Abstract
The active constituent profile in Cape gooseberry (Physalis peruviana L.) juice was determined by GC-MS. Quercetin and kaempferol were active components in the juice. In this study we have evaluated its potential protective effect on hepatic injury and fibrosis induced by carbon tetrachloride (CCl4). Twenty-eight rats divided into 4 groups: Group I served as control group, and Group II received weekly i.p. injection of 2 mL CCl4/kg bwt for 12 weeks. Group III were supplemented with Physalis juice via the drinking water. The animals of Group IV received Physalis juice as Group III and also were intraperitoneally injected weekly with 2 mL CCl4/kg bwt for 12 weeks. Hepatoprotective effect was evaluated by improvement in liver enzymes serum levels, reduction in collagen areas, downregulation in expression of the fibrotic marker MMP-9, reduction in the peroxidative marker malonaldehyde and the inflammatory marker nitric oxide, and restoration of the activity of antioxidant enzymatic and nonenzymatic systems, namely, glutathione content, superoxide dismutase, catalase, glutathione-S-transferase, glutathione peroxidase, and glutathione reductase activities. The results show that the potential hepatoprotective effects of Physalis peruviana may be due to physalis acts by promotion of processes that restore hepatolobular architecture and through the inhibition of oxidative stress pathway.
1. Introduction
Liver diseases are amongst the most serious health problems in the world today and their prevention and treatment options still remain limited despite tremendous advances in modern medicine. The pathogenesis of hepatic diseases as well as the role of oxidative stress and inflammation therein is well established and, accordingly, blocking or retarding the chain reactions of oxidation and inflammation process could be promising therapeutic strategies for prevention and treatment of liver injury [1].
Vitaglione et al. [2] suggested that reactive oxygen species (ROS) including superoxide and hydroxyl radicals are known to play an important role in liver disease's pathology and progression and have been proven to associate with the intoxication by CCl4. Documented evidences suggested that CCl4 has been commonly used as hepatotoxins in experimental hepatopathy [3]. The first metabolite of CCl4 trichloromethyl free radical is believed to initiate the biochemical processes leading to oxidative stress, which is the direct cause of many pathological conditions such as diabetes mellitus, cancer, hypertension, kidney and liver damages, and even death [4, 5].
In recent years, considerably clinical and experimental evidences show that oxidative stress caused by an imbalance between the oxidant and antioxidant systems of the body in favor of the oxidants should be a major apoptotic stimulus in the different types of acute and chronic liver injury and hepatic fibrosis [6].
Hepatic fibrosis induced by CCl4 is associated with the exacerbation of lipid peroxidation and the depletion of antioxidant status [7]. Accordingly, successful antioxidant interventions, which to date has attracted intensive interests from investigators, offer insights into delaying or preventing occurrence and development of hepatic fibrosis and may be a potential and effective therapeutic strategy for prevention and treatment of hepatic fibrosis [8].
There are a number of evidences indicating that natural substances from edible and medicinal plants exhibited strong antioxidant activity that could act against hepatic toxicity caused by various toxicants [9, 10]. One of those candidate plants is Cape gooseberry (Physalis peruviana L.). Various bioactive compounds (withanolides and phenolics) are reported to be present in physalis [11]. Some of these compounds have a strong antioxidant property and prevent peroxidative damage to liver microsomes and hepatocytes [12].
We aimed in this study to evaluate the potential hepatoprotective effect of Physalis peruviana against CCl4-induced hepatotoxicity and liver fibrosis in rats.
2. Materials and Methods
2.1. Chemicals
Carbon tetrachloride (CCl4) and Tris-HCl buffer were purchased from Sigma (St. Louis, MO, USA). Perchloric acid, thiobarbituric acid (TBA), and trichloroacetic acid (TCA) were purchased from Merck. All other chemicals and reagents used in this study were of analytical grade. Double-distilled water was used as the solvent.
2.2. Animals
Adult male Wistar albino rats weighing 200–250 g (8–10 weeks) were obtained from the Holding Company for Biological Products and Vaccines (VACSERA, Cairo, Egypt). The animals were kept in wire bottomed cages in a room under standard condition of illumination with a 12-hour light-dark cycle 55 + 5% relative humidity and at 25 ± 2°C for one week until the beginning of treatment. They were provided with tap water and balanced diet ad libitum. All animals have received human care in compliance with the state authorities following the Egyptian rules of animal protection.
2.3. Plant Material
Physalis peruviana L. fresh fruits were collected from market of East Cairo, Egypt, in the months of February-March, 2012. The plant material was authenticated in Botany Department, Faculty of Science, Helwan University, Cairo, Egypt, on the basis of taxonomic characters and by direct comparison with the herbarium specimens available at the herbarium of the Botany Department.
2.4. Physalis Juice Preparation and Stability
The fresh fruits of Physalis peruviana L. (10 kg) were separated from their calyxes and homogenized. The pulp was filtered off, the filtrate was clear and yellow in colour, and then the filtrate was immediately diluted with distilled water in ratio 1 : 5 (V/V) and stored at 4°C for no longer than 2 months. Physalis juice stability was assessed by measuring initial total phenolic and flavonoid contents and evaluating the alterations after 2 and 3 days of exposure to the same conditions as the juice supplied to the animals.
2.5. Measurement of Flavonoids, Total Polyphenols, and In Vitro Free Radical Scavenging Assays
2.5.1. Determination of Total Phenols
The total polyphenolic contents (TPC) were measured using Folin-Ciocalteau reagent based on the oxidation of polyphenols to a blue colored complex with an absorbance maximum of 750 nm. Calibration curve was prepared using gallic acid as standard for TPC which was measured as mg gallic acid equivalents (GAE) per milliliter of the sample (μg/mL).
2.5.2. Determination of Flavonoid Content
For the assessment of flavonoids a colorimetric method was used. Briefly, 1.50 mL of the deionized water was added to 0.25 mL of the sample and then 90 uL of 5% sodium nitrite (NaNO2) was added. Six min later, after addition of 180 uL of 10% AlCl3, mixture was allowed to stand for another 5 min before adding 0.6 mL of 1 M NaOH. By adding deionized water and mixing well, final volume was made up to 3 mL. The absorbance was measured at a fixed wavelength 510 nm. Calibration curve was prepared using quercetin as standard for total flavonoids which was measured as mg quercetin equivalents (QE) per milliliter of the sample (μg/mL).
2.5.3. Determination of DPPH Radical Scavenging Activity
The free radical scavenging capacity was evaluated by the 2, 2-diphenyl -1-picrylhydrazyl (DPPH) assay. In its radical form, DPPH is monitored at 517 nm but, upon reduction by an antioxidant or a radical species, the absorption decreases. Briefly, 1 mL of 0.25 mM solution of DPPH in methanol was added to 50, 100, 150, and 200 uL of sample in 950, 900, 850, and 800 μL methanol, respectively. After 20 min, the absorbance was measured. Ascorbic acid was used as a positive control. The percentage DPPH decolorisation of the sample was calculated by the equation, % DPPH scavenging = [(A control − A sample)/A control] − 100, where A is the absorbance.
2.5.4. Determination of Superoxide Anion Scavenging Activity
The superoxide anion scavenging activity was determined by the method of Nishikimi et al. [13]. Superoxide anion derived from dissolved oxygen by a phenazine methosulfate (PMS)/NADH coupling reaction reduces nitroblue tetrazolium (NBT), which forms a violet colored complex. A decrease in color after addition of the antioxidant is a measure of its superoxide scavenging activity. To the reaction mixture containing phosphate buffer (100 mM, pH 7.4), NBT (1 mM) solution, NADH (1 mM), 50, 100, 150, and 200 uL of sample in 950, 900, 850, and 800 μL methanol, respectively, and 1 mL of 1 mM PMS were added. After incubation at 25°C for 5 min, the absorbance was measured at 560 nm against a blank. Vit. C was used as a positive control.
2.5.5. Determination of Nitric Oxide Radical Inhibition Activity
The nitric oxide radical inhibition activity was measured using Griess reagent. Briefly, sodium nitroprusside (10 mM) in phosphate buffered saline was mixed with 50, 100, 150, and 200 uL of sample in 950, 900, 850, and 800 μL methanol, respectively, and incubated at room temperature for 150 min followed by addition of 0.5 mL of Griess reagent (1% sulfanilamide, 2% H3PO4, and 0.1% N-(1-naphthyl)ethylenediamine dihydrochloride). The absorbance of the chromophore formed was read at 546 nm.
2.5.6. Determination of Total Antioxidant Potential Activity
The total antioxidant potential was measured by the ability of sample to scavenge thiobarbituric acid reactive substances (TBARS) [14]. Briefly, 50, 100, 150, and 200 uL of the different samples were added to the 10% liver homogenate. Lipid peroxidation was initiated by addition of 100 μL of 15 mM FeSO4 solution to 3 mL of liver homogenate (final concentration was 0.5 mM). After 30 min, 100 μL of this reaction mixture was taken in a tube containing 1.5 mL of 0.67% thiobarbituric acid (TBA) in 50% acetic acid. Samples were incubated at 37°C for 1 hr, and then lipid peroxidation was measured using the reaction with TBA. The absorbance of the organic layer was measured at 532 nm. All reactions were carried out in triplicate. Vitamin C (Vit. C) was used as the positive control. The percentage of inhibition of lipid peroxidation was calculated, by the formula Inhibition (%) = (A control − A sample) × 100/A control.
2.6. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
The GC-MS analysis of physalis juice was performed with Thermo Scientific, Trace GC Ultra & ISQ Single Quadruple MS. The inert gas helium (99.9995%) was used as carrier gas, at flow rate of 1.5 mL/min; split ratio 10 : 1; sample size, 1 μL injected using the splitless injection technique; fused capillary silica column TG-5MS (30 m × 0.25 mm × 0.25 μm). Temperatures: injector: 260°C, detector: 300°C, column: 70°C, 10°C min−1, and 260°C (10 min). The total GC running time is at 60 min. The MS was taken at 70 eV. The MS scan parameters included a mass range of m/z 40–1000, a scan interval of 0.5 s, a scan speed of 2000 amu s−1, and a detector voltage of 1.0 kV. Identification of compounds was conducted using the database of Wiley9 combined with NIST 11 mass spectral database. The name, molecular weight, molecular formula, and area under peak of the components of the test materials were ascertained.
2.7. Experimental Protocol
To study the protective effects of physalis juice on carbon tetrachloride mediated liver toxicity, twenty eight adult male rats were randomly allocated to four groups, seven rats of each. Group I (Con) served as control and received 300 μL of saline by intraperitoneal (i.p.) injection route each week. Group II (CCl4) received weekly i.p. injection of 2 mL CCl4/kg bwt for 12 weeks as described by Sohn et al. [15]. Group III (physalis) received juice supplied on dark water bottles and renewed every 2-3 days. The animals of Group IV (physalis + CCl4) received physalis juice as Group III and were also intraperitoneally injected weekly with 2 mL CCl4/kg bwt for 12 weeks. After one week of the last i.p. injection of CCl4, the animals of all groups were anesthetized with chloroform and blood sampling was performed by cardiac puncture. The collected blood samples were allowed to clot for half an hour at 8°C. Serum was separated by centrifugation at 3000 rpm for 15 min at 4°C to separate serum, stored at −20°C, and used for the estimation of marker enzymes, namely, alanine aminotransferase (ALT), aspartate aminotransferase (AST), γ-glutamyl transpeptidase (γGT), alkaline phosphatase (ALP), and total bilirubin (TB). The livers were dissected out immediately, washed, and homogenized immediately to give 50% (w/v) homogenate in ice-cold medium containing 50 mM Tris-HCl, pH, 7.4. The homogenate was centrifuged at 3000 rpm for 10 min at 4°C. The supernatant (10%) was used for the various biochemical determinations.
2.8. Histopathological Examination
Tissue samples were fixed in 10% neutral formalin for 24 h and paraffin blocks were obtained and routinely processed for light microscopy. Slices of 4-5 μm were obtained from the prepared blocks and stained with hematoxylin and eosin as well as Sirius Red for hepatic fibrosis. The preparations obtained were visualized using a Nikon microscopy at a magnification of 400×.
2.9. Liver Function Tests
Colorimetric determination of alanine aminotransferase or aspartate aminotransferase, ALT or AST, was estimated by measuring the amount of pyruvate or oxaloacetate produced by forming 2, 4-dinitrophenylhydrazine according to the method of Reitman and Frankel [16]. Glutamyl transferase and alkaline phosphatase, γGT and ALP, were assayed using kits provided by Randox Laboratories Co. according to the methods described by Szasz [17] and Belfield and Goldberg [18], respectively. Also, total bilirubin, TB, in serum was assayed according to the method of Garber [19].
2.10. Oxidative Stress Markers
Serum and homogenates of liver were used to determine malondialdehyde (MDA) as indicator of lipid peroxidation by reaction of thiobarbituric acid according to the method of Ohkawa et al. [20], nitrite/nitrate (nitric oxide, NO) was measured using the method of Green et al. [21] and glutathione (GSH) was measured as described by Ellman [22].
2.11. Enzymatic Antioxidant Status
Homogenates of liver were used for determination of superoxide dismutase (SOD) as described by Nishikimi et al. [13], catalase (CAT) as described by Aebi [23], glutathione peroxidase (GPx) as described by Paglia and Valentine [24], glutathione-S-transferase (GST) as described by Habig et al. [25], and glutathione reductase (GRd) as described by Factor et al. [26].
2.12. Real Time PCR
Total RNA was isolated from the liver tissue using an RNeasy Plus Minikit (Qiagen, Valencia, CA). One microgram total RNA and random primers were used for cDNA synthesis using the RevertAid H Minus Reverse Transcriptase (Fermentas, Thermo Fisher Scientific Inc., Canada). For real time PCR analysis, the cDNA samples are run in triplicate and β-actin is used as reference gene. Each PCR amplification includes nontemplate controls containing all reagents except cDNA. Real time PCR reactions were performed using Power SYBR Green (Life Technologies, CA) and was conducted on the Applied Biosystems 7500 Instrument. The typical thermal profile is 95°C for 3 min, followed by 40 cycles of 95°C for 15 s and 56°C for 30 s. After PCR amplification, the ΔCt is calculated by subtraction of the β-actin Ct from each sample Ct. The method of Pfaffl was used for data analysis. The PCR primers for INOS, GPx, and GRd genes were synthesized by Jena Bioscience GmbH (Jena, Germany). Primers were designed using Primer-Blast program from NCBI. For a reference gene, the β-actin is used. The primer sets used the following.
-
iNOS (S): 5′-GAAAGAACTCGGGCATACCT-3′.
-
iNOS (AS): 5′-GGCGAAGAACAATCCACAAC-3′.
-
GPx (S): 5′-CGGTTTCCCGTGCAATCAGT-3′.
-
GPx (AS): 5′-ACACCGGGGACCAAATGATG-3′.
-
GRd (S): 5′-AGCCCACAGCGGAAGTCAAC-3′.
-
GRd (AS): 5′-CAATGTAACCGGCACCCACA-3′.
-
β-Actin (S): 5′-GGCATCCTGACCCTGAAGTA-3′.
-
β-Actin (AS): 5′-GGGGTGTTGAAGGTCTCAAA-3′.
2.13. Immunohistochemistry for Detection of MMP-9
For immunohistochemistry, liver sections (4 μm) were deparaffinized and then boiled to unmask antigen sites; the endogenous activity of peroxidase was quenched with 0.03% H2O2 in absolute methanol. Liver sections were incubated overnight at 4°C with a 1 : 200 dilution of goat polyclonal MMP-9 antibodies (Santa Cruz CA, USA) in phosphate buffered saline (PBS). After removal of the unbound primary antibodies by rinsing with PBS, slides were incubated with a 1 : 500 dilution of biotinylated anti-goat secondary antibody. Bound antibodies were detected with avidin biotinylated peroxidase complex ABC-kit Vectastain and the chromogen 3,3′ diaminobenzidine tetrachloride (DAB) is used as substrate. After appropriate washing in PBS, slides were counterstained with hematoxylin. All sections were incubated under the same conditions with the same concentration of antibodies and at the same time; so the immunostaining was comparable among the different experimental groups.
2.14. Statistical Analysis
Differences between obtained values (mean ± SEM) were carried out by one-way analysis of variance (ANOVA) followed by the Duncan test. A P value of 0.05 or less was taken as a criterion for a statistically significant difference.
3. Results
3.1. Phytochemical Screening and GC-MS Findings
Table 1 shows the flavonoids and total polyphenolic contents of Physalis peruviana L. fruit juice (physalis juice). Flavonoids content in physalis juice was 89.4 μg/mg quercetin equivalents of flavonoids/mL juice. The total polyphenolic content was 121.3 μg/mg gallic acid equivalent of polyphenols/mL juice. According to the present results, no significant changes were observed between the initial and final flavonoids and phenolics contents indicating the stability of physalis juice.
Table 1.
Total flavonoids and polyphenolic contents of physalis juice in different conditions.
| Conditions | Total phenolicsa | Total flavonoidb |
|---|---|---|
| Physalis juice, fresh | 121.3 ± 4.65 | 89.4 ± 4.82 |
| Physalis juice, store* | 113.5 ± 5.31 | 81.7 ± 3.74 |
aFlavonoids are expressed as μg/mg quercetin equivalents of flavonoids/mL juice. bTotal polyphenols are expressed as μg/mg gallic acid equivalent of polyphenols/mL juice. Data are represented as mean ± SEM of two independent experiments each performed in duplicate. *Stored at room temperature for 3 days.
Figure 1 shows the reduction potential of physalis juice. The order of the reduction potential was physalis juice < Vit. C. Analysis of the free radical scavenging activities of the juice revealed a concentration-dependent antiradical activity resulting from reduction of DPPH•, O•, TBARS and nitric oxide radicals to nonradical form [27]. The scavenging activity of Vit. C, a known antioxidant, is used as positive control.
Figure 1.
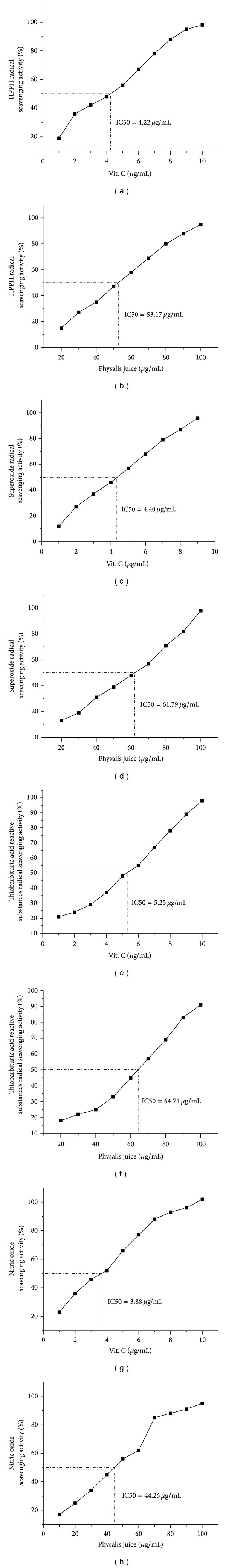
DPPH, superoxide, thiobarbituric acid reactive substances, and nitric oxide radicals scavenging activities of physalis juice and Vit. C. IC 50 values denote the concentration of sample which is required to scavenge 50% of the respective free radicals. Data are represented as mean ± SEM of two independent experiments each performed in triplicate as published in Abdel Moneim and El-Dieb [27].
GC-MS chromatogram of the physalis (Figure 2) showed 29 peaks indicating the presence of 29 phytochemical constituents. On comparison of the mass spectra of the constituents with Wiley9 combined with NIST 11 libraries, the 29 phytoconstituents were characterized and identified (Supplementary data, Table S1, in the Supplementary Material available online at http://dx.doi.org/10.1155/2014/381413). The major phytochemical constituent's mass spectra are kaempferol 3-O-rutinoside (1.40%), Quercetin 3,4′,7-trimethyl ether (3.11%), Folic Acid (0.95%), 1,25-Dihydroxyvitamin D2 (1.27%), Lucenin-2 (1.50%), Betulin (0.62%), (5á)Pregnane-3,20á-diol (0.97%).
Figure 2.
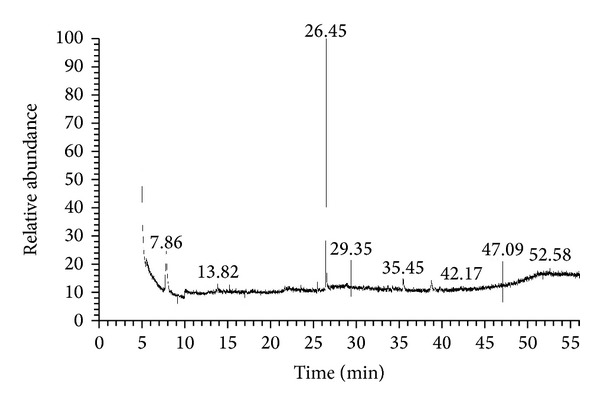
GC-MS chromatogram of physalis juice.
3.2. Histopathologic Findings
Control animals showed no abnormality (Figures 3(a) and 4(a)), whereas liver sections of CCl4-intoxicated rats showed excessive liver damage with necrosis and inflammation of hepatocytes (Figure 3(b)) and collagen accumulation (Figure 4(b)). Physalis-treated animals showed no abnormalities as well (Figures 3(c) and 4(c)), whereas combined treated groups marked decrease in the liver injury and collagen accumulation as compared with CCl4-treated animals (Figures 3(d) and 4(d)).
Figure 3.
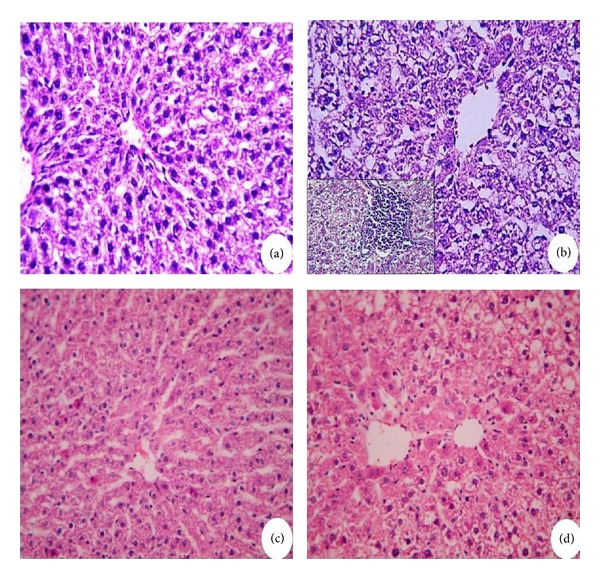
Photomicrographs for liver sections stained with hematoxylin and eosin (H&E). (a) Control rat showing the normal hepatocytes architecture. (b) Rats liver treated with CCl4 showing severe hepatic lesions, degenerated and ballooned/necrotic hepatocytes with lymphocyte cells infiltration (corner). (c) Liver treated with physalis similar to control group. (d) Rat liver treated with physalis + CCl4 showing maintained hepatic architecture, with minimal damage. Original magnification 400x.
Figure 4.
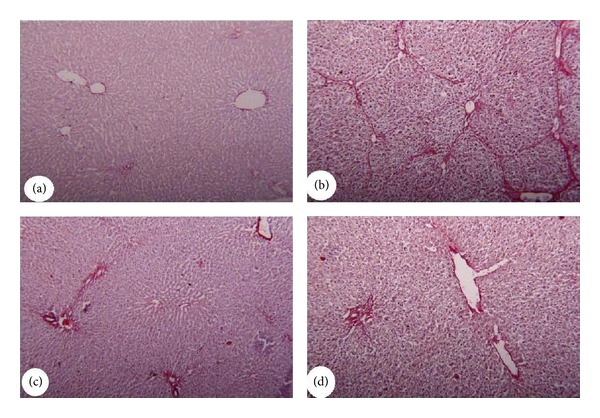
Photomicrographs of liver tissue stained with Sirius Red showing. (a) Control group showing absence of collagen fibers. (b) CCl4 group had developed extensive fibrosis in the periportal area and more fibrillar collagen deposition. (c) Physalis group showing absence of collagen fibers. (d) Physalis + CCl4 group showing sporadic, small fibrotic lesions in the periportal zone and reduction in collagen fibers deposition. Original magnification 200x.
3.3. Biochemical Findings
CCl4 injections to the experimental animals caused hepatotoxicity which is indicated by the elevation of the activities of the sera AST, A LT, γ-GT, ALP, and TB level, while supplementation of physalis juice exhibited a significant decrease in the levels of these marker enzymes and restored it to the control values as shown in Table 2.
Table 2.
Serum levels of liver enzymes in different studied groups.
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| GPT (U/mL) | 70.12 ± 1.88 | 153.91 ± 1.56a | 68.47 ± 1.23 | 79.10 ± 5.12b |
| GOT (U/mL) | 56.08 ± 1.30 | 123.75 ± 7.21a | 56.33 ± 1.21 | 57.08 ± 1.45b |
| γ -GT (U/L) | 38.77 ± 2.66 | 52.80 ± 1.60a | 34.87 ± 1.21 | 35.39 ± 0.56b |
| ALP (IU/L) | 147.73 ± 4.07 | 244.32 ± 7.52a | 116.48 ± 5.31a | 165.28 ± 7.10b |
| TB (mg/dL) | 2.64 ± 0.08 | 4.19 ± 0.08a | 2.81 ± 0.05 | 3.20 ± 0.09ab |
Values are means ± SEM (n = 7).
a P < 0.05, significant change with respect to Group I; b P < 0.05, significant change with respect to Group II.
MDA, an end product of lipid peroxidation, is widely used as a marker of lipid peroxidation. CCl4 injection resulted in a significant increase in MDA in the intoxicated group while treatment withphysalis has significantly reduced the liver injury indicating the hepatoprotective effect of physalis juice (Table 3). The inflammatory marker NO was elevated in the CCl4-treated group compared to other groups; this result was further confirmed by the results of mRNA transcripts which have shown increase in the expression of iNOS, while in the treatment group (Group IV) they showed marked reduction in their expression (Figure 5)
Table 3.
Sera MDA, NO, and GSH and hepatic MDA, NO, GSH, GRd, GST, and GPx in different studied groups.
| Parameters | Group I | Group II | Group III | Group IV |
|---|---|---|---|---|
| Serum MDA (nmol/mL) | 32.37 ± 0.61 | 50.21 ± 1.46a | 23.18 ± 1.51a | 37.52 ± 1.38b |
| Serum NO (μmol/L) | 47.33 ± 1.64 | 101.13 ± 4.79a | 52.30 ± 2.99 | 80.10 ± 2.10ab |
| Serum GSH (mmol/mL) | 53.02 ± 2.86 | 39.13 ± 1.31a | 72.00 ± 4.91a | 63.46 ± 3.65ab |
| Hepatic MDA (nmol/g tissue) | 427.19 ± 4.79 | 591.45 ± 12.47a | 360.75 ± 7.85a | 406.82 ± 2.80b |
| Hepatic NO (μmol/g tissue) | 128.54 ± 2.39 | 226.50 ± 2.93a | 139.33 ± 6.82 | 184.59 ± 8.67ab |
| Liver GSH (mmol/g tissue) | 36.69 ± 2.01 | 25.09 ± 1.47a | 55.51 ± 2.73a | 44.16 ± 5.66b |
| Hepatic GRd (μmol/g tissue) | 102.48 ± 8.97 | 41.5297 ± 3.15a | 105.16 ± 10.95 | 86.4085 ± 5.75b |
| Hepatic GST (μmol/h/g tissue) | 0.27 ± 0.03 | 0.12 ± 0.01a | 0.31 ± 0.02 | 0.22 ± 0.03ab |
| Hepatic GPx (U/g tissue) | 1722.43 ± 69.21 | 616.02 ± 27.76a | 2054.37 ± 54.68a | 1532.68 ± 38.97b |
Values are means ± SEM (n = 7).
a P < 0.05, significant change with respect to Group I; b P < 0.05, significant change with respect to Group II.
Figure 5.
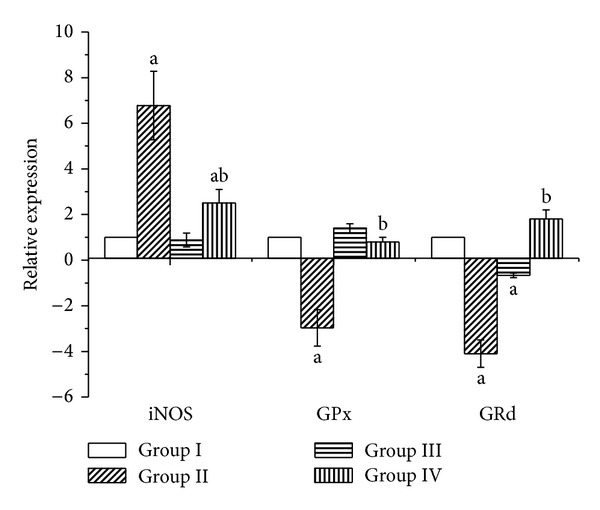
Relative quantification using RT-qPCR of mRNA expression of iNOS, GPx, and GRd genes in liver of rats treated with CCl4 and physalis juice. Values are means ± SEM (n = 7). a P < 0.05, significant change with respect to Group I; b P < 0.05, significant change with respect to Group II.
Compromised hepatic function is always associated with a state of oxidative stress in liver tissues and serum. Thus, the activities of the redox system were measured in liver tissues. Antioxidant enzymes such as SOD, CAT, GPx, GRd, and GST, as well as glutathione as nonenzymatic antioxidant substance, were estimated in the present study. There was a significant decrease in GSH content in the serum and liver homogenates of CCl4-treated groups as compared to the control at P < 0.05. The supplementation of physalis with CCl4 caused a significant increase in GSH when compared with CCl4 group and returned its content to the control level (Table 3). GPx, GRd, and GST activities were also significantly decreased in the liver tissues of rats injected with CCl4 (Table 3). The decreases in GPx and GRd were confirmed by results of real time PCR which showed decreases in mRNA expressions by 2.9-fold for GPx and 4-fold for GRd but supplementation with physalis juice was able to significantly ameliorate these enzymes after 12 weeks of treatment concurrently with CCl4 (Figure 5).
Carbon tetrachloride decreased the activities of SOD and CAT, compared to the control group (Figure 6). Rats supplemented withphysalis juice together with CCl4 for 12 weeks experienced a significant increase in SOD and CAT activities compared to the CCl4-treated group. These improvements indicated thatphysalis juice has antioxidative and beneficial effects for liver recovery from CCl4 injury.
Figure 6.
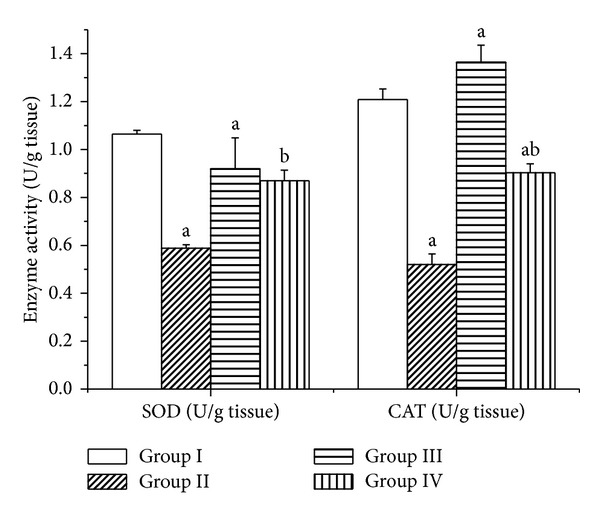
Effects of physalis juice on superoxide dismutase and catalase inhibition in different brain regions of rats treated with CCl4. Values are means ± SEM (n = 7). a P < 0.05, significant change with respect to Group I; b P < 0.05, significant change with respect to Group II.
3.4. Immunohistochemistry Results
Immunohistochemistry revealed a high expression of MMP-9 in the CCl4-treated rats which were reduced in physalis + CCl4 group, indicating the antifibrotic effect of physalisjuice (Figure 7(d)).
Figure 7.
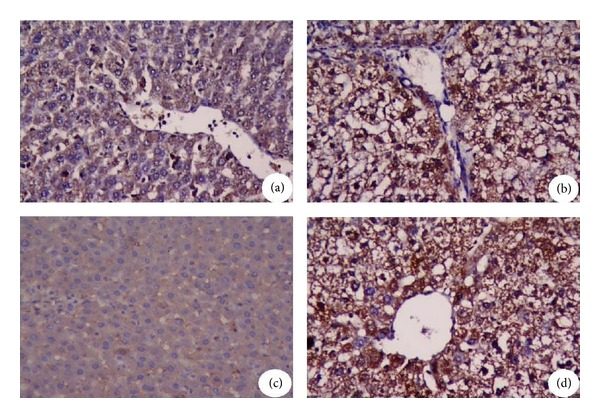
The expression and specific tissue distribution of MMP-9. (a) The liver sections from control rats showed low MMP-9 immunopositive reaction in the hepatic cells. (b) In the livers of rats receiving CCl4 for 12 weeks, high MMP-9 expression. (c) Rats receiving physalis juice for 12 weeks show weak immunopositive reaction for MMP-9 expression in hepatic cells. (d) The livers of rats receiving physalis juice concurrently with CCl4 showed staining pattern similar or more than to the control animals. Original magnification 400x.
4. Discussion
The oxidative stress induced by CCl4, an established model for the evaluation of hepatotoxicity [28–30], was manifested in the hepatic injury observed in the animals treated with CCl4. This is due to the CYP P450-mediated metabolism of CCl4 to reactive metabolites: CCl3 • and CCl3OO•. These radicals bind irreversibly to cellular molecules such as nucleic acid, protein, and lipids, especially the polyunsaturated fatty acids to initiate a process of autocatalytic lipid peroxidation by attacking the methylene bridges of unsaturated fatty acid side chains. This invariably affects the mitochondrial permeability, endoplasmic sequestration, and homeostasis and ultimately in cell damage [31]. Our data show that treatment with CCl4 at a dose of 2 mL/kg body weight one time per week for 12 weeks led to the development of hepatic injury and fibrosis in rats. The results obtained in this work are similar to the findings of Ganie et al. [32] and Breikaa et al. [33] who have shown the hepatotoxic effect of CCl4.
We further tried to evaluate physalis juice hepatoprotection and to show whether it attenuated oxidative stress and inhibited fibrosis in rats treated with CCl4. We found that, after supplementation of physalis juice, liver tissue showed a more or less normal lobular pattern with mild degree of necrosis and lymphocytic infiltration almost comparable to normal control.
The general chemical composition of Physalis peruviana L. fruit juice has many advantages for its use in medicine [34]. Plant extracts of physalis show antioxidant activity [35] as well as antihepatotoxic [36, 37], antiproliferative effects on hepatoma cells [38] and anti-inflammatory activity [39]. The hepatoprotective effect may be due to presence of quercetin which is one of the main phenolic components present inphysalis and a well-known hepatoprotective agent [30, 40, 41].
A first indication of hepatic damage induced by CCl4 was obtained by the evaluation of ALT, AST, ALP, and γ-GT. These enzymes have been identified in cytotoxic and cholestatic hepatic injuries. The reversal of these alterations by physalis juice is a clear indication of the improvement of the functional status of hepatocytes with preservation of cellular architecture [42] indicating the hepatoprotective activity of physalis.
MDA was one of the main lipid peroxidation products, its elevated levels could reflect the degrees of lipid peroxidation injury in hepatocytes [43], and administration ofphysalis juice markedly decreases MDA levels indicating its antiperoxidative effect.
NO plays crucial roles in inflammation and liver injury [44]. It is produced in large quantities by Kupffer cells, endothelial cells, and the hepatocytes themselves in response to tissue damage and inflammation induced by various xenobiotics including CCl4. In addition, its role in oxidative stress cannot be neglected, since high levels of NO have been associated with oxidative injury via lipid peroxide. Our results showed elevated levels of NO in the CCl4 intoxicated rats which is consistent with Breikaa et al. [33]. Our results were supported by the increase in iNOS mRNA gene expression. iNOS is responsible for initiation and propagation of the inflammatory cascade [45];this elevation was significantly decreased after administration of physalis. This may be a potential mechanism by which physalis juice can act as anti-inflammatory and thus protect the liver [39, 46].
Oxidative stress produced by free radicals is the main and primary step in CCl4 toxicity contributing to both onset and progression of fibrosis [31]. This was evidenced by enhanced lipid peroxidation, associated with decreased levels of GSH and the antioxidant enzymes system, namely, SOD, CAT, GPx, GRd, and GST.
Many studies reported that these enzymes constitute a mutually supportive team of defense against ROS [47, 48]. An interesting finding in our study is that treatment withphysalis juice attenuated the intoxications of CCl4 and significantly improved the activity of these enzymes; this indicates the antioxidant and antihepatotoxic effects of physalis which is similar to results obtained by Arun and Asha [36].
MMP-9 is considered a hallmark of fibrosis whose expression increases by tumor necrosis factor alpha and transforming growth factor beta during the onset of liver fibrogenesis [49]. Immunohistochemical detection in the present study revealed high expression of MMP-9 in the intoxicated rats compared to control group. It has been shown that general inhibitors of MMPs attenuate hepatic fibrosis or are useful to inhibit acute and chronic inflammatory or vascular diseases [50].
Another interesting finding of our study is that treatment withphysalis juice has downregulated the expression of MMP-9 and thus inhibiting fibrosis. The antioxidant and antifibrotic effect of physalis may be due to presence of quercetin [41]. Our GC-MS results have shown that it contains high amount of it. Quercetin belongs to an extensive class of polyphenolic flavonoid compounds almost ubiquitous in plants and plant food sources. Quercetin is considered to be a strong antioxidant due to its ability to scavenge free radicals and bind transition metal ions. These properties of quercetin allow it to inhibit lipid peroxidation [51] and have anti-inflammatory properties [52]. In addition to quercetin, physalis juice also contains kaempferol 3-O-rutinoside. Kaempferol is known to be potential antioxidant due to its ability to scavenge free radicals and active oxygen species such as singlet oxygen, superoxide anion radical, and hydroxyl radicals [53]. The presence of these compounds could explain the antioxidant activity found in the crude extract.
5. Conclusion
The findings of the present study indicate the hepatoprotective effect of physalis on CCl4 intoxicated rats. This was accompanied with improvements in liver function, decreasing collagen areas and hepatic fibrosis. The underlying mechanisms for this hepatoprotection may be due scavenging free radicals, as well as decreasing the expression of the well-known fibrotic marker MMP-9. Thus blocking the oxidative stress pathway may be of therapeutic value in treatment of liver injury and fibrosis.
Supplementary Material
GC-MS chromatogram of the physalis showed 29 peaks indicating the presence of 29 phytochemical constituents. On comparison of the mass spectra of the constituents with Wiley9 combined with NIST 11 libraries, the 29 phytoconstituents were characterized and identified as it shown in Table S1. The identified phytochemical constituent's mass spectra are Oleic acid, eicosyl ester (5.04%), Colchifoleine (1.48 %), 3β,11β,21-Trihydroxy-20-oxo-5α-pregnan-18-oic acid 18,11-lactone (0.63%), 1-Hydroxy-2-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)-9H-xanthene-3,6,7-triyl triacetate (0.81%), kaempferol 3-O-rutinoside (1.40%), 25β-Cholan-24-oic acid, 3,12-dioxo- (2.58%), alpha-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate (1.62%), ethyl iso-allocholate (1.99%), Quercetin 3,4',7-trimethyl ether (3.11%), Folic Acid (0.95%), 1,25-Dihydroxyvitamin D2 (1.27%), Docosane (0.93), 3-Hydroxy-4,4-dimethyl-7-oxoandrost-5-en-17-yl acetate (0.71%), (5β)Pregnane-3,20β-diol, 14α,18α-[4-methyl-3-oxo-(1-oxa-4-azabutane-1,4-diyl)], diacetate (1.45%), Pregna-4,6-diene-21-carboxylic acid, 17-hydroxy-3-oxo-, γ-lactone (0.75%), Hexadecatrienoic acid, methyl ester (0.64%), beta-k-strophanthin (2.48%), 2,2,4,9,11,11-Hexamethyl dodecane (0.66%), Cholest-5-en-3-one (0.63%), 9,12,15-Octadecatrienoic acid (2-phenyl-1,3-dioxolan-4-yl)methyl ester (0.89%), 9-cis-Hexadecenoic acid (0.93%), 3,7,11-Trihydroxypregnan-20-one (0.99%), Ceanothine C (1.26%), Methyl-9,9,10,10-D4-octadecanoate (4.01%), Lucenin-2 (1.50%), Betulin (0.62%), (5á)Pregnane-3,20á-diol (0.97%), Anodendroside G, monoacetate (0.64%) and 7,8-Epoxylanostan-11-ol, 3-acetoxy- (0.61%).
Acknowledgment
The authors extend their appreciation to the deanship of scientific research at king Saud University for funding the work through the research group Project no. RGPVPP-074.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Tacke F, Luedde T, Trautwein C. Inflammatory pathways in liver homeostasis and liver injury. Clinical Reviews in Allergy and Immunology. 2009;36(1):4–12. doi: 10.1007/s12016-008-8091-0. [DOI] [PubMed] [Google Scholar]
- 2.Vitaglione P, Morisco F, Caporaso N, Fogliano V. Dietary antioxidant compounds and liver health. Critical Reviews in Food Science and Nutrition. 2004;44(7-8):575–586. doi: 10.1080/10408690490911701. [DOI] [PubMed] [Google Scholar]
- 3.Hsu Y-W, Tsai C-F, Chang W-H, Ho Y-C, Chen W-K, Lu F-J. Protective effects of Dunaliella salina—a carotenoids-rich alga, against carbon tetrachloride-induced hepatotoxicity in mice. Food and Chemical Toxicology. 2008;46(10):3311–3317. doi: 10.1016/j.fct.2008.07.027. [DOI] [PubMed] [Google Scholar]
- 4.Pohl LR, Schulick RD, Highet RJ, George JW. Reductive-oxygenation mechanism of metabolism of carbon tetrachloride to phosgene by cytochrome P-450. Molecular Pharmacology. 1984;25(2):318–321. [PubMed] [Google Scholar]
- 5.Kurata M, Suzuki M, Agar NS. Antioxidant systems and erythrocyte life-span in mammals. Comparative Biochemistry and Physiology B: Biochemistry and Molecular Biology. 1993;106(3):477–487. doi: 10.1016/0305-0491(93)90121-k. [DOI] [PubMed] [Google Scholar]
- 6.Ghatak S, Biswas A, Dhali GK, Chowdhury A, Boyer JL, Santra A. Oxidative stress and hepatic stellate cell activation are key events in arsenic induced liver fibrosis in mice. Toxicology and Applied Pharmacology. 2011;251(1):59–69. doi: 10.1016/j.taap.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fu Y, Zheng S, Lin J, Ryerse J, Chen A. Curcumin protects the rat liver from CCl4-caused injury and fibrogenesis by attenuating oxidative stress and suppressing inflammation. Molecular Pharmacology. 2008;73(2):399–409. doi: 10.1124/mol.107.039818. [DOI] [PubMed] [Google Scholar]
- 8.Deng G, Wang J, Zhang Q, et al. Hepatoprotective effects of phloridzin on hepatic fibrosis induced by carbon tetrachloride against oxidative stress-triggered damage and fibrosis in rats. Biological & Pharmaceutical Bulletin. 2012;35(7):1118–1125. doi: 10.1248/bpb.b12-00057. [DOI] [PubMed] [Google Scholar]
- 9.Othman MS, Nada A, Zaki HS, Abdel Moneim AE. Effect of Physalis peruviana L. on cadmium-induced testicular toxicity in rats. doi: 10.1007/s12011-014-9955-1. Biological Trace Element Research, 2014. [DOI] [PubMed] [Google Scholar]
- 10.Othman MS, Safwat G, Aboulkhair M, Abdel Moneim AE. The Potential Effect of Berberine in Mercury-induced Hepatorenal Toxicity in Albino Rats. doi: 10.1016/j.fct.2014.04.012. Food and Chemical Toxicology, 2014. [DOI] [PubMed] [Google Scholar]
- 11.Fang S-T, Liu J-K, Li B. Ten new withanolides from Physalis peruviana . Steroids. 2012;77(1-2):36–44. doi: 10.1016/j.steroids.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Wang I-K, Lin-Shiau S-Y, Lin J-K. Induction of apoptosis by apigenin and related flavonoids through cytochrome c release and activation of caspase-9 and caspase-3 in leukaemia HL-60 cells. European Journal of Cancer. 1999;35(10):1517–1525. [PubMed] [Google Scholar]
- 13.Nishikimi M, Appaji Rao N, Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochemical and Biophysical Research Communications. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 14.Abdel Moneim AE. The neuroprotective effects of purslane (Portulaca oleracea) on rotenone-induced biochemical changes and apoptosis in brain of rat. CNS Neurological Disorders Drug Targets. 2013;12(6):830–841. doi: 10.2174/18715273113129990081. [DOI] [PubMed] [Google Scholar]
- 15.Sohn DH, Yun Y-P, Park KS, Veech RL, Song BJ. Post-translational reduction of cytochrome P450IIE by CCl4, its substrate. Biochemical and Biophysical Research Communications. 1991;179(1):449–454. doi: 10.1016/0006-291x(91)91391-o. [DOI] [PubMed] [Google Scholar]
- 16.Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. American journal of clinical pathology. 1957;28(1):56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 17.Szasz G. A kinetic photometric method for serum gamma-glutamyl transpeptidase. Clinical Chemistry. 1969;15(2):124–136. [PubMed] [Google Scholar]
- 18.Belfield A, Goldberg DM. Revised assay for serum phenyl phosphatase activity using 4-amino-antipyrine. Enzyme. 1971;12(5):561–573. doi: 10.1159/000459586. [DOI] [PubMed] [Google Scholar]
- 19.Garber CC. Jendrassik—Grof analysis for total and direct bilirubin in serum with a centrifugal analyzer. Clinical Chemistry. 1981;27:1410–1416. [PubMed] [Google Scholar]
- 20.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 21.Green LC, Wagner DA, Glogowski J. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Analytical Biochemistry. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 22.Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 23.Aebi H. Catalase in vitro . Methods in Enzymology. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 24.Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. The Journal of Laboratory and Clinical Medicine. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 25.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. The Journal of Biological Chemistry. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 26.Factor VM, Kiss A, Woitach JT, Wirth PJ, Thorgeirsson SS. Disruption of redox homeostasis in the transforming growth factor-α/c- myc transgenic mouse model of accelerated hepatocarcinogenesis. The Journal of Biological Chemistry. 1998;273(25):15846–15853. doi: 10.1074/jbc.273.25.15846. [DOI] [PubMed] [Google Scholar]
- 27.Abdel Moneim AE, El-Deib KM. The Possible protective effects of Physalis peruviana on carbon tetrachloride-induced nephrotoxicity in male albino rats. Life Science Journal. 2012;9(3):1038–1052. [Google Scholar]
- 28.Yun J-W, Kim C-W, Bae I-H, et al. Determination of the key innate genes related to individual variation in carbon tetrachloride-induced hepatotoxicity using a pre-biopsy procedure. Toxicology and Applied Pharmacology. 2009;239(1):55–63. doi: 10.1016/j.taap.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Adesanoye OA, Farombi EO. Hepatoprotective effects of Vernonia amygdalina (astereaceae) in rats treated with carbon tetrachloride. Experimental and Toxicologic Pathology. 2010;62(2):197–206. doi: 10.1016/j.etp.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 30.Coballase-Urrutia E, Pedraza-Chaverri J, Cárdenas-Rodríguez N, et al. Hepatoprotective effect of acetonic and methanolic extracts of Heterotheca inuloides against CCl4-induced toxicity in rats. Experimental and Toxicologic Pathology. 2011;63(4):363–370. doi: 10.1016/j.etp.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 31.Boll M, Weber LWD, Becker E, Stampfl A. Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Zeitschrift fur Naturforschung C: Journal of Biosciences. 2001;56(7-8):649–659. doi: 10.1515/znc-2001-7-826. [DOI] [PubMed] [Google Scholar]
- 32.Ganie SA, Zargar BA, Masood A, Zargar MA. Hepatoprotective and antioxidant activity of rhizome of Podophyllum hexandrum against carbon tetra chloride induced hepatotoxicity in rats. Biomedical and Environmental Sciences. 2013;26(3):209–221. doi: 10.3967/0895-3988.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 33.Breikaa RM, Algandaby MM, El-Demerdash E, Abdel-Naim AB. Multimechanistic antifibrotic effect of biochanin a in rats: implications of proinflammatory and profibrogenic mediators. PLoS ONE. 2013;8(7) doi: 10.1371/journal.pone.0069276.e69276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramadan MF. Bioactive phytochemicals, nutritional value, and functional properties of cape gooseberry (Physalis peruviana): an overview. Food Research International. 2011;44(7):1830–1836. [Google Scholar]
- 35.Chang JC, Lin CC, Wu SJ, et al. Antioxidative and hepatoprotective effects of Physalis peruviana extract against acetaminophen-induced liver injury in rats. Pharmaceutical Biology. 2008;46(10-11):724–731. [Google Scholar]
- 36.Arun M, Asha VV. Preliminary studies on antihepatotoxic effect of Physalis peruviana Linn. (Solanaceae) against carbon tetrachloride induced acute liver injury in rats. Journal of Ethnopharmacology. 2007;111(1):110–114. doi: 10.1016/j.jep.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 37.El-Gengaihi SE, Hassan EE, Hamed MA, Zahran HG, Mohammed MA. Chemical composition and biological evaluation of Physalis peruviana root as hepato-renal protective agent. Journal of Dietary Supplements. 2013;10(1):39–53. doi: 10.3109/19390211.2012.760509. [DOI] [PubMed] [Google Scholar]
- 38.Wu S-J, Ng L-T, Lin D-L, Huang S-N, Wang S-S, Lin C-C. Physalis peruviana extract induces apoptosis in human Hep G2 cells through CD95/CD95L system and the mitochondrial signaling transduction pathway. Cancer Letters. 2004;215(2):199–208. doi: 10.1016/j.canlet.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 39.Wu SJ, Tsai JY, Chang SP, et al. Supercritical carbon dioxide extract exhibits enhanced antioxidant and anti-inflammatory activities of Physalis peruviana . Journal of Ethnopharmacology. 2006;108(3):407–413. doi: 10.1016/j.jep.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 40.Mandal AK, Das N. Sugar coated liposomal flavonoid: a unique formulation in combating carbontetrachloride induced hepatic oxidative damage. Journal of Drug Targeting. 2005;13(5):305–315. doi: 10.1080/10611860500230278. [DOI] [PubMed] [Google Scholar]
- 41.Janbaz KH, Saeed SA, Gilani AH. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 2004;11(5):424–430. doi: 10.1016/j.phymed.2003.05.002. [DOI] [PubMed] [Google Scholar]
- 42.Tatiya AU, Surana SJ, Sutar MP, Gamit NH. Hepatoprotective effect of poly herbal formulation against various hepatotoxic agents in rats. Pharmacognosy Research. 2012;4(1):50–56. doi: 10.4103/0974-8490.91040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim J, Chehade J, Pinnas JL, Mooradian AD. Effect of select antioxidants on malondialdehyde modification of proteins. Nutrition. 2000;16(11-12):1079–1081. doi: 10.1016/s0899-9007(00)00446-9. [DOI] [PubMed] [Google Scholar]
- 44.Leung T-M, Fung M-L, Liong EC, Lau TYH, Nanji AA, Tipoe GL. Role of nitric oxide in the regulation of fibrogenic factors in experimental liver fibrosis in mice. Histology and Histopathology. 2011;26(2):201–211. doi: 10.14670/HH-26.201. [DOI] [PubMed] [Google Scholar]
- 45.Zhu W, Fung PCW. The roles played by crucial free radicals like lipid free radicals, nitric oxide, and enzymes NOS and NADPH in CCl4-induced acute liver injury of mice. Free Radical Biology and Medicine. 2000;29(9):870–880. doi: 10.1016/s0891-5849(00)00396-8. [DOI] [PubMed] [Google Scholar]
- 46.El-Gengaihi SE, Hamed MA, Khalaf-Allah R, Mohammed MA. Golden berry juice attenuates the severity of hepatorenal injury. Journal of Dietary Supplements. 2013;10(4):357–369. doi: 10.3109/19390211.2013.830675. [DOI] [PubMed] [Google Scholar]
- 47.Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Oxford University Press; 2007. Cellular responses to oxidative stress: adaptation, damage, repair, senescence and death; pp. 187–267. [Google Scholar]
- 48.Valko M, Leibfritz D, Moncol J, Cronin MTD, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. International Journal of Biochemistry and Cell Biology. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 49.Vassiliadis E, Larsen DV, Clausen RE, et al. Measurement of co3-610, a potential liver biomarker derived from matrix metalloproteinase-9 degradation of collagen type iii, in a rat model of reversible carbon-tetrachloride-induced fibrosis. Biomarker Insights. 2011;6:49–58. doi: 10.4137/BMI.S6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kahraman A, Bronk SF, Cazanave S, et al. Matrix metalloproteinase inhibitor, CTS-1027, attenuates liver injury and fibrosis in the bile duct-ligated mouse. Hepatology Research. 2009;39(8):805–813. doi: 10.1111/j.1872-034X.2009.00541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hollman PCH, Van Trijp JMP, Mengelers MJB, De Vries JHM, Katan MB. Bioavailability of the dietary antioxidant flavonol quercetin in man. Cancer Letters. 1997;114(1-2):139–140. doi: 10.1016/s0304-3835(97)04644-2. [DOI] [PubMed] [Google Scholar]
- 52.Boots AW, Wilms LC, Swennen ELR, Kleinjans JCS, Bast A, Haenen GRMM. In vitro and ex vivo anti-inflammatory activity of quercetin in healthy volunteers. Nutrition. 2008;24(7-8):703–710. doi: 10.1016/j.nut.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 53.Tatsimo SJN, Tamokou JDDD, Havyarimana L, et al. Antimicrobial and antioxidant activity of kaempferol rhamnoside derivatives from Bryophyllum pinnatum . BMC Research Notes. 2012;5:p. 158. doi: 10.1186/1756-0500-5-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
GC-MS chromatogram of the physalis showed 29 peaks indicating the presence of 29 phytochemical constituents. On comparison of the mass spectra of the constituents with Wiley9 combined with NIST 11 libraries, the 29 phytoconstituents were characterized and identified as it shown in Table S1. The identified phytochemical constituent's mass spectra are Oleic acid, eicosyl ester (5.04%), Colchifoleine (1.48 %), 3β,11β,21-Trihydroxy-20-oxo-5α-pregnan-18-oic acid 18,11-lactone (0.63%), 1-Hydroxy-2-(2,3,4,6-tetra-O-acetyl-beta-D-glucopyranosyl)-9H-xanthene-3,6,7-triyl triacetate (0.81%), kaempferol 3-O-rutinoside (1.40%), 25β-Cholan-24-oic acid, 3,12-dioxo- (2.58%), alpha-D-Glucopyranoside, methyl 2-(acetylamino)-2-deoxy-3-O-(trimethylsilyl)-, cyclic methylboronate (1.62%), ethyl iso-allocholate (1.99%), Quercetin 3,4',7-trimethyl ether (3.11%), Folic Acid (0.95%), 1,25-Dihydroxyvitamin D2 (1.27%), Docosane (0.93), 3-Hydroxy-4,4-dimethyl-7-oxoandrost-5-en-17-yl acetate (0.71%), (5β)Pregnane-3,20β-diol, 14α,18α-[4-methyl-3-oxo-(1-oxa-4-azabutane-1,4-diyl)], diacetate (1.45%), Pregna-4,6-diene-21-carboxylic acid, 17-hydroxy-3-oxo-, γ-lactone (0.75%), Hexadecatrienoic acid, methyl ester (0.64%), beta-k-strophanthin (2.48%), 2,2,4,9,11,11-Hexamethyl dodecane (0.66%), Cholest-5-en-3-one (0.63%), 9,12,15-Octadecatrienoic acid (2-phenyl-1,3-dioxolan-4-yl)methyl ester (0.89%), 9-cis-Hexadecenoic acid (0.93%), 3,7,11-Trihydroxypregnan-20-one (0.99%), Ceanothine C (1.26%), Methyl-9,9,10,10-D4-octadecanoate (4.01%), Lucenin-2 (1.50%), Betulin (0.62%), (5á)Pregnane-3,20á-diol (0.97%), Anodendroside G, monoacetate (0.64%) and 7,8-Epoxylanostan-11-ol, 3-acetoxy- (0.61%).


