Abstract
Background
Gynaecological cancers are the second most common cancers among women. It has been suggested that centralised care improves outcomes but consensus is lacking.
Objectives
To assess the effectiveness of centralisation of care for patients with gynaecological cancer.
Search methods
We searched the Cochrane Gynaecological Cancer Group Trials Register, CENTRAL (The Cochrane Library, Issue 4, 2010), MEDLINE, and EMBASE up to November 2010. We also searched registers of clinical trials, abstracts of scientific meetings, and reference lists of included studies.
Selection criteria
We included randomised controlled trials (RCTs), quasi‐RCTs, controlled before‐and‐after studies, interrupted time series studies, and observational studies that examined centralisation of services for gynaecological cancer, and used multivariable analysis to adjust for baseline case mix.
Data collection and analysis
Three review authors independently extracted data, and two assessed risk of bias. Where possible, we synthesised the data on survival in a meta‐analysis.
Main results
Five studies met our inclusion criteria; all were retrospective observational studies and therefore at high risk of bias.
Meta‐analysis of three studies assessing over 9000 women suggested that institutions with gynaecologic oncologists on site may prolong survival in women with ovarian cancer, compared to community or general hospitals: hazard ratio (HR) of death was 0.90 (95% confidence interval (CI) 0.82 to 0.99). Similarly, another meta‐analysis of three studies assessing over 50,000 women, found that teaching centres or regional cancer centres may prolong survival in women with any gynaecological cancer compared to community or general hospitals (HR 0.91; 95% CI 0.84 to 0.99). The largest of these studies included all gynaecological malignancies and assessed 48,981 women, so the findings extend beyond ovarian cancer. One study compared community hospitals with semi‐specialised gynaecologists versus general hospitals and reported non‐significantly better disease‐specific survival in women with ovarian cancer (HR 0.89; 95% CI 0.78 to 1.01). The findings of included studies were highly consistent. Adverse event data were not reported in any of the studies.
Authors' conclusions
We found low quality, but consistent evidence to suggest that women with gynaecological cancer who received treatment in specialised centres had longer survival than those managed elsewhere. The evidence was stronger for ovarian cancer than for other gynaecological cancers.
Further studies of survival are needed, with more robust designs than retrospective observational studies. Research should also assess the quality of life associated with centralisation of gynaecological cancer care. Most of the available evidence addresses ovarian cancer in developed countries; future studies should be extended to other gynaecological cancers within different healthcare systems.
Keywords: Adult; Aged; Female; Humans; Middle Aged; Centralized Hospital Services; Centralized Hospital Services/statistics & numerical data; Cancer Care Facilities; Cancer Care Facilities/statistics & numerical data; Genital Neoplasms, Female; Genital Neoplasms, Female/mortality; Genital Neoplasms, Female/therapy; Gynecology; Gynecology/statistics & numerical data; Hospitals, Community; Hospitals, Community/statistics & numerical data; Hospitals, General; Hospitals, General/statistics & numerical data; Hospitals, Teaching; Hospitals, Teaching/statistics & numerical data; Medical Oncology; Medical Oncology/statistics & numerical data; Ovarian Neoplasms; Ovarian Neoplasms/mortality; Ovarian Neoplasms/therapy; Retrospective Studies
Plain language summary
Centralisation of care may prolong survival in women with ovarian cancer, and possibly more generally, gynaecological cancer
Gynaecological cancers are cancers affecting the ovaries, uterus, cervix, vulva, and vagina. They are the second most common cancers among women, after breast cancer. It is often suggested that outcomes are improved by centralising care within highly specialised services that include expert surgeons, radiologists, pathologists, oncologists who specialise in chemotherapy and radiotherapy, specialist nurses and other health professionals. However, consensus is lacking on whether centralisation of care for gynaecological cancer helps patients to live longer. This review investigated this issue by comparing the survival of women diagnosed with gynaecological cancer who received care from specialised and unspecialised centres.
We used a set of tests to ensure that the evidence the five studies identified reached the quality standard for our analysis.The analysis of three studies combined (meta‐analysis), assessing over 9000 women, suggested that institutions with gynaecologic oncologists (specialists in the field of gynaecological cancer treatment) on site may prolong the lives of women with ovarian cancer compared to community or general hospitals. Similarly, another meta‐analysis of three studies which assessed well over 50,000 women, found evidence to suggest that teaching centres or regional cancer centres (specialised centres) may prolong the lives of women with gynaecological cancer compared to community or general hospitals. The largest study in this meta‐analysis assessed all gynaecological cancers in 48,981 women, so it had major influence on the final result; this means that our findings are likely to be relevant to other gynaecological cancers, besides ovarian cancer.
Overall, the findings suggest that centralisation of care may prolong the lives of women with gynaecological cancer, and in particular ovarian cancer. However, the results should be interpreted with caution as all of the studies included in the review could be biased. For example, it is possible that the patients who were treated in specialised centres were less ill to begin with. Another weakness of the review is that only one of the studies included women with gynaecological cancers other than ovarian cancer.
Ideally, further studies in this area are needed. New studies should be designed to avoid the possibility of bias due to the treatment of women at specialist and non‐specialist centres being systematically different. Additionally, studies should assess the impact of centralisation of care on the quality of life of patients.
Most of the available evidence was about ovarian cancer in developed countries; future studies should be extended to other gynaecological cancers and to less developed countries.
Background
Description of the condition
Cancer is a leading cause of death worldwide (WHO 2008). Gynaecological cancers (i.e. cancer affecting the ovaries, uterus, cervix, vulva, vagina, and placental tissue) are among the most common cancers in women. Globally, a woman's risk of developing cancer of the ovaries, uterus or cervix (the most common gynaecological cancer) by the age of 65 is 2.2%; cancers of the vulva and vagina are less common. Gynaecological cancers account for 25% of all new cancers diagnosed amongst women aged up to 65 years in developing countries, compared to 16% in the developed world (GLOBOCAN 2008).
Cervical cancer is the second most common cancer among women up to 65 years of age, and is the most frequent cause of death from gynaecological cancers worldwide; its incidence is twice as high in developing countries (GLOBOCAN 2008). The disparity in incidence is attributed to effective screening programmes in more affluent countries (Macgregor 1994; Nieminen 1999). The treatment of early stage cervical cancer is surgery, while treatment for advanced disease is dependent on radiotherapy. Radiotherapy services are unfortunately not widely and readily available to many patients, particularly in the less developed countries. The introduction of prophylactic human papillomavirus vaccine in many developed countries should reduce the incidence of cervical cancer. However, once again, its impact in less developed countries would be less pronounced.
Uterine cancer tends to be a disease of the elderly and obese female population. More than 80% of these cases arise from the endometrium. Endometrial cancer is the most common genital tract cancer in women in developed countries. Globally, a woman's risk of developing cancer of the uterus by the age of 65 is 0.59%, with the rate twice as high in developed countries compared with developing countries (GLOBOCAN 2008). Obesity has now been shown to be associated with an increased risk of endometrial cancer in the US, Europe and the rest of the world (Linkov 2008). The continuing increases in obesity rates forewarn that endometrial cancer is likely to become more of a public health problem in future years.
Ovarian cancer is the seventh most common cancer among women up to 64 years of age. It often presents late, with widespread intra‐abdominal disease where treatment includes cytoreductive surgery in combination with chemotherapy. A woman's risk of developing ovarian cancer by the age of 65, ranges from 0.36% in developing countries to 0.64% in developed countries (GLOBOCAN 2008). There is evidence to suggest that optimal surgical debulking by trained gynaecological oncologists results in better survival outcomes (Elattar 2011). More recently, studies have shown some improvement in progression‐free and overall survival associated with targeted therapy, and more aggressive chemotherapeutic regimes (Gardner 2011; Katsumata 2009).
Vulval cancer is relatively rare and tends to present late. Aggressive surgery can be mutilating with high morbidity and psychosexual sequelae. Removal of large tumours requires expert reconstructive surgery, whilst new techniques such as sentinel node lymphadenectomy (avoiding radical lymphadenectomy), are often available only in large specialised units.
Choriocarcinomas and other related placental disease are extremely rare. Traditionally, the management of such disease takes place in supra‐regionalised centres in the developed world as expertise in this field is very limited outside such centres.
Gynaecological cancers arise from several different sites with differing cell types and, as such, management principles vary. The treatment options often vary according to the stage of disease, the histological subtype and comorbidity of the woman. Awareness of the diverse disease types, clinical presentations and therapeutic options can potentially be limited in non‐specialised units.
The widespread variation in management of such cancers can present significant diagnostic and therapeutic challenges to both surgeons and oncologists. The focus of this review was to assess whether clinical outcomes differ between centralised specialised centres and local non‐specialised units.
Description of the intervention
International practice in many developed countries is now recommending centralised care for the majority of cancer patients. In the UK, in response to the Calman‐Hine report, cancer networks crossing organisational boundaries, incorporating teaching and non‐teaching hospitals were established (DOH 1995). This model of care assumes that care of most cancers is improved by centralising care within concentrated highly specialised services that include a multidisciplinary team comprising expert surgeons, radiologists, pathologists, medical (chemotherapy) and clinical (radiotherapy) oncologists, palliative care physicians and specialised nursing staff and other health professionals. Previously, in many countries, cancer care at all levels was administered by general surgeons and physicians within non‐specialised hospitals.
How the intervention might work
If centralisation of care results in better outcomes for patients, this could be due to the following.
The effect of higher volume and surgical training might translate directly to better clinical outcomes (Olaitan 2001).
The cancer centres diagnose and manage patients in multidisciplinary teams (MDTs) consisting of professionals with specialised knowledge and surgical expertise.
The experience and training of expert diagnostic pathologists and radiologists might improve the robustness of test results leading to better tailored cancer treatment.
The experience and training of specialised medical and clinical oncologists within high throughput environments might lead to better tailored cancer treatment.
Cancer centres are more likely to have dedicated perioperative support for surgical oncology which might improve outcomes.
Cancer centres are more likely to have specialist cancer support nurses, including palliative care services whereby their support might lead to better psychological outcomes for patients and their families.
Why it is important to do this review
There is great debate on whether centralised care actually improves survival and morbidity (Brookefield 2009; Crawford 2007; Crawford 2008; Engel 2005; Olaitan 2007; Olaitan 2008b; Rachet 2009; Richards 2009; Sikora 2009). The cost of developing such a framework of care is significant and the heavy investment required for such cancer service can only be justified if patients are experiencing better clinical outcomes. Furthermore, a centralised approach often involves patients travelling relatively far away from their local community hospitals, and the social impact on patient wellbeing needs to be justified by evidence of improved care and better outcomes.
In ovarian cancer, the evidence base for improved outcomes is primarily based on studies comparing outcomes of dedicated oncology surgeons with those of general gynaecologists and, more recently, on retrospective comparisons of outcomes between centralised and non‐centralised models of care (Vernooji 2007).
Objectives
To assess the effectiveness of centralisation of care for patients with gynaecological cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included the following types of studies (Shadish 2002).
Randomised controlled trials (RCTs).
As we expected to find few RCTs, we included the following non‐randomised studies with concurrent comparison groups.
Quasi‐RCTs.
-
Controlled before‐and‐after (CBA) studies, i.e. studies that assign participants to intervention and control groups other than at random, and which included assessment of the main outcomes before and after the intervention. We only included these studies if they satisfied certain quality criteria:
contemporaneous data collection (pre‐ and postintervention periods for intervention and control sites are the same); and
intervention and control sites are comparable with respect to patient characteristics.
-
Interrupted time series (ITS) studies, i.e. studies designed to assess whether a change in trend occurred which could be attributable to an intervention. We only included these studies if they satisfy certain quality criteria:
study includes a clearly defined point in time when the intervention occurred; and
at least three data points were recorded before, and three after the intervention.
Observational cohort studies and unselected case series of 200 or more patients which included concurrent comparison groups.
We excluded case‐control studies, studies that did not have concurrent comparison groups, and case series of fewer than 200 patients. We excluded studies if:
the study cohort was not population‐based (i.e. the majority of patients with a specified cancer in a region should be included, or the study population should consist of a random sample form a population‐based registry); and
the study did not include a concurrent comparison group.
In order to minimise selection bias, for non‐randomised studies, we included only studies that used statistical adjustment for baseline case mix using multivariable analyses (e.g. disease severity, age, comorbidity, and type of cancer).
Types of participants
Female adult patients (at least 18 years of age) with a gynaecological malignancy: ovarian, endometrial, cervical, or vulval cancer, or gestational trophoblastic disease.
Types of interventions
Intervention
Management of patients in a gynaecological cancer tertiary/regional referral centre.
Comparison
Management of patients elsewhere.
We excluded studies if they were restricted to:
assessment of the effect of surgeon volume; or
comparison of outcomes for patients treated in gynaecological oncology and general gynaecological centres.
Types of outcome measures
Primary outcomes
Overall survival: survival until death from all causes.
Secondary outcomes
Progression‐free survival.
-
Adverse events classified according to CTCAE 2006:
direct surgical morbidity (e.g. injury to bladder, ureter, vascular system, small bowel, or colon), presence and complications of adhesions, febrile morbidity, haematoma, and local infection;
surgically related systemic morbidity (chest infection, thromboembolic events (deep vein thrombosis and pulmonary embolism), cardiac events (cardiac ischemias and cardiac failure), and cerebrovascular accident;
recovery: delayed discharge, unscheduled re‐admission;
chemotherapy toxicity;
radiotherapy toxicity; and
other.
Search methods for identification of studies
We searched for papers in all languages and carried out translations when necessary.
Electronic searches
See: Cochrane Gynaecological Cancer Group methods used in reviews.
We searched the following electronic databases.
The Cochrane Gynaecological Cancer Collaborative Review Group's Trials Register
The Cochrane Central Register of Controlled Trials (CENTRAL), Issue 4, 2010
MEDLINE to November 2010
EMBASE to November 2010
The MEDLINE, EMBASE, and CENTRAL search strategies are based on terms related to the review topic and are presented in Appendix 1, Appendix 2, and Appendix 3 respectively.
We searched MEDLINE from 1950 to November 2010, and EMBASE from 1980 to November 2010.
We identified all relevant articles found on PubMed and using the 'related articles' feature, we carried out a further search for newly published articles.
Searching other resources
We searched MetaRegister, Physicians Data Query, www.controlled‐trials.com/rct, www.clinicaltrials.gov, and www.cancer.gov/clinicaltrials for ongoing trials. However, we did not find any relevant ongoing trials or active trial groups, and so we did not make any contacts. We had planned to contact the main investigators of any relevant ongoing trials and any major co‐operative trials groups active in this area for further information.
We searched conference proceedings and abstracts through ZETOC (www.zetoc.mimas.ac.uk) and WorldCat Dissertations. We also searched reports of conferences from the following sources.
British Journal of Cancer.
British Cancer Research Meeting.
Annual Meeting of the International Gynecologic Cancer Society.
Annual Meeting of the American Society of Gynecologic Oncologist.
Annual Meeting of European Society of Medical Oncology (ESMO).
Annual Meeting of the American Society of Clinical Oncology (ASCO).
Reference lists and correspondence
We checked the citation lists of included studies and contacted experts in the field, including directors of UK cancer registries, to identify further reports of trials.
Data collection and analysis
Selection of studies
We downloaded all titles and abstracts retrieved by electronic searching to the reference management database, Endnote; we removed duplicates and split up the remaining references for independent examination by four review authors (YLW, AB, TE, MK). We added titles and abstracts retrieved from other sources to Endnote. We excluded those studies which clearly did not meet the inclusion criteria and obtained copies of the full text of potentially relevant references. Three review authors (YLW, AB, MK) independently assessed the eligibility of retrieved papers and resolved disagreements by discussion. We documented reasons for exclusion.
Data extraction and management
For included studies, we extracted the following data.
Author, year of publication and journal citation (including language)
Country
Setting
Inclusion and exclusion criteria
Study design, methodology
-
Study population
total number enrolled
patient characteristics
age
race
comorbidities
type of cancer
stage at diagnosis
-
Intervention details
definition of gynaecological oncology surgeons
definition of specialised cancer centre
-
Comparison
details of gynaecologists/surgeons
details of setting
We recorded any data on changes in management as a result of tertiary level expert opinion on radiology or pathology
Risk of bias in study (see below)
Duration of follow‐up
-
Outcomes: overall survival; progression‐free survival; and adverse events
for each outcome: outcome definition
unit of measurement (if relevant)
for adjusted estimates: variables adjusted for in analysis
results: number of participants allocated to each intervention group
for each outcome of interest: sample size; and missing participants
We extracted data on outcomes as follows.
For time‐to‐event (overall and progression‐free survival) data, we extracted the log of the hazard ratio [log(HR)] and its standard error (SE) from study reports. This HR compared the risk of death among women treated in specialised centres with the risk of death among women treated in non‐specialised centres; hence a HR less than one indicated better survival in specialised centres.
Where possible, the data we extracted were those relevant to an intention‐to‐treat (ITT) analysis, in which participants were analysed in groups to which they were assigned.
We noted the time‐points at which outcomes were collected and reported.
Three review authors (YLW, AB, MK) extracted data independently onto a data extraction form, specially designed for the review. Review authors resolved differences by discussion.
Assessment of risk of bias in included studies
We assessed the risk of bias in included studies using the Cochrane Collaboration's tool (Higgins 2011). This included assessment of:
sequence generation;
allocation concealment;
blinding (restricted to blinding of outcome assessors since it is not possible to blind participants and personnel to type of service provider);
-
incomplete outcome data: we recorded the proportion of participants whose outcomes were not reported at the end of the study; we coded a satisfactory level of loss to follow‐up for each outcome as:
yes, if fewer than 20% of patients were lost to follow‐up and reasons for loss to follow‐up were similar in both treatment arms;
no, if more than 20% of patients were lost to follow‐up or reasons for loss to follow‐up differed between treatment arms; or
unclear, if loss to follow‐up was not reported;
selective reporting of outcomes; and
other possible sources of bias.
As we included observational studies, we assessed risk of bias in accordance with the following additional criteria.
Cohort selection.
-
Were relevant details of criteria for assignment of patients to treatments provided?
Low risk of bias (e.g. yes).
High risk of bias (e.g. no).
Unclear risk of bias.
-
Was the group of women who received the experimental intervention (centralised care) representative?
Low risk of bias (e.g. yes, as they were representative of women with gynaecological cancer).
High risk of bias (e.g. no, as group of patients was selected).
Unclear risk of bias (e.g. selection of group was not described).
-
Was the group of women who received the comparison intervention (non‐centralised care) representative?
Low risk of bias (e.g. yes, as drawn from the same population as the experimental cohort).
High risk of bias (e.g. no, as drawn from a different source).
Unclear risk of bias (e.g. selection of group was not described).
We assessed cohort comparability on the basis of study design or analysis of cohort differences.
-
Were there no differences between the two groups or were differences controlled for, in particular with reference to age, FIGO (International Federation of Gynecology and Obstetrics) stage, histological cell type, and differentiation?
Low risk of bias, if age and at least two other of these characteristics were reported, and any reported differences were controlled for.
High risk of bias, if the two groups differed, and differences were not controlled for.
Unclear risk of bias, if fewer than three of these characteristics were reported, even if there were no other differences between the groups, and other characteristics were controlled for.
Two review authors (YLW, AB) independently applied the risk of bias tool and resolved differences by discussion. Results are presented in both a risk of bias summary (Figure 1) and a risk of bias graph (Figure 2). We interpreted results of meta‐analyses in light of the findings with respect to risk of bias.
1.
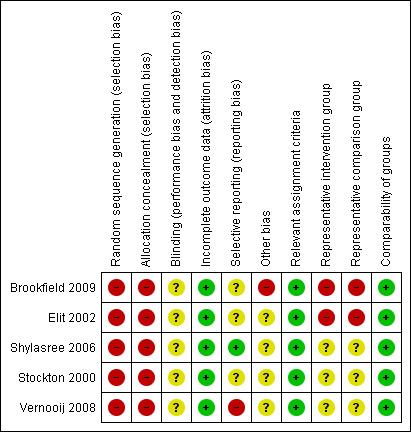
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
2.
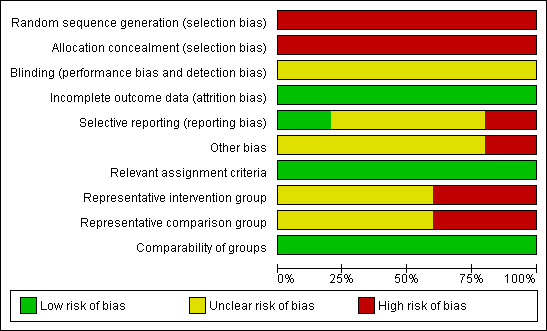
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
For time‐to‐event data, we used the HR.
Dealing with missing data
We did not impute missing outcome data for any of the outcomes.
Assessment of heterogeneity
We assessed heterogeneity between studies by visual inspection of forest plots, and by estimation of the percentage heterogeneity between trials which cannot be ascribed to sampling variation (Higgins 2003).
Assessment of reporting biases
There was an insufficient number of included studies to allow an assessment of small study effects, such as publication bias.
Data synthesis
When sufficient, clinically similar studies were available, we pooled their adjusted results in meta‐analyses; we reported the 95% confidence interval (CI) on the pooled estimate.
For time‐to‐event data, we pooled HRs using the generic inverse variance facility of Review Manager 5 (RevMan 2011). One included study (Elit 2002) compared two types of specialised care with one comparison group; therefore we divided the comparison group in two and treated comparisons between each treatment group and the split comparison group as independent comparisons.
We used random‐effects models with inverse variance weighting for all meta‐analyses (DerSimonian 1986).
Subgroup analysis and investigation of heterogeneity
Where possible, we performed subgroup analyses or separate analyses, grouping the studies by:
tumour site;
-
different types of interventions (specialised centres):
institutions with gynaecologic oncologists on site (specialised centres) versus community or general hospital
teaching or regional cancer centre versus community or general hospital
community hospital with semi‐specialised gynaecologist versus general hospital.
Sensitivity analysis
We performed a sensitivity analysis, comparing the choice of prognostic variables used for adjustment in the trial of Stockton 2000 (see Included studies section for further details).
Results
Description of studies
Results of the search
We identified a total of 8689 unique references from the search strategy. Two review authors (YLW and AB, MK and TE) independently assessed the titles and abstracts: we excluded 8634 irrelevant publications at this stage. We scrutinised and retrieved the remaining 56 potentially eligible articles in full text; we translated non‐English studies. We excluded 49 reports that did not meet the eligibility criteria, leaving seven references, reporting a total of five studies that qualified for the overview. The reasons for exclusion are described in the table Characteristics of excluded studies. The five studies that met our inclusion criteria are described in the table Characteristics of included studies.
Included studies
The five included studies that met our inclusion criteria (Brookfield 2009; Elit 2002; Shylasree 2006; Stockton 2000; Vernooij 2008) enrolled a total of 62,987 women with gynaecological cancer; data were available for 62,191 of these. The women included in this review were diagnosed from 1990 up to 2003, with most being treated from the late 1990s onwards.
Three studies (Elit 2002; Shylasree 2006; Vernooij 2008) reported exclusively on patients with ovarian cancer.
One study (Brookfield 2009) included women with any of five gynaecological cancers (cervical, ovarian, endometrial, uterine sarcoma, and vulval); it reported data for all cancers combined, and by cancer site.
One study (Stockton 2000) reported on various cancer sites including both ovarian cancer and non‐gynaecological malignancies; it reported data separately by cancer site.
The number of patients included varied from 250 patients in the Shylasree 2006 study to 48,981 in the Brookfield 2009 study.
Design
All five eligible studies were retrospective (Brookfield 2009; Elit 2002; Shylasree 2006; Stockton 2000; Vernooij 2008) and used electronic records to identify suitable patients.
The Brookfield 2009 study was a retrospective multicentre study comparing teaching versus non‐teaching facilities and high versus low volume cancer centres. All cases of cervical, ovarian, endometrial and vulval malignancies, and uterine sarcomas diagnosed in the state of Florida from 1990 to 2000 were identified using the 2007 Florida Cancer Data System (FCDS) data set.
The Elit 2002 study was of a population‐based retrospective cohort, set in Ontario. All newly diagnosed epithelial cases of ovarian cancer were identified by electronic records from the hospitals in Ontario.
The Shylasree 2006 study was a funded retrospective audit which included all women undergoing laparotomy for suspected ovarian cancer in 20 hospitals in Wales. The study was initiated prior to introduction of cancer management guidelines in the region of Cardiff.
Stockton 2000 study was a retrospective population‐based study of women with colon, rectal, breast, melanoma, bladder, and ovarian cancer, identified by the East Anglian Cancer Registry. The data were analysed by cancer site and separate results were reported for ovarian cancer.
The Vernooij 2008 study was a retrospective, population‐based cohort of Dutch patients diagnosed with ovarian cancer. Data from the Netherlands Cancer Registry were linked to mortality data from the Statistics Netherlands database to obtain the date and cause of death.
Participant characteristics
The Brookfield 2009 study included 48,981 women, with the distribution of cases as follows. Cervical: 10,175 (20.8%); ovarian: 15,131 (30.9%); endometrial: 21,149 (43.2%); uterine sarcoma: 253 (0.5%); and vulval: 2,273 (4.6%). The median age at diagnosis ranged between 51.9 and 67.6 years in women with different types of gynaecological cancer. There was a difference in the stage and age of patients referred to teaching and non‐teaching facilities (the teaching facility tended to manage younger patients and patients with more advanced disease).
The Elit 2002 study enrolled 3815 women with all stages of epithelial ovarian cancer, and outcome data were available for 3,350 of these women; women in the study had a mean and median age of 58.9 (standard deviation (SD) = 14.4) and 60 years respectively, and had received no prior chemotherapy or surgery.
The Shylasree 2006 study analysed the outcomes of 250 women with ovarian cancer who had a mean age of 63.2 years (SD = 14.1 years; range 13.5 to 91.8). There were no significant differences regarding stage, residual disease, tumour histology, or grade between women managed in the cancer centre and those managed in the peripheral units.
The Stockton 2000 study included a total of 989 women with all stages of ovarian cancer. In this cohort, 719 (73%) were under 75 years of age while 270 (27%) were older than 75.
The Vernooij 2008 study analysed 8621 women with epithelial ovarian cancer. The stage and histology are described in Characteristics of included studies. The median age of patients was 64 years (range 8 to 98).
Interventions
Brookfield 2009 compared the survival outcomes of teaching facilities with those of non‐teaching facilities based on the criteria set by the Association of American Medical Colleges. An additional analysis was performed based on hospital volume. Facilities were classified as high, intermediate, and low volume centres.
Elit 2002 compared the outcomes of patients managed at institutions with gynaecologic oncologists on site, or at teaching hospitals or regional cancer centres with no gynaecological oncologist available on site with outcomes of patients treated at community hospitals. The study also included an analysis according to the hospital volume i.e. high, intermediate, and low volume centres.
Shylasree 2006 compared the outcomes of women treated in two cancer centres with those of women treated in 18 district hospitals.
Stockton 2000 compared the outcomes of women treated in three cancer centres (teaching hospital with radiotherapy and oncology departments) with those of women treated in six district general hospitals.
Vernooij 2008 compared the outcomes based on the hospitals' level of specialisation (general, semi‐specialised, or specialised). The classification was based on the specialist providing the service, within the setting of a regional or community hospital.
Outcomes
The mean duration of follow‐up was 913 days (range 733 to 1101 days) in the Shylasree 2006 study and 3 years (range = 0 to 10 years) in the Vernooij 2008 study. The duration of follow‐up was not reported in the other three studies (Brookfield 2009; Elit 2002; Stockton 2000).
Four studies (Brookfield 2009; Elit 2002; Shylasree 2006; Stockton 2000) reported overall survival; Vernooij 2008 reported disease‐specific survival rather than overall survival. As the coding of death certificates is potentially error‐prone (Ravakhah 2006), death from any cause should ideally have been reported. All five studies reported survival using appropriate statistical techniques (hazard ratios (HRs) to correctly allow for censoring). The Shylasree 2006 study also reported progression‐free survival.
The HR in the Brookfield 2009 study was adjusted for: cancer origin, hospital volume, facility, age (discrete), race, ethnicity (Hispanic versus non‐Hispanic), primary payer, lymph nodes examined, tumour stage, tumour grade, surgical extirpation, chemotherapy, and radiotherapy. This study reported HRs by cancer origin as well as giving an overall HR for all gynaecological cancers, but it was not possible to obtain the 95% CI for the HRs by cancer type or site, and so we could not include these HRs in meta‐analyses. However the HRs for each gynaecological cancer were similar to the HR for cancers of all gynaecological origin combined (see Characteristics of included studies).
The HR in the Elit 2002 study was adjusted for: age, comorbidity, metastatic status, hospital surgical volume (annual), surgeon specialisation and surgeon volume (year of surgery). Comorbidity was defined using a modification of Charlson’s comorbidity index. A cumulative Charlson score was obtained from all hospitalisation data for the patient over a 12‐month period leading up to and including the admission corresponding to initial surgery. This modification did not include the two index elements associated with cancer ‐ primary cancer and metastatic cancer. The study had three arms and used community hospitals in each comparison and so the standard error (SE) of the log(HR) was multiplied by the square root of 2 so that the study was not given undue weight in the meta‐analyses.
The HR in the Shylasree 2006 study was adjusted for: primary surgeon, debulking procedure, postoperative decision regarding chemotherapy, place of postoperative chemotherapy, postoperative chemotherapy, and age.
The HR in the Stockton 2000 study was adjusted for: 10‐year age band in model 1 and 10‐year age band and TNM (tumour, node, metastasis) staging (Union for International Cancer Control (UICC) classification) at diagnosis in model 2. Sex was an additional adjustment factor when the other cancer sites were assessed.
The HR in the Vernooij 2008 study was adjusted for: type of gynaecologist and hospital volume, age, and stage.
Adverse events were not reported by treatment arm in any of the studies.
Excluded studies
After obtaining the full text, we excluded forty‐nine references for the following reasons.
In twenty‐three studies (Carney 2002; Chan 2007; Earle 2006; Eisenkop 1992; Engelen 2006; Ghaemmaghami 2010; Grant 1992; Grossi 2002; Ioka 2004; Ioka 2005; Junor 1999; Kehoe 1994; Kumpulainen 2002; Kwon 2008; Macdonald 2005; Mayer 1992; Nguyen 1993; O'Malley 2003; Petignat 2000; Tanner 2008; Tingulstad 2003; Van der Zee 2009; Woodman 1997), the interventions were not clearly defined. In addition, we excluded studies where only the effect of the surgeon was measured.
Eight studies (Goff 2006; Goff 2007; Munstedt 2003; Paulsen 2006; Pearl 2002; Petignat 1999; Wolfe 1996; Wolfe 1997) did not report pertinent outcomes defined in this review.
Fifteen studies (Bristow 2006; Brolmann 1992; Chan 1999; Du Bois 2009; Giede 2005; Grant 1999; Grant 2000; Lehtovirta 2000; Luesley 2000; Olaitan 2008a; Rich 1993; Savage 2008; Tangjitgamol 2009; Vernooij 2007; Williams 2008) were letters, commentaries, editorials, or reviews.
Three studies (Bailey 2006; Crawford 2002; Diaz‐Montes 2006) did not include a multivariate analysis or use any kind of statistical adjustment for survival outcomes.
For further details of all excluded studies see the table Characteristics of excluded studies.
Risk of bias in included studies
All five studies (Brookfield 2009; Elit 2002; Shylasree 2006; Stockton 2000; Vernooij 2008) reported retrospective observational data and were therefore at high risk of bias: four studies satisfied just three of the ten criteria used to assess the risk of bias in non‐randomised studies and the Shylasree 2006 study satisfied only four (see Figure 1, Figure 2).
Since all of the included studies were non‐randomised, the method of generation of the sequence of random numbers used to allocate women to treatment arms, and concealment of this allocation sequence from patients and healthcare professionals involved in the study was not relevant and these items were scored as indicating high risk of bias. However, all five studies did report details of assignment of patients to treatment centres. None of the studies reported whether the outcome assessors were blinded. It was not certain whether three of the studies (Brookfield 2009; Elit 2002; Stockton 2000) had reported all the outcomes that they assessed, but this was not the case in the Shylasree 2006 study. It seemed highly unlikely that outcomes had been selectively reported in this study as primary outcomes such as overall and progression‐free survival were both reported and clearly defined. Important factors were adjusted for in multivariate analyses so all treatment centres were deemed to be comparable. It was unclear whether any other bias may have been present in any of the included studies, although the Brookfield 2009 study presented imbalances at baseline for almost all prognostic factors for comparisons of hospital volume and medical facility. Although the study adjusted for these factors in a multivariate analysis, selection bias was still likely to be a problem. In two studies (Brookfield 2009; Elit 2002), women who received treatment in the different centres were probably not representative of women with gynaecological (Brookfield 2009) and ovarian (Elit 2002) cancer as stage was distributed slightly differently in the women treated in specialised and non‐specialised centres. It was unclear whether women receiving treatment in the different treatment centres were representative of women with ovarian cancer in the other three studies. Only the Brookfield 2009 study reported overall survival in terms of gynaecological cancer, whereas the other studies included women with ovarian cancer. At least 87% of women who were identified were assessed at endpoint in all five studies.
Effects of interventions
Meta‐analyses of survival are based on HRs that were adjusted for important prognostic variables, although these variables differed between studies (see Included studies).
Institutions with gynaecologic oncologists on site (specialised centres) versus community or general hospital
Overall survival/disease‐specific survival
(See Analysis 1.1)
1.1. Analysis.
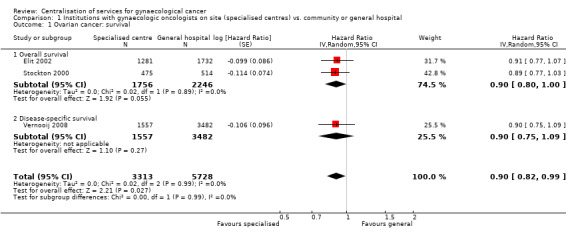
Comparison 1 Institutions with gynaecologic oncologists on site (specialised centres) vs. community or general hospital, Outcome 1 Ovarian cancer: survival.
Meta‐analysis of three studies (Elit 2002; Stockton 2000 (model 2, adjusting for 10‐year age band and TNM tumour stage at diagnosis); Vernooij 2008), assessing 9,041 participants, found that women with ovarian cancer who received treatment from a specialised cancer centre with gynaecologic oncologists on site had significantly better survival than women who received treatment from community or general hospitals (comparing risk of death among women treated in specialised centres with that among women treated in non‐specialised centres: HR 0.90; 95% CI 0.82 to 0.99). The percentage of the variability in effect estimates that was due to heterogeneity rather than sampling error (chance) was not important (I2 = 0%).
This statistically significant pooled result arose although none of the three studies individually found a significant difference in overall survival between the two different hospital settings.
Overall survival/disease‐specific survival: sensitivity analysis
(See Analysis 1.2)
1.2. Analysis.
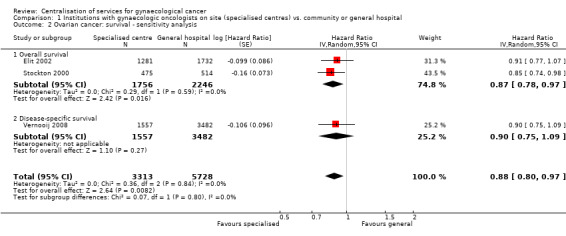
Comparison 1 Institutions with gynaecologic oncologists on site (specialised centres) vs. community or general hospital, Outcome 2 Ovarian cancer: survival ‐ sensitivity analysis.
Analysis 1.1 was repeated using model 1 of Stockton 2000, which adjusted for 10‐year age band but not for TNM tumour stage at diagnosis, and which found that women who received treatment at a hospital with a radiotherapy and oncology department had significantly better survival than those who attended a district general hospital. The results of using this alternative model in the meta‐analysis were similar to those of the previous meta‐analysis (HR 0.88; 95% CI 0.80 to 0.97; I2 = 0%)
Teaching or regional cancer centre versus community or general hospital
Overall survival
(See Analysis 2.1)
2.1. Analysis.
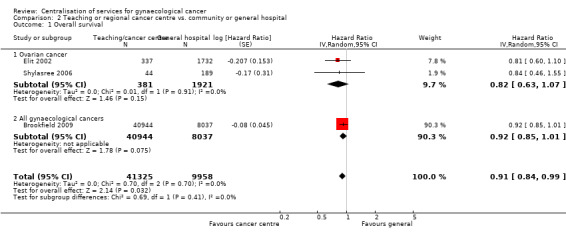
Comparison 2 Teaching or regional cancer centre vs. community or general hospital, Outcome 1 Overall survival.
Meta‐analysis of three studies (Brookfield 2009; Elit 2002; Shylasree 2006), assessing 51,283 participants, found that women who received treatment from a teaching or regional cancer centre had significantly better survival than women who received treatment from community or general hospitals (HR 0.91; 95% CI 0.84 to 0.99). The percentage of the variability in effect estimates that was due to heterogeneity rather than chance was not important (I2 = 0%); two of the studies included only women with ovarian cancer, whereas the Brookfield 2009 study included women with any form of gynaecological cancer. This statistically significant pooled result arose even though two of the three studies (Brookfield 2009; Shylasree 2006) individually found no significant difference in overall survival between the two different hospital settings, and the third (Elit 2002) found a difference of borderline significance (P = 0.05, meta‐analysis).
Progression‐free survival
(See Analysis 2.2)
2.2. Analysis.
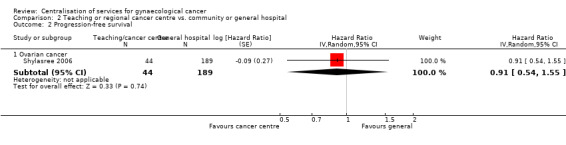
Comparison 2 Teaching or regional cancer centre vs. community or general hospital, Outcome 2 Progression‐free survival.
The Shylasree 2006 study, which assessed 233 participants, found no statistically significant difference in progression‐free survival between women who attended a teaching or regional cancer centre and those who received treatment from a community or general hospital (HR 0.91; 95% CI 0.54 to 1.55).
Community hospital with semi‐specialised gynaecologist versus general hospital
Disease‐specific survival
(See Analysis 3.1)
3.1. Analysis.
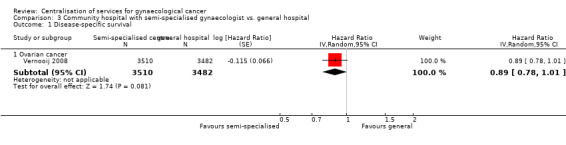
Comparison 3 Community hospital with semi‐specialised gynaecologist vs. general hospital, Outcome 1 Disease‐specific survival.
The Vernooij 2008 study, which assessed 6,992 participants, found better disease‐specific survival in women who attended a community hospital with semi‐specialised gynaecologists than in those who received treatment from a general hospital (HR 0.89; 95% CI 0.78 to 1.01), although this was not statistically significant (P = 0.08).
Discussion
Summary of main results
We found five retrospective observational studies (Brookfield 2009; Elit 2002; Shylasree 2006; Stockton 2000; Vernooij 2008) that adjusted for case mix using a multivariate analysis, and met our inclusion criteria. These studies assessed overall or disease‐specific survival in women with ovarian and/or other gynaecological cancer treated in specialised or semi‐specialised centres versus community or general hospitals. One of these studies (Shylasree 2006) also assessed progression‐free survival. Of the five studies, only one (Brookfield 2009) assessed the evidence for other gynaecological cancers besides ovarian. Outcomes according to the different stages of the different gynaecological cancers within the different hospital settings were not analysed separately.
Meta‐analysis of three of these studies (Elit 2002; Stockton 2000; Vernooij 2008) assessing over 9,000 women, found evidence to suggest that institutions with gynaecological oncologists on site have better survival in women with gynaecological cancer than community or general hospitals (comparing risk of death among women treated in specialised centres with that among women treated in non‐specialised centres: HR 0.90; 95% CI 0.82 to 0.99). Similarly, a different meta‐analysis of three trials (Brookfield 2009; Elit 2002; Shylasree 2006), which assessed over 50,000 women, found evidence to suggest that teaching or regional cancer centres have better overall survival in women with ovarian or any gynaecological cancer than community or general hospitals (HR 0.91; 95% CI 0.84 to 0.99). The largest study in this meta‐analysis assessed 48,981 women, of whom 31% had ovarian cancer and 69% had other gynaecological cancers, and contributed considerably to the pooled estimate. Thus the findings of this review may extend beyond centralisation of care for ovarian cancer. One study reported findings which suggested that community hospitals with semi‐specialised gynaecologists had better disease‐specific survival in women with ovarian cancer than general hospitals (Vernooij 2008), although the difference was not statistically significant (HR 0.89; 95% CI 0.78 to 1.01).
Progression‐free survival was reported in only one study, so was not sufficiently documented to allow firm conclusions to be drawn for this outcome.
Adverse event data were not reported in any of the studies.
Overall completeness and applicability of evidence
The included studies compared outcomes in a range care facilities ‐ cancer centres, teaching hospitals, institutions with gynaecologic oncologists, or semi‐specialised gynaecologists on site ‐ with outcomes in community or general hospitals.
One of the limitations of the review was that all the included studies were performed in developed countries (Canada, Netherlands, UK, and US). As the organisation of care for gynaecological cancer may vary widely between countries, the findings of the review may have limited applicability to developing countries. A further limitation was that only one study (Brookfield 2009) included women with gynaecological cancers other than ovarian cancer. Although this was a large study which assessed women with other gynaecological cancers, evidence from further studies, ideally in other countries, is needed in order to confirm the benefits of centralisation for such women. Finally, the review was unable to assess adverse events as these were not reported by any of the included studies.
While the evidence suggested that women treated in specialised centres had better survival than women treated elsewhere, the means whereby this benefit was achieved remains unclear and, indeed, it was beyond the scope of the review to investigate this issue. Although some authors have argued that centralisation of cancer care encourages a multidisciplinary team approach which has benefits for overall survival (Du 2011; Stephens 2006), the evidence for this remains equivocal (Fleissig 2006).
We did not attempt to estimate the cost‐effectiveness of centralisation of gynaecological cancer which would obviously be important to policy‐makers. We did not attempt to compare quality of life of patients in centralised and non‐centralised care, and none of the included studies reported this outcome. The lack of evidence to inform anything more than the survival outcomes of patients makes it difficult to assess the overall effectiveness of centralisation.
Quality of the evidence
The five included studies assessed a total of 62,191 women, 28,341 of whom had ovarian cancer; one of these studies (Brookfield 2009) also assessed 33,850 women with other gynaecological cancers.
The strengths of the review are that four (Brookfield 2009; Elit 2002; Stockton 2000; Vernooij 2008) of the five included studies were large and one (Brookfield 2009) was very large, including over 40,000 women. The inclusion criteria were strict and we included only studies that adjusted for case‐mix. All studies reported an adjusted HR, which is the best statistic to summarise the difference in survival between two treatment groups over the duration of a study (Altman 1995). The findings of our two meta‐analyses of overall survival were highly consistent, although one analysis assessed institutions with gynaecologists on site (Analysis 1) and the other assessed teaching or regional cancer centres (Analysis 2). Furthermore, although none of the individual studies within the meta‐analyses found a statistically significant benefit of centralisation, their results were consistent and their pooled results were statistically significant, showing better survival in women who received centralised care. The studies that reported disease‐specific survival (Vernooij 2008) and progression‐free survival (Shylasree 2006) likewise reported better outcomes in women receiving centralised care and again, although their findings were not statistically significant, they were quantitatively consistent with the findings of the meta‐analyses.
The main methodological limitation of the review is that all the included studies were at high risk of bias due to their retrospective, observational nature: they satisfied, at most, four of the criteria used to assess risk of bias. We cannot be sure that statistical adjustment for important prognostic factors fully controlled for systematic differences between women who received centralised and non‐centralised care. Ideally, comparisons of centralised and non‐centralised care would be performed using RCTs in order to ensure no systematic differences between women receiving the two types of care. However, it is often not feasible to evaluate organisational interventions in an RCT (EPOC). A recommended alternative design is an interrupted time series (ITS) design in which data are collected at several time‐points before and after the intervention (in this case, centralisation of care), and the intervention effect is estimated by a comparison with the pre‐intervention trend. Another possible design is a controlled before‐and‐after (CBA) study, in which data are collected on the intervention group and on a control group, both before and after the intervention is introduced. This design is less robust because there may be unidentified differences between the intervention and control groups which influence the estimate of the effect of the intervention.
Overall, the evidence favours centres with specialised care rather than community or general hospitals, although the quality of the evidence is low because of the high risk of bias of the included retrospective observational studies (GRADE 2004). Furthermore, although the pooled estimate indicates that centralisation improves overall survival by 10%, this could be as low as 1% or as high as 18%.
Potential biases in the review process
We performed a comprehensive search, including a thorough search of the grey literature, and all studies were sifted and data extracted independently by at least two review authors (YLW, AB). We attempted to ensure that we did not overlook any relevant evidence by searching for studies with a wide range of reasonable quality designs (we excluded case‐control studies and case series of fewer than 200 patients).
We included one study (Vernooij 2008) that did not strictly meet our inclusion criteria (it assessed disease‐specific survival rather than overall survival). Its results were, however, consistent with the pooled estimates from the meta‐analyses.
The greatest threat to the validity of the review is likely to be publication bias: studies that did not find benefits of centralisation may not have been published. We were unable to assess this possibility as we did not find an adequate number of studies that met the inclusion criteria.
Agreements and disagreements with other studies or reviews
Two recent systematic reviews have considered the effect of hospital and physician characteristics on outcomes for ovarian cancer (Du Bois 2009; Vernooij 2007), both reviews differed in scope from our review and neither review adequately assessed the risk of bias in included studies.
Vernooij 2007 reviewed the effect of gynaecological oncologists and specialist centres on a variety of outcomes, searching databases from 1991 to 2006 and including nineteen studies. Six of these studies compared survival in specialised and non‐specialised hospitals; two of these studies were included in our review (Elit 2002; Stockton 2000); the remaining four small studies did not meet our inclusion criteria. The other three studies included in our review (Brookfield 2009; Shylasree 2006; Vernooij 2008) were published simultaneously or subsequent to the searches performed by Vernooij 2007. Despite the differences in included studies, Vernooij 2007 concluded that long‐term survival was better for women treated in specialised hospitals, consistent with our findings.
Vernooij 2007 also concluded that survival was better if surgery was performed by gynaecological oncologists in women with Stage III or greater disease, though this advantage appeared to be lost when all stages were included. However, this effect could be because the studies of Grossi 2002 and Paulsen 2006 adjusted for ‘adequacy of surgery’ as a potential intermediate effect. We would dispute whether this adjustment is logically correct and, indeed, this detail is highlighted by Vernooij 2007.
Vernooij 2007 considered additional outcomes: optimal debulking, staging, and complications. The chance of receiving optimal debulking was higher in specialised hospitals and that, in advanced disease, debulking was more often optimal when performed by a gynaecological oncologist. Similarly, staging was found to be more often performed to an adequate standard by gynaecological oncologists and it was more frequently performed in specialised hospitals. Vernooij 2007 did not find differences in complications between specialist and general gynaecologists. Complications were higher in specialised centres, though these were due to higher rates of minor complications (e.g. blood transfusion), and this discrepancy was not considered to be unexpected given the increased level of complexity of surgery in the specialised centres. The probable improvements in staging and debulking observed in specialised units, with no increase in major complication rates, are likely to be instrumental in improving the overall survival rate.
The review of Du Bois 2009 specifically aimed at correlating institution and physician characteristics with ovarian cancer survival, surgical outcomes, completeness of staging, and compliance to chemotherapy regimes. Although the authors found 17 studies of the impact of hospital characteristics on survival from ovarian cancer, it is difficult to compare their conclusions with ours as the hospital characteristics considered were extremely varied and no meta‐analyses were performed. However, the authors concluded that patients survived longer if operated on by gynaecological oncologists.
Many studies that were excluded from our review because they did not compare outcomes in specialised and non‐specialised centres nevertheless provide evidence which might explain the better survival in specialised centres. For example, several studies have found that patients with ovarian cancer operated on by a gynaecological oncologist are more appropriately staged (Chan 2007; Goff 2006), receive better cytoreduction (Goff 2006; Olaitan 2001), are more likely to receive chemotherapy (Goff 2007) and have better survival outcomes (Brolmann 1992; Chan 2007; Junor 1999; Paulsen 2006; Tingulstad 2003). Furthermore, some large population studies support the notion that university hospitals achieve better outcomes than hospitals without all the necessary support, such as radiotherapy services (Kumpulainen 2002). However, a large population study found no significant survival difference between women who were and were not operated on by gynaecological oncologists (Bailey 2006). While it is often assumed that specialised/university cancer centres are high volume centres, such a correlation cannot be clearly made. Nevertheless, some population studies clearly show a survival advantage for women with ovarian, endometrial, and cervical cancer who are managed in high‐volume centres (Ioka 2004; Ioka 2005; Kumpulainen 2002) although others do not (Schrag 2006; Tanner 2008). For example, in a study that assessed the costs and effects of centralised care and regular care for women with an ovarian malignancy in the Netherlands, it was concluded that not all women suspected of having ovarian cancer should be operated on by a gynaecological oncologist (Geomini 2011).
A consistent finding in many studies is the observation that patients managed at specialised/university cancer centres have different characteristics from those managed at district or non‐teaching hospitals. For example, patients at specialised centres tend to have a different age distribution (Carney 2002; Kumpulainen 2002), to have more advanced disease (Chan 2007; Kumpulainen 2002) and to be demographically different (Carney 2002; Chan 2007). While statistical adjustments are generally made to control for these differences, other unrecorded patient characteristics may influence treatment and prognosis.
We did not find any comprehensive studies on the cost‐effectiveness of centralisation of gynaecological cancer services. However, decision‐analysis modelling suggests that referral of ovarian cancer cases to an expert centre is a cost‐effective measure (Bristow 2007).
Authors' conclusions
Implications for practice.
We found low quality evidence to suggest that women with gynaecological cancer who received treatment from specialised centres or hospitals with specialist resources had longer survival than those managed elsewhere. The evidence was stronger for ovarian cancer than for other gynaecological cancers. We conclude that a centralised service for ovarian cancer may lead to better survival outcomes; evidence from various other sources suggests that this may also be more cost‐effective (Bristow 2006).
Survival from the different gynaecological cancers varies considerably. Survival from uterine cancer is amongst the highest for any cancer in women, while that from ovarian cancer is the lowest of all gynaecological cancers (Cooper 2008a; Cooper 2008b; Jemal 2008). This suggests that in developed countries, it may be most practical to prioritise centralisation of care for advanced ovarian cancer in the first instance.
Many countries do not have the resources to provide centralised specialised multidisciplinary management for all gynaecological cancers. Furthermore, the incidence and burden of gynaecological cancer varies between different countries. Hence the services whose centralisation would benefit most patients are likely to differ between countries. For example, in the developing world, radiotherapy services for advanced cervical cancer are likely be of higher priority than ultra‐radical surgery for ovarian cancer, followed by chemotherapy.
Implications for research.
Ideally, further studies ‐ but with designs that are less prone to bias than retrospective observational studies ‐ are needed to compare survival of women with gynaecological cancer who are managed in centralised and non‐centralised cancer facilities. Realistically, a sufficiently powered RCT comparing outcomes in centralised and non‐centralised cancer facilities for women with gynaecological cancer would be difficult. Nevertheless, in order to reduce the risk of bias due to selective reporting of outcomes or selective inclusion of centres or patients, future studies should be prospective, with a defined protocol and funding for data collection and analysis available before any organisational changes are implemented. ITS designs, which allow comparison of trends in survival before and after centralisation, would be more robust than observational studies that simply compared survival in centralised and non‐centralised facilities. Alternatively, CBA studies should be feasible, especially if centralisation is implemented in some regions of a country but not in others. Additionally, studies of the impact of centralisation of care on quality of life of patients are required, as evidence in this area is lacking.
Most of the available evidence addresses ovarian cancer in developed countries: future studies should be extended to other gynaecological cancers within different healthcare systems. Health economics studies are needed in order to prioritise those aspects of management whose centralisation would deliver most benefit to patients in different healthcare systems.
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2016 | Amended | Contact details updated. |
History
Protocol first published: Issue 3, 2009 Review first published: Issue 3, 2012
| Date | Event | Description |
|---|---|---|
| 11 February 2015 | Amended | Contact details updated. |
| 26 February 2014 | Amended | Contact details updated. |
Acknowledgements
We thank Chris Williams for clinical and editorial advice, Jane Hayes for designing the search strategy and Gail Quinn and Clare Jess for their contribution to the editorial process. We thank the referees for their many helpful suggestions. We also thank Pierre Martin‐Hirsch for many helpful suggestions and being a co‐author in the protocol and Mahmood Shafi for also being a co‐author in the protocol.
Appendices
Appendix 1. MEDLINE search strategy
Medline Ovid 1950 to November week 3, 2010
exp Endometrial Neoplasms
exp Uterine Neoplasms/
exp Uterine Cervical Neoplasms/
exp Vulvar Neoplasms/
exp Ovarian Neoplasms/
((endometr* or uter* or cervi* or ovar* or vulva* or gynae* or gyne*) adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
exp Gestational Trophoblastic Neoplasms/
(gestational adj trophoblastic adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
exp Centralized Hospital Services/
exp Hospitals, Teaching/
exp Hospitals, University/
exp Hospitals, District/
exp Cancer Care Facilities/
exp Oncology Service, Hospital/
exp Gynecology/
exp "Obstetrics and Gynecology Department, Hospital"/
(centrali* adj5 (hospital* or service* or unit* or care)).mp.
(speciali* adj (hospital* or service* or unit* or care)).mp.
((teaching or university) adj hospital*).mp.
((regional or district) adj (hospital* or unit* or service*)).mp.
(cancer adj care adj (facilit* or unit* or hospital* or service*)).mp.
(cancer adj (center* or centre*)).mp.
(tertiary adj referral adj (centre* or center*)).mp.
(gynaecologist* or gynecologist*).mp.
((gynaecologic* or gynecologic*) adj oncologist*).mp.
(surg* adj5 (experience or expertise)).mp.
((hospital* or unit* or service* or facilit* or center* or centre*) adj5 (volume* or workload)).mp.
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
9 and 29
Appendix 2. EMBASE search strategy
Embase Ovid 1980 to 2010 week 47
exp Endometrium Tumor/
exp Uterus Cancer/
exp Uterine Cervix Tumor/
exp Vulva Tumor/
exp Ovary Tumor/
((endometr* or uter* or cervi* or ovar* or vulva* or gynae* or gyne*) adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
exp Trophoblastic Tumor/
(gestational adj trophoblastic adj5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)).mp.
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
exp Hospital Management/
exp Teaching Hospital/
exp University Hospital/
exp Public Hospital/
exp Cancer Center/
exp Gynecology/
(centrali* adj5 (hospital* or service* or unit* or care)).mp.
(speciali* adj (hospital* or service* or unit* or care)).mp.
((teaching or university) adj hospital*).mp.
((regional or district) adj (hospital* or unit* or service*)).mp.
(cancer adj care adj (facilit* or unit* or hospital* or service*)).mp.
(cancer adj (center* or centre*)).mp.
(tertiary adj referral adj (center* or centre*)).mp.
(gynaecologist* or gynecologist*).mp.
((gynaecologic* or gynecologic*) adj oncologist*).mp.
(surg* adj5 (experience or expertise)).mp.
((hospital* or unit* or service* or facilit* or center* or centre*) adj5 (volume* or workload)).mp.
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26
9 and 27
Appendix 3. CENTRAL search strategy
CENTRAL Issue 4, 2010
MeSH descriptor Endometrial Neoplasms explode all trees
MeSH descriptor Uterine Neoplasms explode all trees
MeSH descriptor Uterine Cervical Neoplasms explode all trees
MeSH descriptor Vulvar Neoplasms explode all trees
MeSH descriptor Ovarian Neoplasms explode all trees
(endometr* or uter* or cervi* or ovar* or vulva* or gynae* or gyne*) near/5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)
MeSH descriptor Gestational Trophoblastic Neoplasms explode all trees
gestational next trophoblastic near/5 (cancer* or neoplas* or carcinom* or malignan* or tumor* or tumour*)
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8)
MeSH descriptor Centralized Hospital Services explode all trees
MeSH descriptor Hospitals, Teaching explode all trees
MeSH descriptor Hospitals, University explode all trees
MeSH descriptor Hospitals, District explode all trees
MeSH descriptor Cancer Care Facilities explode all trees
MeSH descriptor Oncology Service, Hospital explode all trees
MeSH descriptor Gynecologyexplode all trees
MeSH descriptor Obstetrics and Gynecology Department, Hospital explode all trees
centrali* near/5 (hospital* or service* or unit* or care)
speciali* next (hospital* or service* or unit* or care*)
(teaching or university) next hospital*
(regional or district) next (hospital* or unit* or service*)
cancer next care next (facilit* or unit* or hospital* or service*)
cancer next (center* or centre*)
tertiary next referral next (centre* or center*)
gynaecologist* or gynecologist*
gynaecologic* or gynecologic* next oncologist*
surg* near/5 (experience or expertise)
(hospital* or unit* or service* or facilit* or center* or centre*) near/5 (volume* or workload)
(#10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR 27 OR #28)
(#9 AND #29)
Data and analyses
Comparison 1. Institutions with gynaecologic oncologists on site (specialised centres) vs. community or general hospital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Ovarian cancer: survival | 3 | 9041 | Hazard Ratio (Random, 95% CI) | 0.90 [0.82, 0.99] |
| 1.1 Overall survival | 2 | 4002 | Hazard Ratio (Random, 95% CI) | 0.90 [0.80, 1.00] |
| 1.2 Disease‐specific survival | 1 | 5039 | Hazard Ratio (Random, 95% CI) | 0.90 [0.75, 1.09] |
| 2 Ovarian cancer: survival ‐ sensitivity analysis | 3 | 9041 | Hazard Ratio (Random, 95% CI) | 0.88 [0.80, 0.97] |
| 2.1 Overall survival | 2 | 4002 | Hazard Ratio (Random, 95% CI) | 0.87 [0.78, 0.97] |
| 2.2 Disease‐specific survival | 1 | 5039 | Hazard Ratio (Random, 95% CI) | 0.90 [0.75, 1.09] |
Comparison 2. Teaching or regional cancer centre vs. community or general hospital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | 51283 | Hazard Ratio (Random, 95% CI) | 0.91 [0.84, 0.99] |
| 1.1 Ovarian cancer | 2 | 2302 | Hazard Ratio (Random, 95% CI) | 0.82 [0.63, 1.07] |
| 1.2 All gynaecological cancers | 1 | 48981 | Hazard Ratio (Random, 95% CI) | 0.92 [0.85, 1.01] |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 2.1 Ovarian cancer | 1 | 233 | Hazard Ratio (Random, 95% CI) | 0.91 [0.54, 1.55] |
Comparison 3. Community hospital with semi‐specialised gynaecologist vs. general hospital.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease‐specific survival | 1 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Ovarian cancer | 1 | 6992 | Hazard Ratio (Random, 95% CI) | 0.89 [0.78, 1.01] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brookfield 2009.
| Methods | Retrospective multi‐centre study assessing TF versus NTF and HVC versus low volume cancer centres. The 2007 FCDS data set was used to identify all incident cases of cervical, ovarian, endometrial, and vulval malignancies, and uterine sarcomas diagnosed in the state of Florida from 1990 to 2000 |
|
| Participants | A total of 48,981 cases of gynaecological cancer, which included cervical, ovarian, endometrial, and vulval malignancies and uterine sarcomas were extracted for analysis. Cases with missing information for any key variable, duplicate cases, carcinomas in situ, and cases treated by community physicians independent of the hospital or ambulatory care centre settings were excluded from the univariate analysis The Median age at diagnosis of gynaecological cancer ranged between 51.9 and 67.6 years. Median age was lowest for cervical cancer and highest for vulval cancer, and was lower for all cancer types in TFs and high volume hospitals (exception being TF for uterine sarcoma, n = 253). Cancer types were as follows: cervical: 10,175 (20.8%); ovarian: 15,131 (30.9%); endometrial: 21,149 (43.2%); uterine sarcoma: 253 (0.5%); and vulvar: 2,273 (4.6%). The majority of patients in the cohort were Caucasian (n = 43,653, 89.1%) and non‐Hispanic (n = 43,901, 89.6%) For cervical, ovarian, and endometrial cancers, patients were treated more frequently at NTFs, and those individuals who were treated at TFs were significantly younger than those treated at NTFs. Regional and distant disease were more commonly treated at TFs, whereas gynaecologic cancer treated at NTFs was more commonly localised disease. For all types of cancer, individuals treated at HVCs were significantly younger than those treated at IVCs or LVCs. For cervical cancer, IVCs treated more regionally advanced disease compared to HVCs and LVCs, but HVCs tended to treat patients with more poorly differentiated cancer. For ovarian cancer, IVCs treated more regionally advanced disease, but HVCs tended to treat patients with more poorly differentiated cancer. For endometrial and vulval cancers, HVCs treated more regional and distant disease and tended to treat patients with poorer differentiated cancer. For uterine sarcomas, IVCs treated more distant stage disease compared to HVCs and LVCs, but HVCs tended to treat patients with more poorly differentiated cancer |
|
| Interventions |
Medical facility (treatment at a TF versus NTF) Medical facilities were defined as TFs or NTFs based on recognition as a teaching institution by the AAMC. The data from FCDS were tabulated to determine the number of treated cancers and surgical resections for five gynaecologic cancer types (cervical, ovarian, endometrial, vulval, and uterine sarcoma) performed at each institution in the state of Florida during the study period Medical facilities were grouped into tertiles based on number of surgeries with curative intent performed during the study period. The upper one‐third of institutions was classified as HVCs, the middle one‐third as IVCs, and the lower one‐third as LVCs. For uterine sarcoma, HVCs operated on an average of 5 cases in the 10‐year study period, IVCs operated on an average of 3 cases in the 10‐year study period, and LVCs operated on an average of 1 case in the 10‐year study period |
|
| Outcomes |
Overall survival Overall survival was calculated by subtracting the date of death or date of last contact from the time of the initial diagnosis: HR adjusted for cancer type, hospital volume, facility, age (discrete), race, ethnicity (Hispanic vs non‐Hispanic), primary payer, lymph nodes examined, tumour stage, tumour grade, surgical extirpation, chemotherapy and radiotherapy using Cox model For all cancer types: NTF vs TF (HR= 1.08; 95% CI 0.99 to 1.18; P = 0.08). We changed the reference group to non‐teaching facility so that it was consistent and could be pooled in the meta‐analysis (HR= 0.92; 95% CI 0.85 to 1.01) By cancer type (HR adjusted for the above covariates with the obvious exception of cancer type) Ovarian cancer: NTF vs TF (HR 1.09; 95% CI was not reported; P = 0.12). We could not change the reference group to NTF without knowing the 95% CI or the number of deaths in each group, so this estimate could not be pooled in the meta‐analysis as other studies in the relevant meta‐analysis included women with ovarian cancer. We used the overall result above and subgrouped Cervical cancer: NTF vs TF (HR= 1.15; P = 0.23) Endometrial cancer: NTF vs TF (HR= 1.02; P = 0.75) Uterine cancer: NTF vs TF (HR= 1.58; P = 0.08) Vulval cancer: NTF vs TF (HR= 1.12; P = 0.44) For all cancer types: the study also reported IVC and LVC vs HVC: HR= 0.96; 95% CI 0.91 to 1.03; P = 0.25) |
|
| Notes | The staging criteria used by the FCDS are consistent with the SEER, National Cancer Institute summary staging and differ from the International Federation of Gynecology and Obstetrics (FIGO) staging guidelines. In this study, local staging represents disease that does not extend beyond the primary organ, while those having positive lymph nodes at the time of resection were classified as having regional disease. Documentation of distant metastases during the peri‐operative period led to classification of affected patients as having distant disease. Univariate analyses and the final multivariate regression was corrected for clustering. Significant variables from the univariate analysis were included in the multivariate regression analysis to determine whether facility characteristics were associated with survival for all gynaecologic malignancies. The 5‐year survival rates for the cohort diagnosed with cervical cancer was 61.7%, for the cohort diagnosed with ovarian cancer was 39.5%, and for the cohort diagnosed with endometrial cancer was 67.3%. As age increased for patients diagnosed with all gynaecological cancers, 5‐year survival rates decreased. For cervical and ovarian cancer, 5‐year survival rates by univariate analysis were significantly greater for patients treated at TFs compared to those treated at NTFs (63.9% vs 60.9% and 43.9% vs 38.8%; P < 0.01, respectively). Among patients diagnosed with cervical cancer, 30‐day and 90‐day surgical mortality rates were significantly greater at NTFs compared to TFs (P = 0.04). Cox regression models adjusting for clustering effects were also created separately for each malignancy, with no difference in survival seen for patients treated at TFs versus NTFs, or HVCs versus LVCs. Independent predictors of survival for all gynaecologic malignancies studied in the 10‐year period were diagnosis of ovarian cancer, age > 40 years, African‐American race, Medicaid payer status, lymph node examination, tumour stage, tumour grade, surgical extirpation, chemotherapy treatment, and lack of radiation therapy. The study found no observed difference in patient survival for any gynaecologic malignancy based upon treating hospital teaching or volume status. It concluded that although instances of improved outcomes may occur, overall further regionalisation would not appear to significantly improve patient survival |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage analysed: 48,981/48,981 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | High risk | There were imbalances at baseline for almost all prognostic factors for comparisons of hospital volume and medical facility. These factors were adjusted for in a multivariate analysis, but selection bias still likely to be a problem |
| Relevant assignment criteria | Low risk | "The 2007 FCDS data set was used to identify all incident cases of cervical, ovarian, endometrial and vulvar malignancies and uterine sarcomas diagnosed in the state of Florida from 1990–2000 ... Medical facilities were defined as TFs or non‐teaching facilities (NTF) based on recognition as a teaching institution by the Association of American Medical Colleges (AAMC). There are currently 11 AAMC‐recognized TFs in the state of Florida. The data from FCDS were tabulated to determine the number of treated cancers and surgical resections for five gynecologic cancer types (cervical, ovarian, endometrial, vulvar, and uterine sarcoma) performed at each institution in the state of Florida during the study period. Medical facilities were grouped into tertiles based on number of surgeries with curative intent performed during the study period" |
| Representative intervention group | High risk | There did appear to be some differences between women treated in hospitals with teaching and non‐teaching faculties. Furthermore, "regional and distant disease were more commonly treated at teaching facilities, whereas gynecologic cancer treated at nonteaching facilities was more commonly localized disease" |
| Representative comparison group | High risk | As above |
| Comparability of groups | Low risk | There were significant differences in many baseline factors between groups, but multivariate analysis was used to adjust for important explanatory variables |
Elit 2002.
| Methods | A retrospective study to determine the relationship among hospital volume of ovarian cancer surgery, academic status of institution, surgical specialty, and outcomes of care (30‐day postoperative mortality, reoperation rate, and overall survival) Study cohort was defined as all women in Ontario (ages 18 and older) with newly diagnosed epithelial ovarian cancer between April 1992 and March 1998. To identify the cohort, electronic records from the hospitals in Ontario were used to create a population‐based cohort |
|
| Participants | A total of 3815 women in Ontario underwent surgery for newly diagnosed ovarian cancer between 1992 and 1998 The mean and median age of patients in the study was 58.9 (SD = 14.4 years) and 60 years respectively. For 12 months prior to the date of surgery, hospital records showed that the majority of women (87.8%) had no documentation of other serious comorbid conditions. Approximately, half of the patients (46.6%) had a hospital diagnostic code of metastatic cancer |
|
| Interventions | The academic status was defined as:
The volumes of ovarian cancer surgeries by hospital were classified as 1 to 5 surgeries per hospital per year (LVCs), 16 to 99 (IVCs),and greater than 100 (HVCs). Hospitals with a gynaecologic oncologist were in the latter group |
|
| Outcomes | The primary outcomes assessed in this study were postoperative mortality (death from any cause within 30 days of the index surgery), the rate of repeat debulking surgery within 3 months of the initial surgical procedure, and total patient survival time The survival time was modelled using both total survival time from the date of surgery, and survival beyond 30 days in order to separate the effects of operative mortality from the effects of surgery on long‐term survival with ovarian cancer. We reported only overall survival from the multivariate analysis. The study had three arms and community hospitals were used in each comparison so the SE of the log(HR) was multiplied by the square root of 2 so that the study was not given undue weight in the meta‐analyses Hospital category Institutions with gynae–oncologist (n = 1281) (HR 0.91; 95% CI 0.80 to 1.02) Other cancer host and teaching (n=337) (HR 0.81; 95% CI 0.66 to 1.00) Remaining institutions (reference group) (n = 1732) (HR 1.00) The study also reported hospital surgical volume (annual) as well as other co‐variates: 100 patients (n = 987) (HR 0.85; 95% CI 0.72 to 1.00) 16 to 99 patients (n = 1378) HR 0.81; 95% CI 0.70 to 0.94) 1 to 15 patients (reference group) (n = 985) (HR= 1.00) |
|
| Notes | Overall survival outcomes better when operated on by gynaecological oncologist and general gynaecologist compared to general surgeons. There was no statistically significant difference between hospital volume and survival outcomes. The postoperative mortality rate did not differ markedly by hospital volume, institution type, or surgeon volume. It is well known that prognostic factors such as stage, grade, histology, and size of residual disease all influence survival. As well, the use of postoperative chemotherapy and the agents used may affect survival. This information was not available for this study. Thus the differences seen by institution and surgical specialty may be biased. In the later publication of 2008 the authors carried out another retrospective study which overlapped with the cohort study reported in 2002 (correspondence with the first author confirmed that the second study was done in more detail but only included a subset of patients in the first study) so was not included in the main text of the review, but details are given below Elit 2008 Methods A population‐based retrospective cohort study of all women in Ontario, Canada, with newly diagnosed ovarian cancer treated initially with abdominal surgery between 1 January 1996, and 31 December 1998. The incident surgical cases were documented using hospital contact data and the Ontario Cancer Registry. Data on patient characteristics, clinical findings, surgical techniques and perioperative care from electronic administrative data records and patient charts were obtained. A regression analysis was performed to assess the influence of the specialty of the surgeon and hospital specialisation and of case volumes on the likelihood of unnecessary repeated abdominal repeat surgery and long‐term patient survival. The authors included a control for the stage of disease and other factors associated with these outcomes. The relation between the adequacy of surgery and adjuvant chemotherapy with survival was also examined Participants A total of 1341 women with epithelial ovarian cancer treated initially with abdominal surgery between January 1996 and December 1998. The median patient age was 60.7 years. More than 90% had no history of cancer and 5% had a comorbidity score of 2 or more. Roughly 25% of the cohort had stage I, 12% had stage 2, 56% had stage 3 and 7% had stage 4 disease at the time of initial surgery. Interventions Surgeons were classified as general surgeon, gynaecologist or other using the Canadian Medical Protective Association code from a provincial care provider database. Gynecologic oncologists were identified within the gynaecologist group using a previously established list of sub‐specialists. Patient volumes for surgeons and facilities were identified within the population‐based cohort (annual number of incident ovarian cancer patients who had surgery during the study period) The surgical centres were classified as:
Outcomes The outcomes of interest were the likelihood of unnecessary repeated abdominal repeat surgery and long‐term patient survival Repeat surgery: Lower surgeon volume and hospital volume were significantly associated with repeated surgery:
The results according to Hospital type were:
Surgeon and hospital specialisation were strongly correlated. Hospital type did not make a significant contribution to the fit of the model; however, adding hospital type caused a marked reduction in the size of the effect associated with surgeon discipline. After adjustment for hospital effects, patients of a general surgeon continued to have an estimated likelihood of repeated surgery that was 6 times greater than that of patients who saw gynaecologic oncologists (RR 5.7; 95% CI 1.17 to 28.46) Survival: Hospital procedure volume > 100: (HR= 1.00) (reference group) 16 to 99: (HR= 1.05; 95% CI 0.84 to 1.31) 1 to 15: (HR= 0.91; 95% CI 0.72 to 1.15) Hospital type: centre with gynaecologic oncologist: (HR= 1.00) (reference group) other regional cancer or teaching centre: (HR= 0.74; 95% CI 0.55 to 1.00) remaining centres: (HR= 0.87; 95% CI 0.75 to 0.99) The analysis showed that repeat surgery was associated with the surgeon's discipline, younger patient age, well‐differentiated tumours and early stage of disease. However, survival was not associated with the surgeon's discipline; rather, it was associated with advanced patient age, increasing co‐morbidities, advanced stage of disease, poorly differentiated tumours, urgent surgery and adjuvant chemotherapy. There was a trend between inadequate surgery and a decreased likelihood of survival |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage analysed: 3350/3815 (88%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias may have been present |
| Relevant assignment criteria | Low risk | "The study cohort was defined as all women in Ontario (ages 18 and older) with newly diagnosed epithelial ovarian cancer between April 1992 and March 1998. Any woman who had an abdominal operation where ovarian cancer was diagnosed (ICD‐9 diagnostic code of 183) was included. To identify the cohort, electronic records from the hospitals in Ontario were used to create a population‐based cohort ... Linkages between patient data and physician specialization data were carried out using exact match of encrypted physician billing numbers ... the volumes of ovarian cancer surgeries by hospital were classified as 1–5 surgeries per hospital per year (low volume centers), 16–99 (intermediate volume centers), and greater than 100 (high‐volume centers). Hospitals with a gynecologic oncologist were in the latter group ... study the following categories were used: (A) institutions where gynecologic oncologists are on site; (B) institutions where no gynecologic oncologists was on site but which were either teaching hospitals or hospitals associated with regional cancer centers; and, (C) community hospitals" |
| Representative intervention group | High risk | "Patients seen at institutions with a gynecologic oncologist were ... slightly more likely to have one or more comorbid conditions ... and more likely to have metastatic disease" |
| Representative comparison group | High risk | As above |
| Comparability of groups | Low risk | "Patients seen at institutions with a gynecologic oncologist were of similar age compared to the other types of institutions". Differences at baseline were noted between patient characteristics, but multivariate analysis was used to adjust for important explanatory variables |
Shylasree 2006.
| Methods | The objective of this retrospective study was to review referral practice, overall management, and survival in women with suspected ovarian cancer in Wales. This study was done prior to introduction of cancer management guidelines in the region The project was funded for 1 year and was undertaken by the Clinical Effectiveness Support Unit (CESU), Llandough Hospital, Cardiff. This was an external review and 20 hospitals in Wales took part in the audit project. Data for the audit were collected between 1 January 1999 and 31 December 1999 on women who were referred to these hospitals between December 1997 and 1998. Data on 287 women were collected |
|
| Participants | The study included 287 consecutive women with suspected ovarian cancer, of which 250 women underwent primary laparotomy Women in the study had to have been referred to the particular hospital with suspected ovarian cancer. Confidential questionnaire comprising information on the management of these women was sent to the participating hospitals. Each individual woman was allocated a number, and each participating hospital allocated a hospital code. The mean age of the women was 63.2 years, (SD 14.1 years; range 13.5 to 91.8 years) Median referral time was 9 days (range 0 to 84 days). The median time between referral and operation was 15 days (range 0 to 219 days) |
|
| Interventions |
Women treated at a teaching hospital At the time of the study, the only designated cancer centres were two teaching hospitals in south Wales District general hospital (peripheral units) These units varied in size and in their catchment areas. At the time of the study, they were not officially recognised as cancer units |
|
| Outcomes | Overall survival and progression‐free survival Place of primary surgery Peripheral unit (district general hospital) vs cancer centre (teaching hospital): (HR= 1.19; 95% CI 0.65 to 2.17; P = 0.56 and (HR= 1.09; 95% CI 0.64 to 1.85; P = 0.75 for overall survival and progression‐free survival, respectively). We changed the reference group to general hospital so that it was consistent and could be pooled in the meta‐analysis (HR= 0.84; 95% CI 0.46 to 1.55) Not recorded/missing data vs cancer centre: (HR= 1.92; 95% CI 0.72 to 5.11; P = 0.19 and (HR= 1.12; 95% CI 0.41 to 3.07; P = 0.82 for overall survival and progression‐free survival, respectively The median follow‐up was 913 days (range 733 to 1101 days) |
|
| Notes | The overall survival time was defined as the time interval from date of diagnosis (date of primary laparotomy) to the date of death. Progression‐free survival was defined as the time interval from the date of diagnosis to the date of an occurrence (death, recurrence, and progression of disease) Referral time was defined as the date the referral letter was received in hospital either by the general practitioner or by other physicians to the date seen in the oncology clinic There were no significant differences in the mean ages of women, and the median referral time between the cancer centre and the peripheral units (61.3 vs 64.2 years, P = 0.30; 5 vs 3 days, P = 0.33), respectively |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage analysed: 250/287 (87%) "Of the 287 women, 250 women underwent a primary laparotomy and staging procedure for ovarian cancer. Twenty‐five were unfit for surgery, seven did not consent, and in five there was no information". |
| Selective reporting (reporting bias) | Low risk | Overall and progression‐free survival was reported and was analysed correctly allowing for censoring, and clear definitions were given. Other outcomes of secondary importance were unlikely to have been selectively reported |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias may have been present |
| Relevant assignment criteria | Low risk | "This was an external review and 20 hospitals in Wales took part in the audit project. Data for the audit were collected ... on women who were referred to these hospitals between December 1997 and 1998. The inclusion criterion for women in the study was that they had been referred to that particular hospital with suspected ovarian cancer. Confidential questionnaire comprising information on the management of these women was sent to the participating hospitals. Each individual woman was allocated a number, and each participating hospital allocated a hospital code. Audit clerks in the participating units entered the data onto the forms. Staff at the CESU then collected the data, checked the data, queried any discrepancies, and attempted to update incomplete data. At the time of the study, the only designated cancer centers were two teaching hospitals in South Wales. The peripheral units consisted of a number of district general hospitals across Wales. These units varied in size and in their catchment areas" |
| Representative intervention group | Unclear risk | "The clinical, pathologic, and treatment characteristics of the women are summarized in Table 3, 4. There were no significant differences regarding stage, residual disease recorded, tumor histology, or grade between women managed in the cancer center and those in the peripheral units". However, "two hundred and eighty‐seven women were referred to the hospital with suspected ovarian cancer". Thus we cannot be sure whether women in the study were representative of women with ovarian cancer. They are certainly not representative of women with gynaecological cancer in general |
| Representative comparison group | Unclear risk | As above |
| Comparability of groups | Low risk | "There were no significant differences in the mean ages of women, and the median referral time between the cancer center and the peripheral units respectively. There were no significant differences regarding stage, residual disease recorded, tumor histology, or grade between women managed in the cancer center and those in the peripheral units". Multivariate analysis was used to adjust for important explanatory variables |
Stockton 2000.
| Methods | A retrospective study in women with colon, rectal, breast, melanoma, bladder, and ovarian cancers. A total of 14,527 cases registered by the East Anglian cancer registry and diagnosed between 1989 and 1993 were included. The data were analysed by cancer type so it was possible to report the results for ovarian cancer (n = 989) | |
| Participants | 989 women with ovarian out of a total of 14,527 women with all cancer types in the study 719 (73%) women were under the age of 75 and 270 (27%) were 75 years of age or older 20% and 24% of women had stage 1, 13% and 11% stage 2, 39% and 34% stage 3, 11% and 12% stage 4 and 17% and 20% were not staged in the specialised and general hospital groups, respectively |
|
| Interventions |
Women treated at a hospital with radiotherapy and oncology departments Addenbrooke's in Cambridge, the Norfolk and Norwich hospital and Ipswich hospital (n = 475) Women treated at a district general hospital without radiotherapy and oncology departments data from six such hospitals was collected (n = 514) |
|
| Outcomes |
Overall survival: District general hospital vs hospital with radiotherapy and oncology department (specialised centre):
|
|
| Notes | The authors concluded that for the patients included in the study, survival up to 5 years after diagnosis was significantly worse for patients with ovarian, rectal, and breast tumours if they were aged under 75 years at diagnosis, and had their main treatment in hospitals without radiotherapy and oncology departments | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage analysed: 989/989 (100%) |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias may have been present |
| Relevant assignment criteria | Low risk | All invasive cancers for the sites fitting our inclusion criteria described below, diagnosed between 1989 and 1993 (to allow survival analyses to be performed for patients followed up until the end of 1998) and registered by the East Anglian Cancer Registry were identified. The inclusion criterion was defined as all cancer sites being considered by the Anglia and Oxford NHS Executive for which the registry has an adequate (at least 70%) proportion of cancers staged over the period. Thus we included colon, rectal, breast, melanoma, bladder and ovarian cancers. For local purposes, the data were initially analysed by individual hospital then grouped so that hospitals with radiotherapy and oncology departments (Addenbrooke’s in Cambridge, the Norfolk and Norwich Hospital, and Ipswich Hospital) [group 1] (7000 patients) could be compared with the six district general hospitals without radiotherapy and oncology departments [group 2] (7527 patients) |
| Representative intervention group | Unclear risk | Authors report Table 2 which shows percentage of patients presenting at each TNM tumour stage (UICC classification) at diagnosis by tumour type and age stratum, but it remains unclear whether the two groups are representative of women with ovarian cancer. They are certainly not representative of women with gynaecological cancer |
| Representative comparison group | Unclear risk | As above |
| Comparability of groups | Low risk | "Cox’s proportional hazards regression models (Cox, 1972) were analysed to investigate survival differences for patients treated at group 1 compared to group 2 hospitals adjusting for sex, age (in 10‐year age bands) and tumour stage at diagnosis" |
Vernooij 2008.
| Methods | A retrospective, population‐based cohort study. Dutch patients diagnosed with ovarian cancer from 1 January 1996, through 31 December 2003. Data from the Netherlands Cancer Registry that were linked to mortality data from the Statistics Netherlands database to obtain the date and cause of death were used | |
| Participants | The study analysed 8621 women with epithelial ovarian cancer The median age of the women in the study was 64 years, (range 8 to 98 years) 2073 (24%) of women had FIGO stage I, 677 (8%) stage II, 3859 (45%) stage III, 1347 (15%) stage IV and in 665 (8%) women stage was unknown. 911 (10%) women had Grade 1 disease, 1870 (22%) had Grade 2, 3157 (37%) had Grade 3 disease and in 117 (1%) grade was undifferentiated and in the remaining 2566 (30%) it was unknown. Histology was adenocarcinoma NOS in 2800 (32%) women, endometrioid in 905 (11%), mucinous in 1022 (12%), serous in 3310 (38%), clear‐cell carcinoma in 417 (5%) and the remaining 167 (2%) women had other histology |
|
| Interventions | Hospitals were classified according to their level of specialisation as a general, semi‐specialised, or specialised hospital. In total, there were 60 general hospitals, 32 semi‐specialised hospitals, and 13 specialised hospitals Specialised hospitals The staff in specialised hospitals include gynaecologic oncologists, and specialised hospitals are regional centres for gynaecologic oncologic care. A physician is recognised as a gynaecologic oncologist by the Dutch Society of Gynecologic Oncology when he or she has received subspecialty training in gynaecologic oncology during a 2‐year fellowship. Of the 13 clinics that fulfilled the criteria for a specialised centre, 8 were university hospitals (n = 1557) Semi‐specialised hospitals Community hospitals with a semi‐specialised gynaecologist were categorised as semi‐specialised hospitals. Semi‐specialised gynaecologists are not trained in oncology but operate on most ovarian and endometrial cancer patients in large community (usually teaching) hospitals (n = 3510) General hospitals General hospitals without semi‐specialised oncologic care were classified as general hospitals (n = 3482) The specialisation level of the institution (general, semi‐specialised, or specialised) was verified by a panel of gynaecologists and gynaecologic oncologists. The hospitals were not additionally characterised according to teaching status because almost all general hospitals were non‐teaching hospitals and almost every semi‐specialised institution and all specialised institutions were teaching hospitals. In The Netherlands, all chemotherapy is provided by medical oncologists in hospital settings |
|
| Outcomes | The primary outcome measure was disease‐specific survival (which included multivariable survival analyses and the assessment of median survival) which was defined as the interval from the date of diagnosis to the date of death from ovarian cancer, as registered in the database of Statistics Netherlands. When the patient died from another cause, she was censored at the date of death. If the patient had not died by 31 December 2005, she was censored on that date. Table 4 which includes the results of the multivariate analysis uses the term 'overall disease‐specific survival'. This term is incorrect as disease‐specific survival and overall survival are different. Overall survival counts all deaths (from whatever cause) as an event; disease‐specific survival counts only deaths from ovarian cancer as an event. We assumed (based on the main text) that this was disease‐specific survival in the multivariate analysis Hospital category General (n = 1281) (HR 1.00) (reference group) Semi‐specialised centre (n = 337) (HR= 0.89; 95% CI 0.78 to 1.01) Specialised centre (n = 1732) (HR= 0.90; 95% CI 0.75 to 1.09) |
|
| Notes | The mean follow‐up time was 3 years (range = 0 to 10 years) Survival increased as the level of specialisation of the hospital increased: 5‐year relative survival ratios of patients treated in general, semi‐specialised, and specialised hospitals were 38.0% (95% CI 36.0% to 39.9%), 39.4% (95% CI 37.5% to 41.4%), and 40.3% (95% CI 37.4% to 43.1%), respectively In multivariable analysis, stage and age were statistically significantly associated with hospital type and ovarian cancer – specific survival, and modified this relationship (P for interaction = 0.001 and 0.002, respectively), whereas histologic tumour type, grade, year of diagnosis, and socioeconomic status were not associated with the relationship between hospital type and survival In the later publication of 2009 the authors carried out another retrospective study which overlapped with the cohort study reported in 2008 (correspondence with the first author confirmed that the patients from the 2009 study were from a subsample of the cohort analysed in the 2008 study. For the 2008 study the authors analysed registry‐data, for the 2009 study they analysed information gathered from patient files) so was not included in the main text of the review, but details are given below Vernooij 2009 Methods A retrospective cohort study on ovarian carcinoma patients newly diagnosed between 1996 and 2003 in The Netherlands. The aim was to assess the influence of hospital and gynaecologist level of specialisation and volume on surgical results and on survival of ovarian cancer patients Participants Data were collected from 1077 ovarian cancer patients treated from 1996 to 2003 in a random sample of 18 Dutch hospitals. Patients with borderline and non‐epithelial tumours, diagnosed postmortem, or in whom the origin of the tumour remained uncertain were excluded Interventions Hospitals and gynaecologists were classified according to specialisation (general, semi‐specialised or specialised) and by volume (≤ 6, 7 to 12, or 12 cases/year) Hospitals and gynaecologists were categorised according to specialisation and volume
Outcomes Outcomes were percentage of adequately staged and optimally debulked patients and length of overall survival Staging: The level of specialisation and the volume of hospitals and of gynaecologists were strongly related to the proportion of adequately staged patients (adjusted OR) specialised hospitals: (3.9; 95% CI 2.0 to 7.6); specialised gynaecologists: (9.5; 95% CI 4.7 to 19) Only 24% of the stages I and IIa patients operated on in general hospitals and about 60% of the patients treated in semi‐specialised and specialised hospitals were adequately staged (P < 0.0001). In most inadequately staged patients, no lymph node sampling was performed. Logistic regression revealed that adequate staging was done about 4 times more often in semi‐specialised hospitals than in general hospitals (OR adjusted for stage and age, compared to general hospitals: (4.6; 95% CI 2.3 to 9.2) in semi‐specialised hospitals, and (3.9; 95% CI 2.0 to 7.6) in specialised hospitals). Patients treated in high‐volume hospitals were 5 times more often adequately staged than patients treated in low‐volume hospitals. Debulking of advanced disease: Patients with stage III disease had a higher chance of optimal debulking when treated in specialised hospitals (adjusted OR= 1.7: 95% CI 1.1 to 2.7) or by high volume gynaecologists (adjusted OR= 2.8; 95% CI 1.4 to 5.7) 37% of the patients with stage III disease treated in general hospitals were optimally debulked, compared to 39% of the patients in semi‐specialised hospitals, and 48% of the patients in specialised hospitals (P = 0.07). In multivariable logistic regression analysis, specialisation of the hospital affected the results of cytoreductive surgery (OR specialised hospital, adjusted for stage and age, compared to general hospitals: 1.7; 95% CI 1.1 to 2.7), whereas the hospital volume did not Overall Survival: Overall survival was best in patients treated in specialised hospitals and by high‐volume gynaecologists. 5‐year survival was highest in patients with FIGO I and II disease treated in specialised hospitals. In multivariable Cox regression analysis, patients in specialised hospitals had an 18% reduction in mortality compared to patients treated in general hospitals (HR adjusted for age and stage: 0.8; 95% CI 0.7 to 1.0). This effect became more pronounced when patients operated on by a specialised gynaecologist in a general hospital and were not included in the analysis (HR= 0.8: 95% CI 0.6 to 0.9). The patient volume of the hospital did not have a significant influence on survival |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Retrospective study |
| Allocation concealment (selection bias) | High risk | Concealment of allocation irrelevant to this study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Percentage analysed: 8621/8915 (97%) "Survival data were missing for 294 (3.3%) of the 8915 patients, and the hospital of treatment was not registered for 283 (3.3%) of the remaining 8621 patients" |
| Selective reporting (reporting bias) | High risk | The study reported disease‐specific survival rather than overall survival (death from any cause) which is more prone to bias Disease‐specific survival is not a good outcome measure to use. The coding of death certificates is error‐prone. If someone dies because of the treatment they receive, this may not be counted as a death from ovarian cancer. But it is just as important to the patient as a death from ovarian cancer and the evaluation of the relative benefits of the treatments should include these deaths |
| Other bias | Unclear risk | Insufficient information to assess whether any additional bias may have been present |
| Relevant assignment criteria | Low risk | "We performed a retrospective cohort study on all patients with ovarian carcinoma who were newly diagnosed from January 1, 1996, through December 31, 2003, in The Netherlands. Patients were categorized according to the hospital in which the initial treatment took place. Data on patients with ovarian cancer from the population‐based Netherlands Cancer Registry were linked to mortality data from the database of Statistics Netherlands. The data from the Netherlands Cancer Registry are gathered by the Comprehensive Cancer Centers that receive lists of all patients with newly diagnosed ovarian cancer from pathology departments and from the National Registry of Hospital Discharge Diagnosis ... Patients were categorized according to the hospital in which treatment was performed. If the patients were not treated surgically or if the hospital of treatment was not known, patients were categorized by the hospital of diagnosis. Hospitals were classified according to their level of specialization as a general, semispecialized, or specialized hospital" |
| Representative intervention group | Unclear risk | "We excluded patients with ovarian tumors of borderline malignancy. We restricted our cohort to patients with epithelial ovarian malignancies ... Of these 8621 patients, 2750 (32%) presented with early‐stage disease (FIGO I and II) ... For the whole cohort, stage of disease at diagnosis did not differ between the different hospital types". However it is still unclear whether this group of women is representative of women with ovarian cancer; they are not representative of women with gynaecological cancer |
| Representative comparison group | Unclear risk | As above |
| Comparability of groups | Low risk | "To allow for statistical adjustments for prognostic variables (ie, stage, age, histologic tumor type, grade of tumor, year of diagnosis, and socioeconomic status), a Cox proportional hazards model was used, and hazard ratios (HRs) with their corresponding 95% confidence intervals (CIs) were calculated" |
AAMC ‐ Association of American Medical Colleges; CI ‐ confidence interval; FCDS ‐ Florida Cancer Data System; FIGO ‐ International Federation of Gynecology and Obstetrics; HR ‐ hazard ratio; HVC ‐ high volume centre; IVC ‐ intermediate volume centre; LVC ‐ ow volume centre; NTF ‐ non‐teaching facility; OR ‐ odds ratio; RR ‐ risk ratio; SD ‐ standard deviation; SE ‐ standard error; SEER ‐ surveillance, epidemiology and end result; TF ‐ teaching facility; vs ‐ versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bailey 2006 | This study evaluates the 5‐year outcome data for the management of advanced ovarian cancer in the four Cancer Networks of the south‐west of England. This region is served by 4 cancer networks. The first year that management of gynaecological cancers by lead clinicians and centralised cancer centres was actively promoted following Improving Outcomes Guidance (IOG) and National Health Service (NHS) Cancer Management Guidelines was 1997. Despite this, in the study period of 1998 only 60% of suspected ovarian cancers were managed by gynaecological oncologists (2nd paragraph of page 27). This study was excluded as it did not use statistical adjustment |
| Bristow 2006 | A review of the literature which included eight studies which we had already identified from the search |
| Brolmann 1992 | Letter correspondence in Dutch that discusses the results of a study performed in an oncology centre of 100 women, who underwent radical hysterectomy and reported only morbidity data and did not distinguish between surgeon expertise |
| Carney 2002 | This population‐based study compares the outcomes of patients who at some point had a gynaecological oncologist involved in their care compared to those who did not. It does not take into account where the patient is managed |
| Chan 2007 | This was a retrospective study assessing influence of gynaecological oncologist versus non‐gynaecological oncologist on treatment outcomes in women with epithelial ovarian cancer from three data bases of the Californian Cancer Registry between January 1994 and December 1996. Patients were categorised into 4 different race/ethnicity groups, their geographical variations and census‐based socioeconomic status. There was no description of the type of institution or hospital patients were managed in |
| Chan 1999 | This was an editorial |
| Crawford 2002 | The analysis was limited to univariate analysis. Therefore, the statistical analysis not sufficiently robust |
| Diaz‐Montes 2006 | This study assessed the impact of surgeon case volume and other prognostic factors on short‐term mortality. Logistic regression model results for in‐hospital death, length of stay and total charges among patients that underwent surgery for uterine cancer was reported. This model included discordant surgeon or attending physician, length of stay, volume of surgeon, volume of hospital and age. However important prognostic factors like stage and grade of tumour were not included in the model, so selection bias was likely to be a problem, especially as the main outcome of in‐hospital death was likely to have been attributable to severe advanced disease as well or rather than volume of surgeon and hospital |
| Du Bois 2009 | This was a meta‐analysis |
| Earle 2006 | Earle 2006 and Schrag 2006 both reported on the same group of women and examined hospital and surgeon specific procedure volumes and expertise of surgeon (gynaecologic oncologist, general gynaecologist and general surgeon) |
| Eisenkop 1992 | This was a retrospective study comparing the outcomes of Stage IIIC and IVA ovarian cancer from 14 hospitals between January 1985 and December 1988. The comparison examined the outcomes of patients operated on by surgeons with gynaecological oncology training compared to others (general obstetrician and gynaecologist, general surgeons, oncologic surgeons and others). The outcomes of 11 gynaecological oncologist were compared to 120 general obstetrician/gynaecologist, 85 general surgeons and 40 non‐specified physicians. The effects of centralisation of services could not be assessed in this paper |
| Engelen 2006 | This study evaluated the effects of primary surgery by a gynaecologic oncologist on treatment outcome. The study population is from the northern part of the Netherlands. Since 1980, gynaecologic oncologists at the regional university hospital regularly have assisted their fellow gynaecologists in the community hospitals when performing surgery on patients with suspected ovarian carcinoma. Therefore, the comparison is strictly based on the effect of gynaecological oncologist versus non‐gynaecological oncologist, and not the type of hospital |
| Ghaemmaghami 2010 | This retrospective analysis only compared the outcomes of general gynaecologist and general gynaecologist in the management of ovarian cancer In Tehran, Iran. It did not assess the effects of hospital volume/teaching vs non‐ teaching |
| Giede 2005 | This was a review article |
| Goff 2006 | This paper describes the patterns of surgery undertaken for ovarian cancer while the primary outcomes were not defined |
| Goff 2007 | The authors did not define the primary outcomes |
| Grant 1992 | This study was excluded as there was inadequate description of the intervention. Furthermore, the retrospective cohort was from 1979 |
| Grant 1999 | This was an editorial |
| Grant 2000 | This was an editorial |
| Grossi 2002 | Retrospective case note review of population‐based sample of all women with ovarian cancer diagnosed during the years 1993 to 1995 was identified from the Victoria Cancer Registry, Australia. There was no definition of gynaecological oncologist. Furtherore, no definition of the type of hospital was available |
| Ioka 2004 | This study examined hospital procedure volume on ovarian cancer survival in Japan. The 'volume' was not correlated to specialty i.e. there was no mention of the expertise of the team in high and low volume centres |
| Ioka 2005 | This study examined hospital procedure volume on uterine cancer survival in Osaka, Japan. The 'volume' was not correlated to specialty i.e. there was no mention of the expertise of the team in high and low volume centres |
| Junor 1999 | This retrospective case note review included all women diagnosed with ovarian cancer in Scotland in 1987, 1992, 1993, 1994. The comparison under investigation was surgery by gynaecological oncologist versus general gynaecologist versus general surgeon and was therefore excluded from further analysis |
| Kehoe 1994 | This retrospective analysis only compared the outcomes of general gynaecologist and general surgeon in the management of ovarian cancer. It did not assess the effects of hospital volume/teaching vs non‐teaching |
| Kumpulainen 2002 | This study is excluded as it compares type of hospitals where the first operation took place (university vs central vs other) and an analysis according to number of operated patients (surgical volume). Furthermore, the statistical adjustments made for the survival analysis were not sufficiently robust. Multivariate analysis was not performed |
| Kwon 2008 | This was a retrospective analysis of endometrial outcomes in relation to healthcare providers ‐ single payer versus public funded versus comprehensive health care system |
| Lehtovirta 2000 | This was an editorial |
| Luesley 2000 | This was a commentary |
| Macdonald 2005 | This retrospective observational study included patients with FIGO stage I‐IIA endometrial adenocarcinoma. It was excluded from further analysis as it compares surgery general gynaecologist versus gynaecologic oncologist. |
| Mayer 1992 | This retrospective observational cohort study included women with stage I‐II epithelial ovarian cancer at Yale University school of Medicine from 1981 to 1989. It was excluded as the comparison includes primary surgery by gynaecologic oncologist versus a non‐oncologic surgeon followed by chemotherapy with cisplatin, doxorubicin and cyclophosphamide in both groups. |
| Munstedt 2003 | The primary outcomes i.e. survival was not reported in this study |
| Nguyen 1993 | This is a national survey from 1230 hospitals in the US including women with ovarian cancer. It was excluded as the comparison included primary surgery operation by gynaecologic oncologist versus obstetrician‐gynaecologists versus general surgeons |
| O'Malley 2003 | The study examined hospital characteristics which included provider (teaching Hospital, ACoS Hospital, gynaecologic oncologist) in multivariate analysis, but only the significant results were presented. This study has been discussed in more detail in Agreements and disagreements with other studies or reviews |
| Olaitan 2008a | This was a letter |
| Paulsen 2006 | Details on survival outcomes were not included |
| Pearl 2002 | Study compared university and community hospitals, but survival outcomes were not reported |
| Petignat 1999 | The primary outcomes were not reported |
| Petignat 2000 | No interventions were defined in this study |
| Rich 1993 | This was a letter |
| Savage 2008 | This was a commentary |
| Tangjitgamol 2009 | This was a Cochrane review |
| Tanner 2008 | Outcomes were defined based on age and surgical volume of surgeon |
| Tingulstad 2003 | Case control study. This was a historical prospective study, where cases were referred between 1995 and 1997 from community hospitals to a teaching hospital for primary surgery. For each referred case, two controls, matched for (FIGO) stage and age, were selected among patients who had had primary surgery at the referral hospitals (non‐teaching) in the years, 1992 to 1995 |
| Van der Zee 2009 | This study was reported in Dutch. The English version and analysis was reported by Engellen 2006 |
| Vernooij 2007 | This was a review article |
| Williams 2008 | This was an editorial |
| Wolfe 1996 | Publication of audit that did not meet the inclusion criteria |
| Wolfe 1997 | Publication of audit that did not meet the inclusion criteria |
| Woodman 1997 | The intervention was not clearly defined. Surgeons were 'arbitrarily' classified as high or low volume |
FIGO ‐ International Federation of Gynecology and Obstetrics; vs ‐ versus
Differences between protocol and review
We had initially stated that all observational cohort studies meeting the inclusion criteria would be included, but we modified this to include unselected case series studies which included concurrent comparison, and placed an additional constraint that studies had to include 200 or more patients to ensure that statistical adjustment was not carried out on sparse data, thus ensuring higher quality evidence.
We did not examine risk of bias in CBA or ITS studies, as the review was restricted to retrospective studies. These retrospective studies assessed risk of bias using the six core items for risk of bias in RCTs, in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), as well as assessing four additional items for non‐randomised studies. We had initially stated that we would apply the following risk of bias criteria to CBA and ITS studies.
"Quality criteria for controlled before‐and‐after (CBA) studies
For CBA studies, we will assess blinding, incomplete outcome data, selective reporting of outcomes and other potential biases, as specified above and additionally assess the following:
Baseline measurement
Yes, if there was no significant difference in baseline measurements between intervention and control groups or if statistical adjustment for possible confounding variables was carried out.
No, if there were significant differences between groups at baseline and these differences were not adjusted for.
Unclear, if baseline measures were not reported, or if it was unclear whether baseline measures differed substantially different between intervention and control groups.
Selection of intervention and control group
Yes, if similar methods were used for the selection of women in the intervention and control group.
No, if the methods used to select groups markedly differed.
Unclear.
Protection against contamination (studies using second site as control)
Yes, if allocation was by community, institution, or practice and it is unlikely that any patients in the control group received the intervention.
No, if it is likely that some patients in the control group received the intervention.
Unclear.
Quality criteria for interrupted time series (ITS) studies
For ITS studies, we will assess blinding, incomplete outcome data, selective reporting of outcomes, baseline measurement, selection of intervention and control group and other potential biases as specified above and additionally assess the following:
The intervention is independent of other changes
Yes, if the intervention was independent of other changes over time that were likely to affect outcomes.
No, if intervention was not independent of other changes in time that were likely to affect outcomes.
Unclear.
Data were analysed appropriately
Yes, if serial correlation was adjusted/tested for (e.g. by using ARIMA models or time series regression models).
No, if serial correlation was not adjusted/tested for.
Unclear.
Reason for the number of points pre‐ and postintervention given
Yes, if rationale for the number of points was stated (e.g. monthly data for 12 months postintervention was used because the anticipated effect was expected to decay), or a sample size calculation was performed.
No, if it is clear that conditions above are not met.
Unclear.
Shape of the intervention effect was specified
Yes, if a rational explanation for the shape of intervention effect was given.
No, if it is clear that the condition above is not met.
Unclear.
Intervention unlikely to affect data collection
Yes, if intervention itself was unlikely to affect data collection (e.g. sources and methods of data collection were the same before and after the intervention).
No, if the intervention itself was likely to affect data collection.
Unclear".
Adverse events were not reported in any of the studies and so we removed sections in the review which discussed the handling of dichotomous data, as they were unnecessary:
"Data extraction and management
For dichotomous outcomes (e.g. adverse events), we will extract the number of patients in each group who experience the outcome of interest and the number of patients assessed at endpoint, in order to estimate a risk ratio.
Measures of treatment effect
For dichotomous outcomes (e.g. adverse events, or time‐to‐event data if it is not possible to use a hazard ratio), we will use the risk ratio.
Data synthesis
For any dichotomous outcomes, the risk ratio will be calculated for each study and these will then be pooled".
We did not produce a funnel plot to assess the potential for small study effects, since there were only three trials in the largest meta‐analysis which assessed overall survival for the comparisons of specialised centres versus community or general hospitals and teaching or regional cancer centres versus community and general hospitals. We removed the following paragraph on reporting biases:
"Assessment of reporting biases
Funnel plots corresponding to meta‐analysis of the primary outcome will be examined to assess the potential for small study effects. When there is evidence of small‐study effects, publication bias will be considered as only one of a number of possible explanations. If these plots suggest that treatment effects may not be sampled from a symmetric distribution, as assumed by the random‐effects model, sensitivity analyses will be performed using fixed‐effects models".
We identified the following subgroups on a post hoc basis and did not specify them a priori in the protocol.
Institutions with gynaecologic oncologists on site (specialised centres) versus community or general hospital.
Teaching or regional cancer centre versus community or general hospital.
Community hospital with semi‐specialised gynaecologist versus general hospital.
The protocol had been more vague but had left scope to include such subgroups in the review.
"Subgroup analysis and investigation of heterogeneity
Where possible subgroup analyses or separate analyses will be performed, grouping the studies by:
tumour type;
intervention setting (centralised services versus non‐specialised); and
treatment provider (i.e. gynaecological oncology specialists versus non‐specialised)".
The review did not identify any RCTs, included only studies that used multivariate analyses and was restricted to studies that were at high risk of bias, so we did not carry‐out the sensitivity analysis specified a priori. We had specified the following in the protocol.
"Sensitivity analysis
Sensitivity analyses will be performed excluding (i) non‐randomised studies if RCTs have been included; (ii) studies at high risk of bias; and (iii) unadjusted results".
Contributions of authors
Yin Ling Woo (YLW), Mahmood Shafi (MS), Thomas Everett (TE), Pierre Martin‐Hirsh (PM‐H) and Maria Kyrgiou (MK) drafted the clinical sections of the protocol; Andrew Bryant (AB) and Heather Dickinson (HD) drafted the methodological sections of the protocol.
YLW and MK drafted the clinical sections of the review; YLW, TE, AB and MK carried out the sift. AB drafted the methodological and statistical sections of the review. TE and HD provided important feedback when the review was at a draft stage. All authors agreed the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Department of Health, UK.
NHS Cochrane Collaboration programme Grant Scheme CPG‐506
Declarations of interest
None.
Edited (no change to conclusions)
References
References to studies included in this review
Brookfield 2009 {published data only}
- Brookfield KF, Cheung MC, Yang R, Byrne MM, Koniaris LG. Will patients benefit from regionalization of gynecologic cancer care?. PLoS ONE [Electronic Resource] 2009;4(1):e4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elit 2002 {published data only}
- Elit LM, Bondy SJ, Paszat L, Przybysz R, Levine, M. Outcomes in surgery for ovarian cancer. Gynecologic Oncology 2002;87(3):260‐7. [DOI] [PubMed] [Google Scholar]
- Elit LM, Bondy SJ, Paszat LP, Holowaty EJ, Thomas GM, Stukel TA, et al. Surgical outcomes in women with ovarian cancer. Canadian Journal of Surgery 2008;51(5):346‐54. [PMC free article] [PubMed] [Google Scholar]
Shylasree 2006 {published data only}
- Shylasree TS, Howells RE, Lim K, Jones PW, Fiander A, Adams M, et al. Survival in ovarian cancer in Wales: Prior to introduction of all Wales guidelines. International Journal of Gynecological Cancer 2006;16(5):1770‐6. [DOI] [PubMed] [Google Scholar]
Stockton 2000 {published data only}
- Stockton D, Davies T. Multiple cancer site comparison of adjusted survival by hospital of treatment: an East Anglian study. British Journal of Cancer 2000;82(1):208‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vernooij 2008 {published data only}
- Vernooij F, Heintz AP, Coebergh JW, Massuger LF, Witteveen PO, Graaf Y. Specialized and high‐volume care leads to better outcomes of ovarian cancer treatment in the Netherlands. Gynecologic Oncology 2009;112(3):455‐61. [DOI] [PubMed] [Google Scholar]
- Vernooij F, Heintz AP, Witteveen PO, Heiden‐Van der Loo M, Coebergh JW, Graaf Y. Specialized care and survival of ovarian cancer patients in The Netherlands: nationwide cohort study. Journal of the National Cancer Institute 2008;100(6):399‐406. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bailey 2006 {published data only}
- Bailey J, Murdoch J, Anderson R, Weeks J, Foy C. Stage III and IV ovarian cancer in the South West of England: five‐year outcome analysis for cases treated in 1998. International Journal of Gynecological Cancer 2006;16:25‐29. [DOI] [PubMed] [Google Scholar]
Bristow 2006 {published data only}
- Bristow RE, Berek JS. Surgery for ovarian cancer: how to improve survival. Lancet 2006;367(9522):1558‐60. [DOI] [PubMed] [Google Scholar]
Brolmann 1992 {published data only}
- Brolmann HA, Dijkhuizen GH. [Morbidity and results of 100 radical hysterectomies performed in an oncology center]. Nederlands Tijdschrift voor Geneeskunde 1992;136(19):940‐1. [PubMed] [Google Scholar]
Carney 2002 {published data only}
- Carney ME, Lancaster JM, Ford C, Tsodikov A, Wiggins CL. A population‐based study of patterns of care for ovarian cancer: who is seen by a gynecologic oncologist and who is not?. Gynecologic Oncology 2002;84(1):36‐42. [DOI] [PubMed] [Google Scholar]
Chan 1999 {published data only}
- Chan KK. Does the surgeon matter in the management of ovarian cancer?. British Journal of Cancer 1999;80(10):1492‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2007 {published data only}
- Chan JK, Kapp DS, Shin JY, Hussain A, Teng NN, Berek JS, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstetrics and Gynecology June 2007;109(6):1342‐50. [DOI] [PubMed] [Google Scholar]
Crawford 2002 {published data only}
- Crawford SC, Caestecker L, Gillis CR, Hole D, Davis JA, Penney G, et al. Staging quality is related to the survival of women with endometrial cancer: a Scottish population based study. Deficient surgical staging and omission of adjuvant radiotherapy is associated with poorer survival of women diagnosed with endometrial cancer in Scotland during 1996 and 1997. British Journal of Cancer 2002;86(12):1837‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diaz‐Montes 2006 {published data only}
- Diaz‐Montes TP, Zahurak ML, Giuntoli RL, Gardner GJ, Bristow RE. Uterine cancer in Maryland: impact of surgeon case volume and other prognostic factors on short‐term mortality. Gynecologic Oncology 2006;103(3):1043‐7. [DOI] [PubMed] [Google Scholar]
Du Bois 2009 {published data only}
- Du Bois A, Rochon J, Pfisterer J, Hoskins WJ. Variations in institutional infrastructure, physician specialization and experience, and outcome in ovarian cancer: a systematic review. Gynecologic Oncology 2009;112(2):422‐36. [DOI] [PubMed] [Google Scholar]
Earle 2006 {published data only}
- Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. Journal of the National Cancer Institute 2006;98(3):172‐80. [DOI] [PubMed] [Google Scholar]
- Schrag D, Earle C, Xu F, Panageas KS, Yabroff KR, Bristow RE, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. Journal of the National Cancer Institute 2006;98(3):163‐71. [DOI] [PubMed] [Google Scholar]
Eisenkop 1992 {published data only}
- Eisenkop SM, Spirtos NM, Montag TW, Nalick RH, Wang HJ. The impact of subspecialty training on the management of advanced ovarian cancer. Gynecologic Oncology 1992;47(2):203‐9. [DOI] [PubMed] [Google Scholar]
Engelen 2006 {published data only}
- Engelen MJ, Kos HE, Willemse PH, Aalders JG, Vries EG, Schaapveld M, et al. Surgery by consultant gynecologic oncologists improves survival in patients with ovarian carcinoma. Cancer 2006;106(3):589‐98. [DOI] [PubMed] [Google Scholar]
Ghaemmaghami 2010 {published data only}
- Ghaemmaghami F, Hassanzadeh M, Karimi‐Zarchi M, Modari‐Gilani M, Behtash A, Mousavi N. Centralization of ovarian cancer surgery: do patients benefit?. European Journal of Gynaecological Oncology 2010;31(4):429‐33. [PubMed] [Google Scholar]
Giede 2005 {published data only}
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence‐based review. Gynecologic Oncology 2005;99(2):447‐61. [DOI] [PubMed] [Google Scholar]
Goff 2006 {published data only}
- Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecologic Oncology 2006;103(2):383‐90. [DOI] [PubMed] [Google Scholar]
Goff 2007 {published data only}
- Goff BA, Matthews BJ, Larson EH, Andrilla CH, Wynn M, Lishner DM, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer 2007;109(10):2031‐42. [DOI] [PubMed] [Google Scholar]
Grant 1992 {published data only}
- Grant PT, Beischer NA, Planner RS. The treatment of gynaecological malignancy in a general public hospital. Medical Journal of Australia 1992;157(6):378‐80. [DOI] [PubMed] [Google Scholar]
Grant 1999 {published data only}
- Grant JM. Specialist gynaecologists and ovarian cancer. British Journal of Obstetrics and Gynaecology 1999;106(11):vii‐viii. [DOI] [PubMed] [Google Scholar]
Grant 2000 {published data only}
- Grant JM. Improving outcomes in gynaecological cancers?. British Journal of Obstetrics and Gynaecology 2000;107(9):vii‐viii. [DOI] [PubMed] [Google Scholar]
Grossi 2002 {published data only}
- Grossi M, Quinn MA, Thursfield VJ, Francis PA, Rome RM, Planner RS, et al. Ovarian cancer: patterns of care in Victoria during 1993‐1995. Medical Journal of Australia 2002;117(1):11‐6. [DOI] [PubMed] [Google Scholar]
Ioka 2004 {published data only}
- Ioka A, Tsukuma H, Ajiki W, Oshima A. Influence of hospital procedure volume on ovarian cancer survival in Japan, a country with low incidence of ovarian cancer. Cancer Science 2004;95(3):233‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ioka 2005 {published data only}
- Ioka A, Tsukuma H, Ajiki W, Oshima A. Influence of hospital procedure volume on uterine cancer survival in Osaka, Japan. Cancer Science 2005;96(10):689‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Junor 1999 {published data only}
- Junor EJ, Hole DJ, McNulty L, Mason M, Young J. Specialist gynaecologists and survival outcome in ovarian cancer: a Scottish national study of 1866 patients. British Journal of Obstetrics and Gynaecology 1999;106(11):1130‐6. [DOI] [PubMed] [Google Scholar]
Kehoe 1994 {published data only}
- Kehoe S, Powell J, Wilson S, Woodman C. The influence of the operating surgeon's specialisation on patient survival in ovarian carcinoma. British Journal of Cancer 1994;70(5):1014‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumpulainen 2002 {published data only}
- Kumpulainen S, Grenman S, Kyyronen P, Pukkala E, Sankila R. Evidence of benefit from centralised treatment of ovarian cancer: a nationwide population‐based survival analysis in Finland. International Journal of Cancer 2002;102(5):541‐4. [DOI] [PubMed] [Google Scholar]
Kwon 2008 {published data only}
- Kwon JS, Carey MS, Cook EF, Qiu F, Paszat LF. Are there regional differences in gynecologic cancer outcomes in the context of a single‐payer, publicly‐funded health care system? A population‐based study. Canadian Journal of Public Health Revue Canadienne de Sante Publique 2008;99(3):221‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lehtovirta 2000 {published data only}
- Lehtovirta P. Surgical treatment of ovarian cancer should be centralized. Duodecim 2000;116(10):1033‐5. [PubMed] [Google Scholar]
Luesley 2000 {published data only}
- Luesley D. Improving outcomes in gynaecological cancers. BJOG: An International Journal of Obstetrics and Gynaecology 2000;107(9):1061‐3. [DOI] [PubMed] [Google Scholar]
Macdonald 2005 {published data only}
- Macdonald OK, Sause WT, Lee RJ, Dodson MK, Zempolich K, Gaffney DK. Does oncologic specialization influence outcomes following surgery in early stage adenocarcinoma of the endometrium?. Gynecologic Oncology 2005;99:730‐5. [DOI] [PubMed] [Google Scholar]
Mayer 1992 {published data only}
- Mayer AR, Chambers SK, Graves E, Holm C, Tseng PC, Nelson BE, et al. Ovarian cancer staging: does it require a gynecologic oncologist?. Gynecologic Oncology 1992;47(2):223‐7. [DOI] [PubMed] [Google Scholar]
Munstedt 2003 {published data only}
- Munstedt K, Georgi R, Misselwitz B, Zygmunt M, Stillger R, Kunzel W. Centralizing surgery for gynecologic oncology ‐ a strategy assuring better quality treatment?. Gynecologic Oncology 2003;89(1):4‐8. [DOI] [PubMed] [Google Scholar]
Nguyen 1993 {published data only}
- Nguyen HN, Averette HE, Hoskins W, Penalver M, Sevin BU, Steren A. National survey of ovarian carcinoma. Part V. The impact of physician's specialty on patients' survival. Cancer 1993;72:3663‐70. [DOI] [PubMed] [Google Scholar]
O'Malley 2003 {published data only}
- O'Malley CD, Cress RD, Campleman SL, Leiserowitz GS. Survival of Californian women with epithelial ovarian cancer, 1994‐1996: A population‐based study. Gynecologic Oncology 2003;91(3):608‐15. [DOI] [PubMed] [Google Scholar]
Olaitan 2008a {published data only}
- Olaitan A, McCormack M. Centralisation of services for the management of ovarian cancer. BJOG: An International Journal of Obstetrics and Gynaecology 2008;115(5):668. [DOI] [PubMed] [Google Scholar]
Paulsen 2006 {published data only}
- Paulsen T, Kjaerheim K, Kaern J, Tretli S, Trope C. Improved short‐term survival for advanced ovarian, tubal, and peritoneal cancer patients operated at teaching hospitals. International Journal of Gynecological Cancer 2006;16 Suppl 1:11‐7. [DOI] [PubMed] [Google Scholar]
Pearl 2002 {published data only}
- Pearl ML, Villella JA, Valea FA, DiSilvestro PA, Chalas E. Outcomes of endometrial cancer patients undergoing surgery with gynecologic oncology involvement. Obstetrics and Gynecology 2002;100(4):724‐9. [DOI] [PubMed] [Google Scholar]
Petignat 1999 {published data only}
- Petignat P, Gaudin G, Vajda D, Stalder M, Rey JP, Faggiano F, et al. Advanced‐stage (FIGO III‐IV) epithelial ovarian cancer: Multivariate analysis of prognostic factors in an area without a tertiary referral oncology center. A population‐based study. Onkologie 1999;22(5):406‐10. [Google Scholar]
Petignat 2000 {published data only}
- Petignat P, Vajda D, Joris F, Obrist R. Surgical management of epithelial ovarian cancer at community hospitals: A population‐based study. Journal of Surgical Oncology 2000;75(1):19‐23. [DOI] [PubMed] [Google Scholar]
Rich 1993 {published data only}
- Rich WM. Ovarian cancer staging: does it require a gynecologic oncologist?. Gynecologic Oncology 1993;51(2):291‐2. [PubMed] [Google Scholar]
Savage 2008 {published data only}
- Savage L. Specialized hospital care associated with better survival in Dutch ovarian cancer patients. Journal of the National Cancer Institute 2008;100(6):375. [Google Scholar]
Tangjitgamol 2009 {published data only}
- Tangjitgamol S, Manusirivithaya S, Laopaiboon M, Lumbiganon P. Interval debulking surgery for advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006014.pub5] [DOI] [PubMed] [Google Scholar]
Tanner 2008 {published data only}
- Tanner EJ, Zahurak ML, Bristow RE, Diaz‐Montes TP. Surgical care of young women diagnosed with ovarian cancer: a population‐based perspective. Gynecologic Oncology 2008;111(2):221‐5. [DOI] [PubMed] [Google Scholar]
Tingulstad 2003 {published data only}
- Tingulstad S, Skjeldestad FE, Hagen B. The effect of centralization of primary surgery on survival in ovarian cancer patients. Obstetrics and Gynecology 2003;102(3):499‐505. [DOI] [PubMed] [Google Scholar]
Van der Zee 2009 {published data only}
- Zee AG, Engelen MJ, Schaapveld M, Karim‐Kos HE, Vries EG, Willemse PH, et al. [Primary surgery by a gynecological oncologist improves the prognosis in patients with ovarian carcinoma]. Nederlands Tijdschrift voor Geneeskunde 2009;153(1‐2):15‐9. [PubMed] [Google Scholar]
Vernooij 2007 {published data only}
- Vernooij F, Heintz P, Witteveen E, Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecologic Oncology 2007;105(3):801‐12. [DOI] [PubMed] [Google Scholar]
Williams 2008 {published data only}
- Williams MV. The Cancer Reform Strategy. Clinical Oncology 2008;20(4):271‐4. [DOI] [PubMed] [Google Scholar]
Wolfe 1996 {published data only}
- Wolfe CD, Tilling K, Bourne HM, Raju KS. Variations in the screening history and appropriateness of management of cervical cancer in South East England. European Journal of Cancer 1996;32A(7):1198‐204. [DOI] [PubMed] [Google Scholar]
Wolfe 1997 {published data only}
- Wolfe CDA, Tilling K, Raju KS. Management and survival of ovarian cancer patients in South East England. European Journal of Cancer 1997;33(11):1835‐40. [DOI] [PubMed] [Google Scholar]
Woodman 1997 {published data only}
- Woodman C, Baghdady A, Collins S, Clyma JA. What changes in the organisation of cancer services will improve the outcome for women with ovarian cancer?. British Journal of Obstetrics and Gynaecology 1997;104(2):135‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 1995
- Altman DG, Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. British Journal of Cancer 1995;72:511‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bristow 2007
- Bristow RE, Santillan A, Diaz‐Montes TP, Gardner GJ, Giuntoli RL, Meisner BC, et al. Centralization of care for patients with advanced‐stage ovarian cancer‐ a cost‐effectiveness analysis. Cancer 2007;109(8):1513‐21. [DOI] [PubMed] [Google Scholar]
Brookefield 2009
- Brookfield KF, Cheung MC, Yang R, Byrne MM, Koniaris LG. Will patients benefit from regionalization of gynecologic cancer care?. Publication Library of Science (PLoS ONE) 2009;4:e4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2008a
- Cooper N, Quinn MJ, Rachet B, Mitry E, Coleman MP. Survival from cancer of the ovary in England and Wales up to 2001. British Journal of Cancer 2008;99(Suppl 1):S70‐S72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cooper 2008b
- Cooper N, Quinn MJ, Rachet B, Mitry E, Coleman MP. Survival from cancer of the uterus in England and Wales up to 2001. British Journal of Cancer 2008;99(Suppl 1):S65‐S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Crawford 2007
- Crawford SM, Brunskill PJ. Centralisation of services for the management of ovarian cancer: arguments against. BJOG: an International Journal of Obstetrics and Gynaecology 2007;114(10):1183‐5. [DOI] [PubMed] [Google Scholar]
Crawford 2008
- Crawford SM, Brunskill PJ. Centralisation of services for the management of ovarian cancer. BJOG: an International Journal of Obstetrics and Gynaecology 2008;115:667. [DOI] [PubMed] [Google Scholar]
CTCAE 2006
- National Cancer Institute. Common terminology criteria for adverse events v3.0. CTCAE 2006:www.ctep.cancer.gov/forms/CTCAEv3.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
DOH 1995
- Department of Health. A policy framework for commissioning cancer services: A report by the expert advisory group on cancer to the chief medical officers of England and Wales. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_4071083 (last accessed 31 January 2012).
Du 2011
- Du CZ, Li J, Cai Y, Sun YS, Xue WC, Gu J. Effect of multidiscipliary team treatment on outcomes of patients with gastointestinal malignancy. World of Gastroenterology 2011;17(15):2013‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elattar 2011
- Elattar A, Bryant A, Winter‐Roach BA, Hatem M, Naik R. Optimal primary surgical treatment for advanced epithelial ovarian cancer. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD007565] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engel 2005
- Engel J, Kerr J, Eckel R, Gűnther B, Heiss M, Heitland W, et al. Influence of hospital volume on local recurrence and survival in a population sample of rectal cancer patients. European Journal of Surgical Oncology 2005;31:512–20. [DOI] [PubMed] [Google Scholar]
EPOC
- Cochrane Effective Practice and Organisation of Care Group (EPOC). http://epoc.cochrane.org/ (last assessed 31 January 2012).
Fleissig 2006
- Fleissig A, Jenkins V, Catt S, Fallowfield L. Multidisciplinary teams in cancer care: are they effective in the UK. Lancet Oncology 2006;7(11):935‐43. [DOI] [PubMed] [Google Scholar]
Gardner 2011
- Gardner GJ, Jewell EL. Current and future directions for clinical trials for ovarian cancer. Cancer Control 2011;18(1):44‐51. [DOI] [PubMed] [Google Scholar]
Geomini 2011
- Geomini PM, Kruitwagen RF, Bremer GL, Massuger L, Mol BW. Should we centralise care for the patient suspected of having ovarian malignancy?. Gynecologic Oncology 2011;122(1):95‐9. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2008
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM, International Agency for Research on Cancer. GLOBOCAN 2008, cancer incidence and mortality worldwide. http://globocan.iarc.fr/ (last accessed 31 January 2012).
GRADE 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics. CA: a Cancer Journal for Clinicians 2008;58(2):71‐96. [DOI] [PubMed] [Google Scholar]
Katsumata 2009
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose‐dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open‐label, randomised controlled trial. The Lancet 2009;374(9698):1331‐8. [DOI] [PubMed] [Google Scholar]
Linkov 2008
- Linkov F, Edwards R, Balk J, Yurkovetsky Z, Stadterman B, Lokshin A, et al. Endometrial hyperplasia, endometrial cancer and prevention: gaps in existing research of modifiable risk factors. European Journal of Cancer 2008;44:1632‐44. [DOI] [PubMed] [Google Scholar]
Macgregor 1994
- Macgregor JE, Campbello MK, Mann EMF, Swanson KY. Screening for cervical intraepithelial neoplasia in north‐east Scotland shows fall in incidence and mortality from invasive cancer with concomitant rise in preinvasive disease. BMJ 1994;308:1407‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nieminen 1999
- Nieminen P, Kallio M, Anttila A, Hakama M. Organised vs. spontaneous pap‐smear screening for cervical cancer: A case‐control study. International Journal of Cancer 1999;83:55‐8. [DOI] [PubMed] [Google Scholar]
Olaitan 2001
- Olaitan A, Weeks J, Mocroft A, Smith J, Howe K, Murdoch J. The surgical management of women with ovarian cancer in the south‐west of England. British Journal of Cancer 2001;85(12):1824‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Olaitan 2007
- Olaitan A, McCormack M. Centralisation of services for the management of ovarian cancer: arguments for. BJOG: an International Journal of Obstetrics and Gynaecology 2007;114:1188–90. [DOI] [PubMed] [Google Scholar]
Olaitan 2008b
- Olaitan A, McCormack M. Centralisation of services for the management of ovarian cancer: author’s Reply. BJOG: an International Journal of Obstetrics and Gynaecology 2008;115:668. [DOI] [PubMed] [Google Scholar]
Rachet 2009
- Rachet B, Maringe C, Nur U, Quaresma M, Shah A, Woods LM, et al. Population‐based cancer survival trends in England and Wales up to 2007: an assessment of the NHS cancer plan for England. Lancet Oncology 2009;10(4):351‐69. [DOI] [PubMed] [Google Scholar]
Ravakhah 2006
- Ravakhah K. Death certificates are not reliable: revivification of the autopsy. Southern Medical Journal 2006;99(7):728‐33. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Richards 2009
- Richards M. Assessment of the NHS cancer plan in England. Lancet Oncology 2009;10(4):311. [DOI] [PubMed] [Google Scholar]
Schrag 2006
- Schrag D, Earle C, Xu F, Panageas KS, Yabroff KR, Bristow RE, et al. Associations between hospital and surgeon procedure volumes and patient outcomes after ovarian cancer resection. Journal of the National Cancer Institute 2006;98(3):163‐71. [DOI] [PubMed] [Google Scholar]
Shadish 2002
- Shadish WR, Cook TD, Campbell DT. Experimental and quasi‐experimental designs for generalized causal inference. Boston: Houghton‐Mifflin, 2002. [Google Scholar]
Sikora 2009
- Sikora K. Was the NHS cancer plan worth the effort?. Lancet Oncology 2009;10(4):312‐13. [DOI] [PubMed] [Google Scholar]
Stephens 2006
- Stephens MR, Lewis WG, Brewster AE, Lord I, Blackshaw GR, Hodzovic I, et al. Multidisciplinary team management is associated with improved outcomes after surgery for esophageal caner. Dis Esophagus 2006;19(3):164‐71. [DOI] [PubMed] [Google Scholar]
Vernooji 2007
- Vernooij F, Heintz P, Witteveen E, Graaf Y. The outcomes of ovarian cancer treatment are better when provided by gynecologic oncologists and in specialized hospitals: a systematic review. Gynecologic Oncology 2007;105:801–12. [DOI] [PubMed] [Google Scholar]
WHO 2008
- World Health Organization. World Health Statistics 2008. http://www.who.int/gho/ncd/mortality_morbidity/cancer/en/index.html (last accessed 31 January 2012).


