Abstract
Background
Cigarette smokers are more susceptible to periodontal diseases and are more likely to be infected with Porphyromonas gingivalis than non-smokers. Furthermore, smoking is known to alter the expression of P. gingivalis surface components and to compromise IgG generation. The aim of this study was to evaluate whether IgG response to P. gingivalis is suppressed in smokers in vivo and whether previously established in vitro tobacco-induced phenotypic P. gingivalis changes would be reflected in vivo.
Methods
We examined the humoral response to several P. gingivalis strains as well as specific tobacco-regulated outer membrane proteins (FimA and RagB) by ELISA in biochemically-validated (salivary cotinine) smokers and non-smokers with chronic (CP, n = 13) or aggressive (AP, n = 20) periodontitis. We also monitored the local and systemic presence of P. gingivalis DNA by PCR.
Results
Smoking was associated with decreased total IgG responses against clinical (10512, 5607, and 10208C; all p < 0.05) but not laboratory (ATCC 33277, W83) P. gingivalis strains. Smoking did not influence IgG produced against specific cell surface proteins, although a non-significant pattern towards increased total FimA-specific IgG in CP subjects, but not AP subjects, was observed. Seropositive smokers were more likely to be infected orally and systemically with P. gingivalis (p < 0.001), as determined by 16S RNA analysis.
Conclusions
Smoking alters the humoral response against P. gingivalis, strengthening the evidence that mechanisms of periodontal disease progression in smokers may differ from non-smokers with the same disease classification.
Keywords: Antigen-Antibody reactions, periodontitis, Porphyromonas gingivalis, smoking, vaccines
Introduction
Cigarette use increases susceptibility to infection with multiple bacteria, including the anaerobic, assacharolyic, Gram negative Porphyromonas gingivalis and the development of more severe and recalcitrant periodontal diseases 1-2. Tobacco smoking has also been shown to lead to a generalized suppression of the antibody response to pathogenic bacteria 2.
P. gingivalis is clearly antigenic in humans 3-4, with differences antibody titers detectable, e.g., upon treatment or in smokers, even in small numbers of subjects 4-6. Indeed, of all tested oral bacterial species, only P. gingivalis-specific antibodies have been associated with disease severity 7-8. In vitro, cigarette smoke extract (CSE) represents an environmental stressor to P. gingivalis 9. Phenotypically, CSE exposure leads to loss of capsule and increased major fimbrial protein (FimA) but not minor fimbrial antigen (Mfa1) expression 10; augmented expression of the outer membrane virulence factors, RagA and RagB 9; promotion of biofilm development; and a suppressed innate response against P. gingivalis 9-11.
We hypothesized that tobacco smoking would lead to a suppression in the overall IgG response to P. gingivalis and that previously reported CSE-induced phenotypic P. gingivalis changes 9-10 would be reflected in vivo. Therefore, we aimed to examine the humoral response to specific tobacco-regulated outer membrane proteins and whole P. gingivalis in biochemically validated human smokers and non-smokers with chronic or aggressive periodontitis and to check for local and systemic P. gingivalis DNA. As large variations in antibody titers against different strains of P. gingivalis are known to occur 12, several isolates were tested. P. gingivalis ATCC 33277, the type strain, and W83 represent the workhorses of molecular oral biology. 10208C, 10512 and 5607 are low passage clinical strains.
Materials and Methods
Study Population
The study was conducted in full accordance with the World Medical Association's Declaration of Helsinki of 1975, as revised in 2000, and was approved by the Institutional Review Board of the University of Louisville and Ege University. Forty-two informed and consenting individuals who sought dental treatment at Ege University were recruited between September 2011 and August 2012; 24 otherwise healthy, according to medical history recorded on recruitment, untreated patients with aggressive periodontitis (AP) and 18 otherwise healthy, untreated individuals with chronic periodontitis (CP). The higher number of AP cases reflects the patient referrals to Ege University. Medical and dental histories, including smoking histories were obtained. Those with antibiotic or periodontal treatment in the last 6 months were excluded. AP and CP was diagnosed using World Workshop in Periodontitis criteria 13 and current smokers (≥10 cigarettes/day; >5 years) and non-smokers included. Inclusion criteria for the AP group was presence of at least six permanent teeth, including incisors and/or first molars, with at least one site with PD and CAL ≥5 mm and six teeth other than first molars and incisors with similar PD and CAL measurements, familial aggregation (all individuals were asked if they had any family member with current or history of severe periodontal disease) and radiographic bone loss of ≥ 30% of root length affecting ≥ 3 permanent teeth other than first molars and incisors. All AP and CP patients were categorized as “generalized.” Those smoking for <5 years and or <10 cigarettes/day for >5 years were excluded.
Saliva and Serum Sampling
Whole, unstimulated saliva samples were obtained by expectoration into polypropylene tubes prior to any clinical measurement or periodontal intervention in the morning following an overnight fast during which subjects were requested not to drink (except water) or chew gum, essentially as reported by Navazesh14. The saliva samples were clarified by centrifugation (800 × g) for 10 min at room temperature and 500 μL amounts were placed in polypropylene tubes and immediately lyophilized. Venous blood (5 ml) was taken from the antecubital vein by standard venipuncture. Serum was separated by centrifugation at 1500 × g for 10 min and immediately frozen at −40°C. Samples were shipped to the University of Louisville for biochemical analysis.
Clinical Periodontal Measurements
Subsequent to saliva and serum sampling, clinical periodontal recordings were performed at 6 sites on each tooth present, except the third molars: plaque index (PI, [0-4]), probing depth (PD, mm), clinical attachment level (CAL, mm) and bleeding on probing (BOP, +/−), as previously described 15.
Quantification of salivary cotinine
Salivary cotinine levels were measured by immunoassay kit*, according to the manufacturer's instructions. Aliquots of frozen saliva samples were thawed, vortexed and centrifuged at 1500 × g for 15 minutes to pellet precipitated mucins. Samples from self-reported smokers were diluted tenfold. Self-reported non-smokers were not diluted. Cotinine standards (0.8 ng ml-1 to 200 ng ml-1), high and low cotinine controls, and samples were tested in triplicates. Plates were measured using a microplate reader†. The reported sensitivity of the assay is 0.15 ng ml-1 and a pre-determined cut-off point of ≥15 ng ml-1 was selected to classify active smokers 16.
Growth of P. gingivalis
P. gingivalis strains W83, 33277, 10208C, 10512 and 5607‡ were grown to mid-to-late exponential phase (OD600 = 1.0) in Gifu Anaerobe Medium (GAM)§ under anaerobic conditions (80% N2, 10% H2, 10% CO2) at 37°C in an anaerobic chamber k. Purity was determined by Gram staining.
Preparation of P. gingivalis antigens
P. gingivalis cells were harvested by centrifugation (1900 × g, 30 min, 4°C) and washed 3x with sterile PBS. Pellets at an OD600 of 1.0 in sterile water were ultrasonically disrupted¶. Protein concentrations in debris-free sonicates were determined by a bicinchoninic acid (BCA) protein assay §, according to the manufacturer's instructions. rFimA and rMfa1 were expressed in and purified from E. coli, as we have described previously 10. rRagB was also expressed in and purified from E. coli TOP10 using a plasmid system# and W83 DNA as a template with ragB amplified using the following primers**; Fwd: CACCGAGCTTGACCGCGACCCC and Rev: TATCGGCCAGTTCTTTATTAACTGCGG.
Quantification of antibody response to P. gingivalis antigens
Sonicates (10 μg/ml) and recombinant proteins (2.5 μg/ml) were used (100 μl) to coat flat-bottomed 96-well microplates overnight at 4°C which were washed 5x with PBS containing 0.05% Tween-20 (PBS-T), blocked with 200 μl of 1x diluent for 1 h, RT and washed again 5x with PBS-T. 100 μl samples diluted in 1x diluent (1:250 for total IgG Fc; 1:100 for IgG subclasses and IgA1 assays, respectively) were added to each well for 2 h at RT. Uncoated wells served as controls. Plates were washed 10 x with PBS-T, and incubated with 100 μl of biotinconjugated detection antibodies‡‡ diluted in 1x ELISA diluent (1:5000 for total IgG; 1:1000 for IgG subclasses; and 1:2500 for IgA1, respectively) for 1 h at RT and washed 10x with PBS-T. 100 μl Avidin-HRP (1:1000 in 1x diluent) was added to each well, 30 min, RT and the plates washed 10x with PBS-T. 100 μl TMB substrate was added and development stopped within 10 mins with 50μl of 2N H2SO4. Absorbances were read at 450 nm using a microplate reader†. Antibody titers are presented as relative absorbance at O.D. 450 nm.
Detection of local and systemic P. gingivalis
The presence or absence of P. gingivalis DNA was established in saliva and plasma samples, essentially as described previously by Matto et al 17 using primers first reported by Slots et al 18. However, samples were centrifuged at 13,000 × g, 6 min, alternate nucleic acids# were employed, and there were 40 amplification cycles. Additionally, 10 ng P. gingivalis W83 DNA served as a positive control. 10ng A. actinomycetemcomitans DNA and water each served as negative controls. Amplicons were separated in 2% agarose/ethidium bromide gels and documented using an imaging system§§.
Statistical analyses
Similar sample sizes have previously been shown to be adequate to determine statistically significant differences in antibody titres between smokers and non-smokers 19-23. Differences in cotinine levels between groups were determined by the Kruskal-Wallace test. Differences in P. gingivalis infection rates were determined using the Fischer's Exact Test. All remaining data were assessed by t-test or Mann-Whitney test, depending on distributionκκ.
Results
Smoking alters clinical parameters in subjects with chronic or aggressive periodontitis
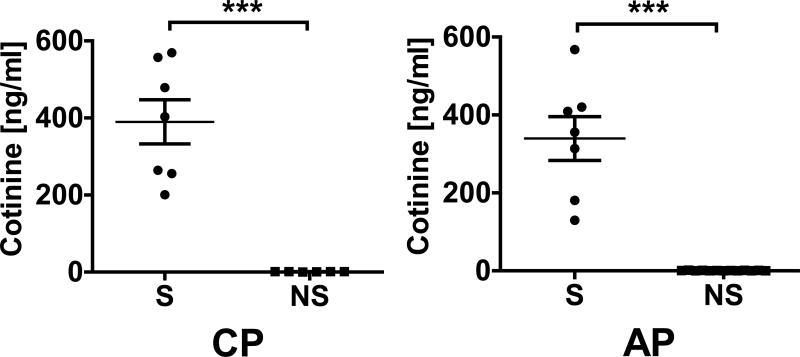
Epidemiological and disease parameters of all subjects whose smoking status was confirmed biochemically, who were seropositive in any assay for P. gingivalis by ELISA and with uncompromised samples and, thus, included in the final data analyses (AP, n = 20; CP, n = 13), are presented in Table 1. Four subjects were excluded from the AP group and 5 from the CP group (smoking status: n = 1 and 1; compromised samples: n = 3 and 4, respectively). As expected, BOP was suppressed in CP smokers relative to CP non-smokers (p < 0.05). CAL was more severe in the subjects with AP who smoked, compared to those who did not (p < 0.05). Otherwise, there were no significant differences in clinical parameters between smokers and non-smokers within periodontal disease groups. As shown in Figure 1, salivary cotinine levels, indicating smoke intake, were elevated in the smokers of both disease groups compared to non-smokers (both p < 0.001).
Table 1.
Clinical data of current smokers and current non-smokers with aggressive or chronic periodontitis.
| CP, S (n = 7) | CP, NS (n = 6) | AP, S (n = 7) | AP, NS (n = 13) | |
|---|---|---|---|---|
| Age | 51.9 (5.5) | 48.7 (6.3) | 35.0 (3.1) | 38.1 (4.2) |
| PD (mm) | 4.9 (1.0) | 5.5 (1.0) | 5.4 (0.9) | 4.5 (1.0) |
| CAL (mm) | 6.0 (0.6) | 6.2 (1.4) | 6.1 (1.2)* | 4.9 (1.1)* |
| Plaque Score (0-4) | 2.7 (0.5) | 2.8 (0.4) | 2.0 (0.6) | 1.8 (0.7) |
| BOP (%) | 74 (20)* | 92 (12)* | 83 (13) | 80 (13) |
Data represents the mean (s.d.)
p < 0.05, smokers vs non-smokers.
CP, chronic periodontitis; AP, aggressive periodontitis; S, smoker; NS, non-smoker.
Figure 1.
Salivary cotinine concentrations (mean ± S.D.) in validated current smokers and current non-smokers with chronic periodontitis and aggressive periodontitis.
Non-detectable cotinine concentrations were entered as 0.0 ng ml-1.
*** p < 0.001.
Smoking suppresses the systemic IgG response to P. gingivalis
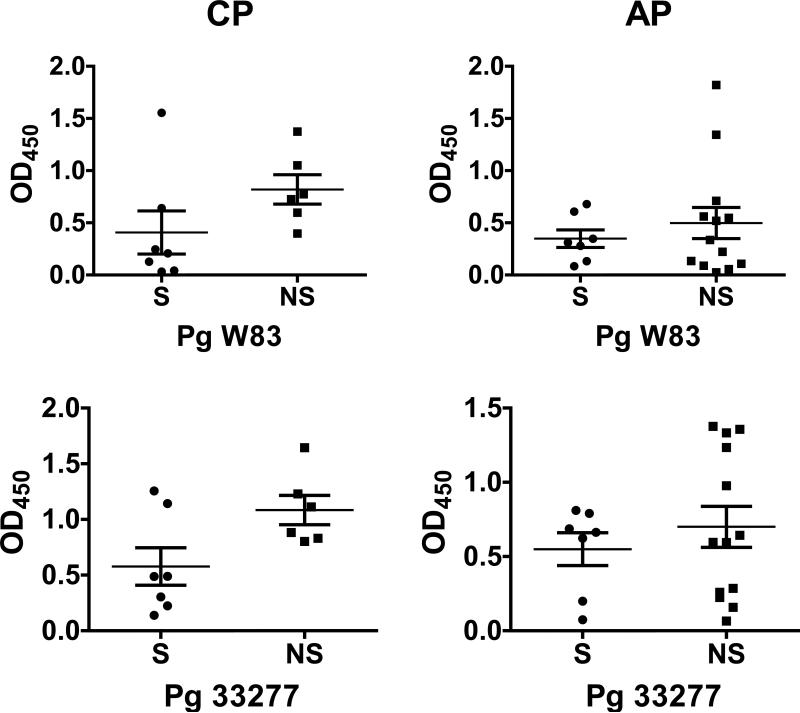
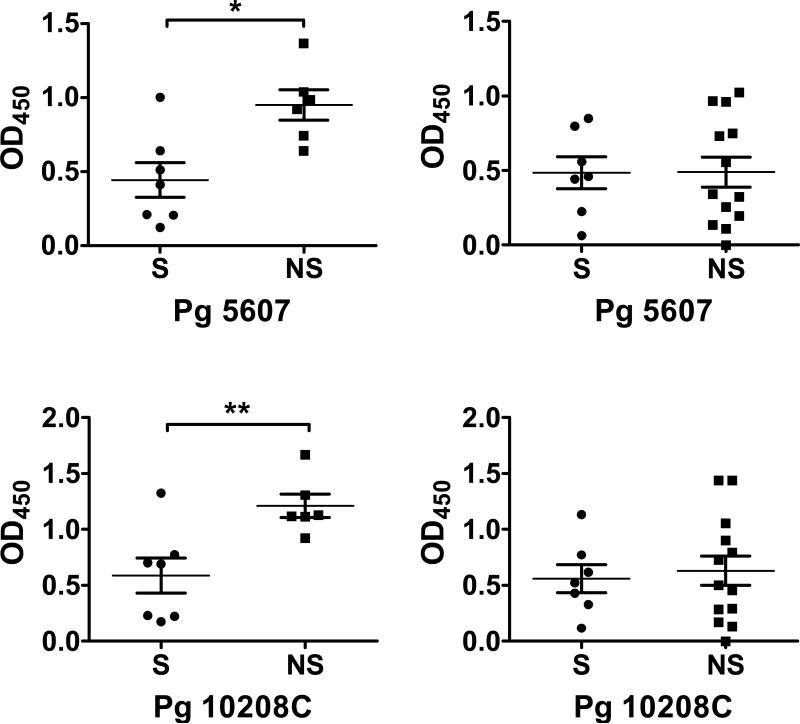
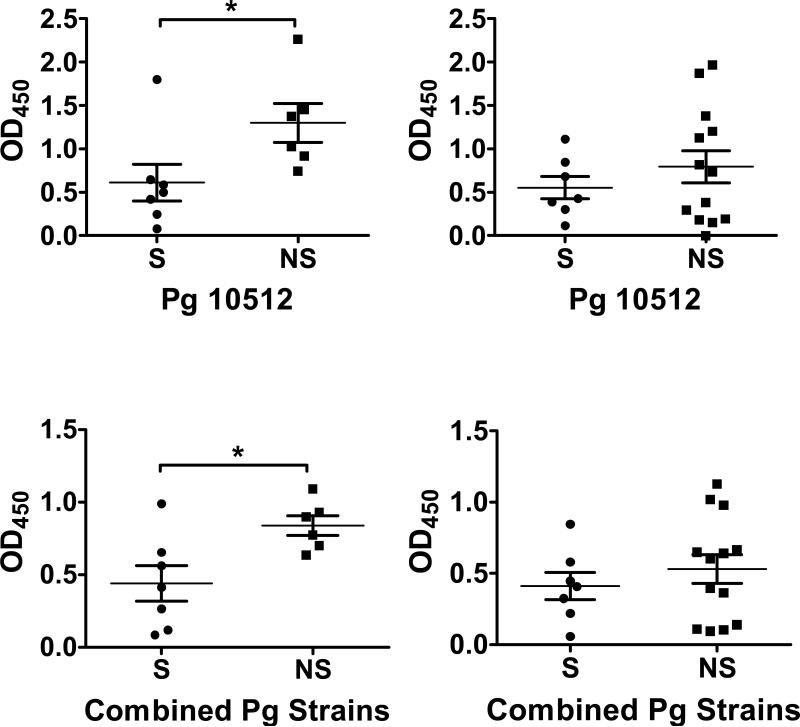
Total IgG titers against two laboratory strains and three recent clinical isolates of P. gingivalis, measured in the serum of individuals with CP or AP are presented in Figure 2. Smoking was associated with decreased total IgG production against low passage clinical isolates (10512, 5607, and 10208C) and all strains combined in subjects with CP (Figure 2), but not in the commonly employed laboratory strains (ATCC 33277 and W83). There were no statistically significant differences in the relative IgG1, IgG2, IgG3, IgG4 and IgA1 antibody titers against W83 or the five combined strains measured in the serum of individuals with CP or AP (data not shown), with the mean relative antibody titer always higher in non-smokers than in smokers.
Figure 2.
Total IgG titers (mean ± S.D.) against laboratory and clinical strains and P. gingivalis strains in smokers and current non-smokers with chronic periodontitis and aggressive periodontitis.
* p < 0.05; ** p < 0.01.
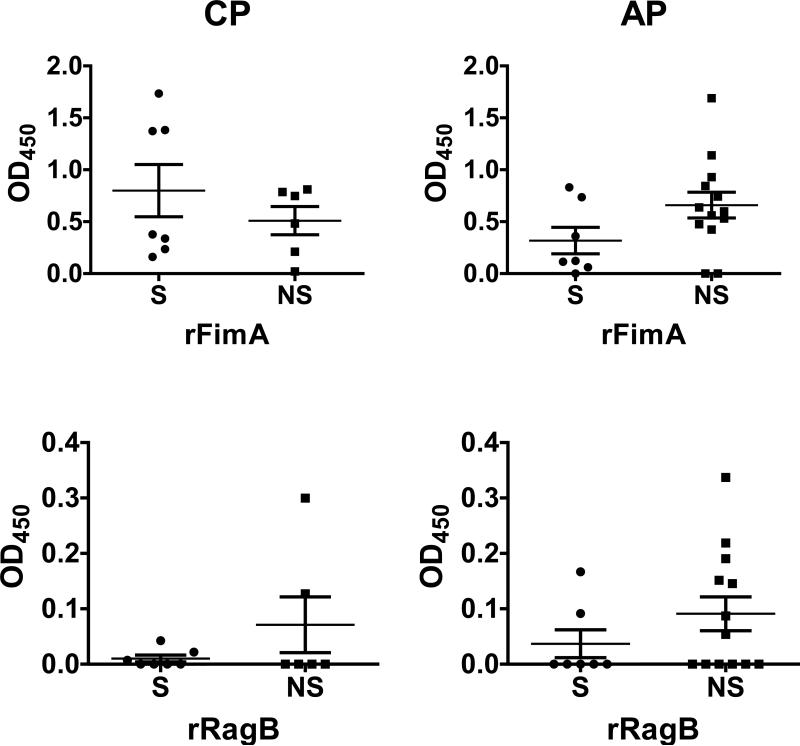
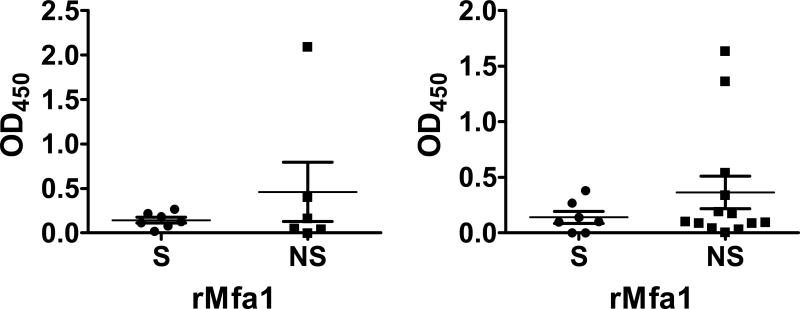
Smoking does not influence the systemic antibody response to specific outer membrane proteins (FimA, Mfa1 and RagB)
While smoking status exerted important effects on the overall IgG response to P. gingivalis cells, smoking did not induce significant differences in antibody production against three major outer membrane proteins of P. gingivalis - FimA and RagB, which are upregulated by CSE in vitro, and Mfa1, which is unaffected 9-11 (Figure 3). Nevertheless, in the CP subjects, the non-significant trend was towards an increased FimA response in smokers (p > 0.05), in contrast to all other comparisons.
Figure 3.
Total IgG titers (mean ± S.D.) against P. gingivalis surface proteins in smokers and current non-smokers with chronic periodontitis and aggressive periodontitis.
Local and systemic P. gingivalis DNA detection
P. gingivalis-specific 16S DNA was detected in most CP saliva samples; 6/7 (86%) and 5/6 (83%) for smokers and non-smokers, alike (p > 0.05). In AP subjects, saliva samples from smokers (5/7, 86%) were more likely to contain P. gingivalis DNA, than non-smoking AP subjects (7/13, 54%, p < 0.001) while CP smokers (1/7, 14%) where more likely than non-smoking CP (0/6, 0%) patients to have serum detectable P. gingivalis DNA (p < 0.001). For AP smokers and non-smokers, systemic infection rates were 1/7 (14%) and 3/13 (23%), respectively (p > 0.05).
Discussion
Smoking is known to alter antibody production 2, 24, but few studies have addressed the influence of smoking on oral pathogens specifically. The great majority 5-6, 22, 25-26, but not all 7, have shown that smoking reduces the antibody response to P. gingivalis, particularly IgG. We show that the total IgG response mounted against low passage clinical isolates (5607, 10208C and 1052), but not laboratory strains, of P. gingivalis (33277 and W83) is significantly suppressed in smokers with CP. Thus, while clearly facilitating impressive molecular microbiology advances, research strains of P. gingivalis may not always be the most relevant to in vivo situations. There were no statistically significant differences in the intensity of the humoral response to P. gingivalis between current smokers and non-smoking subjects with AP, in keeping with the findings of Hayman et al, who have recently reported that correlations between pathogen and commensal antibody ratios in individuals with periodontitis disappear in those with aggressive disease 7. This may be partly explained by our finding that current smokers with AP are more likely to be currently infected with P. gingivalis than non-smokers with AP.
The mechanisms of a generalized suppression of the IgG response in human smokers are not clear. Cigarette smoke inhibits T cell-dependent and -independent antibody responses to a number of antigens in animals 27-28. Furthermore tobacco-induced suppression of the normal inflammatory response could impair leukocyte infiltration into the gingival tissues, negatively influencing the presentation of P. gingivalis antigens. While nicotine-induced periodontal vasoconstriction is often cited, the evidence for this in humans is unprofound 2, 29. Rather, tobacco inhibits plaque-induced angiogenesis 2, 30 in a manner reversible upon cessation 31. Indeed, the key marker of periodontal inflammation, BOP, was significantly lower in smoking CP subjects compared to non-smokers. Thus, angiogenic suppression could also contribute to a reduced humoral response to periodontal pathogens.
Antibody responses to P. gingivalis, particularly IgG2, may vary significantly by race and type of periodontal disease 7, 25-26, 32. All recruits herein were Caucasian and no tobacco-related differences in IgG subtype titers could be ascribed. It is unfortunate that 21% (9/42) of the recruits were excluded from the final analyses. However, smokers often provide false statements on their smoking status, therefore, biochemical detection of cotinine levels is specifically employed to avoid such false reports and to calculate more objectively the real smoking status of the patient 16. Furthermore, while P. gingivalis is clearly a key a key pathogen, not all subjects with periodontitis harbor this particular species 33, thus not all seropositive individuals were positive for oral P. gingivalis DNA.
We had hypothesized that CSE-upregulation of RagB and FimA would be reflected in an increased antibody titer to these surface antigens in smokers. However, this was not validated and the suppressive overall influence of tobacco use on antibody production may have been sufficiently strong to mask any increased antigen specific antibody production in current smokers. Additionally, those who ceased smoking close to the study onset may have passed our cotinine screen. Indeed, ex-smokers are the most likely to be deceitful by claiming to have never smoked 16. Furthermore, it is possible that expression of P. gingivalis proteins in E. coli may have led to alternate post-translational processing that rendered them less efficient antigens. It is interesting, however, that the sole exception to the trend of suppressed antibody production in smokers was total IgG against FimA in CP smokers, which was increased relative to non-smokers. P. gingivalis fimbriae are the bacterial structures first to engage the host. FimA promotes colonization through strong interactions with several host proteins, including collagen and fibronectin, oral epithelia and other plaque bacteria, such as Streptococcus spp. 34-37. More importantly, FimA induces TLR2-specific innate tolerance in an IκBα- and IRAK-1-dependent manner 10 with reduced antibody production one possible downstream consequence of FimA-mediated immune suppression.
Fascinatingly, saliva and serum from smokers were more likely than non-smokers to contain P. gingivalis 16S RNA gene sequences, presumably reflecting current or recent oral infection with this bacterium. Indeed, this report is the first to correlate antibody responses specific to variant P. gingivalis strains and antigens with local and systemic infection in biochemically validated subjects with two types of destructive periodontal disease. However, due to the relatively low number of recruits, these data require validation. Potential variation in the sensitivity of the immunoglobulin assays and uneven distribution of participants also limit the study.
In summary, smoking suppresses total IgG production to P. gingivalis in smokers with CP compared to those who are not current smokers. This association is true for clinical isolates only and not the workhorse strains, ATCC 33277 and W83. All antibody assessments performed herein, and the literature in general, show depressed IgG antibody production in smokers. An exception appears to be a non-significant trend towards an increase in IgG specific for the major fimbrial antigen, FimA, in CP smokers. This may be reflective of the significantly increased FimA expression known to occur in vitro upon exposure to CSE. IgG is considered particularly important in clearing plaque bacteria. Tobacco-induced suppression of IgG production in response to P. gingivalis infection may promote P. gingivalis survival, potentially contributing to increased susceptibility to CP. Furthermore, current oral and systemic infection with P. gingivalis may be more likely in smokers than in non-smokers, helping to explain the differential antibody responses in these two disease classifications and shedding light on systemic sequelae of periodontal diseases that share smoking as a major risk factor.
Acknowledgments
The authors either have no commercial or other associations that might pose a conflict of interest. This study was funded by NIDCR (R01DE019826 to DAS).
Footnotes
Smoking alters the humoral response against P. gingivalis, strengthening the evidence that mechanisms of periodontal disease progression in smokers may differ from non-smokers with the same disease classification.
High sensitivity cotinine immunoassays were obtained from Salimetrics, State College, PA.
Emax Precision Microplate Reader, Molecular Devices, Sunnyvale, CA.
ATCC, Manassas, VA (P. gingivalis W83 and 33277). Laboratory collection (10208C, 10512 and 5607).
Nissui Pharmaceutical, Tokyo, Japan.
Coy Laboratories, Grass Lake, MI.
Vibra-Cell Sonicator, Sonics and Materials, Danbury, CT.
Thermo Scientific, Waltham, MA.
pBAD202/D–TOPO and PCR Supermix, Invitrogen, Carlsbad, CA.
Primers came from BioSynthesis, Lewisville, TX.
Sigma Aldrich, St. Louis, MO (anti-human IgGtotal- and IgG1-4-biotin, Fc-specific monoclonal antibodies [clones HP-6017, 8c/6-39, HP-6014, HP-6050 and HP-6025]). Abcam, Cambridge, MA (anti-human IgA1-biotin antibodies [clone B3506B4]).
BioDoc-It, UVP, Upland, CA.
InStat v3.06, Graphpad Software Inc., La Jolla, CA.
References
- 1.Bagaitkar J, Demuth DR, Scott DA. Increased susceptibility to bacterial infections in tobacco smokers. Tobacco Induced Diseases. 2008:4. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer RM, Wilson RF, Hasan AS, Scott DA. Mechanisms of action of environmental factors--tobacco smoking. J Clin Periodontol. 2005;32(Suppl 6):180–195. doi: 10.1111/j.1600-051X.2005.00786.x. [DOI] [PubMed] [Google Scholar]
- 3.Takeuchi Y, Aramaki M, Nagasawa T, Umeda M, Oda S, Ishikawa I. Immunoglobulin G subclass antibody profiles in Porphyromonas gingivalis-associated aggressive and chronic periodontitis patients. Oral Microbiol Immunol. 2006;21:314–318. doi: 10.1111/j.1399-302X.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- 4.Mooney J, Adonogianaki E, Kinane DF. Relative avidity of serum antibodies to putative periodontopathogens in periodontal disease. J Periodontal Res. 1993;28:444–450. [PubMed] [Google Scholar]
- 5.Graswinckel JE, van der Velden U, van Winkelhoff AJ, Hoek FJ, Loos BG. Plasma antibody levels in periodontitis patients and controls. J Clin Periodontol. 2004;31:562–568. doi: 10.1111/j.1600-051X.2004.00522.x. [DOI] [PubMed] [Google Scholar]
- 6.Califano JV, Schifferle RE, Gunsolley JC, Best AM, Schenkein HA, Tew JG. Antibody reactive with Porphyromonas gingivalis serotypes K1-6 in adult and generalized early-onset periodontitis. J Periodontol. 1999;70:730–735. doi: 10.1902/jop.1999.70.7.730. [DOI] [PubMed] [Google Scholar]
- 7.Hayman L, Steffen MJ, Stevens J, et al. Smoking and periodontal disease: discrimination of antibody responses to pathogenic and commensal oral bacteria. Clin Exp Immunol. 2011;164:118–126. doi: 10.1111/j.1365-2249.2010.04314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dye BA, Herrera-Abreu M, Lerche-Sehm J, et al. Serum antibodies to periodontal bacteria as diagnostic markers of periodontitis. J Periodontol. 2009;80:634–647. doi: 10.1902/jop.2009.080474. [DOI] [PubMed] [Google Scholar]
- 9.Bagaitkar J, Scott DA, Williams LR, et al. Tobacco-induced alterations to Porphyromonas gingivalis-host interactions. Environmental Microbiology. 2009;11:1242–1253. doi: 10.1111/j.1462-2920.2008.01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bagaitkar J, Demuth DR, Daep CA, Renaud DE, Pierce DL, Scott DA. Tobacco upregulates P. gingivalis fimbrial proteins which induce TLR2 hyposensitivity. PloS one. 2010;5:e9323. doi: 10.1371/journal.pone.0009323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagaitkar J, Daep CA, Patel CK, Renaud DE, Demuth DR, Scott DA. Tobacco smoke augments Porphyromonas gingivalis - Streptococcus gordonii biofilm formation. PLOS One. 2011;6:e27386. doi: 10.1371/journal.pone.0027386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takahashi T, Muro S, Tanabe N, et al. Relationship between periodontitis-related antibody and frequent exacerbations in chronic obstructive pulmonary disease. PLoS One. 2012;7:e40570. doi: 10.1371/journal.pone.0040570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 14.Navazesh M. Methods for collecting saliva. Ann N Y Acad Sci. 1993;694:72–77. doi: 10.1111/j.1749-6632.1993.tb18343.x. [DOI] [PubMed] [Google Scholar]
- 15.Ozcaka O, Nalbantsoy A, Buduneli N. Interleukin-17 and interleukin-18 levels in saliva and plasma of patients with chronic periodontitis. J Periodontal Res. 2011;46:592–598. doi: 10.1111/j.1600-0765.2011.01377.x. [DOI] [PubMed] [Google Scholar]
- 16.Scott DA, Palmer RM, Stapleton JA. Validation of smoking status in clinical research into inflammatory periodontal disease. J Clin Periodontol. 2001;28:715–722. doi: 10.1034/j.1600-051x.2001.280801.x. [DOI] [PubMed] [Google Scholar]
- 17.Matto J, Saarela M, Alaluusua S, Oja V, Jousimies-Somer H, Asikainen S. Detection of Porphyromonas gingivalis from saliva by PCR by using a simple sample-processing method. J Clin Microbiol. 1998;36:157–160. doi: 10.1128/jcm.36.1.157-160.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slots J, Ashimoto A, Flynn MJ, Li G, Chen C. Detection of putative periodontal pathogens in subgingival specimens by 16S ribosomal DNA amplification with the polymerase chain reaction. Clin Infect Dis. 1995;20(Suppl 2):S304–307. doi: 10.1093/clinids/20.supplement_2.s304. [DOI] [PubMed] [Google Scholar]
- 19.Olayanju AO, Rahamon SK, Arinola OG. Salivary immunoglobulin classes in Nigerian cigarette smokers: Indication for increased risk of oral diseases. Dent Res J (Isfahan) 2012;9:531–534. doi: 10.4103/1735-3327.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waszkiewicz N, Zalewska A, Szajda SD, et al. The effect of chronic alcohol intoxication and smoking on the output of salivary immunoglobulin A. Folia Histochem Cytobiol. 2012;50:605–608. doi: 10.5603/19709. [DOI] [PubMed] [Google Scholar]
- 21.Olayanju OA, Rahamon SK, Joseph IO, Arinola OG. Salivary immunoglobulin classes in Nigerian smokers with periodontitis. World J Biol Chem. 2012;3:180–183. doi: 10.4331/wjbc.v3.i10.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apatzidou DA, Riggio MP, Kinane DF. Impact of smoking on the clinical, microbiological and immunological parameters of adult patients with periodontitis. J Clin Periodontol. 2005;32:973–983. doi: 10.1111/j.1600-051X.2005.00788.x. [DOI] [PubMed] [Google Scholar]
- 23.Mooney J, Hodge PJ, Kinane DF. Humoral immune response in early-onset periodontitis: influence of smoking. J Periodontal Res. 2001;36:227–232. doi: 10.1034/j.1600-0765.2001.036004227.x. [DOI] [PubMed] [Google Scholar]
- 24.Gulsvik A, Fagerhoi MK. Smoking and immunoglobulin levels. Lancet. 1979;1:449. doi: 10.1016/s0140-6736(79)90934-6. [DOI] [PubMed] [Google Scholar]
- 25.Quinn SM, Zhang JB, Gunsolley JC, Schenkein HA, Tew JG. The influence of smoking and race on adult periodontitis and serum IgG2 levels. J Periodontol. 1998;69:171–177. doi: 10.1902/jop.1998.69.2.171. [DOI] [PubMed] [Google Scholar]
- 26.Quinn SM, Zhang JB, Gunsolley JC, Schenkein JG, Schenkein HA, Tew JG. Influence of smoking and race on immunoglobulin G subclass concentrations in early-onset periodontitis patients. Infect Immun. 1996;64:2500–2505. doi: 10.1128/iai.64.7.2500-2505.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geng Y, Savage SM, Johnson LJ, Seagrave J, Sopori ML. Effects of nicotine on the immune response. I. Chronic exposure to nicotine impairs antigen receptor-mediated signal transduction in lymphocytes. Toxicol Appl Pharmacol. 1995;135:268–278. doi: 10.1006/taap.1995.1233. [DOI] [PubMed] [Google Scholar]
- 28.Sopori ML, Cherian S, Chilukuri R, Shopp GM. Cigarette smoke causes inhibition of the immune response to intratracheally administered antigens. Toxicol Appl Pharmacol. 1989;97:489–499. doi: 10.1016/0041-008x(89)90254-8. [DOI] [PubMed] [Google Scholar]
- 29.Meekin TN, Wilson RF, Scott DA, Ide M, Palmer RM. Laser Doppler flowmeter measurement of relative gingival and forehead skin blood flow in light and heavy smokers during and after smoking. J Clin Periodontol. 2000;27:236–242. doi: 10.1034/j.1600-051x.2000.027004236.x. [DOI] [PubMed] [Google Scholar]
- 30.Rezavandi K, Palmer RM, Odell EW, Scott DA, Wilson RF. Expression of ICAM-1 and E-selectin in gingival tissues of smokers and non-smokers with periodontitis. J Oral Pathol Med. 2002;31:59–64. doi: 10.1046/j.0904-2512.2001.joptest.doc.x. [DOI] [PubMed] [Google Scholar]
- 31.Nair P, Sutherland G, Palmer RM, Wilson RF, Scott DA. Gingival bleeding on probing increases after quitting smoking. J Clin Periodontol. 2003;30:435–437. doi: 10.1034/j.1600-051x.2003.20039.x. [DOI] [PubMed] [Google Scholar]
- 32.Burrows B, Halonen M, Barbee RA, Lebowitz MD. The relationship of serum immunoglobulin E to cigarette smoking. Am Rev Respir Dis. 1981;124:523–525. doi: 10.1164/arrd.1981.124.5.523. [DOI] [PubMed] [Google Scholar]
- 33.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–144. doi: 10.1111/j.1600-051x.1998.tb02419.x. [DOI] [PubMed] [Google Scholar]
- 34.Nakagawa I, Inaba H, Yamamura T, et al. Invasion of epithelial cells and proteolysis of cellular focal adhesion components by distinct types of Porphyromonas gingivalis fimbriae. Infect Immun. 2006;74:3773–3782. doi: 10.1128/IAI.01902-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umeda JE, Missailidis C, Longo PL, Anzai D, Wikstrom M, Mayer MP. Adhesion and invasion to epithelial cells by fimA genotypes of Porphyromonas gingivalis. Oral Microbiol Immunol. 2006;21:415–419. doi: 10.1111/j.1399-302X.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- 36.Yilmaz O, Watanabe K, Lamont RJ. Involvement of integrins in fimbriae-mediated binding and invasion by Porphyromonas gingivalis. Cell Microbiol. 2002;4:305–314. doi: 10.1046/j.1462-5822.2002.00192.x. [DOI] [PubMed] [Google Scholar]
- 37.Maeda K, Nagata H, Yamamoto Y, et al. Glyceraldehyde-3-phosphate dehydrogenase of Streptococcus oralis functions as a coadhesin for Porphyromonas gingivalis major fimbriae. Infect Immun. 2004;72:1341–1348. doi: 10.1128/IAI.72.3.1341-1348.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]