Abstract
Benzodiazepines are widely used clinically to treat anxiety and insomnia. They also induce muscle relaxation, control epileptic seizures, and can provoke amnesia. Moreover, benzodiazepines are often abused after chronic clinical treatment but also for recreational purposes. Within weeks, tolerance to the pharmacological effects can develop, in addition to dependence and even addiction in vulnerable individuals. Here, we review recent observations from animal models regarding the cellular and molecular basis that may underlie the addictive properties of benzodiazepines. These data reveal how benzodiazepines, acting through specific GABAA receptor subtypes, activate midbrain dopamine neurons and how this may hijack the mesolimbic reward system. Such findings have important implications for the future design of benzodiazepines with reduced or even absent addiction liability.
Introduction
Four benzodiazepines (BDZs), alprazolam (Xanax), clonazepam (Klonopin), diazepam (Valium), and lorazepam (Ativan) are listed among the 200 most commonly prescribed drugs in the U.S. [1,2]. Since the discovery of the first BDZ, chlordiazepoxide (Librium) in 1955 [3], about thirty other BDZs have been introduced for clinical use. They are typically categorized according to their pharmacokinetic properties as either short-, intermediate- or long-acting, and prescribed to obtain one of the following major effects: decrease of sleep latency, reduction of anxiety, suppression of epileptic seizures or relaxation of muscle spasms (Box 1). BDZs can also induce anterograde amnesia, which can be considered as a side-effect at times, but loss of memory for unpleasant events may also be a useful effect, for example, during invasive medical procedures (Box 1). In general, BDZs are safe and effective for short-term treatment; however, long-term use is controversial due to the development of tolerance (Glossary) and their liability for physical dependence [4]. The Drug Abuse Warning Network, which monitors prescription and illicit drug use, found that two of the most frequently reported prescription medications in drug abuse-related cases are opioid-based pain relievers and BDZs (http://www.nida.nih.gov). Furthermore, BDZ abuse often occurs in conjunction with the abuse of another substance (e.g., alcohol or cocaine), making treatment approaches even more difficult. The knowledge of how BDZs induce addiction might help in the development of anxiolytics and hypnotics with lower addictive liability.
Box 1. BDZs and their pharmacological effects.
BDZs have a number of clinically approved uses, in addition to some adverse and unwanted side-effects. Their main pharmacological actions are outlined below:
Clinical uses
Sleep Disorders: BDZs are used in the treatment of insomnia. They can help to initiate sleep (ie. reduce latency) and to maintain sleep [90].
Anxiety Disorders: Many studies have demonstrated the effective use of BDZs in the treatment of generalized anxiety disorder and other related anxiety disorders [90], such as panic attacks. These studies have also shown that BDZs are superior to other medications used to treat anxiety disorders, such as barbiturates and antipsychotic agents [91]. Antidepressants (such as selective serotonin reuptake inhibitors) are also used for the treatment of anxiety disorders, however, BDZs have the advantage of a rapid onset of action.
Convulsive Disorders: BDZs can be used in the treatment of epilepsy in children and adults [92]. However, a significant limitation is that tolerance to the anticonvulsant effects of BDZ’s often develops in patients. Nevertheless, BDZs are often administered for the treatment of convulsive emergencies and can reduce the mortality rate that is associated with severe epileptic seizures [91].
Muscle spasms: BDZs are also effectively used for muscle relaxation [91].
Side effects and recreational use
Anterograde amnesia: BDZs can induce anterograde amnesia at low concentrations. This property of BDZs can be used for beneficial purposes (such as during surgical procedures to reduce psychological trauma [93]), however, it can also be misused for illegal purposes. For instance, flunitrazepam (Rohypnol) is often cited as a date rape drug, due to its rapid pharmacokinetics and ability to induce strong amnesia.
Dependence and addiction: During initial administration of BDZs, a person may feel sleepy and uncoordinated, but a tolerance to these sedative effects rapidly develops. After a few weeks, physical dependence takes place, which manifests as a withdrawal syndrome when use of the drug is reduced or abruptly stopped. A protracted withdrawal syndrome may develop in a proportion of individuals and can include sleep disturbances, irritability, increased tension and anxiety, panic attacks, sweating, and a host of other perceptual changes [94]. In a small number of people, these symptoms can be severe and resemble serious psychiatric and neurological conditions, such as schizophrenia and seizure disorders [95]. A serious side effect of benzodiazepine withdrawal is suicide [96]. Addiction is less frequent than dependence, but is associated with chronic use and relapse after withdrawal. Unfortunately, BDZ abuse often occurs in conjunction with the abuse of another substance, such as alcohol or cocaine, making treatment approaches even more difficult.
Our understanding of the specific neural mechanisms underlying these pharmacological actions has developed to the point where specific GABAAR subtypes have been identified that mediate each of these specific effects (Figure I).
All addictive drugs, as well as natural rewards, increase dopamine (DA) levels in the mesolimbic dopamine (DA) system, also termed the reward system (Box 2). Several landmark studies with monkeys have shown that DA neurons play a role in the signaling of the “reward error prediction”, and thus, are involved in learning processes related to reward and intrinsic value. Specifically, DA neurons are excited following the presentation of an unexpected reward. Once this reward becomes predictable (by an experimentally-controlled cue), DA neurons shift their phasic activation from the reward to the cue. Finally, when the cue is present but the reward is withheld, DA neurons are inhibited [5,6]. Unlike natural rewards, addictive drugs always cause an increase in DA levels upon drug exposure even after repeated trials [7]. This interruption of normal DA signaling mechanisms may allow addictive drugs to hijack the reward system and lead to the malfunction of mechanisms controlling learning and memory.
Box 2. The neurobiology of addiction.
Evidence from animal models and humans have converged to demonstrate that the mesolimbic DA system is critically involved in mediating the rewarding properties in response to drugs of abuse [11,12] (Figure I).
Despite their chemical heterogeneity and distinct molecular targets, one common feature and property of all addictive drugs is that they increase DA concentrations in target structures of the mesolimbic projections [11,12]. Another feature common to addictive drugs is that they are capable of inducing synaptic plasticity changes. A single injection of cocaine is sufficient to induce plasticity at excitatory synapses onto DA neurons in the VTA [58,59]. This synaptic plasticity is mediated in part by the increased insertion of GluA2-lacking AMPARs [97], which results in a long-term potentiation-like state being induced in VTA DA neurons [58,98-100].
Drug-induced alterations in the GABA inhibitory regulation of DA neurons have also been implicated for opiates [101,102], cocaine [103], and more recently, BDZs [9], as discussed in this review. While these early adaptive changes are certainly not sufficient to explain the long-term development of addiction, they are believed to represent one of the key and necessary permissive steps involved in the progression of the disease. Moreover, long-term synaptic changes and cell morphology changes have been observed after repeated exposure to drugs in other nuclei of the mesolimbic DA system. For example, in the NAc, the AMPAR levels are increased during cocaine withdrawal [104]. Other studies have shown that repeated treatments with either amphetamine or cocaine lead to an increase in the number of dendritic branches and the density of dendritic spines on medium spiny neurons in the shell of the NAc, and on apical dendrites of layer V pyramidal cells in the prefrontal cortex. Cocaine also increased dendritic branching and spine density on the basilar dendrites of pyramidal cells. In addition, both drugs doubled the incidence of branched spines on medium spiny neurons [105].
Addiction has been proposed to arise from a sequence of drug-evoked synaptic plasticity stages [63, 106, 107]. In this model, DA release represents a coincident detector to trigger synaptic plasticity first in the VTA, followed by changes in the ventral striatum, the substantia nigra and eventually the dorsal striatum. Over time, addictive drugs hijack the reward system and other brain circuitry that modulate learning and memory processes, such that behavior becomes automatic and compulsive.
Recent findings have demonstrated that BDZs engage pharmacological and cellular mechanisms in the mesolimbic DA system [8, 9] that are similar in nature to those which have previously been identified for other drugs of abuse [10–12]. In this review, we provide an overview of the current understanding of the molecular mechanisms that may underlie the addictive properties of BDZs. Furthermore, we discuss how such knowledge of the neural basis of BDZ could be harnessed to design new BDZs with lower addiction liability.
BDZs positively modulate GABAA receptor function
GABAA receptor diversity
BDZs are positive allosteric modulators of the γ-aminobutyric acid type A receptors (GABAARs) (Box 3). GABAARs are ligand-gated chloride-selective ion channels that are physiologically activated by GABA, the major inhibitory neurotransmission in the brain. In addition to GABAARs, GABA also activates GABABRs and GABACRs. GABABRs are metabotropic receptors involved in slow inhibitory neurotransmission [13]. GABACRs are ionotropic receptors composed of ρ (rho) subunits that are related to - and classified by the International Union of Basic and Clinical Pharmacology (IUPHAR) as - GABAAR subunits. In vertebrates, the ρ1 subunit is only expressed in retinal bipolar or horizontal cells [14], whereas the ρ2 and ρ3 subunits are also found elsewhere [15]. Both types of receptors are insensitive to BDZs and therefore will not be further discussed here.
Box 3. BDZs are modulators of GABAARs.
Three groups of drugs which bind at the BDZ-binding site can be distinguished based on the type of modulation: positive allosteric modulators (also called agonists), negative allosteric modulators (or inverse agonists) and antagonists (Table 1).
Positive allosteric modulators (e.g., diazepam, zolpidem) potentiate the function of GABAARs. This potentiating action is thought to be induced by a change in conformation that allows for an increase in receptor affinity for GABA. The mechanism of such an increase in the affinity for GABA is still debated, but many studies in various models (e.g., dissociated cell cultures and heterologous expression systems) have demonstrated that diazepam increases single channel conductance [108] and single channel open probability [109]. Flunitrazepam also decreases receptor deactivation by ~50 % along with increased receptor desensitization [110]. Other studies have shown that BDZs increase the association rate of GABA, as well as decreasing the dissociation rate of GABA [109-111]. Finally, it has also been demonstrated that diazepam increases the apparent affinity of the receptor, not by altering the affinity of the closed state, but rather by shifting the equilibrium towards the high affinity open state [112].
The negative allosteric modulators (e.g., Ro15-4543, which is sometimes also referred to as an inverse agonist, because it binds to the BDZ site) have opposite effects to the positive allosteric modulators at the BDZ site. They decrease the affinity for GABA and are anxiogenic and pro-convulsant.
The antagonists (e.g., flumazenil) do not affect the GABA-elicited response; therefore they do not modify the affinity of GABA for the receptor. However, flumazenil binds to the BDZ site and competes with the binding of positive allosteric modulators. In this fashion it acts as a competitive antagonist and inhibits allosteric modulation of GABAARs. Flumazenil is routinely used in the clinic to counteract BDZ overdoses.
Functional GABAARs comprise a combination of five subunits derived from seven main receptor subunit families (α, β, γ, δ, ε, π and θ). Many of these subunits exist as multiple isoforms (e.g., α1–6, β1–3 and γ1–3) [16–18]. In the central nervous system (CNS), the most common receptor (~ 43% of all GABAARs) is comprised of α1, β2 and γ2 subunits, with a defined stoichiometry of 2α:2β:1γ [19,20]. The various subunit isoforms are expressed with regional specificity [18], as well as in a cell-type specific manner (e.g., the α6 subunit isoform is largely expressed in cerebellar granule cells [21]). GABAARs often reside at synaptic sites, mostly post-synaptically but also presynaptically [22], and some are expressed largely exclusively at peri- and extra-synaptic sites (e.g., the δ subunit [23,24]).
BDZ binding site within GABAA receptors
While the possibility of a distinct BDZ receptor has been suggested, it has become clear that BDZs bind to a specific site present on most GABAARs [25,26]. BDZs bind to a pocket formed by the α and the γ subunits that is distinct from the agonist binding site, which is located between one α and one β subunit [27,28]. Furthermore, it has been demonstrated that a specific histidine residue (H101) within the α1 subunit [29] and the homologous residues within the α2, α3 and α5 subunits (H101, H126 and H105, respectively) are crucial for BDZ action [30]. GABAARs containing α4 and α6 subunit isoforms are insensitive to BDZs, since they contain an arginine residue at this critical position [31,32]. This knowledge has been exploited to generate several lines of knock-in (KI) mice in which the relevant histidine residue has been mutated to an arginine within various α subunits [33–37]. GABAARs containing such mutant α subunit isoforms are largely insensitive to BDZs [29,30,33,35] and therefore are ideal tools to study the contribution of individual GABAAR subtypes to the pharmacological effects of BDZs.
Towards a GABAAR subtype-specific BDZ pharmacology
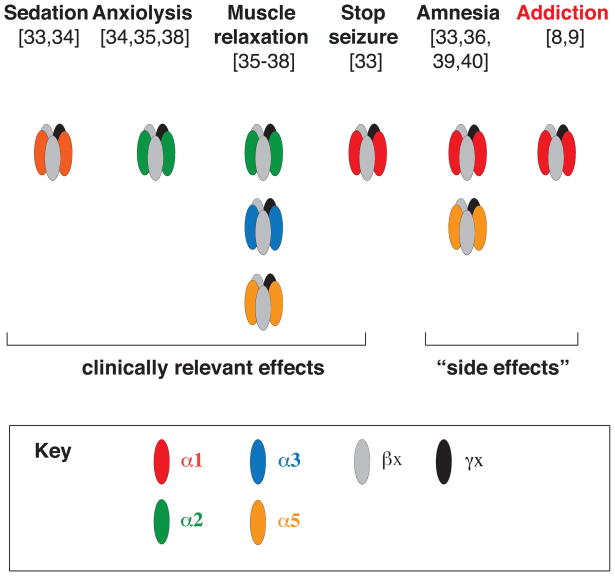
Pharmacological and behavioral studies in KI mice have led to correlations between a specific α subunit isoform with one or several of the five major effects of BDZs described above (Box 1). These studies have revealed that GABAARs containing the α1 subunit mediate the sedative, the anterograde amnesic effects, and partly the anticonvulsive effects, of diazepam [33,34]. α2-containing GABAARs mediate the anxiolytic actions, and to a large degree the myorelaxant effects [35,38]. α3- and α5-containing GABAARs also contribute to the myorelaxant actions [35,36,38], while GABAARs expressing the α5 subunit were shown to modulate the temporal and spatial memory effects of BZDs [36,39,40]. Recently, the addictive properties of BDZs have been shown to require the α1-containing GABAARs [8,9] (Box 1).
The latter findings rely on the cell type-specific GABAARs and BDZ pharmacology in the ventral tegmental area (VTA). The VTA is a key brain region involved in the mesolimbic DA pathway (Box 2). It is mainly comprised of DA neurons (~ 70%), GABA interneurons (~20%) and glutamatergic neurons [41,42]. Both GABA and DA neurons express GABAARs. Specifically, DA neurons express mRNA for α2, α3, α4, β1, β3 and γ2 subunit isoforms [43], while interneurons express α1, β2 and γ2 subunit isoforms [44,45]. Cell type-specific expression of the α3 and α1 isoforms in DA and GABA neurons, respectively, has recently been confirmed with immunohistochemistry [9] (Figure 1). Furthermore, such cell-specific expression patterns have been confirmed by electrophysiological studies that have assessed the functional properties of inhibitory postsynaptic currents (IPSCs) using wild type (WT) and α1-H101R and α3-H126R KI mice [9], which are crucial for the understanding of how BDZs activate DA neurons of the VTA (see next section).
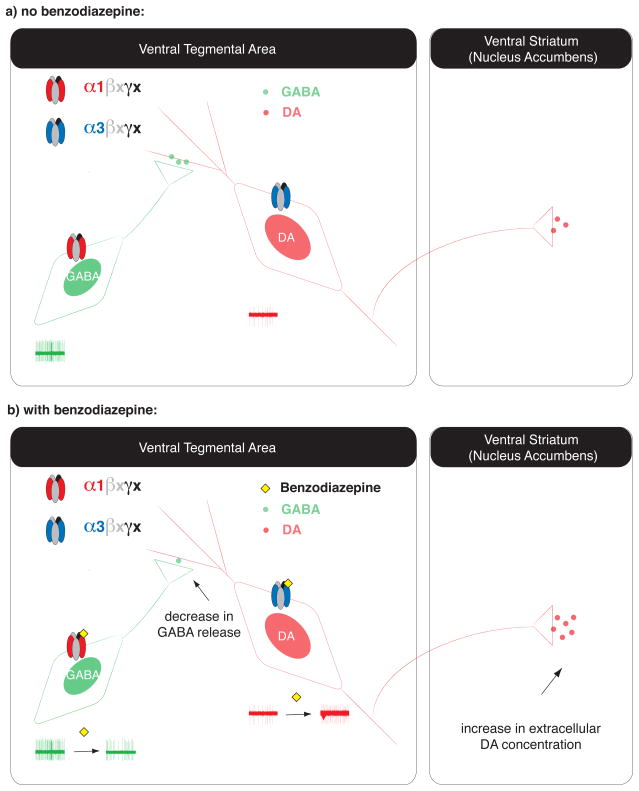
Fig. 1. Schematic representation of the mechanism of action of BDZs in the VTA.
(a) The VTA is composed of two major cell types, DA neurons and GABA interneurons. DA neurons receive local inhibitory inputs from the GABA interneurons, and send projections to the ventral striatum (NAc). While interneurons express α1-containing GABAARs, the DA neurons express GABAARs that contain other subunits, such as the α3 isoform. (b) BDZs such as diazepam bind to GABAARs on both cell types. In the presence of BDZs, inhibitory GABAAR currents are potentiated in both sets of neurons; however, the overall impact is much stronger in the interneurons, since GABAARs in interneurons cause larger unitary currents than in DA neurons. Such potentiation of the inhibitory currents in the interneurons leads to an overall hyperpolarization and a decrease in their activity (as depicted by the slower rate of neuronal excitability, seen in the single unit reading of neuronal activity in green). These results imply a decrease in the release of GABA from these neurons, and as a consequence, DA neurons are disinhibited and their firing rate increases (see example single unit recording in red). This results in more DA being released in the striatum and other target regions of the VTA [9].
Disinhibition: a mechanism shared among several addictive drugs to increase DA
Benzodiazepines
It was demonstrated that BDZs have a stronger impact on GABA neurons than on DA neurons. At baseline, miniature IPSCs of GABA neurons are slower and bigger than those in DA neurons. In the presence of BDZs, the spontaneous IPSC frequency increased in GABA neurons and decreased in DA neurons [9]. As a result, GABA neurons are more strongly hyperpolarized in the presence of BDZs and no longer inhibit DA neurons as seen with the in vivo single unit recordings [9] (Figure 1). This cellular mechanism is called the disinhibition of DA neurons and has also been demonstrated for other addictive drugs, such as morphine [46] and γ-hydroxybutyrate (GHB) [47–49](discussed further below).
A previous study in rats had already suggested that VTA DA neurons may be disinhibited after intravenous (i.v.) injection of diazepam [50]. However, a microdialysis study in rats contradicted these earlier findings [51]. Indeed, subcutaneous acute or chronic (twice a day for 14 days) injections of midazolam decreased the extracellular DA concentrations in the nucleus accumbens (NAc) (as measured 40 min after the injection). Similar results were obtained in rats when midazolam [52] or flurazepam [53] was locally injected in the NAc. However, in this latter case, since the drug is restricted to the NAc, GABAARs of VTA cells are not potentiated, which may explain this result. Moreover, the time resolution of the microdialysis assay may be too slow to detect an early increase in DA levels. In line with this interpretation, fast-scan cyclic voltammetry (FSCV) studies have shown that activation of GABAARs by direct administration of the GABAAR agonist muscimol into the VTA significantly increased DA release in the NAc [54].
Opioids
Opioids activate μ opioid receptors selectively located on GABA interneurons in the VTA [46]. They are metabotropic receptors coupled to Gi/o proteins. Their activation leads to the hyperpolarization of GABA interneurons and a concomitant reduction of release probability at their terminals, which subsequently induces the disinhibition of DA neurons, resulting in their excitation [46].
γ-hydroxybutyrate
GHB has two binding sites in the brain. One is an orphan G protein-coupled receptor (GPCR) [55] and the other is the GABABR [56]. GABABRs are expressed on both DA and GABA neurons of the VTA [47]. At recreationally relevant doses, GHB preferentially activates GABA neurons because they express G protein-coupled inwardly-rectifying potassium (GIRK)1/2 heteromeric effector channels, which couple more tightly to GABABRs than the GIRK2/3 channels found in DA neurons [49]. In fact, the EC50 value for GHB is an order of magnitude higher in DA neurons than GABA neurons [47]. Furthermore, this difference is amplified by the regulatory G protein signaling protein RGS2, selectively expressed in DA neurons [48,49]. RGS proteins are GTPase-accelerating proteins, which can modulate the coupling of GIRK channels to their respective GPCRs [57]. As a consequence, only GABA neurons are hyperpolarized at GHB concentrations below 1 mM, causing a disinhibition of DA neurons [48,49].
Taken together, these findings suggest that opioids, GHB and BDZs, which act at separate molecular targets, all increase mesolimbic DA levels through activation of the same cellular mechanism, namely the disinhibition of DA neurons.
Drug-evoked synaptic plasticity in the VTA
Past the actual presence of the drug in the brain, drug-evoked synaptic plasticity of glutamatergic transmission represents a first trace of drug exposure; it is in fact a hallmark feature of all addictive drugs [58,59]. A single dose of cocaine, nicotine, or morphine induces a rectification of the current/voltage (I/V) curve of AMPAR-mediated excitatory postsynaptic currents (EPSCs), and increases the AMPA/NMDA ratio [58,59]. Electrophysiological investigations along with Electron microscopy (EM) studies have confirmed that such inward rectification is due to a change in the subunit composition of AMPARs – specifically, GluA2-containing AMPARs are exchanged for GluA2-lacking AMPARs at synaptic sites within the VTA [59,60] (Box 2).
Role of α1-GABAARs in addiction
A single intra-peritoneal (i.p.) injection of a BDZ (such as midazolam or diazepam) in mice is also sufficient to induce synaptic plasticity at excitatory glutamatergic synapses onto DA neurons of the VTA [8, 9] (Figure 1). Intra-VTA injections of BDZs were also observed to result in the induction of synaptic plasticity in mice [9]. Furthermore, it was shown that such BDZ-evoked synaptic plasticity depends on α1-containing GABAARs, since the plasticity was abolished in α1-H101R KI mice [9].
The synaptic plasticity observed in the VTA after one single injection of a drug of abuse is believed to represent a necessary permissive step to trigger longer-term synaptic plasticity changes that eventuate in addiction, if injections are repeated and drug use becomes chronic. Several studies have observed adaptive synaptic plasticity in the NAc and the prefrontal cortex (PFC) after chronic drug exposure [61,62]. These investigations focused on cocaine- and morphine-evoked plasticity and future studies will have to address the question of whether similar changes also occur with BDZs (Box 4). Since drug-evoked plasticity in the VTA and NAc have been proposed to be hierarchically organized [63,64], it is feasible that chronic exposure to BDZs is likely to eventually also induce synaptic plasticity alterations in the NAc and PFC.
Box 4. Outstanding questions.
Many important questions remain to be answered in relation to BDZs and the neural basis of their addictive properties. A number of the most important areas for future research in this topic are highlighted below:
• The physiology of inhibition in the VTA
The basic work on BDZ effects on the mesolimbic system also teaches us something about the physiology of GABA transmission in the VTA. Future studies will help to address the question as to the extent that DA neuron diversity is also associated with specific (inhibitory) circuitry. For example, is the firing frequency of DA neurons, which are activated by salient, but aversive stimuli, similarly controlled by interneurons and therefore also subject to disinhibition by BDZs?
• BDZ addiction: beyond the VTA
The remodeling and plasticity of brain reward circuitry induced by addictive drugs is not limited to the VTA, but also involves the NAc, the PFC, and many other brain regions. This has been demonstrated to be the case with many drugs of abuse, including cocaine, morphine and nicotine, but has not really yet been investigated after chronic BDZ exposure. Such experiments will be important for determining the commonalities that BDZ share with other addictive drugs, as well as for identifying the specific attributes that make them unique.
• Use of BDZs in addicts: cross-talk between drugs
BDZs are often prescribed for patients who abuse other drugs, for example, to dampen withdrawal symptoms or to decrease anxiety in these patients. It will be important to reassess such use in the light of our increasing understanding of the addictive properties of BDZs.
• Subunit-specific pharmacology of BDZ: the clinical reality
Despite the availability of experimental subunit-specific GABAergic drugs for more than a decade [113], no selective BDZs have yet been introduced into clinical practice. The reasons for this are largely unknown. However, it appears that the failures that have so far been observed during the drug development and clinical trials are unrelated to the main mechanism of action of these experimental compounds. For instance, it has been shown that L-838,417 has unfavorable pharmacokinetics in rodents [114]. Therefore, more efforts should be directed towards addressing the pharmacokinetics and the toxicity of these compounds, to render them safe and effective for future clinical use.
Interestingly, BDZ-evoked synaptic plasticity of excitatory transmission was observed in the hippocampus [65,66]. Using electrophysiological recordings of AMPAR-mediated currents, immunofluorescence and EM, these studies reported an enhanced synaptic insertion of GluA2-lacking AMPARs in Schaffer collateral synapses in rats withdrawn from chronic flurazepam exposure, similar to the changes observed in the VTA after a single injection of BDZ [9] and other drugs [58,59].
Role of non α1-GABAARs in addiction
In addition to α1-containing GABAARs, which mediate BDZ-evoked disinhibition and synaptic plasticity in the VTA, other lines of evidence suggest a potential role for anxiolytic effects in BDZ addiction. The anxiolytic actions of BDZs are mediated by α2- [35] and possibly α3-containing GABAARs [37]. Support for this conclusion comes from behavioral studies in monkeys, which were first trained to self-administer (i.v. administration) the short-acting barbiturate methohexital, and were then offered different classes of drugs that act at the BDZ site [67,68]. These drugs include diazepam and midazolam (i.e., classical non-selective BDZs which act at α1-/α2-/α3- and α5-containing GABAARs), zolpidem (a non classical BDZ site ligand that exerts higher affinity for α1-containing GABAARs as compared to α2-/α3-/α5-containing GABAARs) and L-838,417 (a positive BDZ site modulator of α2-/α3- and α5-containing GABAARs but an antagonist at α1-containing GABAARs) (Table 1 and Box 3). Under these conditions, the monkeys self-administered all four compounds, hence the authors concluded that each of these compounds had reinforcing properties [67,68]. However, in a direct comparison using a progressive ratio self-administration paradigm, the breakpoint was highest for zolpidem, intermediate for both midazolam and diazepam, and lowest for L-838,417. The breakpoint represents the number of lever presses that is made by the animal to obtain an injection of drug; this reflects how much the animal would work for the drug. In this study, zolpidem was more reinforcing than midazolam/diazepam whereas L-838,417 was the least reinforcing drug (ie. the monkeys were more motivated to perform the task to obtain zolpidem as compared to L-838,417) [67,68]. From the results of this study, it was concluded that α1-containing GABAARs are sufficient, but not necessary, for mediating the addictive properties of BDZs.
Table 1.
Representative examples of clinical and experimental drugs that act at the BDZ-binding site of GABAARs.
| Generic name | Trade name (example) | use | Classical BDZ | Non classical BDz | Positive modulator | Negative modulator | Antagonist | Ki (nM) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Diazepam | Valium | clinical | √ | all | 15–17 | 115 | |||
| Flunitrazepam | Rohypnol | clinical | √ | α1/α2/α5 α3 |
2–7 15 |
115 | |||
| Midazolam | Hypnovel | clinical | √ | all | 2–10 | 116 | |||
| Flumazenil | Anexate | clinical | √ | all | 0.5–1.5 | 115 | |||
| Zolpidem | Stilnox | clinical | √ | α1 α2/α3 |
17 290–360 |
115 | |||
| Ro15-4513 | experimental | √ | all | 0.5–15 | 115 | ||||
| L-838,417 | experimental | √ | α2/α3 α5 |
α1 | 0.8 2 |
34,117 | |||
| TPA023 | experimental | √ | α2/α3 | α1/α5 | 0.2–0.5 | 118 | |||
| Imidazenil | experimental | √ | α2/α3 | α1/α5 | N/A | 119 |
The subunit-selectivity for each compound is listed, along with the relative drug binding affinity (Ki). In this context, the term “experimental” refers to compounds that are not currently in clinical use. The term “clinical” refers to the fact that these drugs have been approved for therapeutic use, but does not exclude experimental uses for these drugs.
It should be noted, however, that in this study, L-838,417 was still self-administered by the monkeys, therefore α1-sparing compounds may not be completely devoid of an addiction liability. Furthermore, in these particular studies, the monkeys had been trained with barbiturates before being tested with BDZs. Therefore, the reward system had already been primed and modified by another addictive drug, hence, potentially confounding any subsequent conclusions regarding the reinforcing effect of a drug such as L-838,417. It would be of high interest to repeat these experiments with naïve animals. It should also be noted that another study using TPA023 (a positive modulator at α2-/α3- containing GABAARs but essentially an antagonist at α1-/α5-containing GABAARs) [69] (Table 1 and Box 3) showed that compounds sparing (or antagonizing) α1-containing GABAARs may have a low abuse liability only when the same compound also has a reduced efficacy at GABAARs containing α2-/α3-subunit isoforms [70].
Taken together, these findings propose that α1-sparing compounds can contribute to BDZ addiction, albeit through DA-independent mechanisms. This component may be related to the anxiolytic effect that is mediated by α2- and/or α3-containing GABAARs. However the neural basis of this latter effect still remains to be determined.
Other drugs which have pharmacological actions at GABAARs
In addition to the GABA site to which the experimental compound muscimol binds, GABAARs possess additional allosteric modulatory sites at which various other drugs, such as barbiturates and ethanol exert their effects. Barbiturates were largely replaced by BDZs, in the 1960s, for clinical purposes [71] because BDZs have a significantly lower potential for overdose. In addition, barbiturates have a much strong liability for dependence and addiction, which was not observed with BDZs at that time. Ethanol is also well known to induce addiction and it is co-abused with BDZs. Muscimol is not known to induce addiction, however, several studies have shown that, depending on the concentration, it activates the VTA system in a somewhat similar way as BDZs do. In this section, we discuss our current knowledge of how these three substances activate the mesolimbic system.
Barbiturates
Barbiturates have been widely used clinically for a range of indications, including the treatment of anxiety, insomnia, seizure disorders and as muscle relaxants and general anesthetic agents. Although largely replaced by BDZs, barbiturates are still used in general anesthesia, as well as in the treatment of epilepsy [72]. At low concentrations (μM), barbiturates act as positive allosteric modulators of GABAARs. At significantly higher concentrations, barbiturates can directly activate the receptor (ie. directly gate the channel even in the absence of GABA) [62,63], however such concentrations are generally higher than those required for clinically effective anesthesia. The mechanism by which they cause these effects remains unknown, as no direct binding site for barbiturates on GABAARs has yet been identified. However, it was shown recently that specific segments of the δ subunit are, at least in part, involved in mediating the allosteric actions of pentobarbital at GABAARs [73]. It is particularly interesting to compare BDZs to barbiturates since both of these families of drugs exhibit addictive properties and both of them modulate GABAARs. However, how barbiturates induce addiction at the circuit level remains unknown. It will be of particular interest to see whether barbiturates also induce synaptic plasticity changes in the VTA, similar to what is observed with BDZs [9].
Ethanol
Studies over the last decade have shown that ethanol can specifically alter the function of several ligand-gated ion channels including GABAARs and other ionotropic receptors, including NMDA, serotonin (5-HT3) and glycine receptors. A high-affinity ethanol binding site on GABAARs that contains a combination of α4 (or α6), β3, and δ subunits has been proposed [74]. However, the idea that specific GABAARs display high sensitivity to ethanol is still controversial [75], and a specific molecular target for ethanol action in the VTA has not been definitively identified.
Several studies have investigated the physiological and pharmacological effects of ethanol on VTA neurons. One study reported an ethanol-induced increase of DA neuron activity in a VTA rodent slice preparation [76]. Furthermore, ethanol was shown to directly excite VTA DA neurons both in a dissociated VTA neuron culture model [77] and in vivo [78]. It was subsequently proposed that ethanol may actually enhance the intrinsic pacemaker activity of DA neurons [79]. In addition to modulating intrinsic properties of DA neurons, ethanol may also affect synaptic transmission mediated by GABAARs. For example, in an in vivo study with halothane-anesthetized rats, ethanol applied locally in the VTA decreased the spontaneous firing rate of GABA neurons [80]. Specifically, systemic ethanol injection produced an early inhibition followed by a late excitation at 30 to 60 min. Thus, this study suggests that ethanol modulation of an extrinsic input may excite GABA neurons [80].
Taken together, these studies indicate that ethanol and BDZs both act through GABAARs to increase extracellular DA levels in the VTA, although different subtypes may be implicated. It would be interesting to further elaborate and potentially confirm this shared mechanism. The identification of a specific target for ethanol in VTA cells would be an important first-step towards this goal. In addition, it would be important to measure whether changes in synaptic plasticity at excitatory afferences onto DA cells were observed in the presence of ethanol, as was observed to be the case with BDZs [9].
Muscimol
Muscimol is a non-addictive GABAAR agonist. Even though it does not have addictive properties, its pharmacological effects on the VTA are of interest here, particularly when contrasted to the effects of the BDZs. When administered directly into the VTA, low doses of muscimol cause an increase of DA levels in the NAc [54] via an increase of DA neuron firing that presumably results from a selective inhibition of GABA inputs [50,81] and the resultant disinhibition of DA neurons [82]. However, at higher concentrations, muscimol inhibits DA neurons [83]. Such an inverse dose-dependent response is in contrast with the observations following BDZ administration. One reason for this might be that muscimol is an agonist at the GABA site (rather than a positive modulator like BDZs), therefore it can inhibit DA neurons (via actions at GABAARs expressed on these cells) even when the afferent GABA interneurons are silent and no longer releasing GABA.
Blueprint for designing a BDZ without addiction liability
In summary, recent work on the neural basis of the addictive properties of BDZs suggests that receptors that contain the GABAAR α1 subunit are important for triggering drug-evoked synaptic plasticity in the VTA – an important first step underlying drug reinforcement. While many outstanding questions remain to be addressed in future experiments (Box 4), the current findings suggest that BDZs that act non-selectively at GABAAR subtypes, or drugs which have a selectivity that includes actions at α1-containing GABAARs, have a high addiction liability. This conclusion is in line with the results of studies where monkeys preferred to self-administer the α1-selective compound zolpidem [67] over midazolam, a non-selective BDZ [68]. These animal experiments are mirrored by clinical case reports confirming the dependence and abuse liability of zolpidem [84–86]. α1-sparing compounds, on the other hand, could represent promising candidates in the search for BDZs with a lower addiction liability. For example, the non-classical BDZ, L-838,417, fails to induce a form of synaptic plasticity in the VTA that is generally considered as a permissive step towards the development of addiction [9].
When considering the development of new compounds that spare, or are antagonists, at α1-containing GABAARs, one must take into account whether such drugs will still be effective in acting at GABAAR subtypes that mediate the sedative, anterograde amnesic and anticonvulsant actions of classical BDZs. Therefore α1-sparing compounds will certainly not be suitable for all indications. However, imidazo-BDZ carboxamide derivatives (e.g., imidazenil) are α1-sparing compounds that have been shown to have anxiolytic and anticonvulsant actions without (at least so far) demonstrating tolerance and dependence liabilities in monkeys [87] and in rodents [88,89] (Table 1). As such, it may therefore be possible to dissociate anxiolysis from addictive liability, which would have important clinical ramifications for the effective treatment of anxiety-related disorders.
Box 1. Figure I. GABAAR BDZ-specific pharmacology.
Over the past decade, an emerging understanding of the specific GABAAR subtypes that are responsible for mediating the diverse spectrum of BDZ pharmacological effects. For example, the sedative actions are known to be mediated by α1-containing GABAARs, whereas the anxiolytic actions are mediated by α2-containing GABAARs.
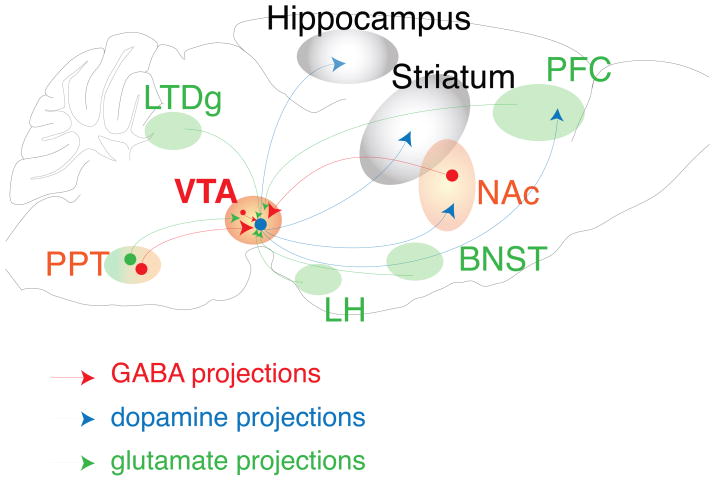
Box 2. Figure I. Schematic cartoon depicting the circuitry of the mesolimbic DA system.
The reward system originates in the VTA, which is composed of two major cell types, the DA neurons and the GABA neurons. The DA neurons (blue) project to the ventral striatum, the NAc, the PFC and the hippocampus. The VTA receives inhibitory projections (red) back from the NAc and from the pedunculopontine nucleus (PPT) and excitatory projections (green) from the PFC, the PPT, the amygdala, the lateral hypothalamus (LH), the laterodorsal tegmental nucleus (LDTg) and the bed nucleus of the stria terminalis (BNST), as well as receiving local inhibitory feedback from the GABA interneurons in the VTA.
Acknowledgments
This work is supported by the National Institute on Drug Abuse (NIDA; DA019022; C. Lüscher., P. Slesinger), the Swiss National Science Foundation and the Swiss Initiative in Systems Biology (Neurochoice). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDA or the National Institutes of Health.
Glossary
- AMPA/NMDA ratio
ratio of the amplitudes of EPSCs mediated by AMPARs and NMDARs, respectively. An increase in the AMPA/NMDA ratio may be due to enhanced AMPAR transmission, reduced NMDAR transmission, or a concomitant change in both components. Changes in the AMPA/NMDA ratio are considered a hallmark of synaptic plasticity
- Deactivation of receptor
Process by which activated receptors relax toward the resting state
- Desensitization of receptor
decay in receptor effect in the continuous presence of the agonist
- Dependence
an adaptive state induced by chronic drug use that becomes apparent when abrupt cessation of drug-use induces withdrawal syndrome, which is typically preceded by tolerance
- Drug addiction
A chronic disorder characterized by compulsive drug-seeking and drug-taking behavior that persists despite negative consequences
- I/V curve
current/voltage relationship that is determined by plotting the EPSC amplitude as a function of the membrane potential. For AMPAR-mediated EPSCs in VTA DA neurons, the I/V curve is linear and reverses at 0 mV in slices from naïve or saline injected mice. After administration of an addictive drug, the I/V curve is inwardly rectifying (i.e., currents at positive potential are smaller than currents recorded at mirrored negative potential). Inward rectification is the biophysical signature of GluA2-lacking AMPARs and is also considered a hallmark of synaptic plasticity
- Self-administration
A behavioral paradigm that is used to evaluate the reinforcing properties of a substance. Animals learn to press a lever in order to obtain an injection of a drug. The number of lever presses is taken to quantify the reinforcement of the substance. Particularly reinforcing substances are tested using a so-called progressive ratio scheme (i.e., the animal has to press the lever several times to receive one dose). During the test, the ratio between lever presses and drug injection is gradually increased until the animal stops pressing the lever, which is defined as the breakpoint
- Tolerance
reduction in the response to the drug upon repeated administration. A progressive increase in drug dosage is therefore necessary to experience the desired effects
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Salzman C. Addiction to benzodiazepines. Psy Quart. 1998;69:251–261. doi: 10.1023/a:1022125929946. [DOI] [PubMed] [Google Scholar]
- 2.Schull P. Nursing Spectrum Drug Handbook. McGraw Hill; 2009. [Google Scholar]
- 3.Sternbach LH. The benzodiazepine story. J Psychoact drugs. 1983;15:15–17. doi: 10.1080/02791072.1983.10472118. [DOI] [PubMed] [Google Scholar]
- 4.Woods JH, et al. Benzodiazepines: use, abuse, and consequences. Pharmacol Rev. 1992;44:151–347. [PubMed] [Google Scholar]
- 5.Schultz W, et al. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 6.Schultz W. Dopamine signals for reward value and risk: basic and recent data. Behav Brain Functions: BBF. 2010;6:24–32. doi: 10.1186/1744-9081-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, et al. Effect of chronic “binge cocaine” on basal levels and cocaine-induced increases of dopamine in the caudate putamen and nucleus accumbens of C57BL/6J and 129/J mice. Synapse. 2003;50:191–199. doi: 10.1002/syn.10251. [DOI] [PubMed] [Google Scholar]
- 8.Heikkinen AE, et al. Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacol. 2009;34:290–298. doi: 10.1038/npp.2008.89. [DOI] [PubMed] [Google Scholar]
- 9.Tan KR, et al. Neural bases for addictive properties of benzodiazepines. Nature. 2010;463:769–774. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ungless MA, et al. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- 11.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci. 2005;8:1445–1449. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 12.Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 13.Pinard A, et al. GABAB receptors: physiological functions and mechanisms of diversity. Adv Pharmacol. 2010;58:231–255. doi: 10.1016/S1054-3589(10)58010-4. [DOI] [PubMed] [Google Scholar]
- 14.Chebib M. GABAC receptor ion channels. Clin Exp Pharmacol Physiol. 2004;31:800–804. doi: 10.1111/j.1440-1681.2004.04083.x. [DOI] [PubMed] [Google Scholar]
- 15.Olsen RW, Sieghart W. International Union of Pharmacology. LXX Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnard EA, et al. International Union of Pharmacology. XV Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- 17.Moragues N, et al. cDNA cloning and expression of a gamma-aminobutyric acid A receptor epsilon-subunit in rat brain. Eur J Neurosci. 2000;12:4318–4330. [PubMed] [Google Scholar]
- 18.Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Current topics in medicinal chemistry. 2002;2:795. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- 19.Chang Y, et al. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baumann SW, et al. Forced subunit assembly in alpha1beta2gamma2 GABAA receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 21.Lüddens H, et al. Cerebellar GABAA receptor selective for a behavioural alcohol antagonist. Nature. 1990;346:648–651. doi: 10.1038/346648a0. [DOI] [PubMed] [Google Scholar]
- 22.Kullmann DM, et al. Presynaptic, extrasynaptic and axonal GABAA receptors in the CNS: where and why? Prog Biophys Mol Biol. 2005;87:33–46. doi: 10.1016/j.pbiomolbio.2004.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nusser Z, et al. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 25.Möhler H, Okada T. Benzodiazepine receptor: demonstration in the central nervous system. Science. 1977;198:849–851. doi: 10.1126/science.918669. [DOI] [PubMed] [Google Scholar]
- 26.Tallman JF, Hutchison A. Molecular biological insights into GABA and benzodiazepine receptor structure. Prog Clin Biol Res. 1990;361:231–255. [PubMed] [Google Scholar]
- 27.Sigel E, Buhr A. The benzodiazepine binding site of GABAA receptors. Trends Pharmacol Sci. 1997;18:425–429. doi: 10.1016/s0165-6147(97)01118-8. [DOI] [PubMed] [Google Scholar]
- 28.Sigel E. Mapping of the benzodiazepine recognition site on GABA(A) receptors. Cur Top Med Chem. 2002;2:833–839. doi: 10.2174/1568026023393444. [DOI] [PubMed] [Google Scholar]
- 29.Wieland HA, et al. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- 30.Benson JA, et al. Pharmacology of recombinant gamma-aminobutyric acidA receptors rendered diazepam-insensitive by point-mutated alpha-subunits. FEBS let. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 31.Korpi ER, Seeburg PH. Natural mutation of GABAA receptor alpha 6 subunit alters benzodiazepine affinity but not allosteric GABA effects. Eur J Pharmacol. 1993;247:23–27. doi: 10.1016/0922-4106(93)90133-t. [DOI] [PubMed] [Google Scholar]
- 32.Yang W, et al. Cloning and characterization of the human GABAA receptor alpha 4 subunit: identification of a unique diazepam-insensitive binding site. Eur J Pharmacol. 1995;291:319–325. doi: 10.1016/0922-4106(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 33.Rudolph U, et al. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 34.McKernan RM, et al. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype. Nat Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- 35.Löw K, et al. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 36.Crestani F, et al. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dias R, et al. Evidence for a significant role of alpha 3-containing GABAA receptors in mediating the anxiolytic effects of benzodiazepines. J Neurosci. 2005;25:10682–10688. doi: 10.1523/JNEUROSCI.1166-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crestani F, et al. Molecular targets for the myorelaxant action of diazepam. Mol Pharmacol. 2001;59:442–445. doi: 10.1124/mol.59.3.442. [DOI] [PubMed] [Google Scholar]
- 39.Collinson N, et al. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the alpha 5 subunit of the GABAA receptor. J Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng VY, et al. Alpha5GABAA receptors mediate the amnestic but not sedative-hypnotic effects of the general anesthetic etomidate. J Neurosci. 2006;26:3713–3720. doi: 10.1523/JNEUROSCI.5024-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamaguchi T, et al. Glutamatergic neurons are present in the rat ventral tegmental area. Eur J Neurosci. 2007;25:106–118. doi: 10.1111/j.1460-9568.2006.05263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dobi A, et al. Glutamatergic and nonglutamatergic neurons of the ventral tegmental area establish local synaptic contacts with dopaminergic and nondopaminergic neurons. J Neurosci. 2010;30:218–229. doi: 10.1523/JNEUROSCI.3884-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada H, et al. Identification of GABAA receptor subunit variants in midbrain dopaminergic neurons. J Neurochem. 2004;89:7–14. doi: 10.1111/j.1471-4159.2004.02271.x. [DOI] [PubMed] [Google Scholar]
- 44.Gao B, et al. Neuron-specific expression of GABAA-receptor subtypes: differential association of the alpha 1- and alpha 3-subunits with serotonergic and GABAergic neurons. Neuroscience. 1993;54:881. doi: 10.1016/0306-4522(93)90582-z. [DOI] [PubMed] [Google Scholar]
- 45.Gao B, Fritschy JM. Selective allocation of GABAA receptors containing the alpha 1 subunit to neurochemically distinct subpopulations of rat hippocampal interneurons. Eur J Neurosci. 1994;6:837–853. doi: 10.1111/j.1460-9568.1994.tb00994.x. [DOI] [PubMed] [Google Scholar]
- 46.Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cruz HG, et al. Bi-directional effects of GABA(B) receptor agonists on the mesolimbic dopamine system. Nat Neurosci. 2004;7:153–159. doi: 10.1038/nn1181. [DOI] [PubMed] [Google Scholar]
- 48.Labouèbe G, et al. RGS2 modulates coupling between GABAB receptors and GIRK channels in dopamine neurons of the ventral tegmental area. Nat Neurosci. 2007;10:1559–1568. doi: 10.1038/nn2006. [DOI] [PubMed] [Google Scholar]
- 49.Lomazzi M, et al. Addictive drugs modulate GIRK-channel signaling by regulating RGS proteins. Trends Pharmacol Sci. 2008;29:544–549. doi: 10.1016/j.tips.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O’Brien DP, White FJ. Inhibition of non-dopamine cells in the ventral tegmental area by benzodiazepines: relationship to A10 dopamine cell activity. Eur J Pharmacol. 1987;142:343–354. doi: 10.1016/0014-2999(87)90072-0. [DOI] [PubMed] [Google Scholar]
- 51.Finlay JM, et al. Benzodiazepine-induced decreases in extracellular concentrations of dopamine in the nucleus accumbens after acute and repeated administration. Psychopharmacol. 1992;106:202–208. doi: 10.1007/BF02801973. [DOI] [PubMed] [Google Scholar]
- 52.Murai T, et al. Opposite effects of midazolam and beta-carboline-3-carboxylate ethyl ester on the release of dopamine from rat nucleus accumbens measured by in vivo microdialysis. Eur J Pharmacol. 1994;261:65–71. doi: 10.1016/0014-2999(94)90301-8. [DOI] [PubMed] [Google Scholar]
- 53.Pei Q, et al. Both systemic and local administration of benzodiazepine agonists inhibit the in vivo release of 5-HT from ventral hippocampus. Neuropharmacol. 1989;28:1061–1066. doi: 10.1016/0028-3908(89)90118-4. [DOI] [PubMed] [Google Scholar]
- 54.Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res. 1998;798:156–165. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- 55.Andriamampandry C, et al. Cloning and characterization of a rat brain receptor that binds the endogenous neuromodulator gamma-hydroxybutyrate (GHB) FASEB J. 2003;17:1691–1693. doi: 10.1096/fj.02-0846fje. [DOI] [PubMed] [Google Scholar]
- 56.Kaupmann K, et al. Specific gamma-hydroxybutyrate-binding sites but loss of pharmacological effects of gamma-hydroxybutyrate in GABA(B)(1)-deficient mice. Eur J Neurosci. 2003;18:2722–2730. doi: 10.1111/j.1460-9568.2003.03013.x. [DOI] [PubMed] [Google Scholar]
- 57.Doupnik CA, et al. Measuring the modulatory effects of RGS proteins on GIRK channels. Meth Enzymol. 2004;389:131–154. doi: 10.1016/S0076-6879(04)89009-8. [DOI] [PubMed] [Google Scholar]
- 58.Saal D, et al. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- 59.Brown MTC, et al. Drug-Driven AMPA Receptor Redistribution Mimicked by Selective Dopamine Neuron Stimulation. PLoS ONE. 2010. ( http://www.plosone.org) [DOI] [PMC free article] [PubMed]
- 60.Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- 61.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 62.Thomas MJ, et al. Neuroplasticity in the mesolimbic dopamine system and cocaine addiction. Br J Pharmacol. 2008;154:327–342. doi: 10.1038/bjp.2008.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lüscher C, Bellone C. Cocaine-evoked synaptic plasticity: a key to addiction? Nat Neurosci. 2008;11:737–738. doi: 10.1038/nn0708-737. [DOI] [PubMed] [Google Scholar]
- 64.Mameli M, et al. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 65.Song J, et al. Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing alpha-amino-3-hydroxy-5- methylisoxazole-4-propionic acid receptors in Hippocampal CA1 neurons. J Pharmacol Exp Ther. 2007;322:569–581. doi: 10.1124/jpet.107.121798. [DOI] [PubMed] [Google Scholar]
- 66.Das P, et al. Increased AMPA receptor GluR1 subunit incorporation in rat hippocampal CA1 synapses during benzodiazepine withdrawal. J Comp Neurol. 2008;511:832–846. doi: 10.1002/cne.21866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rowlett JK, et al. Different GABAA receptor subtypes mediate the anxiolytic, abuse-related, and motor effects of benzodiazepine-like drugs in primates. Proc Natl Aca Sci U S A. 2005;102:915–920. doi: 10.1073/pnas.0405621102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowlett JK, Lelas S. Comparison of zolpidem and midazolam self-administration under progressive-ratio schedules: consumer demand and labor supply analyses. Exp Clin Psychopharmacol. 2007;15:328–337. doi: 10.1037/1064-1297.15.4.328. [DOI] [PubMed] [Google Scholar]
- 69.Atack JR, et al. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- 70.Ator NA, et al. Reducing abuse liability of GABAA/benzodiazepine ligands via selective partial agonist efficacy at alpha1 and alpha2/3 subtypes. J Pharmacol Exp Ther. 2010;332:4–16. doi: 10.1124/jpet.109.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Birdwood G, Craven M. Letter: Outmoded barbiturates. Brit Med J. 1975;1:148. doi: 10.1136/bmj.1.5950.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Isbell H, Fraser HF. Addiction to analgesics and barbiturates. J Pharmacol Exp Ther. 1950;99:355–397. [PubMed] [Google Scholar]
- 73.Feng HJ, Macdonald RL. Barbiturates require the N terminus and first transmembrane domain of the delta subunit for enhancement of alpha1beta3delta GABAA receptor currents. J Biol Chem. 2010;285:23614–23621. doi: 10.1074/jbc.M110.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wallner M, et al. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Aca Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lovinger DM, Homanics GE. Tonic for what ails us? high-affinity GABAA receptors and alcohol. Alcohol. 2007;41:139–143. doi: 10.1016/j.alcohol.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brodie MS, et al. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- 77.Brodie MS, et al. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcoholism Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- 78.Foddai M, et al. Acetaldehyde increases dopaminergic neuronal activity in the VTA. Neuropsychopharmacol. 2004;29:530–536. doi: 10.1038/sj.npp.1300326. [DOI] [PubMed] [Google Scholar]
- 79.Okamoto T, et al. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallegos RA, et al. Adaptive responses of gamma-aminobutyric acid neurons in the ventral tegmental area to chronic ethanol. J Pharmacol Exp Ther. 1999;291:1045–1053. [PubMed] [Google Scholar]
- 81.Kalivas PW, et al. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- 82.Grace AA, Bunney BS. Paradoxical GABA excitation of nigral dopaminergic cells: indirect mediation through reticulata inhibitory neurons. Eur J Pharmacol. 1979;59:211–218. doi: 10.1016/0014-2999(79)90283-8. [DOI] [PubMed] [Google Scholar]
- 83.Klitenick MA, et al. Regulation of somatodendritic dopamine release in the ventral tegmental area by opioids and GABA: an in vivo microdialysis study. J Neurosci. 1992;12:2623–2632. doi: 10.1523/JNEUROSCI.12-07-02623.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Aragona M. Abuse, dependence, and epileptic seizures after zolpidem withdrawal: review and case report. Clin Neuropharmacol. 2000;23:281–283. doi: 10.1097/00002826-200009000-00008. [DOI] [PubMed] [Google Scholar]
- 85.Boulanger-Rostowsky L, et al. Dependence on zolpidem: a report of two cases. L’Encéphale. 2004;30:153–155. doi: 10.1016/s0013-7006(04)95426-7. [DOI] [PubMed] [Google Scholar]
- 86.Svitek J, et al. Extensive craving in high dose zolpidem dependency. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:591–592. doi: 10.1016/j.pnpbp.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 87.Thompson DM, et al. Imidazenil, a new anxiolytic and anticonvulsant drug, attenuates a benzodiazepine-induced cognition deficit in monkeys. J Pharmacol Exp Ther. 1995;273:1307–1312. [PubMed] [Google Scholar]
- 88.Auta J, et al. Imidazenil: a low efficacy agonist at alpha1- but high efficacy at alpha5-GABAA receptors fail to show anticonvulsant cross tolerance to diazepam or zolpidem. Neuropharmacol. 2008;55:148–153. doi: 10.1016/j.neuropharm.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Auta J, et al. Anticonvulsant, anxiolytic, and non-sedating actions of imidazenil and other imidazo-benzodiazepine carboxamide derivatives. Pharmacol Biochem Behav. 2010;95:383–389. doi: 10.1016/j.pbb.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 90.Anderson KN, Shneerson JM. Drug treatment of REM sleep behavior disorder: the use of drug therapies other than clonazepam. J Clin Sleep Med. 2009;5:235–239. [PMC free article] [PubMed] [Google Scholar]
- 91.Hollister LE, et al. Clinical uses of benzodiazepines. J Clin Psychopharmacol. 1993;13:1S–169S. [PubMed] [Google Scholar]
- 92.McMullan J, et al. Midazolam versus diazepam for the treatment of status epilepticus in children and young adults: a meta-analysis. J Soc Acad Emerg Med. 2010;17:575–582. doi: 10.1111/j.1553-2712.2010.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Koht A, Moss JI. Does midazolam cause retrograde amnesia, and can flumazenil reverse that amnesia? Anesth Analg. 1997;85:211–212. doi: 10.1097/00000539-199707000-00037. [DOI] [PubMed] [Google Scholar]
- 94.Pétursson H. The benzodiazepine withdrawal syndrome. Addiction. 1994;89:1455–1459. doi: 10.1111/j.1360-0443.1994.tb03743.x. [DOI] [PubMed] [Google Scholar]
- 95.Gabbard GO. Gabbard’s treatments of psychaitric disorders. American Psychiatric Publishing; 2007. [Google Scholar]
- 97.Colvin R. Overcoming Prescription Drug Addiction: Prescription drug Abuse: the hidden epidemic: a guide to coping and understanding. Addicus Book; 2008. pp. 74–76. [Google Scholar]
- 98.Mameli M, et al. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- 99.Borgland SL, et al. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- 101.Nugent FS, et al. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- 102.Nugent FS, Kauer JA. LTP of GABAergic synapses in the ventral tegmental area and beyond. J Physiol. 2008;586:1487–1493. doi: 10.1113/jphysiol.2007.148098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Q, et al. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacol. 2004;47(Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 106.Belin D, et al. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacol. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- 108.Eghbali M, et al. Hippocampal GABA(A) channel conductance increased by diazepam. Nature. 1997;388:71–75. doi: 10.1038/40404. [DOI] [PubMed] [Google Scholar]
- 109.Rogers CJ, et al. Benzodiazepine and beta-carboline regulation of single GABAA receptor channels of mouse spinal neurones in culture. J Physiol. 1994;475:69–82. doi: 10.1113/jphysiol.1994.sp020050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mellor JR, Randall AD. Frequency-dependent actions of benzodiazepines on GABAA receptors in cultured murine cerebellar granule cells. J Physiol. 1997;503:353–369. doi: 10.1111/j.1469-7793.1997.353bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Goldschen-Ohm MP, et al. An epilepsy-related region in the GABA(A) receptor mediates long-distance effects on GABA and benzodiazepine binding sites. Mol pharmacol. 2010;77:35–45. doi: 10.1124/mol.109.058289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Campo-Soria C, et al. Mechanism of action of benzodiazepines on GABAA receptors. Br J Pharmacol. 2006;148:984–990. doi: 10.1038/sj.bjp.0706796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rudolph U, Möhler H. GABA-based therapeutic approaches: GABAA receptor subtype functions. Curr Opin Pharmacol. 2006;6:18–23. doi: 10.1016/j.coph.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 114.Scott-Stevens P, et al. Rodent pharmacokinetics and receptor occupancy of the GABAA receptor subtype selective benzodiazepine site ligand L-838417. Biopharm Drug Dispos. 2005;26:13–20. doi: 10.1002/bdd.423. [DOI] [PubMed] [Google Scholar]
- 115.Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995;47:181–234. [PubMed] [Google Scholar]
- 116.Kucken AM, et al. Structural requirements for imidazobenzodiazepine binding to GABAA receptors. Mol Pharmacol. 2003;63:289–296. doi: 10.1124/mol.63.2.289. [DOI] [PubMed] [Google Scholar]
- 117.Buhr A, Sigel E. A point mutatin in the gamma2 subunit of gamma- aminobutyric acid type A receptors results in altered benzodiazepine binding site specificity. Proc Natl Aca Sci U S A. 1997;94:8824–8829. doi: 10.1073/pnas.94.16.8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Atack JR, et al. TPA023 [7-(1,1-dimethylethyl)-6-(2-ethyl-2H-1,2,4-triazol-3-ylmethoxy)-3-(2-fluorophenyl)-1,2,4-triazolo[4,3-b]pyridazine], an agonist selective for alpha2- and alpha3-containing GABAA receptors, is a nonsedating anxiolytic in rodents and primates. J Pharmacol Exp Ther. 2006;316:410–422. doi: 10.1124/jpet.105.089920. [DOI] [PubMed] [Google Scholar]
- 119.Giusti P, et al. Imidazenil: a new partial positive allosteric modulator of gamma aminobutyric acid (GABA) action at GABAA receptors. J Pharmacol Exp Ther. 1993;266:1018–1028. [PubMed] [Google Scholar]