al., 2012).
Y. pestis produces multiple virulence factors to suppress the initial host inflammatory response, including a tetra-acylated form of LPS, anti-phagocytic surface proteins, and a type III secretion system (T3SS) that injects multiple toxins into host cells (Montminy et al., 2006; Perry and Fetherston, 1997). These effects are broad - for instance, the T3SS effector protein YopK induces macrophage apoptosis early during infection, while YopM inhibits the activation of caspase-1 to prevent cell death via pyroptosis (LaRock and Cookson, 2012; Peters et al., 2013). Indeed, these concerted functions permit Y. pestis to evade early detection by the host even in the presence of otherwise stimulatory signals such as PAMPs. As the infection transitions into the pro-inflammatory phase, however, it is believed that host cell apoptosis declines while pyroptosis or other forms of inflammatory cell death increase (Bergsbaken and Cookson, 2007). In spite of these anti-inflammatory factors the bacteria are eventually detected by the host and massive inflammation ensues.
Previous work has shown that the Y. pestis bacterial plasminogen activator protease Pla also contributes to the manipulation of host inflammation during pneumonic plague (Lathem et al., 2007), although the mechanisms by which this occurs have no t yet been elaborated. Pla is a member of the omptin family of bacterial outer membrane proteases and has been shown to cleave a number of host substrates in vitro, including plasminogen, α2-antiplasmin, and other factors integral to coagulation and fibrinolysis (Caulfield and Lathem, 2012). Indeed, Pla is required for the full virulence of Y. pestis during both bubonic and pneumonic plague (Lathem et al., 2007; Sodeinde et al., 1992). Pla activates host fibrinolysis during bubonic plague to enable dissemination from the skin to distal sites (Degen et al., 2007), and during pneumonic plague, Pla is required for the massive outgrowth of bacteria observed in the lower airways and is maximally expressed during the pro-inflammatory phase of disease (Lathem et al., 2007; Lathem et al., 2014). The mechanisms by which Pla contributes to bubonic vs. pneumonic plague may be distinct, however, and the host proteins cleaved by Pla in the lungs to alter inflammation and enhance disease during pneumonic plague are not yet known.
FasL is a type-II membrane protein within the TNF family of death factors (Nagata, 1997). By engaging with the canonical death receptor Fas on cell surfaces, FasL initiates host cell death through the downstream activation of caspase-8 followed by caspase-3/7 as part of the extrinsic apoptosis pathway (Nagata, 1997). FasL has traditionally been recognized to play roles in T cell homeostasis and the elimination of tumor cells, yet is also present on airway epithelial cells and infiltrating immune cells in the lungs during infection (Hamann et al., 1998; Xerri et al., 1997). FasL has recently been appreciated for its protective role in the lungs during bacterial pneumonias by mediating inflammation and innate immune cell recruitment (Yeretssian et al., 2008). Via Fas signaling, the host induces apoptotic cell death as a mechanism to optimize innate inflammatory responses for defense while avoiding excessive pulmonary damage (Labbe and Saleh, 2008). For instance, apoptosis is associated with increased bacterial clearance during infection with Streptococcus pneumoniae (Daigneault et al., 2012), and infection with either Pseudomonas aeruginosa or Helicobacter pylori leads to increased FasL-dependent apoptosis of host cells and increased disease severity among FasL-deficient mice as compared to wild-type controls (Grassme et al., 2000; Jones et al., 2002). Here we report that through the activity of Pla, Y. pestis inactivates the host signaling molecule Fas ligand (FasL) to alter host cell death pathways and to modulate innate inflammatory responses during pneumonic plague, which results in increased virulence of the pathogen during infection.
RESULTS
Identification of Fas Ligand as a Substrate of Pla
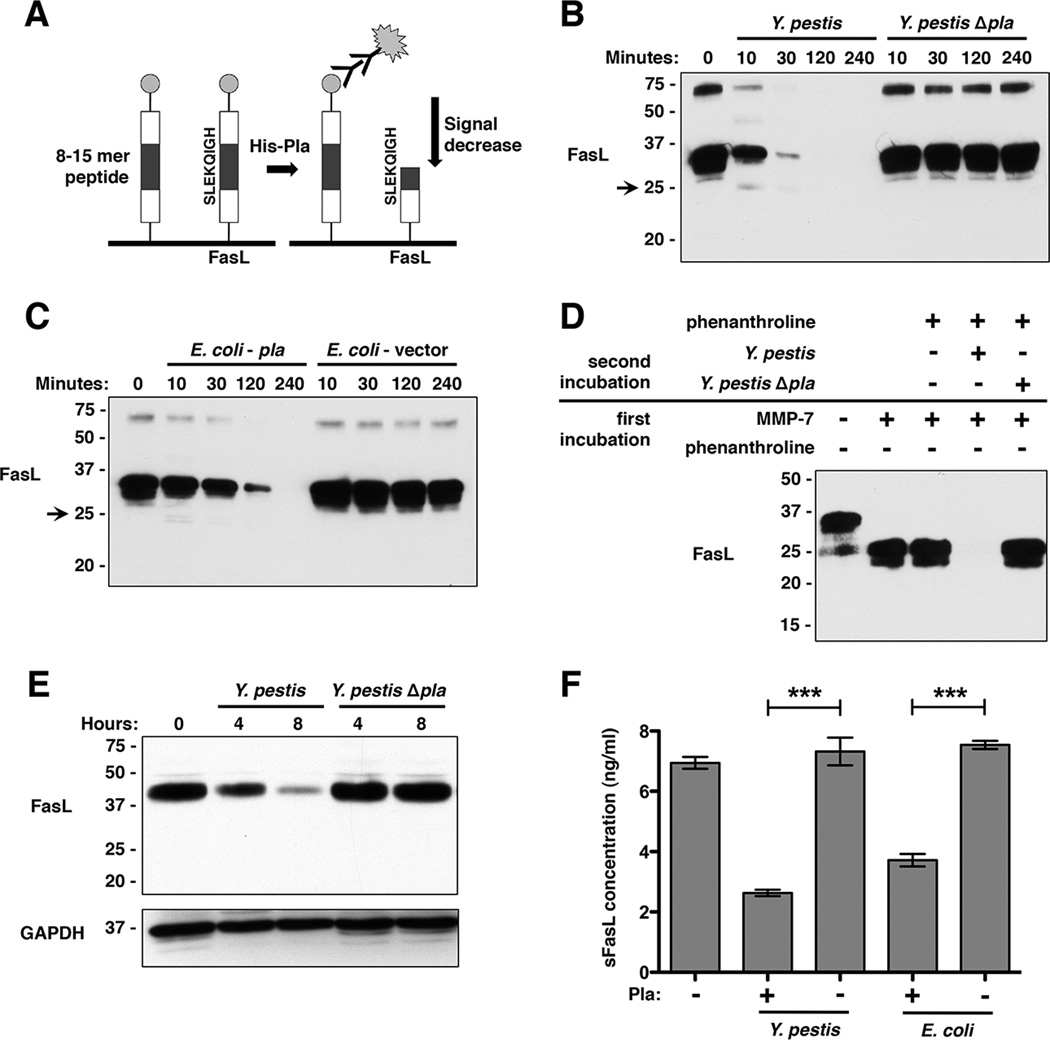
In order to identify host factors that, when cleaved by Pla, could influence the host inflammatory response to Y. pestis infection in the lungs, we undertook a proteomic-scale approach as a means of substrate discovery. Specifically, we used commercially available microarray slides that contained 3,525 unique 8–15 residue peptides of random amino acid sequence as well as sequences from previously annotated protease cleavage sites (Figure 1A). In order to obtain active Pla protein to use with this array, we purified a recombinant form of full-length Pla with an N-terminal 6xHis-tag. His-Pla protein was subsequently activated by incubation with rough LPS as previously described (Figures S1A and S1B) (Kukkonen et al., 2001).
Figure 1. Pla is necessary and sufficient for degradation of full-length and soluble FasL (sFasL).
(A) Diagram of peptide array. After incubation with purified His-Pla for peptide cleavage, a decrease in signal intensity from a given peptide represents its cleavage by Pla. The peptide on the array corresponding to FasL is shown. (B) Pla-dependent degradation of full-length FasL by Y. pestis. FasL processing was assessed over time by immunoblot with an anti-FasL antibody. The transient 25 kDa product produced by Pla is indicated with an arrow. Numbers to the left of each blot indicate molecular weight in kDa. (C) Pla is sufficient for degradation of FasL. FasL was incubated with E. coli either induced to produce Pla or carrying the corresponding empty expression vector. (D) Pla-dependent processing of sFasL generated by MMP-7 following inhibition of MMP-7 using phenanthroline. (E) Pla-dependent degradation of FasL from KFL-9 cells. Following detection of FasL, the immunoblot was stripped and GAPDH was subsequently detected (as a loading control). (F) Degradation of sFasL endogenously shed from KFL-9 cell surfaces. sFasL was purified from conditioned media, incubated with either Y. pestis or E. coli induced to produce Pla, and assessed by sFasL-specific ELISA. n = 4 for all groups and this experiment was repeated 3 times. ***P < 0.001; Student’s t test. See also Figure S1 and Table S1.
After incubation of the microarray with His-Pla and subsequent detection with fluorescently conjugated antibodies, we found that 4.8% (170 of 3,525) of the peptides were cleaved by Pla (Table S1). This approach for substrate discovery was internally validated by the cleavage of peptides representing the previously identified Pla substrates plasminogen and α2-antiplasmin. In addition, we observed a reduction in fluorescence from spots with sequences corresponding to amino acids 126–133 of the host apoptotic signaling protein FasL, suggesting that this molecule may represent a previously unknown Pla substrate.
Pla is Necessary and Sufficient to Degrade Fas Ligand
To validate FasL as a substrate of Pla under native conditions (i.e. in the presence of whole bacteria), we incubated purified human FasL, consisting of the extracellular domain of the protein, with either Y. pestis or an isogenic mutant of Y. pestis lacking Pla (Y. pestis Δpla). We observed a time-dependent loss of detectable FasL by immunoblot when treated with Y. pestis, but not the Δpla mutant, confirming that the degradation of FasL is Pla-dependent (Figure 1B). To show the specificity of processing by Pla, we confirmed that Y. pestis is also able to cleave plasminogen, but not TNFα or GPIb-α (Figure S1C). In addition, the requirement for proteolytically active Pla to degrade FasL was demonstrated using a Pla active site point mutant, as well as the ability of Pla to degrade both the human and mouse variants of the protein (Figure S1D). To show sufficiency of Pla for FasL degradation, we used E. coli to artificially produce Pla, and found that this pla-expressing strain is also able to degrade FasL (Figure 1C).
The host matrix metalloprotease-7 (MMP-7) regulates FasL-dependent apoptotic and inflammatory signaling by cleaving FasL within its extracellular domain between amino acids K129 – Q130 to release a 25 kDa, soluble FasL (sFasL) fragment from cell surfaces with minimal apoptotic activity (Hohlbaum et al., 2000; Schneider et al., 1998; Tanaka et al., 1998). These amino acids are contained within the Pla-cleaved array peptide SLEKQIGH. While Pla also generates an initial 25 kDa fragment similar to that produced by MMP-7, we observed that Pla is able to further degrade this protein fragment beyond that produced by MMP-7 (Figure 1B). The absence of accumulating cleavage fragments and the loss of detectable FasL together suggest that Pla cleaves FasL at multiple sites within its extracellular domain that may fully inactivate FasL. Indeed, Y. pestis is also able to degrade sFasL generated directly by MMP-7 in a Pla-dependent manner (Figure 1D).
As FasL is a transmembrane protein localized to the surface of host cells, it is likely that Pla would encounter this form of FasL during infection following host cell contact by the bacteria. Therefore, we incubated Y. pestis with KFL-9 cells, a mammalian lymphoblast cell line that produces membrane-bound FasL, and examined the ability of Pla to degrade FasL associated with these cells. We observed a Pla-dependent reduction of FasL associated with whole cells, indicating that Pla is able to degrade the membrane-bound variant of this protein in its native context (Figure 1E). Since these cells also naturally release sFasL into the culture medium, we determined that Pla is able to degrade sFasL shed from host cell surfaces as an alternative form of FasL that could be encountered by Y. pestis within the host (Figure 1F).
Degradation of FasL by Pla Prevents Induction of Apoptosis
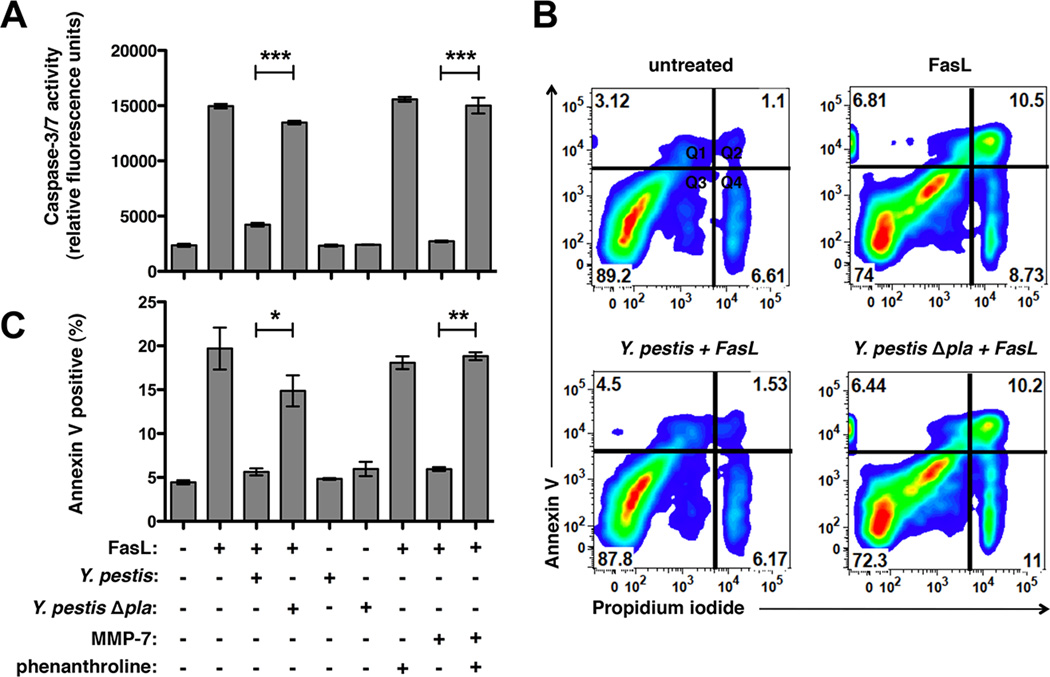
A major consequence of the engagement of the Fas receptor by FasL is the activation of caspase-3/7 as part of the extrinsic pathway of apoptosis, resulting in death of the cell (Peter et al., 2007). Therefore, to assess the functional impact of the Pla-FasL interaction, we asked if Y. pestis-treated FasL is no longer able to activate caspase-3/7 and induce apoptosis in eukaryotic cells. FasL was untreated or incubated with either Y. pestis or purified MMP-7, bacteria were removed by centrifugation, and any remaining FasL was added to Jurkat cells, a human leukemic T cell line widely used for the study of Fas signaling, or A549 cells, a human type II pneumocyte cell line similar to cells that are encountered by Y. pestis during pneumonic plague. We found that, following treatment with either wild-type Y. pestis or MMP-7, FasL is no longer able to induce caspase-3/7 activation in either cell type (Figures 2A and S2). Conversely, FasL retains its ability to activate caspase-3/7 under conditions where it remains intact, including treatments with Y. pestis Δpla or MMP-7 in the presence of the metalloprotease inhibitor phenanthroline.
Figure 2. Pla abrogates FasL-induced caspase-3/7 activation and cellular apoptosis.
(A) FasL was pretreated with Y. pestis or MMP-7 before addition to Jurkat cells. Caspase-3/7 activation was measured based on binding of the fluorescent substrate Ac-DEVD-AMC. Phenanthroline was used as an MMP-7 inhibitor. (B) Jurkat cells were incubated with FasL and stained with the apoptosis marker Annexin V and propidium iodide (PI) as a viability dye. Representative plots are shown. Percentage of total cells is indicated in each quadrant as viable (Q3; Annexin V-, PI-), early apoptotic (Q1; Annexin V+, PI-), late-stage apoptotic (Q2; Annexin V+, PI+), or necrotic (Q4; Annexin V-, PI+). (C) Percentage of apoptotic Annexin V+ cells (Q1 + Q2) for each treatment. (A-C) n = 3 for all groups and these experiments were repeated 3 times. *P < 0.05, **P < 0.01, ***P < 0.001; Student’s t test. See also Figure S2.
To test if the inability of Pla-treated FasL to activate caspase-3/7 corresponds with decreased cellular apoptosis, we incubated Y. pestis- or MMP-7-treated FasL with Jurkat cells as above and then assessed changes in Annexin V staining, a marker of host cell apoptosis, by flow cytometry. The processing of FasL by either Pla or MMP-7 abrogates the induction of FasL-mediated apoptosis, consistent with the lack of caspase-3/7 activation (Figures 2B and 2C). When incubated with MMP-7 in the presence of phenanthroline or with Y. pestis Δpla, however, FasL retains the ability to induce apoptosis. In total, these data indicate that Y. pestis, through the activity of Pla, degrades the extracellular domain of FasL, which prevents the downstream activation of caspase-3/7 and the subsequent induction of Fas-mediated apoptotic cell death.
Pla Alters FasL Abundance in the Lungs During Pneumonic Plague
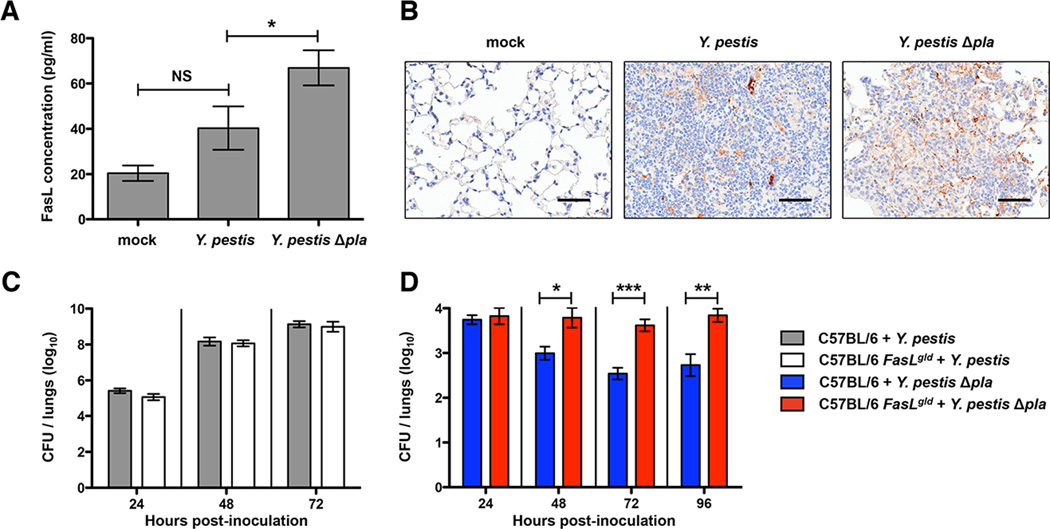
While it is known that Pla is required for the progression of pneumonic plague, it is not yet clear which substrates of this protease are relevant in vivo. As our data suggest that Pla may degrade FasL during infection, we assessed the abundance and distribution of FasL in the lungs of mice infected with Y. pestis. Since the Y. pestis Δpla strain is attenuated during respiratory infections, to eliminate differences in CFU between the wild-type and Δpla mutant we adjusted the input inoculum of the Δpla strain given to mice. Because pulmonary infection with Y. pestis Δpla leads to relatively steady-state CFU levels in the lungs regardless of bacterial dose given (Figure S3A), C57BL/6 mice were infected via the intranasal route with either 104 CFU wild-type or 108 CFU Δpla Y. pestis, doses that result in relatively equivalent bacterial loads after 48 h. At this time point, mice were sacrificed, the lungs were perfused, removed and homogenized for the quantification of FasL abundance by ELISA. Although FasL is an acute-phase responsive protein and is induced during infection (Kitamura et al., 2001; Neff et al., 2005), we measured significantly reduced FasL levels in the lungs of mice infected with wild-type Y. pestis compared to Y. pestis Δpla (Figure 3A). We also assessed the distribution of FasL at the local level by immunohistochemistry (IHC) and observed reduced staining of FasL within the inflammatory lesions of the lungs (where bacteria are found) during the wild-type infection as compared to Δpla (Figure 3B). These results are not due to differences in FasL transcript (Figure S3B), thus in total these data demonstrate that Y. pestis alters FasL protein levels in the lungs, consistent with the hypothesis that Pla cleaves FasL during pneumonic plague.
Figure 3. Loss of Fas-FasL signaling leads to increased Y. pestis Δpla CFU in the lungs.
(A) Total FasL abundance in the lungs during pneumonic plague. At 48 h post-inoculation with PBS (mock), Y. pestis, or Y. pestis Δpla, FasL concentration was measured from perfused lung homogenates by ELISA. (B) Immunohistochemical staining of FasL in lung tissue. At 48 h post-inoculation, lung sections were stained with an anti-FasL antibody (brown). Hematoxylin was used as a counter-stain (blue). Representative images of inflammatory lesions are shown. Scale bars represent 50 µm. (C-D) Wild-type C57BL/6 and functionally FasL-deficient C57BL/6 FasLgld mice were inoculated intranasally with Y. pestis (C) or Y. pestis Δpla (D). At various time points post-inoculation, mice were sacrificed and kinetics of infection determined by enumerating CFU per whole lungs. n = 10 for all groups representing data combined from two independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and NS = not significant; one-way ANOVA (ELISA), Mann-Whitney U test (CFU). See also Figure S3.
Degradation of FasL by Pla Enables Optimal Outgrowth of Y. pestis in the Lungs
To test the influence of FasL-mediated signaling on the virulence of Y. pestis in the presence or absence of Pla, we utilized C57BL/6 FasLgld mice, which lack functional FasL and are unable to activate Fas (Takahashi et al., 1994). The absence of Fas-FasL signaling in FasLgld mice can be either protective or detrimental to the host during various bacterial infections (Grassme et al., 2000; Matute-Bello et al., 2005b). We hypothesized that if Pla contributes to the virulence of Y. pestis by inactivating FasL, then no difference in bacterial burden would be observed between wild-type and FasLgld mice when infected with fully virulent Y. pestis. As expected, there is equivalent bacterial outgrowth in the lungs since FasL inactivation by Pla in wild-type mice is mimicked in FasLgld mice (Figure 3C). However, upon infection with Y. pestis Δpla, the FasLgld mice would specifically compensate for the loss of Pla-mediated FasL degradation and is predicted to result in increased bacterial load compared to wild-type mice. Indeed, the number of bacteria in the lungs is significantly greater in FasLgld mice compared to wild-type mice when infected with the Δpla strain at 48 h and later (Figure 3D). On the other hand, infection of FasLgld mice with the attenuated pgm- strain of Y. pestis does not result in significant differences in CFU compared to wild-type mice, indicating that FasLgld mice are not generally immunodeficient towards Y. pestis (Figure S3C), and demonstrates the specific contribution of Pla-dependent FasL inactivation to virulence during pneumonic plague.
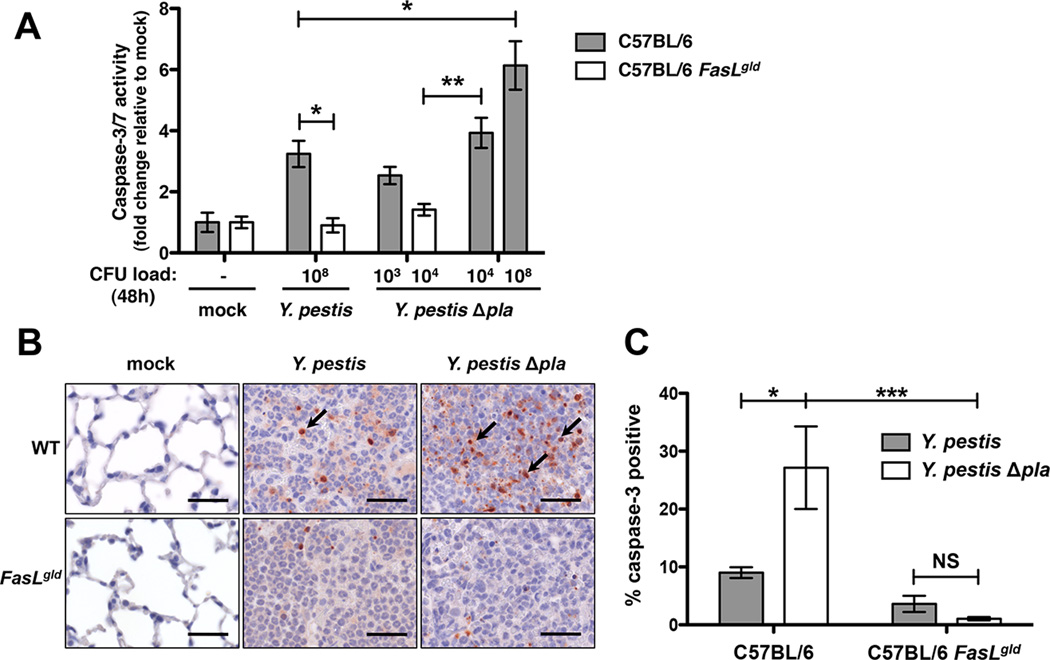
Pla Reduces FasL-Dependent Caspase-3/7 Activation in the Lungs
As our data indicate that Y. pestis degrades FasL, we hypothesized that Y. pestis may manipulate caspase-3/7 activation in the lungs during the pro-inflammatory phase of pneumonic plague. Wild type or FasLgld mice were mock-infected or infected with wild-type or Δpla Y. pestis and after 48 h the lungs were perfused, homogenized, and active caspase-3/7 levels were determined. We found that during the pro-inflammatory phase of pneumonic plague, caspase-3/7 activation relies on the presence of FasL and is reduced to mock-infected levels in FasLgld mice (Figure 4A). To examine caspase activation independent of bacterial burden, mice were given increased doses of Y. pestis Δpla to adjust output CFU in the lungs at 48 h. We observed significantly reduced capase-3/7 activation in the lungs of mice infected with wild-type Y. pestis as compared to CFU-normalized Y. pestis Δpla (108 CFU), indicating that the presence of Pla decreases caspase-3/7 activation by preventing the induction of Fas signaling (Figure 4A).
Figure 4. Caspase-3/7 activation in the lungs requires FasL signaling and is reduced by Pla.
(A) Caspase-3/7 activity from perfused lung homogenates of mice infected with Y. pestis or Y. pestis Δpla at standard or load-matched doses after 48 h. n = 5 for all groups and the experiment was repeated 3 times. “CFU load (48 h)” indicates the approximate numbers of bacteria in the lungs under each condition at the time of analysis. (B) Active caspase-3 IHC of lung tissue from mice infected with Y. pestis or Y. pestis Δpla for 48 h. Representative images of inflammatory lesions are shown with positively stained cells for active caspase-3 indicated with arrows. Scale bars represent 30 µm. (C) Quantification of active caspase-3 IHC as the percent of positively stained cells for each condition. n = 3 for all groups and the experiment was repeated 3 times. *P < 0.05, **P < 0.01, and NS = not significant; one-way ANOVA. See also Figure S4.
To determine the spatial induction of caspase-3 activation in the lungs during infection with Y. pestis, we examined active caspase-3 by IHC using fixed lung sections from mice infected for 48 h with Y. pestis or Y. pestis Δpla. Few caspase-3 positive cells were found outside of the inflammatory lesions in wild-type mice and in any pulmonary location in the FasLgld mice, confirming that caspase-3 activation is FasL-dependent and localized to the lesions where bacteria are present (Figure 4B). In addition, we observed that caspase-3 activation is significantly reduced in the presence of wild-type Y. pestis as compared to the Δpla mutant (Figure 4C). Thus, the requirement of apoptotic signaling to optimize innate host defenses during infection may explain why decreased caspase-3/7 activity in FasLgld mice correlates with increased Y. pestis Δpla CFU.
As previous studies have shown that the Yersinia T3SS effector YopJ enhances apoptosis under in vitro conditions (Matsumoto and Young, 2009; Yeretssian et al., 2008), to determine the relative impact of YopJ vs. Pla on apoptosis during pneumonic plague, we generated ΔyopJ and Δpla ΔyopJ mutants of Y. pestis and examined caspase-3/7 activation in the lungs of infected mice. We found that YopJ has no significant effect on caspase-3/7 activation during the pro-inflammatory phase of pneumonic plague, regardless of the presence or absence of Pla (Figure S4).
Pla Manipulates the Host Inflammatory Response Via FasL Degradation
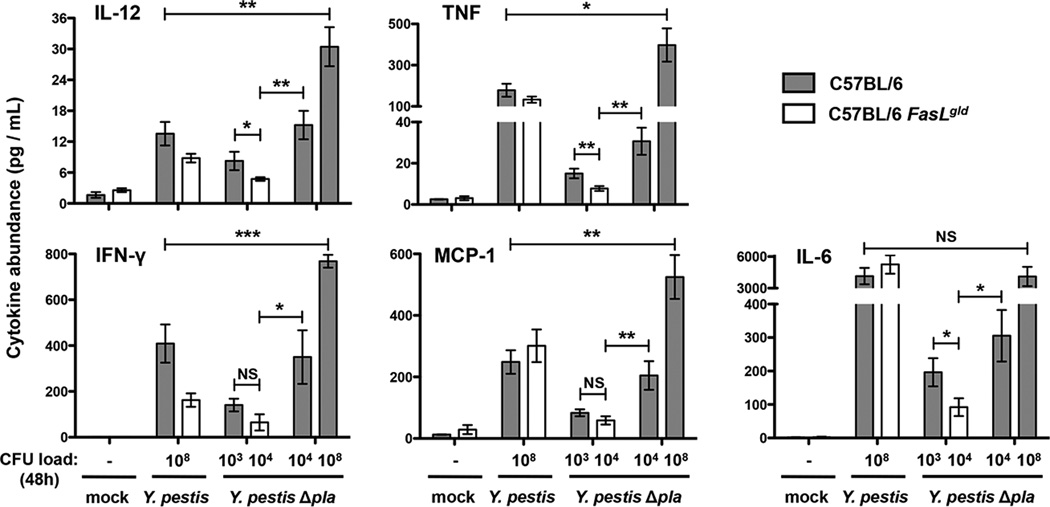
In addition to inducing cellular apoptosis, FasL signaling in the lungs has been shown to mediate immune cell recruitment and the secretion of cytokines (Hagimoto et al., 1999; Matute-Bello et al., 2005b; Matute-Bello et al., 2001; Neff et al., 2005; Park et al., 2003). Therefore, we hypothesized that inactivation of FasL by Pla may be used by Y. pestis to subvert these immune responses. To test this, we infected wild-type or FasLgld mice and after 48 h measured the abundance of inflammatory cytokines in the lungs. We found that the overall inflammatory response is dampened in FasLgld mice infected with Y. pestis Δpla, which produce lower levels of IL-12, TNF, IFN-γ, MCP-1, and IL-6 compared to wild-type mice (Figure 5), suggesting that the loss of FasL signaling redirects the host immune response during pneumonic plague. In addition, when the bacterial loads are normalized between the strains, we observe significantly lower cytokine levels in the lungs of mice infected with wild-type Y. pestis compared to the Δpla bacteria. This indicates that it is the presence of Pla that alters the host inflammatory response, in part by preventing the induction of Fas signaling, rather than simply due to differences in bacterial load.
Figure 5. Loss of FasL signaling leads to a dampened host cytokine response upon infection with Y. pestis Δpla.
At 48 h post-inoculation with the indicated bacterial strains, lungs were perfused and homogenized. Levels of IL-12p70, TNF, IFN-γ, MCP-1, and IL-6 were measured in lung homogenates by cytometric bead array. Cytokine levels are represented as fold change relative to mock infection values. n = 10 for all groups representing data combined from two independent experiments. “CFU load (48 h)” indicates the approximate numbers of bacteria in the lungs under each condition at the time of analysis. *P < 0.05, **P < 0.01, and ***P < 0.001; Student’s t test. See also Figure S5.
Although we observed little difference in overall lung pathology between wild-type and FasLgld mice when infected with Y. pestis or the Δpla mutant (Figure S5A), we performed flow cytometry to quantify the Pla-dependent recruitment of immune cells to the lungs during pneumonic plague. At 48 h post-infection with Y. pestis Δpla, we observed a lower percentage of monocytes within the lungs of FasLgld mice compared to wild-type mice (Figure S5B and S5C), consistent with the FasL-dependent production of MCP-1 (Figure 5). To test whether these FasL-dependent immune responses also affect lung injury, we measured alveolar permeability and found reduced albumin leakage in the broncho-alveolar lavage fluid of FasLgld mice compared to wild-type animals, consistent with reports of FasL-dependent lung injury in response to inflammatory stimuli (Figure S5D) (Matute-Bello et al., 2005a; Neff et al., 2005). These alterations of cytokines, immune cell populations, and tissue permeability reinforce the model that the Pla-dependent manipulation of FasL signaling disrupts the normal immune response, which correlates with increased bacterial outgrowth and tissue damage during primary pneumonic plague.
DEVDase Inhibition Recapitulates the Loss of FasL During Pneumonic Plague
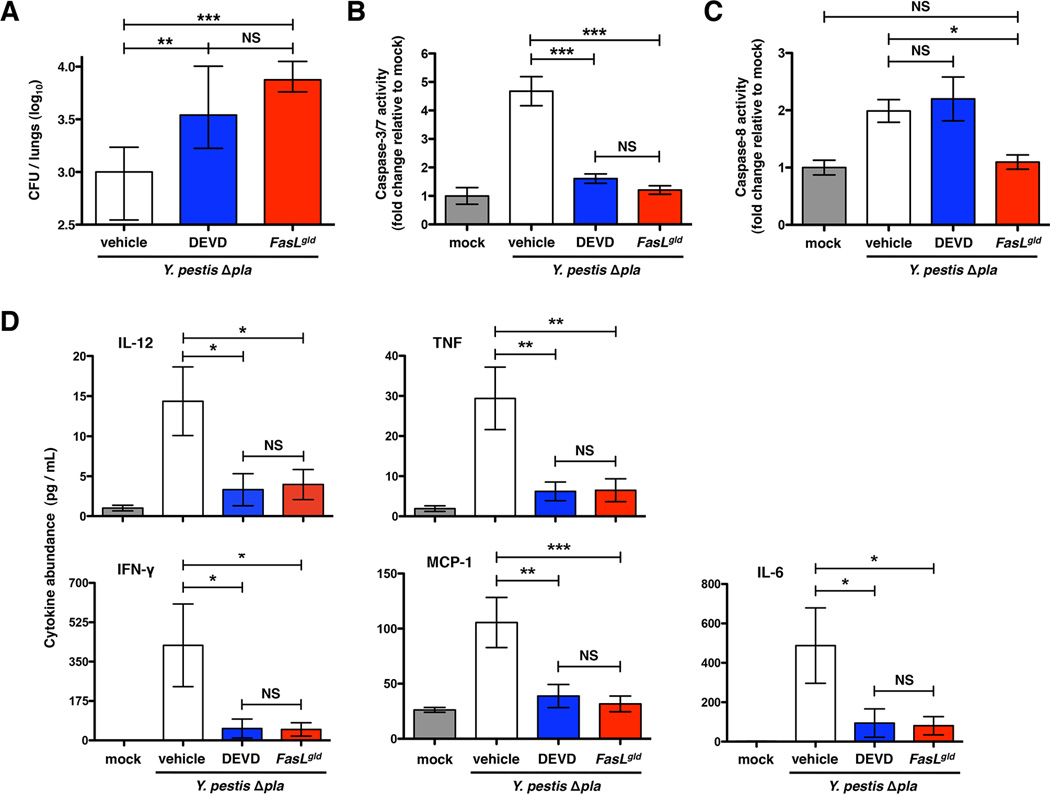
It is known that caspase-3/7-dependent apoptosis enhances host defenses and restricts the outgrowth of various bacterial pathogens during infection (Daigneault et al., 2012; Grassme et al., 2000; Jones et al., 2002). Therefore, to determine if the outgrowth and cytokine phenotypes of FasL inactivation by Pla are a result of the modulation of downstream DEVDase activity (which includes caspase-3/7), we treated mice with the inhibitor Ac-DEVD-CMK and assessed caspase activation, bacterial load, and cytokine levels in the lungs of infected mice (Ekert et al., 1999).
To confirm specificity of inhibition, we measured the activation of capases-3/7, -8, and -1 in the lungs of mice. Caspase-8 is activated through Fas signaling upon infection, yet only downstream caspase-3/7 is specifically inhibited by DEVD (Figure 6C). The pyroptosis factor caspase-1 is activated during infection in a FasL-independent manner and thus is not affected by DEVD treatment (Figure S6). We found that reduced DEVDase activity results in increased CFU in the lungs following infection with Y. pestis Δpla in a manner that recapitulates that observed with FasLgld mice (Figures 6A and 6B). Likewise, the inflammatory response to Y. pestis infection is dampened during DEVD treatment to similar levels as seen in FasLgld mice (Figure 6D). Therefore, the alteration of DEVDase-dependent factors, likely caspase-3, is a major consequence of FasL inactivation by Pla.
Figure 6. Inhibition of DEVDase activity enhances bacterial outgrowth and reduces cytokine production.
(A-D) Mice were infected with Y. pestis Δpla and treated with either the caspase inhibitor DEVD or vehicle alone via the intraperitoneal route. Bacterial burden (A), caspase-3/7 activity (B), caspase-8 activity (C), and cytokine levels (D) were determined in the lungs at 48 h post-inoculation as described above. For analysis of bacterial burden measurements, n = 10 for all groups representing data combined from two independent experiments. For caspase activity and cytokine abundance measurements, n = 9 per group representing data combined from three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001, and NS = not significant; one-way ANOVA. See also Figure S6.
DISCUSSION
Y. pestis employs multiple strategies to dampen early immune responses during pneumonic plague. For example, several T3SS Yop effectors restrict the ability of epithelial cells to upregulate proinflammatory cytokines and chemokines (Price et al., 2012), resulting in a delay in the induction of pulmonary inflammation compared to that caused by other Gram-negative bacteria (Li and Yang, 2008). During the first 36 h of the respiratory infection, the lungs are relatively non-inflammatory despite rapid bacterial proliferation (Lathem et al., 2005). By 48 h, however, the pulmonary environment becomes highly pro-inflammatory as the host responds to bacterial loads approaching 108 CFU. This biphasic disease supports two distinct pathogenic strategies: in the early non-inflammatory phase, Y. pestis avoids detection by innate immunity to allow for initial bacterial replication and to establish infection in a new host environment. As bacteria continue to replicate, detection by the host becomes inevitable and a pro-inflammatory phase commences. Therefore, the bacterial virulence strategy shifts from evading to overwhelming innate immunity. Within this pro-inflammatory phase, pla expression increases (Lathem et al., 2014) and represents an additional method for Y. pestis to modulate host immune responses.
Caspase-3/7 activation is known to mediate host inflammation in the lungs during bacterial pneumonias (Hagimoto et al., 1999; Park et al., 2003), however our data show that Y. pestis modulates these caspase-3/7-mediated immune responses via the Pla protease (Figure 7). Specifically, through the degradation of both FasL and sFasL, Pla inactivates FasL-dependent signaling, and the Pla-dependent reduction in FasL abundance and caspase-3 activation during infection is consistent with FasL degradation by Pla in vivo. Cell death via apoptosis modulates inflammatory cytokine production during infection and is required for the host to initiate a fully effective innate immune response (Labbe and Saleh, 2008; Matute-Bello et al., 2005a), although this is likely to occur indirectly, downstream of FasL-mediated cell death. We found that during pneumonic plague, the production of several inflammatory cytokines is FasL-dependent and is affected by the presence of Pla. This cytokine response presumably aids in the recruitment and/or activation of immune cells as part of the host defense against infection to control bacterial outgrowth, which Pla manipulates via the degradation of FasL (Figure 7).
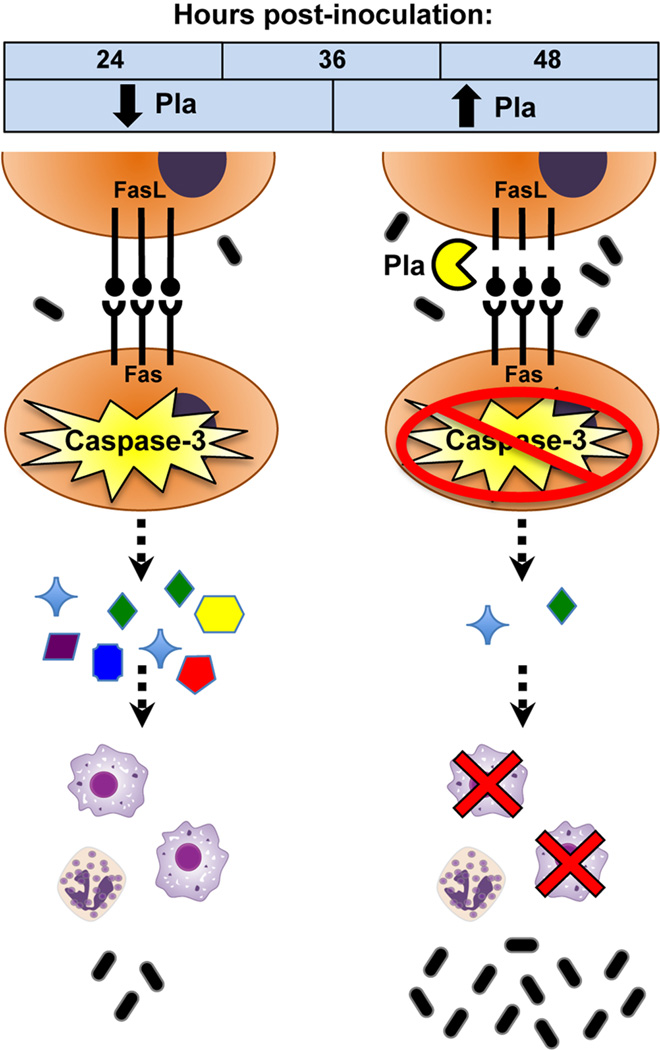
Figure 7. Model of FasL inactivation by Pla during pneumonic plague.
Upon pneumonic infection with Y. pestis, Fas-FasL signaling is induced to activate caspase-3 in the lungs. Caspase-3 activation is required for the host to elicit an optimal inflammatory cytokine response as part of host innate defenses. As part of its pathogenic strategy, Y. pestis utilizes Pla, which is upregulated during later stages of disease to degrade FasL and prevent caspase-3 activation. Downstream immune responses are altered, including cytokine secretion and macrophage recruitment, resulting in the enhanced virulence and outgrowth within the lungs of this bacterial pathogen.
Consistent with our results using the caspase inhibitor DEVD, prior studies have found increased bacterial burden in the lungs during Streptococcus pneumoniae infection following treatment with the pancaspase inhibitor zVADfmk (Ali et al., 2003; Dockrell et al., 2003). Specifically, monocytes may play a role in host defense here since their recruitment is partially FasL-dependent. As seen by histology and flow cytometry in this and prior work (Lathem et al., 2007; Pechous et al., 2013), neutrophils are the most abundant cell type recruited to the lungs in a Pla-dependent manner. Neutrophils accumulate around foci of bacterial replication to form inflammatory lesions in the lungs and are at least one cell type in which the activation of caspase-3 is limited. It is not yet known, however, what other cell types may be affected by the degradation of FasL or if other mechanisms of cell death, such as pyroptosis or programmed necrosis, are induced due to FasL degradation during infection.
Our findings demonstrate unappreciated protective roles for FasL and caspase-3/7 signaling in the induction of host defenses during Y. pestis infection of the lungs. The degradation of FasL by Pla actively modulates these host defenses as part of the overall approach to virulence of Y. pestis to alter host immune responses that allow for the increased fitness of this pathogen during respiratory infection. The inactivation of the extrinisic apoptosis signaling pathway by Pla provides a mechanism by which Y. pestis may redirect the biphasic inflammatory response to reduce apoptosis, potentially allowing for pyroptosis or programmed necrosis to predominate during the later stages of pneumonic plague. In addition, by reducing apoptosis during infection, Pla may prevent the control and resolution of inflammation. Indeed, it is becoming clear that the manipulation of cell death is a major strategy by which by bacterial pathogens enhance virulence, although the specific mechanisms by which this occurs appear to be different from species to species. For instance, it was r ecently reported that the enteropathogenic E. coli T3SS effector NleB disrupts FADD-mediated apoptosis downstream of the engagement of Fas by FasL to counteract host defenses and enhance colonization (Li et al., 2013; Pearson et al., 2013).
Given the host benefit of FasL signaling during pneumonia, the use of Pla inhibitors, exogenous FasL, or agonistic anti-Fas antibodies may serve as possible therapeutics (Linkermann et al., 2005). The related pro-apoptotic ligand TRAIL has been shown to be a therapeutic during pneumococcal pneumonia, and TRAIL-deficient mice display increased bacterial burden in the lungs, reduced apoptosis, and reduced caspase-3/7 activation (Steinwede et al., 2012). Thus, a greater understanding of how apoptotic host cell death pathways initiate innate immune responses in the lungs will aid the study of other bacterial pneumonias. This work describes a mechanism by which highly virulent pathogens such as Y. pestis control host cell death pathways during pneumonia by manipulating the activation of Fas signaling through the degradation of FasL, and in part explains how Pla contributes to the development of primary pneumonic plague.
EXPERIMENTAL PR OCEDURES
Reagents, bacterial strains, and growth conditions
Reagents were obtained from Sigma or VWR, unless otherwise indicated. Bacterial strains and plasmids used in this study are listed in Table S2. All experiments using select agent strains of Y. pestis were conducted in an approved BSL-3/ABSL-3 facility at Northwestern University; experiments described in Figures 1–2 and corresponding supplementary figures used the avirulent, pCD1- variant of Y. pestis CO92. Y. pestis strains were cultured in liquid brain heart infusion (BHI) medium at 37°C with 2.5 mM CaCl2 when appropriate. E. coli strains were cultured in Luria-Bertani (LB) medium supplemented with ampicillin (100 µg/mL). Plasmids pSE380 and pMRK1 were introduced into E. coli strain BL21. IPTG (100 µM) was added to induce pla expression from pMRK1 (Kukkonen et al., 2001).
Purification and activation of His-Pla
The gene encoding the mature form of Pla was cloned into the pQE30 expression vector (Qiagen) to generate plasmid pWL223 and His-tagged Pla protein was purified from E. coli BL21 (pREP4) under denaturing conditions according to the QIAexpressionist kit protocol (Qiagen). Following refolding by dialysis in the presence of the detergent N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate, Pla was activated by agitation with E. coli K12 LPS as previously described (Kukkonen et al., 2001).
Peptide microarray
The protease profiler peptide microarray (JPT Peptide Technologies) contains 1,989 8-meric peptides derived from annotated protease cleavage sites and 1,536 randomly generated 15-meric peptides. Peptide substrates were spotted in triplicate on each microarray (10,575 total data points) and distally tagged with a phospho-tyrosine moiety. To cleave peptides, a microarray slide was incubated with purified and activated His-Pla (8 µg) for 5 h at 37°C. A control slide was incubated with an equal amount of BSA-LPS mixture. Fluorescence signal was detected by incubation with a mouse anti-phospho-tyrosine antibody (Cell Signaling) followed by a fluorescently conjugated secondary antibody (goat anti-mouse DyLight 649; Pierce). Relative peptide cleavage was determined by calculating fluorescence ratios between His-Pla and BSA treated slides. A 2-fold signal reduction threshold was used to identify peptides as candidate Pla substrates.
Cell culture
Jurkat cells and KFL-9 cells (undifferentiated lymphoblast cell line that constitutively expresses human FasL) were grown in RPMI 1640 medium (Cellgro; Mediatech) supplemented with penicillin-streptomycin (100 µg/mL) and 10% heat-inactivated fetal bovine serum (HyClone; Thermo). A549 cells were grown in minimal essential medium (MEM) supplemented with antibiotics and 10% serum. Cells were grown at 37°C in a 5% CO2 environment.
Immunoblot analyses
Human recombinant FasL consisting of the extracellular portion of FasL (aa 103–281) with N-terminal FLAG-tag (Enzo Life Sciences) was used for in vitro proteolysis assays. FasL protein (0.25 µg) was incubated at 37°C with either Y. pestis (4×107 CFU) or E. coli BL21 (8×107 CFU) in 20 mM HEPES buffer, pH 7. At various times, bacteria were removed by centrifugation and supernatants and bacterial lysates separated by SDS-PAGE and analyzed for FasL content by immunoblot using the anti-FasL antibody N2C1 (GeneTex). To assess the degradation of sFasL by Pla, MMP-7 (0.5 µg; Genway Biotech) was first used to specifically process FasL (0.15 µg) into sFasL by incubating for 1 h at 37°C. MMP-7 was then inhibited by the addition of 1,10-phenanthroline (1 mM), followed by a second 1 h incubation with Y. pestis (6×107 CFU). To detect processing of FasL from cell surfaces, KFL-9 cells were incubated with Y. pestis at an MOI of 50, followed by lysis with RIPA buffer. Cell lysates (50 µg) were analyzed by immunoblot using anti-FasL (BD Biosciences, clone G247-4) and anti-GAPDH (Sigma, #G9545) antibodies. Mouse FasL, GPIb-α (both 0.15 µg; R&D Systems), and TNFα (0.15 µg; Invitrogen) were incubated with Y. pestis (4×107 CFU) for 1 h and degradation assessed with anti-HA (Roche, clone 3F10) or anti-TNFα (BioLegend, clone MAb1) antibodies. An anti-Pla antibody was used as previously described (Houppert et al., 2013). All immunoblot experiments were performed a minimum of 2 times.
FasL quantification by ELISA
To quantify soluble FasL (sFasL), KFL-9 cells were cultured overnight in serum-free RPMI to a final density of 1.4×105 cells/mL. The media supernatant containing sFasL released by endogenous proteases was collected and concentrated approximately 30-fold using 10 kDa MWCO Centriprep YM-10 filters (Millipore). This concentrated sFasL (50 µL) was incubated with Y. pestis or E. coli (8×108 CFU) for 3 h at 37°C in 20 mM HEPES, pH 7. The supernatant was then used to perform an ELISA specific for human sFasL (eBioscience). To assess FasL levels in vivo, lungs were perfused, removed, and homogenized in PBS. FasL in lung homogenates was assessed using the FasL Quantikine ELISA (R&D Systems). Samples were analyzed as duplicates from 5 mice per treatment and experiments were performed at least twice.
Caspase activity assays
FasL was treated with Y. pestis or MMP-7 as described above. Treated FLAG-FasL (2 µg/mL) was incubated with monoclonal anti-FLAG M2 antibody (1.4 µg; Sigma) for 15 min to allow for FasL trimerization before incubation with Jurkat (2×105) or A549 (2.5×105) cells, in triplicate, at 37°C for 4 h. MMP-7 activity was inhibited with phenanthroline as indicated. Cells were assessed using the caspase-3/7 activity assay kit (Sigma). To assess caspase-3/7, caspase-1, and caspase-8 activities in vivo, lungs were perfused and whole lung homogenates collected at 48 h post-infection with Y. pestis. Cells were washed with PBS, fixed, and analyzed with the corresponding FLICA caspase detection kit (ImmunoChemistry Technologies). Samples were analyzed as duplicates from 5 mice per treatment and experiments were performed at least twice.
Annexin V staining
FasL was treated with Y. pestis or MMP-7 and incubated with anti-FLAG M2 antibody as described above. MMP-7 activity was inhibited with phenanthroline as indicated. Treated FasL (0.5 µg) was incubated with Jurkat (1×105) cells, in duplicate, at 37°C for 16 h. Cells were washed with PBS and stained with FITC-Annexin V and propidium iodide following the manufacturer’s instuctions (eBioscience). Samples were analyzed using a BD FACSCanto II flow cytometer.
Animal infections
All procedures involving animals were carried out in compliance with protocols approved by the Northwestern University institutional animal care and use committee. Pathogen-free C57BL/6 and C57BL/6 FasLgld mice were obtained from the Jackson Laboratory or bred at Northwestern University and were infected with strains of Y. pestis as previously described (Lathem et al., 2005). Mice (6–8 weeks old) were anesthetized with ketamine and xylazine and inoculated by the intranasal route with Y. pestis or Y. pestis Δpla. A standard dose of approximately 1×104 CFU was administered except when matching for bacterial load in the lungs at 48 h, in which case mice were given increased doses of Y. pestis Δpla. Infection of wild-type mice with 105 CFU of Y. pestis Δpla approximates FasLgld mice given 104 CFU at 48 h. Likewise, infection of mice with 108 CFU of Y. pestis Δpla yields approximately the same bacterial load after 48 h as inoculating with 104 CFU of fully virulent Y. pestis. Animals were sacrificed at various times post-infection, lungs homogenized in sterile PBS, and homogenates serially diluted onto BHI agar for CFU enumeration. All animal infections were repeated at least twice and the data combined. For caspase-3/7 inhibition studies, 200 µg of the caspase inhibitor Ac-DEVD-CMK (American Peptide Company) or vehicle control was injected via the intraperitoneal route at 0 and 24 h post-inoculation.
Cytokine analysis
At 48 h post-inoculation with mock PBS, Y. pestis, or Y. pestis Δpla, levels of IL-12p70, TNF, IFN-γ, MCP-1, IL-10, and IL-6 were quantitatively established from perfused lung tissue homogenates using the cytometric bead array technique (BD Cytometric Bead Array Mouse Inflammation Kit, BD Biosciences) as specified by the manufacturer. Prior to analysis, homogenates were centrifuged at 16,000×g for one min to pellet tissue debris, and supernatants were passed through a 0.22-µm filter for sterilization. Data were analyzed using BD Cytometric Bead Array Software.
Histopathology and immunohistochemistry
At 48 h post-inoculation with PBS, Y. pestis, or Y. pestis Δpla, mice were sacrificed and lungs inflated with 10% neutral-buffered formalin via cannulation of the trachea. Lungs were removed and fixed in 10% formalin overnight before being embedded in paraffin for sectioning and staining. FasL (Enzo Life Sciences, clone A11) and active caspase-3 (Cell Signaling, #9661) antibodies were used for IHC. Caspase-3 activation was enumerated by counting the fraction of positive cells in 4 biological replicate fields per set of conditions. In all cases, 3 mice were used within each experimental group.
Statistical Analysis
All experiments were performed at least twice as indicated in figure legends, and no data were excluded from analyses. In all cases, statistical means are graphed and error bars represent SEM. Sample sizes (n) given represent biological replicates in independent experiments unless stated otherwise. Statistical significance was calculated by performing a two-tailed Mann-Whitney U test for bacterial burden measurements, a one-way ANOVA with Dunnett test for multiple comparisons, and Student’s two-tailed unpaired t-test for other analyses. P values of ≤ 0.05 are considered significant. *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
Supplementary Material
HIGHLIGHTS.
FasL is a host substrate of the Pla protease of Y. pestis
FasL degradation prevents pulmonary caspase-3/7 activation and host cell apoptosis
Y. pestis manipulates host immune responses during pneumonic plague via FasL degradation
FasL degradation by Pla enables full virulence of Y. pestis in the lungs
ACKNOWLEDGEMENTS
We thank Joseph Connor for providing the KFL-9 cell line, Nicholas Cianciotto for the A549 cell line, and Chyung-Ru Wang for the Jurkat cell line. We also thank Timo Korhonen for the inducible pla plasmids, Roger Pechous for technical assistance, and Marcus Peter, Chyung-Ru Wang, and Peter Sporn for helpful discussions. Technical assistance was provided by the Northwestern Univ. Interdepartmental Immunobiology Center, the Northwestern Univ. Mouse Histology and Phenotyping Laboratory, and the Northwestern Univ. Cell Imaging Facility (NCI CCSG P30 CA060553 awarded to the Robert H. Lurie Comprehensive Cancer Center). This work was supported by National Institute of Health grants T32 AI007476 to AJC and R01 AI093727 to WWL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ali F, Lee ME, Iannelli F, Pozzi G, Mitchell TJ, Read RC, Dockrell DH. Streptococcus pneumoniae-associated human macrophage apoptosis after bacterial internalization via complement and Fcγ receptors correlates with intracellular bacterial load. J. Infect. Dis. 2003;188:1119–1131. doi: 10.1086/378675. [DOI] [PubMed] [Google Scholar]
- Bergsbaken T, Cookson BT. Macrophage activation redirects Yersinia-infected host cell death from apoptosis to caspase-1-dependent pyroptosis. PLoS Pathog. 2007;3:e161. doi: 10.1371/journal.ppat.0030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield AJ, Lathem WW. Substrates of the plasminogen activator protease of Yersinia pestis . Adv Exp Med Biol. 2012;954:253–260. doi: 10.1007/978-1-4614-3561-7_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daigneault M, De Silva TI, Bewley MA, Preston JA, Marriott HM, Mitchell AM, Mitchell TJ, Read RC, Whyte MK, Dockrell DH. Monocytes regulate the mechanism of T-cell death by inducing Fas-mediated apoptosis during bacterial infection. PLoS Pathog. 2012;8:e1002814. doi: 10.1371/journal.ppat.1002814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degen JL, Bugge TH, Goguen JD. Fibrin and fibrinolysis in infection and host defense. J Thromb Haemost. 2007;5(Suppl 1):24–31. doi: 10.1111/j.1538-7836.2007.02519.x. [DOI] [PubMed] [Google Scholar]
- Dockrell DH, Marriott HM, Prince LR, Ridger VC, Ince PG, Hellewell PG, Whyte MK. Alveolar macrophage apoptosis contributes to pneumococcal clearance in a resolving model of pulmonary infection. J. Immunol. 2003;171:5380–5388. doi: 10.4049/jimmunol.171.10.5380. [DOI] [PubMed] [Google Scholar]
- Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–1086. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- Grassme H, Kirschnek S, Riethmueller J, Riehle A, von Kurthy G, Lang F, Weller M, Gulbins E. CD95/CD95 ligand interactions on epithelial cells in host defense to Pseudomonas aeruginosa . Science. 2000;290:527–530. doi: 10.1126/science.290.5491.527. [DOI] [PubMed] [Google Scholar]
- Hagimoto N, Kuwano K, Kawasaki M, Yoshimi M, Kaneko Y, Kunitake R, Maeyama T, Tanaka T, Hara N. Induction of interleukin-8 secretion and apoptosis in bronchiolar epithelial cells by Fas ligation. Am J Respir Cell Mol Biol. 1999;21:436–445. doi: 10.1165/ajrcmb.21.3.3397. [DOI] [PubMed] [Google Scholar]
- Hamann KJ, Dorscheid DR, Ko FD, Conforti AE, Sperling AI, Rabe KF, White SR. Expression of Fas (CD95) and FasL (CD95L) in human airway epithelium. Am J Respir Cell Mol Biol. 1998;19:537–542. doi: 10.1165/ajrcmb.19.4.3100. [DOI] [PubMed] [Google Scholar]
- Hohlbaum AM, Moe S, Marshak-Rothstein A. Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. J Exp Med. 2000;191:1209–1220. doi: 10.1084/jem.191.7.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houppert AS, Bohman L, Merritt PM, Cole CB, Caulfield AJ, Lathem WW, Marketon MM. RfaL is required for Yersinia pestis type III secretion and virulence. Infect Immun. 2013;81:1186–1197. doi: 10.1128/IAI.01417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones NL, Day AS, Jennings H, Shannon PT, Galindo-Mata E, Sherman PM. Enhanced disease severity in Helicobacter pylori-infected mice deficient in Fas signaling. Infect Immun. 2002;70:2591–2597. doi: 10.1128/IAI.70.5.2591-2597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura Y, Hashimoto S, Mizuta N, Kobayashi A, Kooguchi K, Fujiwara I, Nakajima H. Fas/FasL-dependent apoptosis of alveolar cells after lipopolysaccharide-induced lung injury in mice. Am J Respir Crit Care Med. 2001;163:762–769. doi: 10.1164/ajrccm.163.3.2003065. [DOI] [PubMed] [Google Scholar]
- Kukkonen M, Lahteenmaki K, Suomalainen M, Kalkkinen N, Emody L, Lang H, Korhonen TK. Protein regions important for plasminogen activation and inactivation of α2-antiplasmin in the surface protease Pla of Yersinia pestis . Mol Microbiol. 2001;40:1097–1111. doi: 10.1046/j.1365-2958.2001.02451.x. [DOI] [PubMed] [Google Scholar]
- Labbe K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15:1339–1349. doi: 10.1038/cdd.2008.91. [DOI] [PubMed] [Google Scholar]
- LaRock CN, Cookson BT. The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe. 2012;12:799–805. doi: 10.1016/j.chom.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem WW, Crosby SD, Miller VL, Goldman WE. Progression of primary pneumonic plague: a mouse model of infection, pathology, and bacterial transcriptional activity. Proc Natl Acad Sci USA. 2005;102:17786–17791. doi: 10.1073/pnas.0506840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathem WW, Price PA, Miller VL, Goldman WE. A plasminogen-activating protease specifically controls the development of primary pneumonic plague. Science. 2007;315:509–513. doi: 10.1126/science.1137195. [DOI] [PubMed] [Google Scholar]
- Lathem WW, Schroeder JA, Bellows LE, Ritzert JT, Koo JT, Price PA, Caulfield AJ, Goldman WE. Post-transcriptional regulation of the Yersinia pestis cAMP receptor protein Crp and impact on virulence. mBio. 2014 doi: 10.1128/mBio.01038-13. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Yang R. Interaction between Yersinia pestis and the host immune system. Infect Immun. 2008;76:1804–1811. doi: 10.1128/IAI.01517-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Zhang L, Yao Q, Li L, Dong N, Rong J, Gao W, Ding X, Sun L, Chen X, et al. Pathogen blocks host death receptor signalling by arginine GlcNAcylation of death domains. Nature. 2013;501:242–246. doi: 10.1038/nature12436. [DOI] [PubMed] [Google Scholar]
- Linkermann A, Qian J, Lettau M, Kabelitz D, Janssen O. Considering Fas ligand as a target for therapy. Expert Opin Ther Targets. 2005;9:119–134. doi: 10.1517/14728222.9.1.119. [DOI] [PubMed] [Google Scholar]
- Matsumoto H, Young GM. Translocated effectors of Yersinia . Curr Opin Microbiol. 2009;12:94–100. doi: 10.1016/j.mib.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute-Bello G, Lee JS, Liles WC, Frevert CW, Mongovin S, Wong V, Ballman K, Sutlief S, Martin TR. Fas-mediated acute lung injury requires fas expression on nonmyeloid cells of the lung. J Immunol. 2005a;175:4069–4075. doi: 10.4049/jimmunol.175.6.4069. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Liles WC, Frevert CW, Dhanireddy S, Ballman K, Wong V, Green RR, Song HY, Witcher DR, Jakubowski JA, et al. Blockade of the Fas/FasL system improves pneumococcal clearance from the lungs without preventing dissemination of bacteria to the spleen. J Infect Dis. 2005b;191:596–606. doi: 10.1086/427261. [DOI] [PubMed] [Google Scholar]
- Matute-Bello G, Winn RK, Jonas M, Chi EY, Martin TR, Liles WC. Fas (CD95) induces alveolar epithelial cell apoptosis in vivo: implications for acute pulmonary inflammation. Am J Pathol. 2001;158:153–161. doi: 10.1016/S0002-9440(10)63953-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizgerd JP. Lung infection--a public health priority. PLoS Med. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, Conlon JE, Fukase K, Kusumoto S, Sweet C, Miyake K, et al. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol. 2006;7:1066–1073. doi: 10.1038/ni1386. [DOI] [PubMed] [Google Scholar]
- Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- Neff TA, Guo RF, Neff SB, Sarma JV, Speyer CL, Gao H, Bernacki KD, Huber-Lang M, McGuire S, Hoesel LM, et al. Relationship of acute lung inflammatory injury to Fas/FasL system. Am J Pathol. 2005;166:685–694. doi: 10.1016/S0002-9440(10)62290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DR, Thomsen AR, Frevert CW, Pham U, Skerrett SJ, Kiener PA, Liles WC. Fas (CD95) induces proinflammatory cytokine responses by human monocytes and monocyte-derived macrophages. J Immunol. 2003;170:6209–6216. doi: 10.4049/jimmunol.170.12.6209. [DOI] [PubMed] [Google Scholar]
- Pearson JS, Giogha C, Ong SY, Kennedy CL, Kelly M, Robinson KS, Lung TW, Mansell A, Riedmaier P, Oates CV, et al. A type III effector antagonizes death receptor signalling during bacterial gut infection. Nature. 2013;501:247–251. doi: 10.1038/nature12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechous RD, Sivaraman V, Price PA, Stasulli NM, Goldman WE. Early host cell targets of Yersinia pestis during primary pneumonic plague. PLoS Pathog. 2013;9:e1003679. doi: 10.1371/journal.ppat.1003679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RD, Fetherston JD. Yersinia pestis--etiologic agent of plague. Clin Microbiol Rev. 1997;10:35–66. doi: 10.1128/cmr.10.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter ME, Budd RC, Desbarats J, Hedrick SM, Hueber AO, Newell MK, Owen LB, Pope RM, Tschopp J, Wajant H, et al. The CD95 receptor: apoptosis revisited. Cell. 2007;129:447–450. doi: 10.1016/j.cell.2007.04.031. [DOI] [PubMed] [Google Scholar]
- Peters KN, Dhariwala MO, Hughes Hanks JM, Brown CR, Anderson DM. Early apoptosis of macrophages modulated by injection of Yersinia pestis YopK promotes progression of primary pneumonic plague. PLoS Pathog. 2013;9:e1003324. doi: 10.1371/journal.ppat.1003324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price PA, Jin J, Goldman WE. Pulmonary infection by Yersinia pestis rapidly establishes a permissive environment for microbial proliferation. Proc Natl Acad Sci USA. 2012;109:3083–3088. doi: 10.1073/pnas.1112729109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J. Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med. 1998;187:1205–1213. doi: 10.1084/jem.187.8.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, Goguen JD. A surface protease and the invasive character of plague. Science. 1992;258:1004–1007. doi: 10.1126/science.1439793. [DOI] [PubMed] [Google Scholar]
- Steinwede K, Henken S, Bohling J, Maus R, Ueberberg B, Brumshagen C, Brincks EL, Griffith TS, Welte T, Maus UA. TNF-related apoptosis-inducing ligand (TRAIL) exerts therapeutic efficacy for the treatment of pneumococcal pneumonia in mice. J Exp Med. 2012;209:1937–1952. doi: 10.1084/jem.20120983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Itai T, Adachi M, Nagata S. Downregulation of Fas ligand by shedding. Nature Med. 1998;4:31–36. doi: 10.1038/nm0198-031. [DOI] [PubMed] [Google Scholar]
- Xerri L, Devilard E, Hassoun J, Mawas C, Birg F. Fas ligand is not only expressed in immune privileged human organs but is also coexpressed with Fas in various epithelial tissues. Mol Pathol. 1997;50:87–91. doi: 10.1136/mp.50.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeretssian G, Labbe K, Saleh M. Molecular regulation of inflammation and cell death. Cytokine. 2008;43:380–390. doi: 10.1016/j.cyto.2008.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.