Summary
Polysaccharide A (PSA), the archetypical immunomodulatory molecule of the gut commensal Bacteroides fragilis, induces regulatory T cells to secrete the anti-inflammatory cytokine interleukin 10 (IL-10). The cellular mediators of PSA’s immunomodulatory properties are incompletely understood. In a mouse model of colitis, we find that PSA requires both innate and adaptive immune mechanisms to generate protection. Plasmacytoid DCs (PDCs) exposed to PSA do not produce proinflammatory cytokines but instead they specifically stimulate IL-10 secretion by CD4+ T cells and efficiently mediate PSA-afforded immunoprotection. PSA induces and preferentially ligates Toll-like receptor 2 on PDCs but not on conventional DCs. Compared with other TLR2 ligands, PSA is better at enhancing PDC expression of co-stimulatory molecules required for protection against colitis. PDCs can thus orchestrate the beneficial immunoregulatory interaction of commensal microbial molecules, such as PSA, through both innate and adaptive immune mechanisms.
Keywords: anti-inflammation, commensal molecule, plasmacytoid dendritic cells, polysaccharide A, co-stimulatory molecules
Introduction
Studies in animal models and patients show that oral antigen administration represents a highly tolerogenic route linked to the gut and its associated lymphoid tissues —sites presumably rich in commensal microbes with immunomodulatory molecules and in immune cells with regulatory potential (Pabst and Mowat, 2012). Since commensal microbes have immunoregulatory capacities, the microbiota is viewed as important in inducing a tolerogenic outcome when an antigen encounters the immune system. In the few commensal–immune associations known to cause immunoregulation, some microbes have been identified at the genus (or uncommonly the species) level, but little information has been obtained at the microbial molecular level (Atarashi et al., 2013; Atarashi et al., 2011; Hall et al., 2008; Smith et al.). An exception is capsular polysaccharide A (PSA) of the gut commensal Bacteroides fragilis, which induces regulatory T cells (Tregs) to secrete the potent anti-inflammatory cytokine interleukin 10 (IL-10), thereby limiting pathologic inflammation in the gut and more distant tissues (e.g., brain) (Mazmanian et al., 2008; Ochoa-Reparaz et al., 2010; Round and Mazmanian, 2010).
In the absence of antigen-presenting cells (APCs), PSA-induced IL-10 secretion by CD4+ T cells in vitro depends on Toll-like receptor 2 (TLR2) (Round et al., 2011). We hypothesized that this direct PSA–T cell interaction may not suffice for immunoprotection; and that APCs may be necessary. Studies using PSA-containing outer-membrane vesicles from B. fragilis rather than purified PSA showed that adoptively transferred dendritic cells (DCs) confer PSA-specific immunoprotection, whereas those from PSA-depleted B. fragilis do not (Shen et al., 2012). Outer-membrane vesicles contain immunostimulatory molecules other than PSA—e.g., lipopolysaccharide (LPS), lipids, and membrane proteins. We asked whether purified PSA can induce tolerogenic properties in DCs and, if so, whether these DCs are required for immunoprotection in vivo.
Tolerogenic DCs are a major functional class of APCs influencing Tregs (Steinman et al., 2003). Their exact characteristics, including subset phenotype, vary with the inflammatory setting (Maldonado and von Andrian, 2010). Two broad DC categories thought to be distinct in transcriptional control of DC lineage commitment and function are conventional DCs (CDCs) and plasmacytoid DCs (PDCs) (Miller et al., 2012; Young et al., 2008). Using purified PSA as a prototypical commensal microbial molecule, we compared tolerogenic potential of DC subsets against inflammation-induced pathologic outcomes. We found a vital role for PDCs in PSA-mediated induction of tolerance and immunoprotection. Our findings raise the possibility of PDC use in commensal-based immunoregulatory therapies.
Results
DC-dependent augmentation of IL-10 production in CD4+ T cells by PSA in vitro
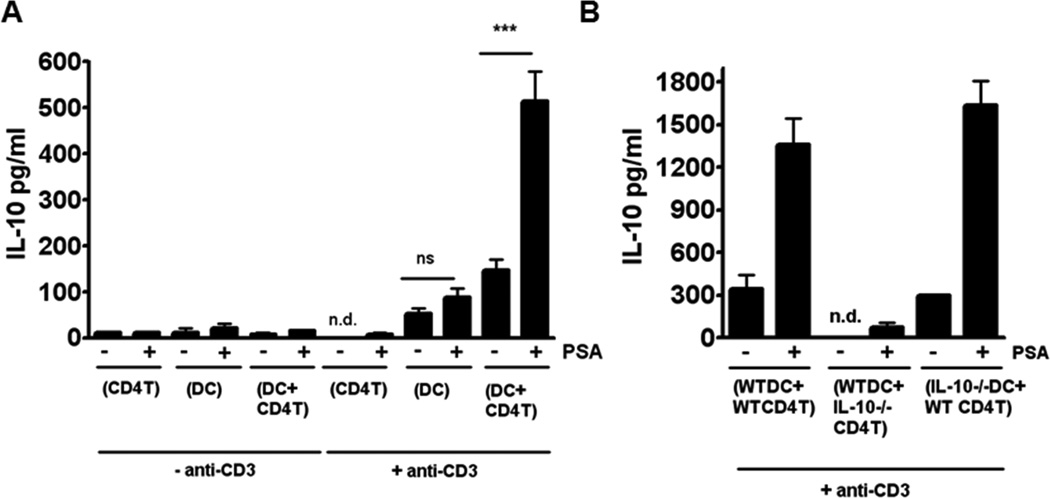
We used primary splenic DC–CD4+ T cell co-culture to investigate DC relevance in PSA-mediated IL-10 augmentation (Figure 1A). Only in the presence of DCs did CD4+ T cells from wild-type (WT) mice yield significant amounts of IL-10. The yield was significantly greater with than without PSA. Co-culture of DCs with CD4+ T cells from IL-10−/− mice showed that CD4+ T cells were the source of IL-10 (Figure 1B). These results suggested an APC requirement for IL-10 production by CD4+ T cells and differed from those obtained by different methodology (Round et al., 2011). We set out to determine whether PSA mediated immunoregulation of inflammatory diseases requires DC help and, if so, to identify the required DC subset.
Figure 1.
PSA-stimulated IL-10 secretion by CD4+ T cells in splenic DC–CD4+ T cell co-culture is dependent on DCs. (A) IL-10 levels in culture supernatants (as measured by ELISA) are significantly higher in wells with PSA than in wells without PSA and suggest DC dependence of IL-10 secretion by CD4+ T-cells. Data represent the average of 4 independent experiments analyzed by Student's t-test. ***p<0.001; n.d., not detected; ns, not significant. (B) In co-cultures with IL-10−/− CD4+ T cells and WT DCs but not with WT CD4+ T cells and IL-10−/− DCs, essentially no IL-10 is produced; thus the major source of IL-10 in these co-cultures is CD4+ T cells. n.d., not detected.
PDCs are linked with PSA-mediated protection in TNBS-induced colitis
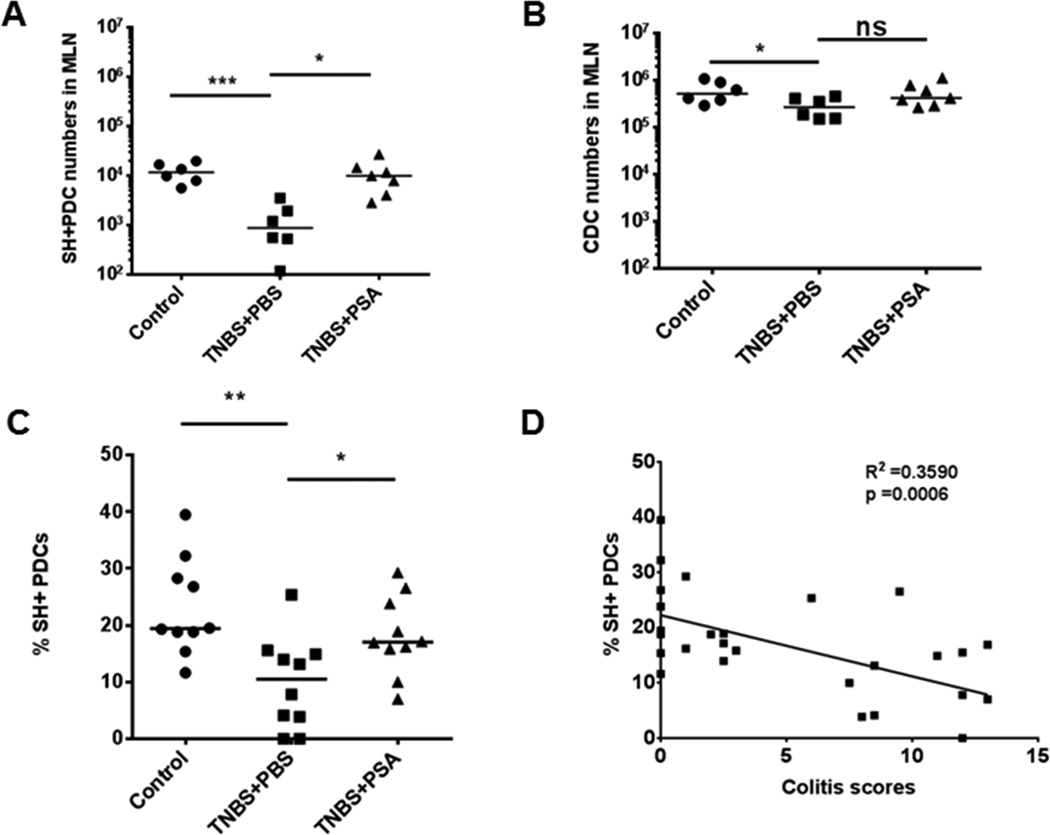
Mesenteric lymph nodes (MLNs), the major gut-draining lymph nodes, are immunologically affected by orally delivered antigens (Pabst and Mowat, 2012; Spahn et al., 2001; Worbs et al., 2006) and are a potential site of DC–CD4+ T cell interaction after PSA oral gavage. We reported that fluorescently labeled PSA given by oral gavage is associated with CD11c+ DCs but not CD4+ T cells in MLNs (Mazmanian et al., 2005). To characterize the role of DC subsets in PSA-mediated immunoregulation, we studied these cells in MLNs during 2,4,6-trinitrobenzenesulfonic acid (TNBS)–induced colitis. Before giving TNBS intrarectally, we pretreated mice by gavage with PSA or PBS. First we counted absolute PDC and CDC numbers with phenotypic markers and Flow-Count Fluorospheres. During inflammation, we used the marker Siglec H (SH, an I-type lectin receptor expressed with high fidelity on PDCs); (Swiecki and Colonna, 2010; Zhang et al., 2006) to follow PDCs (Figures S1A and S1B).
At peak inflammation, PDC numbers were drastically reduced in MLNs of diseased mice (the TNBS+PBS group) from counts in controls (no TNBS), while CDC numbers were minimally affected in MLNs. At the inflammation apex, PDC numbers in MLNs of PSA-pretreated mice (the TNBS+PSA group) but not of the diseased group were nearly normal (Figures 2A and 2B). PDC immunomodulation during disease was not seen in colonic LP. However, PSA significantly increased PDC numbers without significantly altering CDC numbers from those in TNBS+PBS mice (Figures S1C and S1D). Given the clear modulation of PDCs in lymphoid and gut tissues of PSA-pretreated mice, we investigated whether PDCs were directly associated with PSA’s immunoprotective action.
Figure 2.
PDCs exhibit a protective phenotype in TNBS-induced colitis after PSA pretreatment. (A) Mice treated with PSA orally (100 μg/dose, TNBS+PSA) before intrarectal challenge with TNBS in a colitis model had a significantly greater (8.52-fold) increase in PDC numbers in MLNs than did mice pretreated with PBS (TNBS+PBS). Each dot represents one mouse on day 3 after TNBS or control buffer administration. PDCs were identified by gating of SH+CD11b−B220+CD11c+ cells. (B) Numbers of CDCs (CD11b−B220+ population gated out from CD11c+ population) in MLNs were not significantly changed in disease or in PSA-treated mice. (C) Augmentation of PDC frequency in MLNs after TNBS challenge (% of SH+ PDCs in the CD11b−B220+CD11c+ population) was significantly greater in mice pretreated with oral PSA (50 μg/dose) than in PBS-treated mice. (D) In TNBS colitis, SH+ PDC frequency in MLNs is significantly but inversely correlated with colitis scores. Horizontal bars in scatter plots represent median values. Unpaired Student’s t-test: *p<0.05; **p<0.01; ***p<0.001; ns, not significant. See also Figure S1.
In MLNs, both PDC frequency and SH expression (measured as mean fluorescence intensity) were significantly lower in TNBS+PBS mice than in mice not given TNBS. In contrast, TNBS+PSA mice had significantly higher PDC frequency and SH expression than TNBS+PBS mice (Figures 2C and S1E). In addition to affecting PDC numbers, PSA maintained immune status at preinflammatory levels, as assessed by PDC frequency and SH expression. Both of the latter parameters were significantly but inversely correlated with cumulative colitis scores (Figures 2D and S1F)—results strongly supporting a role for PDCs in immunoprotection.
Depeletion of PDCs abrogates PSA-mediated protection against TNBS-induced colitis
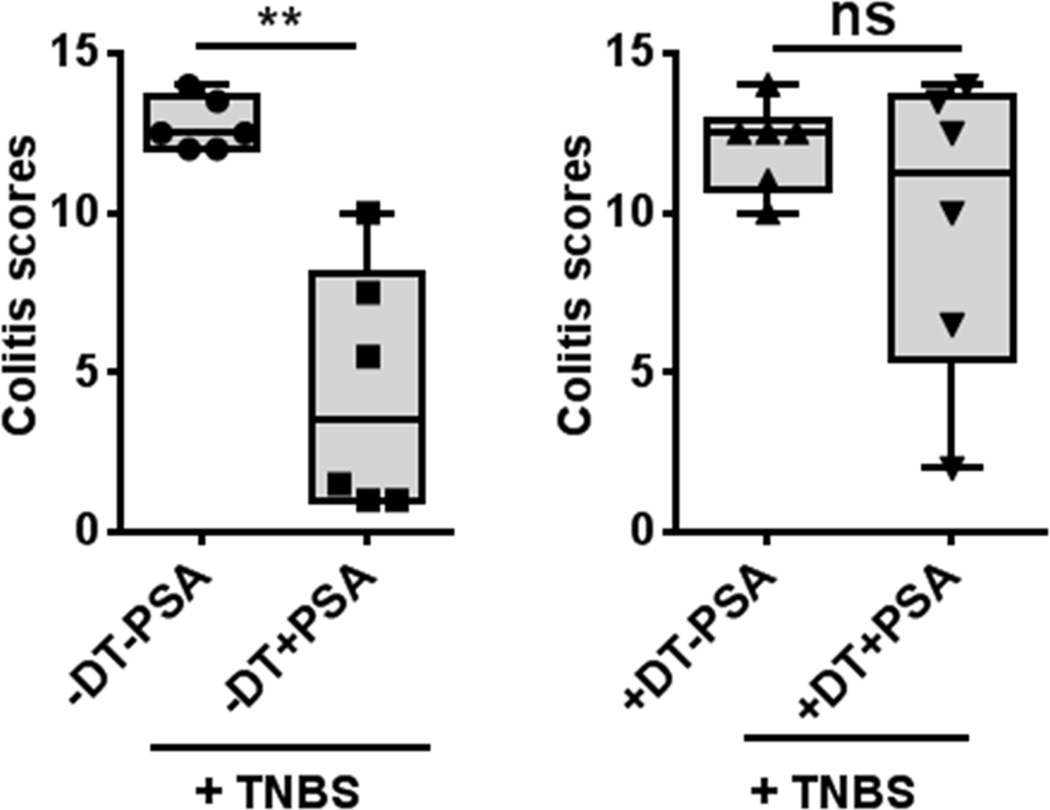
To determine whether PDCs are required for PSA-mediated protection, we depleted PDCs by treating BDCA2-DTR mice—i.e., human diphtheria toxin receptor (DTR) transgenic mice expressing DTR under the blood DC antigen 2 (BDCA2) promoter—with diphtheria toxin (DT). This approach has been validated for specificity of PDC depletion (Swiecki et al., 2010). We verified that DT treatment of BDCA2-DTR mice depletes PDCs in MLNs and spleen (Figure S2B). PSA-treated controls not given DT were protected. However, specific PDC removal by DT treatment rendered PSA-mediated protection insignificant (Figure 3).
Figure 3.
PDC depletion abrogates PSA protection in a TNBS colitis model. PDC depletion by DT administration (200 ng/dose, 6 doses) to BDCA2-DTR mice before inflammation onset inhibits PSA-mediated protection. Boxplots show median (horizontal bar inside box) and quartile distributions in the TNBS model. Left and right panels show colitis scores with and without DT, respectively. Each dot in the boxplots represents an individual mouse. Scores were assessed for statistical significance by two-tailed nonparametric Mann-Whitney test. **p<0.01; ns, not significant. See also Figure S2.
As an alternative approach of PDC depletion, we treated mice with monoclonal antibodies (mAbs) to PDCA-1 on each of the 2 days before intrarectal TNBS administration (Figure S2A). In addition to clinical and pathologic scores, we used CD11b+CD11c− cell frequency in colonic LP as a cellular correlate of pathology. We found a significant direct correlation between cell counts and colitis scores (Figures S2C and S2D). A pathogenic role for similar cells in TNBS colitis has been described (Kanai et al., 2001; Leon et al., 2006; Palmen et al., 1995). Among diseased mice not treated with PSA, neither cumulative disease score nor accumulation of potentially pathogenic CD11b+CD11c− cells in the colon after mAb treatment differed from values in isotype controls. These results suggest that PDCs do not play a pathogenic role in this colitis model. Values for both parameters (disease score and CD11b+CD11c− cell counts) were significantly lower in PSA-treated mice than in controls. However, PSA did not protect mice or reduce CD11b+CD11c− cell frequency when PDCs were depleted with mAbs (Figures S2E–S2G). Despite concern about the anti–PDCA-1 approach (i.e., PDCA-1 is expressed during inflammation on cells other than PDCs) (Swiecki and Colonna, 2010), our very similar results with BDCA2-DTR mice support a requirement for PDCs in vivo in PSA-mediated immunoregulation.
We used the experimental autoimmune encephalomyelitis (EAE) model to validate PDCs’ immunoregulatory role, assessing PSA-mediated immunoprotection at a site distant from the gut. We induced EAE in four groups of SJL/J mice with myelin proteolipid protein (PLP) and adjuvant; PSA is protective in this model (Ochoa-Reparaz et al., 2010). Two groups received a mAb to PDCA-1 to deplete PDCs. One of these groups also received PSA; the other received PBS. The remaining two groups were given isotype control IgG. One of these groups also received PSA and the other PBS. In the two groups given mAb to PDCA-1, 15 of 16 mice died on day 7, irrespective of PSA treatment. These deaths preceded clinical symptom onset, and no sign of progression or spinal-cord histopathologic damage was visually detectable (Figure S2H). The one surviving mouse in the group given PSA and PDCA-1 mAb developed disease on day 12. In contrast, early deaths occurred among PDCA-1 mAb–treated mice but not isotype control IgG–treated mice (Figure S2H). The mortality rate was higher, clinical symptoms developed earlier, and EAE scores indicated more severe disease in mice given PBS and isotype control (Figures S2H–S2J) than in counterparts given PSA and isotype control. Thus PDCs are critical to PSA-mediated immunoprotection in the gut and distant tissues. We could not use the DT approach to investigate EAE because DT is toxic to WT mice when given during EAE induction (Meyer Zu Horste et al., 2010).
PSA-pretreated PDCs—but not CDCs—confer protection after adoptive transfer
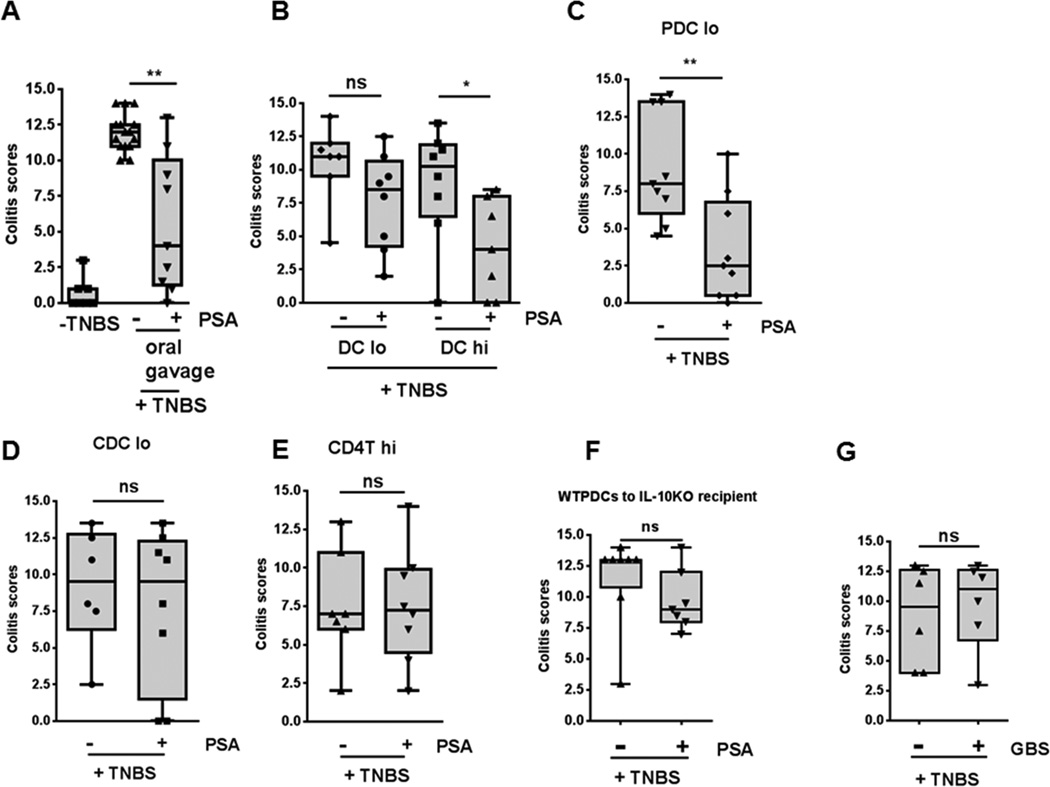
To establish a mechanistic role for PDCs in PSA-mediated immunoregulation, we first investigated whether PSA-treated DCs confer protection by adoptive transfer in the TNBS-induced colitis model. We used bone marrow–derived DCs (BMDCs) enriched with CD11c microbeads. After isolation, DCs from WT mice were cultured with or without PSA, washed, and adoptively transferred to mice of the same background. Two DC doses (1–1.5 × 105 and 6–6.5 × 105, administered twice) were used. After intrarectal TNBS administration, mice adoptively receiving in vitro PSA–treated DCs had significantly lower cumulative clinical scores, with a dose-response trend, than mice receiving PBS-treated DCs. The median colitis score in TNBS+PSA controls was similar to that in mice given the higher dose of PSA-treated DCs (Figures 4A and 4B). To compare PDC and CDC efficiency in protection against TNBS colitis, we compared adoptive transfer of relatively few (1–1.5 × 105) bone marrow–derived PDCs and CDCs after incubation with PSA or PBS. In contrast to PBS-pretreated PDCs, PSA-pretreated PDCs offered significant protection, whereas this dose of PSA-pretreated CDCs did not (Figures 4C and 4D). When we adoptively transferred PSA-treated WT CD4+ T cells but not DCs, CD4+ T-cell recipients were not protected (Figure 4E). This result indicated that CD4+ T cells require DCs previously exposed to PSA to mediate protection in vivo.
Figure 4.
Adoptive transfer of PSA-treated PDCs protects mice in a TNBS colitis model. (A) Protection was seen with oral PSA treatment (100 μg/dose). (B) Colitis scores of WT mice after treatment with CD11c+ BMDCs show protection following adoptive transfer of the higher of two doses of PSA-pretreated, CD11c microbead–selected BMDCs (6–6.5 × 105/dose, 2 doses; PSA-DC hi) but not the lower dose (1–1.5 × 105/dose, 2 doses; PSA-DC lo) when results were compared to those with the respective PBS-pretreated BMDCs. (C) Protection was seen with adoptively transferred lower-dose PSA-pretreated PDCs when results were compared to those with PBS-pretreated PDCs. (D) No protection was seen with low-dose PSA-pretreated CDCs when results were compared to those with PBS-pretreated CDCs. (E) PSA-pretreated CD4+ T cells (5 × 105/dose, 2 doses) did not protect mice when results were compared to those with PBS-pretreated CD4+ T-cell controls. (F) After adoptive transfer to IL-10−/− mice, low-dose PDCs from WT mice pretreated with PSA or PBS failed to confer significant protection. (G) Pretreatment of PDCs with a control polysaccharide (type II polysaccharide from group B Streptococcus) failed to protect mice from TNBS-induced colitis after adoptive transfer. Each dot represents one mouse. Scores were assessed for statistical significance by two-tailed nonparametric Mann-Whitney test. *p<0.05; **p<0.01; ns, not significant. See also Figure S3.
To demonstrate that adoptively transferred DCs were not the source of IL-10 required for protection, we transferred 1–1.5 × 105 PSA-pretreated WT bone marrow–derived PDCs (BMPDCs) to IL-10−/− mice and challenged them with TNBS; no significant protection occurred (Figure 4F). To confirm the specificity of PSA-mediated protection, we pretreated BMPDCs with a control non-zwitterionic polysaccharide—type II group B streptococcal polysaccharide—rather than PSA. GBSII polysaccharide–treated PDCs failed to confer protection (Figure 4G).
To assess whether the lack of protection by low-dose PSA-treated CDCs was due to failure of adoptively transferred CDCs to localize in the gut and gut-associated lymphoid tissue, we transferred unsorted donor PSA-pretreated (in vitro) BMDCs from CD45.1+ B6 mice to CD45.2+ B6 recipientsand determined the cells’ distribution . BMDCs localize and function in MLNs when transferred IP (Creusot et al., 2009). We found varying quantities of donor PDCs and CDCs in spleen, MLNs, and colon of recipient mice after 48 h and even 9 days (Figure S3). Thus failure of CDCs to localize to gut tissues does not explain poor function in the adoptive transfer model. In fact, CD11c microbead–purified BMDCs—a population that consists primarily of CDCs—are protective at a higher dose (6–6.5 × 105). PSA-mediated protection may not be solely attributable to PDCs, but PDCs are certainly significantly more efficient at conferring protection than CDCs, especially in low numbers.
PDCs and CDCs vary in response to PSA
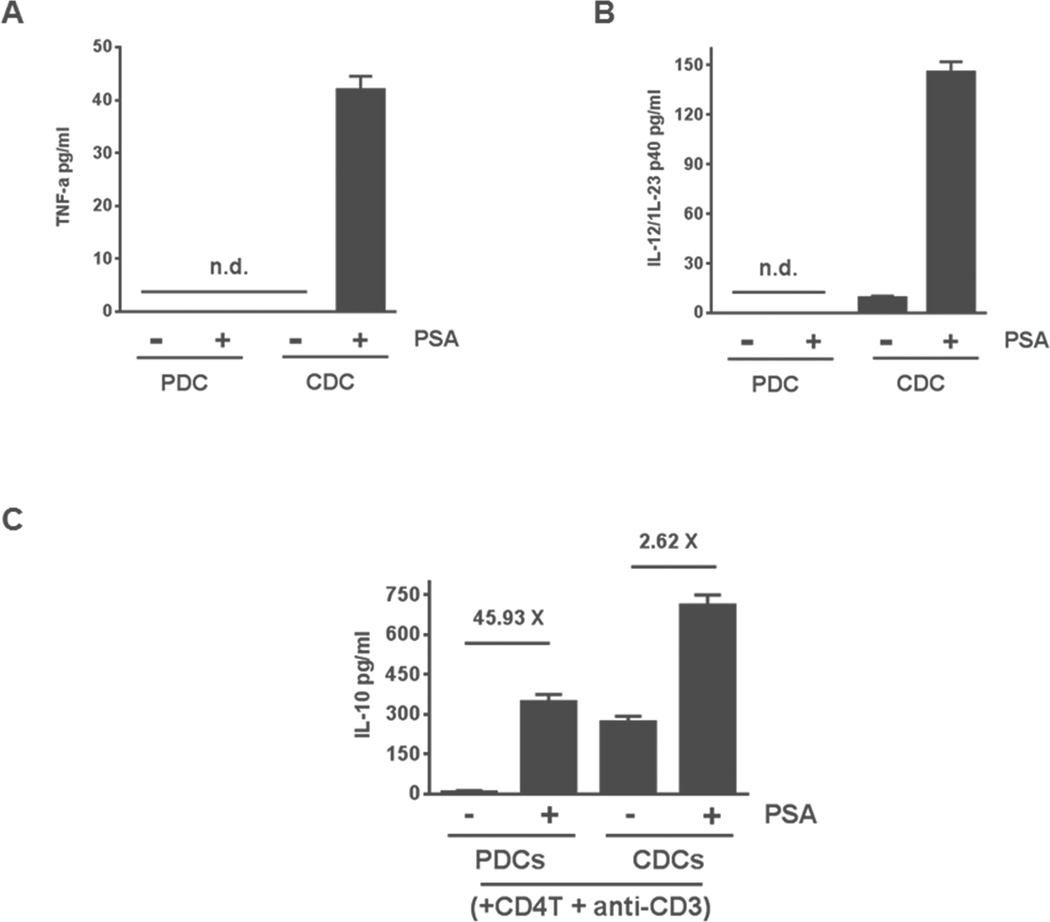
We reported that proinflammatory cytokines tumor necrosis factor α (TNF-α) and IL-12/IL-23 are liberated by unsorted DCs after PSA stimulation (Wang et al., 2006). Both cytokines are implicated in inflammatory bowel disease in humans and mouse models (Bouma and Strober, 2003). We studied their secretion in PDCs and CDCs after incubation with PSA. In response to PSA, CDCs but not PDCs liberated TNF-α and IL-12/IL-23 (Figures 5A and 5B). We co-cultured these DC subsets with splenic CD4+ T cells, with or without PSA. In both settings, CDCs induced higher IL-10 production by CD4+ T cells than did PDCs (Figure 5C). However, PSA-induced IL-10 production by CD4+ T cells, as indicated by the fold-increase of IL-10 (ratio of the cytokine level detected with PSA to that without), was significantly higher when T cells were cultured with PDCs rather than CDCs (Figure 5C). These results show greater specificity of PDCs than CDCs in induction of IL-10 and are consistent with the greater observed ability of PDCs to confer protection.
Figure 5.
PSA differentially affects diverse DC subsets. (A, B) Levels of proinflammatory cytokines TNF-α (A) and IL-12/IL-23 (B) were measured in supernatants of monocultures containing PDCs or CDCs (5 × 104 cells/ml) that were incubated for 24 h with or without PSA (100 μg/ml). Data represent 2 experiments. (C) IL-10 liberation from CD4+ T cells co-cultured with CDCs and PDCs. IL-10 levels were measured by ELISA of culture supernatants of PDCs or CDCs co-cultured with CD4+ T cells for 5 days in the presence of anti-CD3. Co-cultures were either treated or not treated with PSA (50 μg/ml). Horizontal bars show the fold increase in mean IL-10 production in PSA-treated co-cultures. Data represent the average of 7 independent experiments. Error bars indicate SEM values. See also Figure S6.
Enhanced TLR2 expression on PDCs and association of PDCs with TLR2-dependent PSA protection
The presence of TLR2 on APCs is reportedly essential for PSA stimulation of interferon γ (IFNγ) production by CD4+ T cells (Round et al., 2011; Wang et al., 2006). PDCs reportedly mediate immunoregulation both in vitro and in vivo as well as the response to viral and some bacterial nucleic acid antigens via TLRs 7, 8, and 9 (Swiecki and Colonna, 2010), but whether they play a role in TLR2-dependent immune responses is unknown. Tracking PDCs in MLNs and spleen through SH expression without other stimulation, we found higher-frequency TLR2 expression in PDCs from MLNs than in splenic PDCs (Figure S4A). Our results indicated the need to further seek a TLR2-dependent PDC mechanism prompting PSA-mediated immunoregulation. PDC-mediated immunoregulation has not previously been linked to the microbiome.
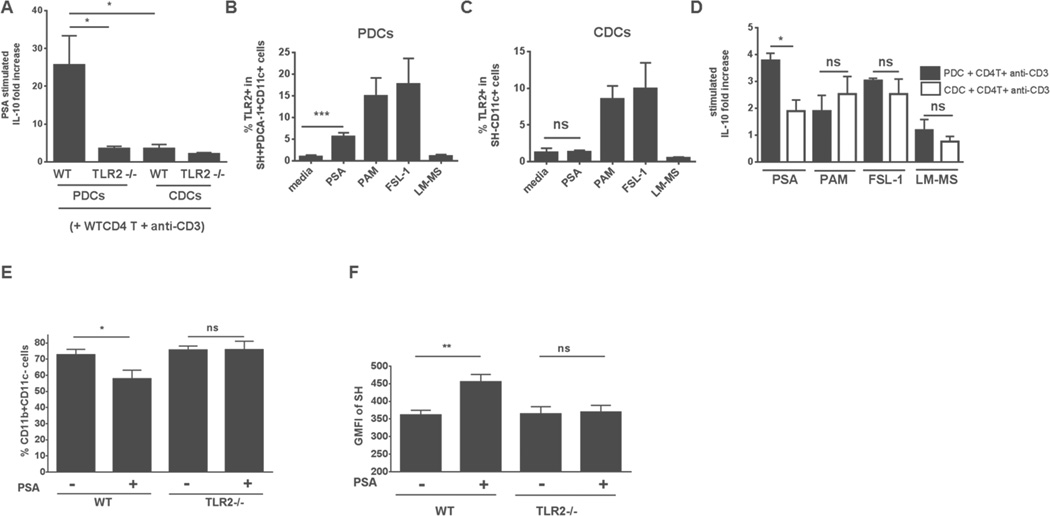
We investigated whether TLR2 provides a pathway in PDCs for invocation of immunoregulatory molecules in CD4+ T cells. Co-culture of PDCs or CDCs with CD4+ T cells showed a dependence of IL-10 production by CD4+ T cells on TLR2 expression by PDCs but not by CDCs (Figure 6A). PDCs can be characterized by specific surface markers, such as SH and PDCA-1. Induction of TLR2+ PDCs (Figures 6B and S4B), but not CDCs (Figures 6C and S4B), increased significantly when BMDCs were incubated with PSA rather than without it. Other TLR2 ligands (e.g., Pam3CSK4, FSL-1) induced TLR2 in both PDCs and CDCs. Another reported TLR2 agonist, lipomannan from Mycobacterium smegmatis, failed to significantly induce TLR2 in any DC subset beyond control levels. In another unique aspect of PSA–PDC interaction, PDCs treated with PSA but not with other TLR2 ligands induced significantly higher IL-10 production by CD4+ T cells than occurred in the respective CDC-containing wells (Figure 6D). This finding strongly suggests that immunoregulatory effects of PSA-treated PDCs on CD4+ T cells are not limited to TLR2 ligation and that additional signaling pathways are required.
Figure 6.
PDCs have an immunoregulatory phenotype that is TLR2-dependent. (A) The presence of TLR2 on PDCs (but not on CDCs) augments PSA-generated IL-10 production by CD4+ T cells, as measured by ELISA (average of 3 experiments). (B, C) Elevated frequency of TLR2+ PDCs (SH+PDCA-1+CD11c+) (B), but not of TLR2+ CDCs (SH−CD11c+) (C), after PSA treatment (60 μg/ml). Pam3CSK4 (PAM, 0.1 μg/ml) and FSL-1 (0.1 μg/ml)—but not lipomannan (LM-MS, 10 ng/ml)—augment both TLR2+ PDCs and TLR2+ CDCs. Data are from 2 independent experiments. (D) PDCs treated with PSA but not with other TLR2 ligands show a significant increase (as measured by ELISA) in fold-induction of IL-10 production by CD4+ T cells over that seen with PSA-treated CDCs (average of 3 independent experiments). Error bars in A–D indicate SEM values. (E, F) PSA-induced diminution of colonic lamina propria CD11b+CD11c− cells (E) and augmentation of GMFI of SH in MLNs (F) in TNBS-induced colitis in WT mice but not TLR2−/− mice a similar situation. Unpaired Student’s t-test: *p<0.05; **p<0.01; ns, not significant. Error bars indicate SEM values. See also Figure S4.
Our comparison of mice from WT and TLR2−/− backgrounds in the TNBS colitis model revealed a failure of PSA to confer protection in the TLR2−/− background (Round and Mazmanian, 2010) (Figure S4C). Neither the significant reduction in CD11b+CD11c− cell frequency in colonic LP nor the augmented SH expression seen in WT mice after PSA treatment was seen in TLR2−/− mice (Figures 6E and 6F).
Central role of cognate PDC–CD4+ T cell interactions in PSA-mediated immunoregulation in vitro and in vivo
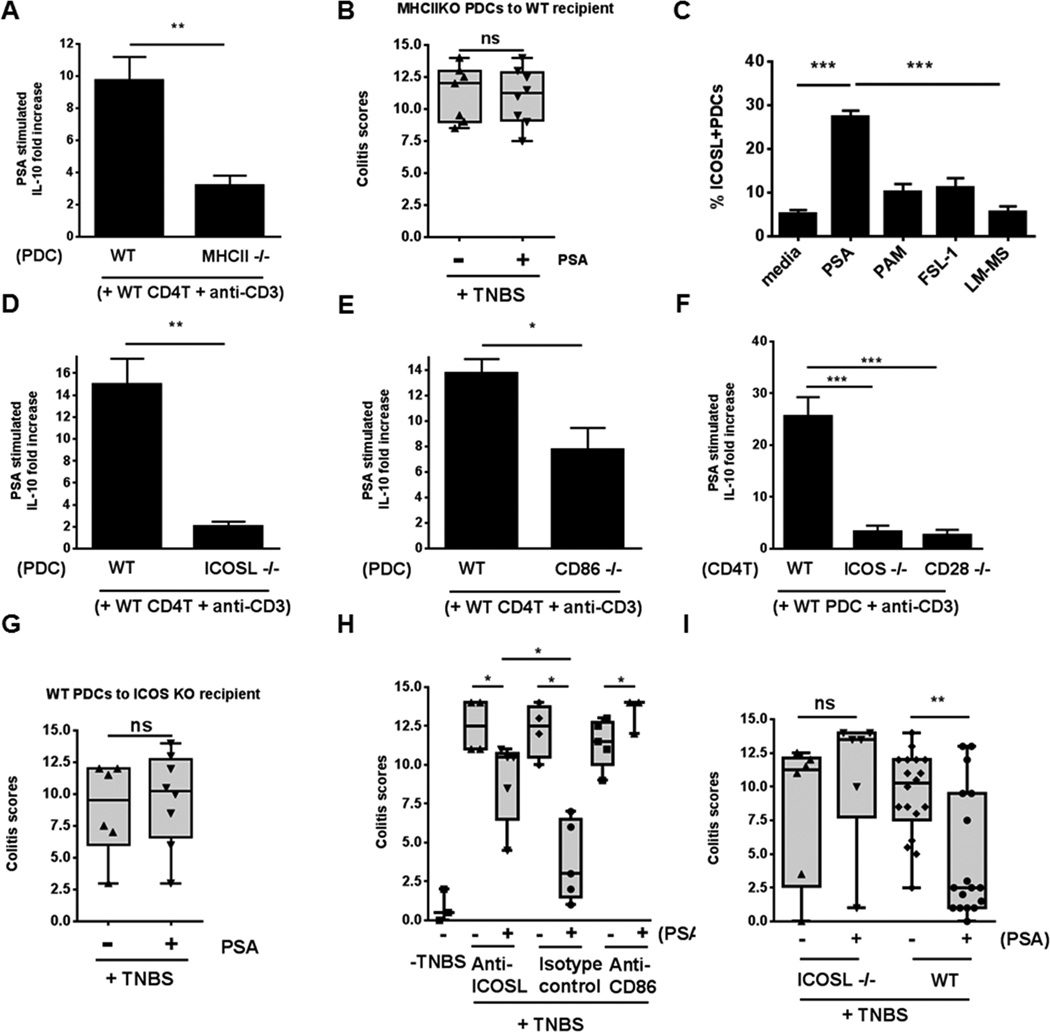
DCs reportedly use several strategies to induce immunoregulatory activity in CD4+ T cells (Maldonado and von Andrian, 2010). We isolated PDCs from BMDCs and examined two known molecular strategies by co-culture. First, we assessed the use of indoleamine 2,3-dioxygenase (IDO) to break down tryptophan into metabolites that can activate immunoregulatory functions in CD4+ T cells (Sharma et al., 2007). After addition of 1-methyl-D-tryptophan (an inhibitor of IDO) to PDC–CD4+ T cell co-culture, IL-10 levels were not perturbed—a result indicating that this pathway does not participate in PSA-mediated immunoregulation (Figure S5A). Second, we explored DC antigen presentation to CD4+ T cells. To determine whether PSA-mediated cognate DC–CD4+ T cell interactions are essential for immunoregulation, we examined the requirement for major histocompatibility class II (MHCII) involvement (usually referred to as signal 1) and co-stimulation (signal 2). We determined IL-10 production in PDC–WT CD4+ T cell co-culture, comparing PDCs from WT and MHCII−/− mice (Figure 7A). IL-10 production was substantially lower with MHCII−/− PDCs, and these cells failed to confer protection when pretreated with PSA and adoptively transferred to WT mice in the TNBS-induced colitis model (Figure 7B).
Figure 7.
Cognate interactions are essential for PSA-mediated generation of IL-10 in vitro and for protection in the TNBS colitis model. (A) WT CD4+ T cells co-cultured with PDCs from WT and MHCII−/− mice were compared. Co-cultures with WT PDCs had significantly higher levels of IL-10 than those with MHCII-deficient PDCs. (B) Adoptive transfer of PSA-pretreated BMPDCs from MHCII−/− mice (1.5 × 105/dose, 2 doses) did not confer significant protection to WT mice. (C) Bone marrow–derived PDCs (SH+PDCA-1+CD11c+; gating used for evaluation) included a significantly higher frequency of ICOSL+SH+ cells when treated with PSA (60 μg/ml) than when treated with other TLR2 ligands [Pam3CSK4 (PAM), 0.1 μg/ml; FSL-1, 0.1 μg/ml; and lipomannan (LM-MS), 10 ng/ml] or left untreated (medium control). Bar graph shows average of 5 independent experiments. (D, E) Co-cultures of WT CD4+ T cells with PDCs from ICOSL−/− mice (D) or CD86−/− mice (E) produced significantly less IL-10 than co-cultures of WT CD4+ cells with WT PDCs. (F) Enhanced ability of PDCs from WT mice to stimulate IL-10 production more strongly in WT CD4+ T cells than in CD4+ T cells from ICOS−/− or CD28−/− mice. Data are the average of 2 independent experiments. (G) Adoptive transfer of PSA-pretreated WT PDCs did not confer protection in ICOS−/− recipients. (H) Anti-CD86 IgG and anti-ICOSL IgG blocked PSA-mediated protection and significantly reduced protection from that in IgG isotype control–treated mice. (I) WT mice—but not ICOSL−/− mice—were significantly protected after oral PSA treatment. Data shown in A, C, and D–F were analyzed by unpaired Student's t-test. *, **, and *** denote p<0.05, p<0.01, and p<0.001, respectively. Clinical scores shown in B, G, H, and I were assessed for statistical significance by two-tailed nonparametric Mann-Whitney test. *p<0.05; **p<0.01; ns, not significant. Each dot represents one mouse. Error bars indicate SEM values. See also Figure S5.
To investigate the role of signal 2, we monitored expression of the inducible co-stimulator ligand (ICOSL) in BMPDCs cultured with PSA or the TLR2 ligands used above. Expression of ICOSL in SH+PDCA-1+CD11c+ PDCs was significantly higher in PSA-containing wells than with any other treatment (Figures 7C and S5C). CD4+ T-cell production of IL-10 decreased substantially in co-culture with PDCs from ICOSL−/− or CD86−/− mice but not CD80−/− mice (Figures 7D, 7E and S5B). ICOS and CD28 are the receptors on CD4+ T cells that interact with ICOSL and CD86 on APCs, respectively (Greenwald et al., 2005). Accordingly, in co-culture with WT PDCs, IL-10 production was significantly lower with CD4+ T cells from ICOS−/− or CD28−/− mice than with CD4+ T cells from WT mice (Figure 7F). Thus cognate interactions involving MHCII and co-stimulation by PSA-activated PDCs are required for initiation of IL-10 production by CD4+ T cells. PSA’s ability to stimulate cognate interactions explains why pure TLR2 ligands, while inducing high-level expression of TLR2 in PDCs, fail to stimulate higher-fold increases in IL-10 production by CD4+ T cells in co-culture (Figures 6B and 6D). The importance of co-stimulation in PSA-treated PDCs was further validated by a lack of protection when PSA-treated WT BMPDCs were transferred to ICOS−/− mice in the TNBS colitis model (Figure 7G); this result contrasted with that in WT recipients (Figure 4C).
We determined the state of PDC activation during active TNBS-induced colitis after oral treatment of mice with PSA or PBS. In diseased TNBS+PBS mice, the PDCs remaining after drastic reduction in PDCs (Figure 2A) retained their antigen-presenting and co-stimulatory molecules. The percentage of marker-bearing PDCs (i.e., the proportion of antigen-presenting molecule–bearing PDCs among total PDCs) was actually higher in PBS-treated mice than in PSA-treated animals. However, when absolute numbers of antigen-presenting molecule–bearing PDCs were determined, PSA-protected mice (TNBS+PSA) had higher counts of active PDCs than TNBS+PBS mice. Expression of markers as represented by GMFI did not change appreciably in the treatment groups (Figures S5D–S5G). Finally, we investigated whether adaptive immune co-stimulation-dependent cognate interaction is essential for PSA-mediated immunoregulation in vivo. We treated mice with inhibitory mAb to either ICOSL or CD86 or with an isotype control antibody before intrarectal administration of TNBS. The mice received PBS or PSA by oral gavage. The lack of change in colitis scores of mice treated with mAb and PBS indicated that inhibition by blocking antibodies did not alter the disease process. Mice treated with PSA and isotype control antibody had significantly lower colitis scores than mice in the corresponding PBS-treated group. Inhibition of CD86, however, completely negated PSA protection. Inhibition of ICOSL had an intermediate effect, with colitis scores lower than those in CD86-inhibited mice but higher than those in isotype controls (Figure 7H). To further investigate ICOSL participation, we compared PSA’s protective effect in WT and ICOSL−/− mice. PSA’s failure to protect ICOSL−/− mice contrasted with PSA-mediated protection in WT animals (Figure 7I). Taken together, our data show the importance of cognate PDC–CD4+ T cell interactions mediated by MHCII and co-stimulatory molecules on PDCs in PSA-mediated protection.
Discussion
B. fragilis PSA provides a unique opportunity to explore immunoregulation mediated by commensal microbes on a molecular level. We have found that in vivo PSA-mediated protection in an inflammatory bowel disease model requires DC involvement and that one DC subset, PDCs, is particularly active. CDCs and PDCs arise from common precursors that have lost the potential to differentiate into monocytes and macrophages (Miller et al., 2012). PDCs rank high in the tolerogenic DC hierarchy, with a greater capacity to generate Tregs (Maldonado and von Andrian, 2010), but their role in containing colonic inflammation has not been described.
In adoptive transfer experiments, we used BMPDCs rather than primary tissue (e.g., MLN)–derived murine PDCs. We acknowledge that the two may behave differently. To establish a direct link between PSA and PDCs, we first showed the dynamics of PDCs (absolute numbers, frequency, and GMFI of SH) in MLNs and colonic LP in TNBS-induced colitis. As a proof of principle, we validated PSA’s direct influence on PDCs, using BMPDCs both in vitro and in vivo. PDCs preincubated with PSA were protective in TNBS colitis after adoptive transfer; equivalent numbers of PSA-preincubated CDCs were not. This result suggests that PDCs may be unique in the ability to generate PSA-induced immunoprotection. The lack of induction of proinflammatory cytokines after PSA treatment and the significant ability to induce the PSA-dependent anti-inflammatory cytokine IL-10 in CD4+ T cells may account for fewer PDCs than CDCs mediating protection after adoptive transfer. PDCs generate IFNγ+IL-10+CD4+ T cells in vivo through Notch signaling (Kassner et al., 2010). That PSA induces the generation of both IFNγ and IL-10 supports PDC involvement in PSA-mediated immunoregulation. Although a role for other DC subsets in PSA function cannot be ruled out, our results indicate that PSA’s effect on PDCs, with consequent augmentation of Treg function, represents a central host–microbial strategy in the gut that limits the extent and severity of inflammatory diseases.
Molecular mediators in murine and human PDCs that reportedly generate Tregs include ICOSL, IDO, retinoic acid, CD40L, Δ-like ligand 4 (a Notch ligand), and MHCII (Chen et al., 2008; Colvin et al., 2009; Fallarino et al., 2004; Irla et al., 2010; Ito et al., 2007; Kassner et al., 2010; Lombardi et al., 2012; Manches et al., 2008; Martin-Gayo et al., 2010; Sharma et al., 2007). Some of these mediators may act in tandem to control pathologic inflammation. PSA uses some of these mediators in PDCs (e.g., MHCII, ICOSL, CD86) but not others (e.g., IDO). Our data support the hypothesis that PSA—acting through DCs, particularly PDCs—is presented to T cells and that its presentation requires both signals 1 and 2. PSA-mediated induction of IFNγ in CD4+ T cells in vitro depends on synergy of TLR2 activation and antigen presentation pathways (Wang et al., 2006). We have reported that PSA is a unique polysaccharide presented in the context of MHCII by APCs (Cobb et al., 2004). In mouse models of abscess formation and surgical adhesion development, the immunostimulatory activity of PSA and related zwitterionic polysaccharides depends on co-stimulatory molecules of the B7 family (Stephen et al., 2005). Immunoprotection by PSA in those models also depends on ICOS–ICOSL interaction (Ruiz-Perez et al., 2005), although a specific APC mediating these interactions with T cells was not identified. Antigen presentation and co-stimulation alone are not sufficient for PSA-induced immunoprotection; innate immune pathways, particularly those mediated through TLR2, are also required. PSA-mediated induction of T-cell immunoregulatory activity illustrates innate–adaptive immune system cooperation. PSA’s induction of its immunosensitive receptor TLR2 on PDCs opens avenues for study of other commensal microbial molecules in terms of reactivity to PDCs and subsequent immunoprotection.
A major feature of PDCs after TLR7/TLR9-mediated activation is type 1 IFN secretion, a response usually culminating in an immunity-enhancing proinflammatory outcome (Swiecki et al., 2010). However, TLR9 activation–mediated type 1 IFNs are protective in experimental colitis (Katakura et al., 2005). Thus PDCs may promote protection against colitis via type 1 IFN secretion. We considered this possibility but did not detect IFNα secretion from BMDCs or isolated PDCs in the presence of PSA in vitro (Figure S6). We observed an association of SH expression on PDCs with protection. In PDCs, SH activates DAP-12-generated immunoregulatory pathways that oppose type 1 IFN–driven proinflammatory pathways initiated by TLR9 ligation (Blasius et al., 2006). Whether association of SH with colitis has biological relevance must be examined in future studies.
One pathway by which PDCs may help protect animals against inflammation is promotion of tolerance to pathology-inducing antigens. PDCs from MLNs and liver play important roles in oral tolerance to antigens and haptens. Tolerance in this setting is due to deletion of antigen presentation–induced anergic or antigen-specific T cells (Goubier et al., 2008). We wonder whether PDCs can play such a role in mounting tolerance to TNBS-haptenized antigens so that, in the absence of PDCs, tolerogenic potential is compromised and this deficiency manifests as augmented inflammation exceeding PSA’s capacity to mediate protection. Indeed, PDCs are tolerogenic to antigen-induced immune pathology in EAE and airway hyperreactivity (Irla et al., 2010; Lombardi et al., 2012) and play a role in promoting central tolerance to peripheral antigens (Hadeiba et al., 2012). Of interest is whether the “human microbial education” of this vital DC subset, which includes induction of innate immune receptor TLR2 and communication with CD4+ T cells through co-stimulatory molecules and which leads to immunoregulation, has important clinical consequences. Our observation that adoptively transferred PSA-treated PDCs confer protection may serve as a “proof of concept” for future PDC-dependent therapeutics.
Herein we show that PSA, the archetypical immunoregulatory microbial molecule from the microbiome, requires both innate and adaptive immunity to generate protection; that DCs are needed in initiating production of IL-10—a critical immunomodulator in this process—by CD4+ T cells; that PDCs are more efficient and specific than CDCs in initiating this process; that PDCs play a fundamental role in commensal molecule–CD4+ T cell interaction; and that this interaction results in immunoregulation—a biological outcome critical to health. This mechanistic insight has potential therapeutic utility and offers a pathway by which to search for therapeutic molecules expressed by commensals.
Materials and methods
Animals
Unless stated otherwise, all mice were CD45.2+ C57BL/6 and were purchased from Jackson Laboratory (Bar Harbor, ME) or Taconic Farms (Germantown, NY).
PSA extraction
PSA was purified from B. fragilis mutant strain Δ44 (Tzianabos et al., 1992). No LPS was detectable in the PSA preparation by gel electrophoresis, 1H-NMR analysis, or endotoxin assay.
PDC and CDC preparation
PDCs were isolated from 10-day-old BMDCs by positive selection (mPDCA-1 microbeads; Miltenyi Biotec, Boston, MA) or negative selection (PDC Isolation Kit II; Miltenyi). The flow-through of the positive-selection column was further positively selected with CD11c microbeads to obtain CDCs. The cells separated out at each step were scrutinized with flow cytometry, which revealed a difference of ~6- to 11-fold in PDC frequency between the PDC-enriched fraction [% SH+CD11c+ (mean ± s.d.): 36.02 ± 2.2 by positive selection, 69.15 ± 7.07 by negative selection] and the CDC fraction (% SH+CD11c+: 6.71 ± 1.36).
DC–CD4+ T cell co-culture
DCs and CD4+ T cells were isolated from spleens of untreated mice with CD11c microbeads (Miltenyi) and a Mouse T Cell CD4 Subset Column Kit (R&D Systems), seeded simultaneously (2 × 104 and 1 × 105, respectively), and cultured with PSA (50 μg/ml) and anti-CD3 (1 μg/ml) for 5 days at 37°C in 5% CO2 in 96-well round-bottom plates. Conditions of PDC/CDC−CD4+ T cell co-culture were similar to those of splenic DC−CD4+ T cell co-culture.
TNBS-induced colonic inflammation and PSA treatment
Animals were bought at the age of 5 weeks and maintained in our facility for at least 1 week before experiments. Colitis was induced with TNBS (Sigma) as previously reported but with some modifications (te Velde et al., 2006; Wirtz et al., 2007). Mice were presensitized with 1% TNBS in acetone–olive oil (4:1) buffer, which was placed on the shaved back; 7 days later, mice received TNBS in 50% ethanol (145–160 μg of TNBS, or ~3% wt/vol for a 20-g mouse) intrarectally (3.5-Fr catheter; Instech Solomon, Plymouth Meeting, PA). Controls were treated similarly but without TNBS. On alternate days before TNBS administration, mice received six oral doses of PSA (50–100 μg in 200 μl of PBS/dose) or PBS. Mice were weighed each day thereafter and sacrificed after 3–4 days. Tissue samples were collected for flow cytometry and histologic analysis after scoring of gross clinical findings. The colitis score was calculated as the sum of the percentage of weight lost (0–4% = 0, >4–10% = 1, >10–15% = 2, >15–20% = 3, and >20% = 4), stool softness (scored blindly; 0 = well-formed hard stool, 1 = soft stool, 2 = very soft stool with fluid, and 3 = completely diluted stool), apparent colonic thickness (scored blindly; 0 = visually normal colon, cut with no resistance; 1 = slightly enlarged-diameter colon, cut with no resistance; 2 = enlarged-diameter colon, cut with little resistance; and 3 = enlarged-diameter colon, cut only with great resistance), and histopathology (scored blindly; 0, 1, 2, and 3 = no, mild, moderate, and severe inflammation; 4 = severe inflammation and necrosis).
Adoptive transfer
CD11c+ whole DCs, PDCs and CDCs, were isolated from BMDCs by a magnetic bead–based method involving CD11c microbeads, a PDC Isolation Kit II, mPDCA-1 microbeads followed by CD11c microbeads, respectively and splenic CD4+ T cells with CD4 (L3T4) microbeads (Miltenyi). After incubation in culture medium with PSA (300 μg/ml) or PBS for 6 h at 37°C in 5% CO2, cells were washed and resuspended in cold PBS for adoptive transfer. CD11c+ whole DCs (high dose: 6–6.5 × 105/dose; low dose: 1–1.5 × 105/dose), PDCs (1–1.5 × 105/dose), CDCs (1–1.5 × 105/dose), or CD4+ T cells (5 × 105/dose) were transferred IP to recipients in 300 μl in PBS. Injections were given 12 and 2 days before intrarectal administration of TNBS. All mice received PBS by mouth. Controls included mice given oral PSA (100 μg/ml) along with IP PBS and intrarectal TNBS . TNBS-untreated controls received PBS both orally and IP.
EAE induction and PSA treatment
Female SJL/J mice (Jackson Lab oratory) were purchased at 6 weeks of age and maintained in our facility for at least 1 week before experiments. Mice were treated with PSA, and EAE was induced with PLP as previously reported but with some modifications (Ochoa-Reparaz et al., 2010). In brief, mice received 100 μg of purified PSA by oral gavage every 3 days, starting 6 days before EAE induction and ending 9 days afterward. Mice were challenged SC with 250 μg of PLP 139−151 (Peptides International, Louisville, KY) in 250 μl of complete Freund’s adjuvant (Sigma) fortified with Mycobacterium tuberculosis H37 Ra antigen (5 mg/ml; BD, Difco). On days 0 and 2 challenged mice received 250 ng of Bordetella pertussis toxin IP (List Biological, Campbell, CA). Disease progression was scored daily up to day 14 after PLP administration (0 = no clinical signs; 0.5, partially limp tail; 1, paralyzed tail; 2, loss ofcoordination and hind-limb paresis; 2.5, paralysis of one hind limb; 3, paralysis of both hind limbs; 3.5, paralysis of both hind limbs and forelimb weakness; 4, forelimb and hind-limb paralysis; and 5, moribund state) (Stromnes and Goverman, 2006). Mice scoring ≥4 were sacrificed. Spinal-cord histopathology scores were assessed blindly (0, 1, 2, and 3 = no, mild, moderate, and severe inflammation). EAE scores were calculated as the sum of disease progression scores at death or sacrifice and corresponding spinal-cord histopathology scores. For percentage survival, each mouse was scored 1 when found dead or ill enough to be sacrificed or 0 in all other cases. For severity of illness, each mouse was scored 1 at first symptom detection or when found dead before symptom detection. In all other cases, the score was 0.
Histology
Mouse tissues were fixed and stored in Bouin's solution (VWR Scientific, West Chester, PA). Fixed tissues were embedded in paraffin, sectioned, mounted onto slides, and stained with hematoxylin and eosin. Sections were assessed blindly by a pathologist (Dr. R. T. Bronson, Harvard Medical School).
Statistical analysis
All p values except clinical scores and survival rates were calculated by unpaired two-tailed Student's t-test. For comparisons of clinical scores, the two-tailed nonparametric Mann-Whitney test was used. To calculate p values for survival rates, the log-rank test was used.
Supplementary Material
Highlights.
Plasmacytoid DCs are required for B. fragilis PSA-mediated protection against colitis
In vivo protection is induced by adoptive transfer of PDCs exposed to PSA in vitro
PSA-induced PDC immunoregulatory phenotype is TLR2-dependent
Cognate PDC–CD4+ T cell interactions are critical for PSA-mediated immunoregulation
Acknowledgments
This work was supported by a Senior Research Award from the Crohn’s and Colitis Foundation of America and R21 NIAID (AI1090102) to D.L.K. We thank B. Reinap, R. T. Bronson, and J. McCoy for PSA purification, histopathology discussions, and editorial expertise, respectively; N. Reading, H. Chun, E. Troy, and N. Surana for technical help; and Diane Mathis (Harvard Medical School) for manuscript review and helpful suggestions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, Fukuda S, Saito T, Narushima S, Hase K, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–236. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- Chen W, Liang X, Peterson AJ, Munn DH, Blazar BR. The indoleamine 2,3-dioxygenase pathway is essential for human plasmacytoid dendritic cell-induced adaptive T regulatory cell generation. J Immunol. 2008;181:5396–5404. doi: 10.4049/jimmunol.181.8.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb BA, Wang Q, Tzianabos AO, Kasper DL. Polysaccharide processing and presentation by the MHCII pathway. Cell. 2004;117:677–687. doi: 10.016/j.cell.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin BL, Sumpter TL, Tokita D, Salati J, Mellor AL, Thomson AW. Allostimulatory activity of bone marrow-derived plasmacytoid dendritic cells is independent of indoleamine dioxygenase but regulated by inducible costimulator ligand expression. Hum Immunol. 2009;70:313–320. doi: 10.1016/j.humimm.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creusot RJ, Yaghoubi SS, Chang P, Chia J, Contag CH, Gambhir SS, Fathman CG. Lymphoid-tissue-specific homing of bone-marrow-derived dendritic cells. Blood. 2009;113:6638–6647. doi: 10.1182/blood-2009-02-204321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol. 2004;173:3748–3754. doi: 10.4049/jimmunol.173.6.3748. [DOI] [PubMed] [Google Scholar]
- Goubier A, Dubois B, Gheit H, Joubert G, Villard-Truc F, Asselin-Paturel C, Trinchieri G, Kaiserlian D. Plasmacytoid dendritic cells mediate oral tolerance. Immunity. 2008;29:464–475. doi: 10.1016/j.immuni.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Bouladoux N, Sun CM, Wohlfert EA, Blank RB, Zhu Q, Grigg ME, Berzofsky JA, Belkaid Y. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irla M, Kupfer N, Suter T, Lissilaa R, Benkhoucha M, Skupsky J, Lalive PH, Fontana A, Reith W, Hugues S. MHC class II-restricted antigen presentation by plasmacytoid dendritic cells inhibits T cell-mediated autoimmunity. J Exp Med. 2010;207:1891–1905. doi: 10.1084/jem.20092627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Yang M, Wang YH, Lande R, Gregorio J, Perng OA, Qin XF, Liu YJ, Gilliet M. Plasmacytoid dendritic cells prime IL-10-producing T regulatory cells by inducible costimulator ligand. J Exp Med. 2007;204:105–115. doi: 10.1084/jem.20061660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, Ishii H, Totsuka T, Iiyama R, Okamoto R, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn's disease. Gastroenterology. 2001;121:875–888. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- Kassner N, Krueger M, Yagita H, Dzionek A, Hutloff A, Kroczek R, Scheffold A, Rutz S. Cutting edge: Plasmacytoid dendritic cells induce IL-10 production in T cells via the Delta-like-4/Notch axis. J Immunol. 2010;184:550–554. doi: 10.4049/jimmunol.0903152. [DOI] [PubMed] [Google Scholar]
- Katakura K, Lee J, Rachmilewitz D, Li G, Eckmann L, Raz E. Toll-like receptor 9-induced type I IFN protects mice from experimental colitis. J Clin Invest. 2005;115:695–702. doi: 10.1172/JCI22996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon F, Contractor N, Fuss I, Marth T, Lahey E, Iwaki S, la Sala A, Hoffmann V, Strober W, Kelsall BL. Antibodies to complement receptor 3 treat established inflammation in murine models of colitis and a novel model of psoriasiform dermatitis. J Immunol. 2006;177:6974–6982. doi: 10.4049/jimmunol.177.10.6974. [DOI] [PubMed] [Google Scholar]
- Lombardi V, Speak AO, Kerzerho J, Szely N, Akbari O. CD8alpha(+)beta(−) and CD8alpha(+)beta(+) plasmacytoid dendritic cells induce Foxp3(+) regulatory T cells and prevent the induction of airway hyper-reactivity. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado RA, von Andrian UH. How tolerogenic dendritic cells induce regulatory T cells. Adv Immunol. 2010;108:111–165. doi: 10.1016/B978-0-12-380995-7.00004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manches O, Munn D, Fallahi A, Lifson J, Chaperot L, Plumas J, Bhardwaj N. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Meyer Zu Horste G, Zozulya AL, El-Haddad H, Lehmann HC, Hartung HP, Wiendl H, Kieseier BC. Active immunization induces toxicity of diphtheria toxin in diphtheria resistant mice--implications for neuroinflammatory models. J Immunol Methods. 2010;354:80–84. doi: 10.1016/j.jim.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Miller JC, Brown BD, Shay T, Gautier EL, Jojic V, Cohain A, Pandey G, Leboeuf M, Elpek KG, Helft J, et al. Deciphering the transcriptional network of the dendritic cell lineage. Nat Immunol. 2012;13:888–899. doi: 10.1038/ni.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Mielcarz DW, Wang Y, Begum-Haque S, Dasgupta S, Kasper DL, Kasper LH. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010;3:487–495. doi: 10.1038/mi.2010.29. [DOI] [PubMed] [Google Scholar]
- Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5:232–239. doi: 10.1038/mi.2012.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmen MJ, Dijkstra CD, van der Ende MB, Pena AS, van Rees EP. Anti-CD11b/CD18 antibodies reduce inflammation in acute colitis in rats. Clin Exp Immunol. 1995;101:351–356. doi: 10.1111/j.1365-2249.1995.tb08363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Lee SM, Li J, Tran G, Jabri B, Chatila TA, Mazmanian SK. The Toll-like receptor 2 pathway establishes colonization by a commensal of the human microbiota. Science. 2011;332:974–977. doi: 10.1126/science.1206095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Round JL, Mazmanian SK. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez B, Chung DR, Sharpe AH, Yagita H, Kalka-Moll WM, Sayegh MH, Kasper DL, Tzianabos AO. Modulation of surgical fibrosis by microbial zwitterionic polysaccharides. Proc Natl Acad Sci USA. 2005;102:16753–16758. doi: 10.1073/pnas.0505688102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, Azuma M, Blazar BR, Mellor AL, Munn DH. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe. 2012;12:509–520. doi: 10.1016/j.chom.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science. 2013 doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn TW, Fontana A, Faria AM, Slavin AJ, Eugster HP, Zhang X, Koni PA, Ruddle NH, Flavell RA, Rennert PD, Weiner HL. Induction of oral tolerance to cellular immune responses in the absence of Peyer's patches. Eur J Immunol. 2001;31:1278–1287. doi: 10.1002/1521-4141(200104)31:4<1278::aid-immu1278>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Stephen TL, Niemeyer M, Tzianabos AO, Kroenke M, Kasper DL, Kalka-Moll WM. Effect of B7-2 and CD40 Signals from Activated Antigen-Presenting Cells on the Ability of Zwitterionic Polysaccharides To Induce T-cell stimulation. Infect Immun. 2005;73:2184–2189. doi: 10.1128/IAI.73.4.2184-2189.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nat Protoc. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- Swiecki M, Colonna M. Unraveling the functions of plasmacytoid dendritic cells during viral infections, autoimmunity, and tolerance. Immunol Rev. 2010;234:142–162. doi: 10.1111/j.0105-2896.2009.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde AA, Verstege MI, Hommes DW. Critical appraisal of the current practice in murine TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:995–999. doi: 10.1097/01.mib.0000227817.54969.5e. [DOI] [PubMed] [Google Scholar]
- Tzianabos AO, Pantosti A, Baumann H, Brisson JR, Jennings HJ, Kasper DL. The capsular polysaccharide of Bacteroides fragilis comprises two ionically linked polysaccharides. J Biol Chem. 1992;267:18230–18235. [PubMed] [Google Scholar]
- Wang Q, McLoughlin RM, Cobb BA, Charrel-Dennis M, Zaleski KJ, Golenbock D, Tzianabos AO, Kasper DL. A bacterial carbohydrate links innate and adaptive responses through Toll-like receptor 2. J Exp Med. 2006;203:2853–2863. doi: 10.1084/jem.20062008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Wilson NS, Schnorrer P, Proietto A, ten Broeke T, Matsuki Y, Mount AM, Belz GT, O'Keeffe M, Ohmura-Hoshino M, et al. Differential MHC class II synthesis and ubiquitination confers distinct antigen-presenting properties on conventional and plasmacytoid dendritic cells. Nat Immunol. 2008;9:1244–1252. doi: 10.1038/ni.1665. [DOI] [PubMed] [Google Scholar]
- Zhang J, Raper A, Sugita N, Hingorani R, Salio M, Palmowski MJ, Cerundolo V, Crocker PR. Characterization of Siglec-H as a novel endocytic receptor expressed on murine plasmacytoid dendritic cell precursors. Blood. 2006;107:3600–3608. doi: 10.1182/blood-2005-09-3842. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.