Abstract
Fusarium and Aspergillus species of mould are major causes of corneal infections in the USA and worldwide, resulting in severe visual impairment and blindness. As there is evidence for T cell responses to these pathogenic fungi in infected individuals, we examined the role of IL-17A (IL-17) and IFN-γ in murine models of fungal keratitis. We found that C57BL/6 mice given intratracheal or subcutaneous immunization of conidia prior to corneal infection exhibited enhanced fungal killing and lower corneal opacity compared with unimmunized mice. Protective immunity was associated with temporal recruitment of IL-17 producing neutrophils, Th17 and Th1 cells, and was dependent on production of IL-17 but not IFN-γ. Protection was also impaired in neutrophil depleted and in Rag2−/− mice. Together, the results of these studies identify an essential role for IL-17 producing neutrophils and Th17 cells in regulating the growth of fungal hyphae and the severity of corneal disease.
Keywords: Neutrophil, IL-17, Th17, fungal infection, Aspergillus, Fusarium, cornea, keratitis
Aspergillus and Fusarium are filamentous fungi that are ubiquitous in the environment and can cause life-threatening systemic diseases. Potentially fatal pulmonary aspergillosis can occur in patients given hematopoietic stem cell transplants, and in patients with chronic pulmonary obstruction (1, 2). Pulmonary and systemic aspergillosis and fusariosis can occur in patients with immune suppression due to HIV/AIDS, hematopoietic stem cell transplants, organ transplants and cancer therapy (3-5). These organisms are also the major cause of blinding corneal infections in immune competent individuals following corneal injury by plant material containing fungal spores (conidia) (6-8). Fusarium was also found to be the causative organism in an outbreak of contact lens associated keratitis in the USA, Europe and Singapore, with over 300 cases of corneal infections in a 1-year period in the USA alone (9-11). Once in the corneal stroma, the conidia germinate, and hyphae spread through the tissue and can penetrate into the anterior chamber. Both the hyphae and the ensuing cellular infiltrate cause severe corneal opacification, visual impairment and blindness.
Utilizing mouse models of cornea infection, we reported that dormant conidia in the cornea stroma do not recruit neutrophils or induce keratitis as the external hydrophobin protein layer is not recognized by the immune system (12). However, following germination, conidia express β-glucan and α-mannan, which activate the c-type lectins Dectin-1 and Dectin-2, respectively, on resident corneal macrophages. IL-1β and CXC chemokines are then produced, which recruit neutrophils from peripheral, limbal blood vessels to the peripheral corneal stroma, which then migrate to the infected area of the cornea (12-14). Given that neutrophils cannot ingest hyphae, their ability to inhibit hyphal growth is dependent on reactive oxygen species production and iron sequestration (15, 16).
In addition to neutrophils, infected corneas examined after patients had undergone transplant were found to contain CD3+ and CD4+ T cells and to express IL-17A and IFN-γ (17). Given that T cells were likely sensitized following inhalation of fungal spores, experiments described in the current study sensitized mice to Aspergillus and Fusarium by intratracheal or subcutaneous injection of killed, swollen conidia prior to corneal infection with live conidia. We found that Th17 and Th1 cells are recruited to the corneal stroma, and that IL-17, but not IFNγ is required to kill the fungi. We also demonstrate that neutrophils are recruited prior to T cells, that a sub-population of neutrophils also produce IL -17, and that optimal protective immunity requires T cells and IL-17 producing neutrophils.
Materials and Methods
Source of mice
All animals were treated in accordance with the guidelines provided in the Association for Research in Vision and Ophthalmology ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by Case Western Reserve University IACUC. Female C57BL/6 mice (6–12 wk old) and Rag2−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Il17−/− mice were kindly provided by Dr Yoichiro Iwakura, University of Tokyo.
Aspergillus and Fusarium strains
Aspergillus fumigatus strain Af-BP is a clinical isolate from a fungal keratitis patient treated at Bascom Palmer Eye Institute (Miami, FL) provided by Dr Darlene Miller and used in our earlier studies (13). Fusarium oxysporum strain 8996 is a fungal keratitis clinical isolate from a patient treated at the Cole Eye Institute, Cleveland Clinic Foundation (Cleveland, OH), which we also used previously in mouse models of keratitis (14, 18). The RFP expressing strain of A. fumigatus (Af-dsRed) has a gpdA promoter and constitutively express monomeric dsRed protein, and was generated in collaboration with Dr Michelle Momany at the University of Georgia in Athens (13). GFP expressing Fusarium oxysporum were obtained from Dr Seogchan Kang, Penn State University.
Subcutaneous and intratracheal immunization with heat-killed, swollen conidia
A. fumigatus and F. oxysporum were harvested from plate-grown cultures, and conidia were obtained by passing the culture suspension through sterile PBS-soaked cotton gauze positioned at the tip of a 10 ml syringe as described (13). Conidia were incubated 6h in Sabouraud dextrose broth (SDB) to allow germination and expression of β-glucan, which initiates the host response (13). Conidia suspensions were centrifuged, diluted in PBS to 3 × 108 conidia/100 μl, and then killed by boiling for 5 min.
Mice were immunized with heat-killed swollen conidia by the intratracheal or subcutaneous routes. For airborne sensitization, mice were given a single trans-tracheal instillation 10 days prior to corneal infection. The trachea was surgically exposed, and 10 μl of 3 × 107 A. fumigatus spores suspended in PBS were inoculated through the tracheal wall into the lumen as described for a model of sarcoid (19). For subcutaneous immunization, 3 × 108 heat-killed swollen conidia in 100 μl PBS were injected into the base of the tail 10 days and 3 days prior to corneal infection.
Aspergillus and Fusarium hyphal extracts
Aspergillus and Fusarium conidia were added to Sabouraud broth in a shaking incubator at 37°C (Aspergillus) or 34°C (Fusarium) and incubated 18h to generate hyphae. After harvesting by passing through an aspiration filter, hyphae were then frozen in a bath of liquid nitrogen, pulverized using a mortar and pestle, and passed through a 30μm filter. Protein concentration was adjusted to 1 mg/ml protein and stored at −20°C.
Mouse model of Aspergillus and Fusarium keratitis
Conidia were harvested from A. fumigatus and F. oxysporum cultures as described above, and adjusted to a final concentration of 5 × 104 conidia/μl in PBS. Mice were anaesthetized by intraperitoneal injection of tribromoethanol, and the corneal epithelium was abraded using a 30-gauge needle. A 33-gauge Hamilton syringe was inserted into the abrasion, and 2 μl of 1 × 105 conidia in PBS was injected into the corneal stroma as described (13, 14). Mice were photographed daily using a stereomicroscope, and corneal opacity was visualized in the intact cornea using a high-resolution stereo fluorescence MZFLIII microscope (Leica Micro- systems) and Spot RT Slider KE camera (Diagnostics Instruments). All images were captured using SpotCam software (RT Slider KE; Diagnostics Instruments). Corneal opacity was quantified using Metamorph software as described previously (13, 14) and in Supplemental Figure S2.
Quantification of Aspergillus and Fusarium in infected corneas
Metamorph was also used to quantify total fungal mass in mice infected with the Af-dsRed and GFP as described (13). For assessment of fungal viability, whole eyes were homogenized under sterile conditions in 1 ml PBS, using the Mixer Mill MM300 (Retsch, Qiagen, Valencia, CA) at 33 Hz for 4 min. Subsequently, serial log dilutions were performed and plated onto bacteriologic grade Sabouraud dextrose agar plates (Becton Dickenson). A.fumigatus was cultured at 37°C and F.oxysporum was cultured at 34°C. The number of colony forming units (CFU) was determined by direct counting.
Cytokine quantification by ELISA
For cytokine production, corneas were dissected and placed in 0.5ml reagent diluent and homogenized using a Retsch MM 300 ball miller at 33 Hz for 4 min (Qiagen). Soluble protein extracts were then diluted and cytokine production was measured by ELISA according to the manufacturer’s directions (R & D Systems, Minneapolis, MN).
Flow cytometry
Corneas were carefully dissected and incubated for one hour at 37°C in 80 units of collaganese (C0130, Sigma-Aldrich), and washed in FACS buffer (PBS + 1% FBS + 0.5% Na azide). Spleens were removed and a single cell suspension was generated and incubated in erythrocyte lysis buffer (eBioscience) for five minutes at 37°C. Cells were incubated with anti-mouse CD16/32 antibody (Fc block, clone 93 eBioscience), washed and incubated with anti-mouse NIMP-R14 antibody, or anti-mouse CD4 antibody (eBioscience.) For intracellular staining, cells from ten corneas were pooled and incubated in fixation buffer (eBioscience), resuspended in permeabilization buffer (eBioscience), incubated in Fc block, and stained with anti-mouse IL-17A, or IFN–γ (eBioscience) conjugated antibodies and analyzed using an Accuri C6 flow cytometer (BD Bioscience). Cell populations were not gated, so that all cells were analyzed. Gates for positive antibody responses were determined based on isotype controls. To sort neutrophils in infected corneas, we used a FACSAria III (BD Bioscience) yielding >98% pure NIMP-R14+ neutrophils (which was also confirmed by Wrights-Giemsa staining, not shown).
Quantitative PCR
Neutrophil RNA was extracted using the RNAeasy mini kit according to manufacturer’s directions (Qiagen, Valencia, CA). Samples with an OD260/280 ratio of 2.0 were used to generate cDNA using the SuperScript First Strand synthesis system (Invitrogen), and quantitative PCR (qPCR) was performed using SYBR green (Applied Biosystems, Carlsbad, CA) as the detecting probe. (Il17a gene NM_010552 primers: 5′-TCAGCGTGTCCAAACACTGAG-3′, 3′-CGCCAAGGGAGTTAAAGACTT-5′). Quantitative PCR products were also detected by agarose gel electrophoresis and compared to Actb (which encodes the β-actin gene) as the loading control.
IL-17 and IFN–γneutralization in vivo
Mice were given a single injection of 25 μg of mouse anti-IL-17A (R&D) or 200μg (2 × 104 neutralizing units) anti-IFN–γ(R&D) in 5μl PBS into the conjunctival sac 3h prior to corneal infection. Antibodies diffuse into the corneal stroma and have neutralizing activity, which we confirmed by cytokine analysis of infected corneas.
In vivo neutrophil depletion
To deplete neutrophils systemically, 200 μg rat anti-mouse NIMP-R14 in 200 μl PBS (prepared in-house) or rat IgG (ctrl) was injected into the intraperitoneal cavity. After 24 hours, the number of bone marrow neutrophils was examined by flow cytometry.
In vitro chemokine production
The MK/T-1 murine corneal fibroblast cell line was maintained in DMEM-low glucose media containing 10% FBS and 50 μg/ml hygromycin (Invitrogen) at 37°C as described (20, 21), and cells were harvested when 70% confluent. Bone marrow derived macrophages were obtained from naïve C57BL/6 mice as described (12). Briefly, total bone marrow cells were collected from the femurs and tibias of mice, incubated in erythrocyte lysis buffer (eBioscience), and cultured in a 6ml bacteriologic-grade Petri dish containing Macrophage Growth Media (DMEM with L-glutamine, Na-pyruvate, HEPES, penicillin/streptomycin, 10% FBS, and 30% L929 cell-conditioned media) at 37°C. Growth medium was replaced on day 5 and adherent cells were isolated on day 7. MK/T-1 cells and macrophages were then cultured with media alone, or media containing100 μg/ml Aspergillus hyphal extract, 10ng/ml of recombinant mouse IL-17A (R&D), or both for three hours at 37°C. Supernatants were collected and chemokines were analyzed by ELISA.
Statistical analysis
Statistical analysis was performed for each experiment using an unpaired t-test or Tukey’s one-way ANOVA analysis (Prism, GraphPad Software). A p-value <0.05 was considered significant.
RESULTS
Protective immunity in Fusarium and Aspergillus keratitis is associated with elevated local and systemic IL-17 and IFN-γ
As IL-17A and IFN-γ are elevated in corneas of Fusarium and Aspergillus infected patients (17), we determined if these cytokines are generated in immunized mice. C57BL/6 mice were immunized with heat-killed, swollen conidia by the intratracheal or subcutaneous routes, infected intrastromally with live Fusarium and Aspergillus conidia, and cytokines were examined.
Splenocytes from immunized and unimmunized mice were incubated 3h with soluble hyphal extracts from Aspergillus (AspHE) or Fusarium (FusHE), and cytokines were measured by ELISA. As shown in Figure 1a, both cytokines were elevated in FusHE or AspHE stimulated splenocytes from C57BL/6 mice immunized by either the intratracheal or subcutaneous routes, but not from unimmunized mice. Th17 and Th1 cells were also detected in the spleens of immunized mice 13 days after the initial immunization (Supplemental Figure S1). These cells also expressed cell surface CD44 (Supplemental Figure S1), which together with cytokine production, is consistent with a peripheral memory phenotype (22). To examine cytokine production during corneal infection, corneas were dissected and homogenized, and IL-17A and IFN-γ in the soluble fractions were measured by ELISA. Figure 1b shows that production of IL-17A was maximal at 24h and 48h, but was lower after 72h. In contrast, IFN-γ was elevated in immunized mice at 48h and further increased at 72h post-infection. Neither cytokine was detected in infected corneas from unimmunized mice.
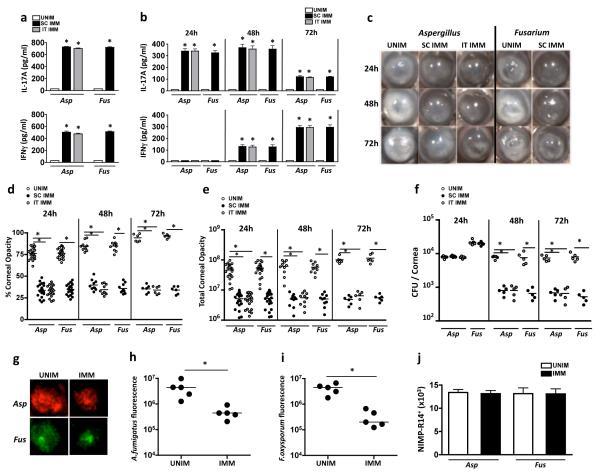
Figure 1. IL-17A (IL-17) and IFN-γ production in Aspergillus and Fusarium keratitis.
(a) IL-17 and IFN-γ production by splenocytes recovered from C57BL/6 mice 10 d after subcutaneous (SC IMM) or intratracheal (IT IMM) injection of heat-killed, swollen Aspergillus fumigatus (Asp) or Fusarium oxysporum (Fus) conidia (UNIM: splenocytes from naïve, unimmunized mice). IL-17 and IFN-γ in splenocyte supernatants were quantified by ELISA after 3h stimulation with Aspergillus or Fusarium hyphal extracts. (b) Aspergillus and Fusarium infected corneas were dissected and homogenized, and total IL-17 and IFN-γ was measured. (c) Representative infected corneas in unimmunized and immunized, mice; original magnification, 20×. (d, e) Percent and total corneal opacity quantified by image analysis. (f) Viable fungi in homogenized eyes quantified by CFU. (g) Representative corneas showing dsRed expressing A. fumigatus and GFP expressing F. oxysporum 24h post infection. (h, i) Quantification of total RFP and GFP as a measure of total fungal mass in infected corneas. (j) Total NIMP-R14+ neutrophils in collagenase digested corneas. (a,b,j) mean +/− SD of five mice per group. (d-f, h-i) Data points represent individual corneas. *p<0.01, by student’s t-test. Data are representative of three experiments with similar results.
Corneal opacity in Fusarium and Aspergillus infected mice are shown in Figure 1c. As we reported earlier (13, 14), naïve C57BL/6 mice receiving live Fusarium or Aspergillus conidia develop pronounced corneal opacification over 72h. In marked contrast, infected corneas of mice immunized by either the intratracheal or subcutaneous routes had less corneal disease. Total and percent corneal opacification was quantified by image analysis of infected corneas as described previously (13) and in Supplemental Figure S2. There was significantly less total and percent corneal opacity at each time point in infected mice that had been immunized by either route compared with unimmunized mice, with no differences between intratracheal and subcutaneous immunization (Figure 1d, e). Consistent with less severe corneal disease, significantly lower CFU values were observed in immunized compared with unimmunized mice at 48h and 72h, but not 24h (Figure 1f).
We, and others reported that measuring CFU in filamentous fungi does not necessarily reflect total fungal mass as a single CFU can represent hyphae of different lengths (13, 23), we infected mice with a dsRed expressing A. fumigatus or F. oxysporum expressing GFP strain, and quantified fungal mass by image analysis as before (13, 16). Aspergillus and Fusarium hyphae were present throughout the corneas of unimmunized mice at 24h post infection (Figure 1g). In contrast, there was significantly less RFP Aspergillus and GFP Fusarium fungal mass in the corneas of immunized mice (Figure 1 g-i). To determine if the enhanced fungal killing was due to increased numbers of infiltrating neutrophils, infected corneas were collaganese digested, cells were incubated with NIMP-R14, and the total number of neutrophils in each cornea was assessed by flow cytometry. However, as shown in Figure 1j, there was no significant difference in the number of infiltrating neutrophils recovered from corneas of unimmunized compared with immunized mice infected with either Aspergillus or Fusarium.
Together, these data show that intra-tracheal or subcutaneous immunization results in enhanced fungal clearance and elevated local and systemic IL-17A and IFN-γ, but not increased neutrophil infiltration.
Temporal recruitment of Th17 and Th1 cells to Aspergillus and Fusarium infected corneas
Given that CD4 cells are present and IL-17A and IFN-γ are expressed in Aspergillus and Fusarium infected human corneas (17), we examined Th17 and Th1 cell infiltration in Aspergillus and Fusarium infected corneas. Although there is a CD4 −, IL-17+ population 24h post-infection, Th17 (CD4+, IL-17+) cells were not detected at this time, but were present 48h and 72h post-infection, where they represented ~85% and ~35%, respectively of total CD4 cells in the cornea (Figure 2a). In contrast, Th1 (CD4+, IFN-γ+) cells were not detected until 72h post-infection (Figure 2a). Although there were fewer Th17 cells at 72h compared with 48h, the mean fluorescence intensity of IL-17 appeared higher at the later time point. CD4 cells were not detected in corneas of unimmunized mice at any time (data not shown).
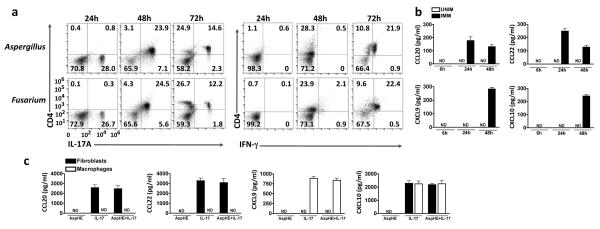
Figure 2. Recruitment of Th1 and Th17 cells to infected corneas.
(a) IL-17 (left) and IFN-γ (right) expressing CD4+ cells in A. fumigatus and F. oxysporum infected corneas from immunized C57BL/6 mice; corneas were examined 24h, 48h and 72h after infection. (b) CCL20 and CCL22 (Th17 recruiting chemokines), and CXCL9 and CXCL10 (Th1 recruiting chemokines) in A. fumigatus infected corneas of unimmunized (UNIM) and immunized (IMM) mice. (c) T cell chemokines in culture supernatants of murine corneal fibroblasts and bone marrow derived macrophages stimulated for 3h with AspHE, recombinant mouse IL-17, or both. Data are representative of two experiments (mean and s.d. in b,c).
To determine if the apparent biphasic recruitment of Th17 and Th1 cells in infected corneas is related to chemokine production, corneas were dissected and homogenized 6h, 24h, and 48h post-infection, and T cell chemokines were measured by ELISA. Figure 2b shows elevated production of CCL20 and CCL22, which are specific for Th17 cells, at 24h post infection. Conversely, CXCL9 and CXCL10, which recruit Th1 cells, were elevated 48h after infection. T cell chemokines were not detected in unimmunized, infected mice at any time point. To determine the source of T cell chemokines, mouse cornea fibroblasts and bone marrow derived macrophages were stimulated in vitro with Aspergillus hyphal extract and/or recombinant mouse for 3h and supernatants were assayed for T cell recruiting chemokines by ELISA. Figure 2c shows IL-17 – stimulated mouse corneal fibroblasts selectively produced CXCL10, CCL20 and CCL22, whereas IL-17 – stimulated macrophages produced CXCL9 and CXCL10; however, Aspergillus hyphal extract did not induce production of these chemokines in either cell type.
IL-17 producing neutrophils are recruited to infected corneas prior to T cells
As neutrophils are recruited to the cornea within hours of infection with Aspergillus or Fusarium (12-14), we next ascertained if the CD4 negative, IL-17+ cells in corneas of immunized mice 24h post-infection were neutrophils. Total cells from infected corneas were therefore incubated with the Ly6G specific NIMP-R14 antibody, and the presence of intracellular IL-17A in these cells was assessed by flow cytometry. As shown in Figure 3a, although there was a distinct population of NIMP-R14+ cells in unimmunized, infected corneas, these were IL-17A negative; in contrast, 16.7-19.6% total corneal cells from immunized, infected mice were NIMP-R14+ / IL-17A+, which is ~46% of the total NIMP-R14+ cells in the cornea. At this time point, all the IL-17A producing cells in infected corneas were NIMPR-14+ (Figure 3a), with no detectable NK1.1+ cells or γδ T cells (Supplemental Figure S3). There was no significant difference between immunized and unimmunized mice in either the total number of neutrophils in infected corneas (Figure 3b), or in total CXCL1 and CXCL2 (Figure 3c). CXCL1 and CXCL2 were produced by Aspergillus hyphal extract (AspHE) and / or rIL-17 stimulated macrophages, whereas corneal fibroblasts produced only CXCL1 (Figure 3d). No chemokines were detected in unstimulated cells.
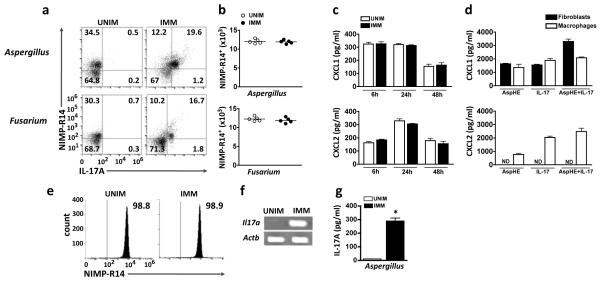
Figure 3. Recruitment of IL-17 producing neutrophils to infected corneas.
(a) Intracellular IL-17 in NIMP-R14+ neutrophils 24h after Aspergillus and Fusarium corneal infection of unimmunized and immunized C57BL/6 mice. (b) Total number of NIMP-R14+ neutrophils per cornea quantified by flow cytometry. Data points represent individual corneas. (c) CXCL1 and CXCL2 production in infected corneas. (d) Chemokine production by corneal fibroblasts and bone marrow macrophages after 3h stimulation with AspHE, recombinant mouse IL-17, or both. (e) Cell sorted NIMP-R14+ neutrophils 24h after A. fumigatus corneal infection of unimmunized (UNIM) and immunized (IMM) C57BL/6 mice. (f) Il17a gene expression, and (g) total IL-17 protein in cell lysates from cell sorted neutrophils from infected corneas 24h after Aspergillus infection. Actb (which encodes β-actin) serves as a loading control. *p<0.001 (unpaired Student’s t-test). Data are representative of two experiments with similar results, with five mice per group (c,d,g: mean and s.d.).
To examine if neutrophils in infected corneas also express Il17a transcripts, cells from infected corneas were tagged with NIMP-R14, isolated by fluorescence cell sorting, and Il17a transcripts were identified by quantitative PCR. Figure 3e shows a highly purified population of cell-sorted neutrophils from immunized and unimmunized mice; however, Il17a gene expression was only detected in neutrophils from immunized, infected mice (Figure 3f). Similarly, total IL-17A protein was detected in a cell lysates of flow sorted corneal neutrophils from immunized, but not unimmunized mice (Figure 3g).
These findings demonstrate that neutrophils are an early source of IL-17A in fungal infected corneas of immunized mice.
IL-17 but not IFN-γ regulates protective immunity in fungal keratitis
To determine the relative contribution of IL-17A and IFN-γ in the protective immune response to fungal keratitis, C57BL/6 mice were immunized subcutaneously, and neutralizing antibodies to IL-17A or IFN-γ were injected into the conjunctiva two hours prior to infection with live Aspergillus or Fusarium conidia. We found that infected corneas from mice given neutralizing antibodies had no detectable IL-17A (Figure 4a). Further, anti-IL-17A treated C57BL/6 mice exhibited significantly elevated corneal opacity, similar to unimmunized mice (Figure 4b, Supplemental Figure S4a). CFU were also significantly higher in mice receiving anti-IL-17A compared with mice given control IgG (Figure 4c).
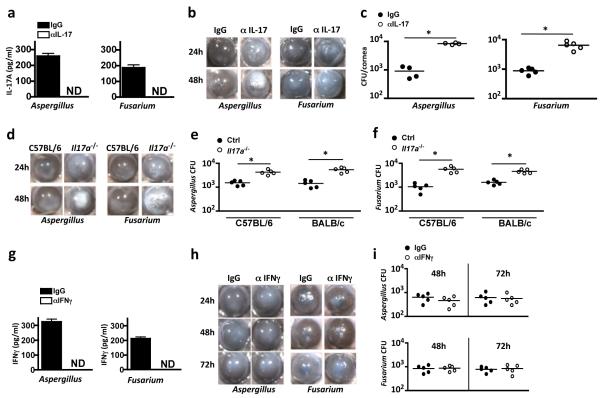
Figure 4. The role of IFN-γ and IL-17 in fungal keratitis in immunized mice a-f: Effect of IL-17 neutralization or deletion on fungal keratitis.
Immunized C57BL/6 mice were given a sub-conjunctival injection of neutralizing anti-IL-17 or isotype (IgG) control one day prior to Aspergillus or Fusarium corneal infection, and examined 24h post infection. (a) IL-17 in homogenized corneas showing effective antibody neutralization. (b-c) Representative corneas from immunized mice given anti-IL-17 (αIL-17) or control antibodies (IgG) 24h and 48h post infection (b) (quantification is shown in Supplementary Figure S4). (c) CFU quantification 48h post infection. (d) Representative corneas from immunized C57BL/6 and Il17a−/− mice at indicated time points after Aspergillus or Fusarium cornea infection. (e-f) CFU quantification of corneas from immunized Il17a−/− mice on C57BL/6 background with C57BL/6 controls (left) or Il17a−/− mice on BALB/c background with BALB/c controls (right), 48h after Aspergillus (e) or Fusarium (f) infection. g-i: IFNγ neutralization. (g) IFN-γ in homogenized corneas from immunized mice showing effective antibody neutralization. (h) Representative corneas from immunized mice given anti-IFN-γ (αIFNγ) or control antibodies (IgG). (i) CFU in corneas of Aspergillus (upper panel) or Fusarium (lower panel) infected corneas at indicated time points. c,e,f,i: Data points represent individual corneas; a,g: data are mean and s.d. of five mice per group; *p<0.01 (unpaired Student’s t-test). Data are representative of three repeat experiments with five mice per group.
As a second approach to determine the role of IL-17 in fungal keratitis, Il17a−/− mice were immunized and infected with Aspergillus or Fusarium conidia. Il17a−/− mice exhibited significantly elevated corneal opacity (Figure 4d, Supplemental Figure S4b), similar to the effect of IL-17A neutralizing antibody. CFU were also significantly elevated in infected Il17a−/− mice on either a C57BL/6 or a BALB/c background (Figure 4e, f).
In contrast, although IFN-γ was not detected in infected mice given a sub-conjunctival injection of anti-IFN-γ (Figure 4g), there was no significant difference in corneal opacity between mice given neutralizing IFN-γ and those given control IgG (Figure 4h and Supplemental Figure S4c, d). There was also no significant difference in CFU at 48h or 72h after Aspergillus or Fusarium infection (Figure 4i). Taken together, these data show that IL-17A but not IFN-γ is required for the protective immune response in fungal keratitis.
Immunized Rag2−/− mice exhibit intermediate protective immunity in fungal keratitis
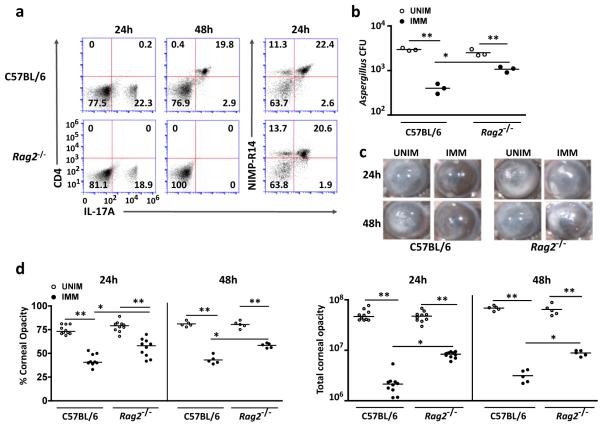
To examine the role of T cells in IL-17 – dependent protective immunity, Rag2−/− mice, which have no functional T or B cells, were immunized subcutaneously and infected intrastromally with A. fumigatus. At 48h post infection, Th17 cells (CD4+ IL-17+) were present in C57BL/6, but not in Rag2−/− corneas; however, there was no significant difference in the number of IL-17A+ neutrophils in infected corneas of Rag2−/− compared to C57BL/6 mice (Figure 5a).
Figure 5. The role of Th17 cells in protective immunity to fungal infection.
(a) Intracellular IL-17 in CD4+ cells and NIMP-R14+ neutrophils from infected corneas of immunized C57BL/6 and Rag2−/− mice 24h and 48h after Aspergillus infection. (b) Quantification of CFU in corneas from unimmunized and immunized C57BL/6 and Rag2−/− mice 48h after Aspergillus infection. (c) Representative corneas from Aspergillus infected unimmunized (UNIM) and immunized (IMM) C57BL/6 and Rag2−/− mice 24h and 48h post-infection. (d) Total and percent corneal opacity quantified 24h and 48h after Aspergillus infection. (a) Representative plots are shown. (b,d) Data points represent individual corneas. *p<0.01, **p<0.001 (unpaired Student’s t-test). Data are representative of three experiments, with ten mice per group.
There were significantly less CFU in immunized C57BL/6 and Rag2−/− mice compared with unimmunized counterparts, indicating enhanced killing activity in the absence of T cells (Figure 5b); however, protection was only partial as CFU recovered from immunized, infected Rag2−/− corneas was significantly higher than immunized C57BL/6. Total and percent corneal opacity were also significantly higher in immunized, infected Rag2−/− compared with C57BL/6 mice, consistent with the impaired fungal clearance (Figure 5c,d).
Taken together, these finding demonstrate that immunized, infected Rag2−/− mice have an intermediate phenotype between unimmunized and immunized C57BL/6 mice, and also indicate that Th17 cells have a significant role in fungal keratitis.
Depletion of neutrophils ablates protective immunity in fungal keratitis
To determine the role of neutrophils in fungal keratitis in immunized mice, neutrophils were depleted by intra-peritoneal injection of 200 μg anti-NIMP-R14 after immunization and 24h prior to corneal infection. We found that anti-NIMP-R14 treated mice had no detectable neutrophils in the bone marrow (Figure 6a); further, IL-17A production in Fusarium or Aspergillus infected corneas was undetectable in neutrophil depleted compared with mice given control IgG (Figure 6b), consistent with neutrophils being the predominant source of IL-17A at this time point. Further, CFU were significantly higher in neutrophil - depleted mice compared with mice given control IgG (Figure 6c), and the percent and total corneal opacity was significantly higher, (Figure 6d, and Supplemental Figure S4e).
Figure 6. Role of neutrophils in IL-17 production and on Aspergillus and Fusarium keratitis.
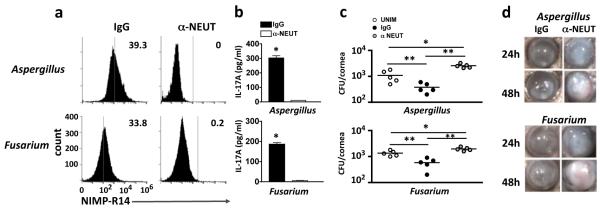
(a) Flow cytometry showing the absence of neutrophils in total bone marrow cells recovered from immunized C57BL/6 mice 48h after intraperitoneal injection of 200 μg NIMP-R14 (α-NEUT) or isotype IgG control (IgG). (b) Concentration of IL-17 in corneal lysates obtained from immunized C57BL/6 mice 24h after Aspergillus (upper panel) or Fusarium (lower panel) infection (data are mean and s.d. of 5 mice per group). (c) CFU of Aspergillus (upper panel) and Fusarium (lower panel) infected corneas from neutrophil depleted (αNEUT) immunized mice 48h post infection. Data points represent individual corneas. (d) Representative corneas from immunized, neutrophil depleted (αNEUT) C57BL/6 mice 24h and 48h post infection. *p<0.05, **p<0.001 (unpaired Student’s t-test). Data are representative of two experiments with similar results, with five mice per group.
These data clearly demonstrate that in immunized mice, neutrophils are the major source of IL-17A 24h after corneal infection with Fusarium or Aspergillus, and that neutrophils have an essential role in limiting fungal growth and corneal disease.
DISCUSSION
Filamentous moulds, which are the major worldwide cause of fungal keratitis, are ubiquitous in the environment, and conidia are present in the air we breathe. The report that Aspergillus specific T cell IFN-γ responses can be detected in healthy individuals demonstrates that we can respond to airborne spores in the absence of disease manifestations (24). Based on this finding and on reports that the concentration of spores in the air can reach very high levels in some environments (25, 26), a report by Karthikeyan and colleagues examined the possibility that T cell responses contribute to the outcome of fungal keratitis in regions with high airborne spore counts (17). As the prevalence of fungal keratitis is very high in rural India and China, and the incidence increases further during harvest seasons (6, 7, 27), the study by Karthikeyan and colleagues showed evidence of T cell involvement in infected corneas from patients in southern India who had undergone corneal transplantation (17). CD3+ and CD4+ cells were detected in Aspergillus and Fusarium infected corneas, which also express elevated IFN-γ and IL-17A compared with uninfected corneas, whereas IL-4 expression was low (17). Although non-CD4 cells can also produce IL-17 (28), these observations are consistent with the presence of Th1 and Th17 cells.
To examine the role of IL-17 and IFN-γ in fungal keratitis, we developed a murine model in which Th17 and Th1 cells are induced systemically by either intratracheal or subcutaneous immunization with heat-killed, swollen conidia. As with human corneas, CD4 cells, IL-17A and IFN-γ were detected in Aspergillus and Fusarium infected mouse corneas. However, we extended the human studies by showing that although Th1 and Th17 cells are generated systemically following immunization, Th17 cells are recruited to infected corneas earlier than Th1 cells, consistent with production of the Th17 chemokines CCL20 and CCL22, which were detected prior to the Th1 chemokines CXCL9 and CXCL10 and produced by corneal fibroblasts in response to IL-17. We also found that CD4 cells are not the only source of IL-17 in fungal keratitis, as approximately 50% total neutrophils recruited to the cornea 24h post-infection expressed intracellular IL-17A, and were the predominant source of IL-17A at this time point. The number of neutrophils in infected corneas of immunized mice was the same as unimmunized mice, indicating that immunization does not affect neutrophil recruitment. Consistent with this finding, we found elevated production of neutrophil chemokines CXCL1 and CXCL2 in both groups of animals and were produced by corneal fibroblasts and macrophages. Neutrophils also produce CXC chemokines (29).
Although IFN-γ was produced in infected corneas, there was no effect of IFN-γ neutralization on either CFU or corneal disease. In contrast, neutralization or deletion of IL-17A ablated the protective immune response, indicating an essential role for this cytokine in regulating fungal growth. Depletion of neutrophils or eliminating lymphoid cells (T cells and γδ T cells) as a source of IL-17 by using Rag2−/− mice each impaired fungal killing, indicating an essential role for both cell types.
Individuals who have impaired IL-17 responses due to production of autoantibodies to IL-17 are more susceptible to fungal infections, especially mucosal candidiasis (30, 31). Similarly, mutations in STAT3, which is required for IL-17 production result in increased susceptibility to Candida infections (32, 33). Murine models of oral candidiasis also show a requirement for IL-17, with the cell sources being Th17 and innate lymphoid cells (34-36). Th17 cells and neutrophils were also found to be a source of IL-17 in a model of pulmonary aspergillosis and systemic histoplasmosis (23, 37, 38), and IL-17 producing neutrophils were reported in models of Bacillus anthracis and Yersinia pestis infection, and LPS induced lung inflammation (39-41). In addition to microbial infections, IL-17 producing neutrophils have been reported in murine models of kidney ischemia reperfusion, and early stage collagen induced arthritis, and in human psoriasis tissue and T cell lymphomas (42-45). Taken together, these reports not only support our current findings, but also indicate that IL-17 producing neutrophils are involved in multiple infectious and inflammatory diseases. Although T cells reportedly contribute to IL-17 production by neutrophils in pulmonary aspergillosis (38), the current study clearly shows IL-17 producing neutrophils in Rag2−/− mice, indicating that T cells are not required. IL-23 has been implicated in IL-17 production by neutrophils (38, 46); however, our recent studies show that in addition to IL-23, IL-6 is required for IL-17A production, and that these cytokines also induce expression of a functional IL-17 receptor (47).
IL-17 also mediates corneal infections with herpes simplex virus, Pseudomonas aeruginosa, and Candida albicans (48-50). The latter study shows neutrophils and CD4 cells as a source of IL-17 during Candida infection (50), which is consistent with our findings; however, in marked contrast to results presented here, these investigators reported increased fungal killing and less corneal disease in the absence of CD4 cells or IL-17. The differences between their study and ours may relate to infection with Candida compared with Aspergillus or Fusarium, or to differences between the models as they examined unimmunized IL-17A−/− mice on a BALB/c background. However, although this group had reported differences between BALB/c and C57BL/6 mice (51), we show in the current study that C57BL/6/IL-17A−/− and BALB/c/IL-17A−/− both exhibited impaired fungal clearance compared with the parent strains. The difference between our results and theirs has therefore yet to be determined.
A broadly accepted role for IL-17 in infection and autoimmunity is that IL-17 produced by Th17 and innate lymphoid cells such as NK-T cells and γδ T cells activate fibroblasts and epithelial cells, which constitutively express the IL-17RA and IL-17RC subunits (52). IL-17A then induces production of pro-inflammatory and chemotactic cytokines, which mediate neutrophil recruitment, resulting in microbial killing and tissue damage. In fungal keratitis, IL-17 produced by neutrophils and Th17 cells likely activates both these mechanisms; however, we reported that IL-17 producing neutrophils also express functional IL-17 receptors, and showed that IL-17 producing neutrophils generate more ROS and have increased fungal killing activity than their IL-17-negative counterparts (47). Therefore IL-17 produced by neutrophils and by Th17 cells can contribute to the increased ROS production and fungal killing by these cells.
IL-17 has multiple functions in inflammation, including inducing lymphatic endothelial cells to produce VEGF-D, and IL-17 can stimulate lymphangiogenesis in the normally avascular cornea (53). This mechanism could also contribute to the pathogenesis of fungal keratitis by promoting cellular infiltration to the corneal stroma. Future studies will examine the role of IL-17 in promoting inflammation that leads both to microbial clearance and to IL-17 associated tissue damage in the cornea and in other tissues in which IL-17 regulates the severity of disease.
Supplementary Material
Acknowledgements
The authors gratefully acknowledge the outstanding technical assistance of the core modules of the Visual Sciences Research Center core facilities, including Scott Howell, Denice Major and Dawn Smith.
This work was supported by NIH grants F32EY022278 (PRT), F31EY019841 (SML), RO1 EY018612 (EP), P30 EY011373 (EP), and by the Research to Prevent Blindness Foundation and the Ohio Lions Eye Research Foundation (EP).
REFERENCES
- 1.Barnes PD, Marr KA. Risks, diagnosis and outcomes of invasive fungal infections in haematopoietic stem cell transplant recipients. Br J Haematol. 2007;139:519–531. doi: 10.1111/j.1365-2141.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 2.Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J. 2007;30:782–800. doi: 10.1183/09031936.00062206. [DOI] [PubMed] [Google Scholar]
- 3.Boutati EI, Anaissie EJ. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years’ experience at a cancer center and implications for management. Blood. 1997;90:999–1008. [PubMed] [Google Scholar]
- 4.Carneiro HA, Coleman JJ, Restrepo A, Mylonakis E. Fusarium infection in lung transplant patients: report of 6 cases and review of the literature. Medicine (Baltimore) 2011;90:69–80. doi: 10.1097/MD.0b013e318207612d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson GR, 3rd, Patterson TF. Pulmonary aspergillosis. Semin Respir Crit Care Med. 2008;29:103–110. doi: 10.1055/s-2008-1063849. [DOI] [PubMed] [Google Scholar]
- 6.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–69. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 7.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–1948. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Leck AK, Thomas PA, Hagan M, Kaliamurthy J, Ackuaku E, John M, Newman MJ, Codjoe FS, Opintan JA, Kalavathy CM, Essuman V, Jesudasan CA, Johnson GJ. Aetiology of suppurative corneal ulcers in Ghana and south India, and epidemiology of fungal keratitis. Br J Ophthalmol. 2002;86:1211–1215. doi: 10.1136/bjo.86.11.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang DC, Grant GB, O’Donnell K, Wannemuehler KA, Noble-Wang J, Rao CY, Jacobson LM, Crowell CS, Sneed RS, Lewis FM, Schaffzin JK, Kainer MA, Genese CA, Alfonso EC, Jones DB, Srinivasan A, Fridkin SK, Park BJ. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–963. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 10.Gaujoux T, Chatel MA, Chaumeil C, Laroche L, Borderie VM. Outbreak of contact lens-related Fusarium keratitis in France. Cornea. 2008;27:1018–1021. doi: 10.1097/ICO.0b013e318173144d. [DOI] [PubMed] [Google Scholar]
- 11.Khor WB, Aung T, Saw SM, Wong TY, Tambyah PA, Tan AL, Beuerman R, Lim L, Chan WK, Heng WJ, Lim J, Loh RS, Lee SB, Tan DT. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA. 2006;295:2867–2873. doi: 10.1001/jama.295.24.2867. [DOI] [PubMed] [Google Scholar]
- 12.Carrion Sde J, Leal SM, Jr., Ghannoum MA, Aimanianda V, Latge JP, Pearlman E. The RodA hydrophobin on Aspergillus fumigatus spores masks dectin-1- and dectin-2-dependent responses and enhances fungal survival in vivo. J Immunol. 2013;191:2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leal SM, Jr., Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tarabishy AB, Aldabagh B, Sun Y, Imamura Y, Mukherjee PK, Lass JH, Ghannoum MA, Pearlman E. MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J Immunol. 2008;181:593–600. doi: 10.4049/jimmunol.181.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leal SM, Jr., Roy S, Vareechon C, Carrion SD, Clark H, Lopez-Berges MS, Dipietro A, Schrettl M, Beckmann N, Redl B, Haas H, Pearlman E. Targeting Iron Acquisition Blocks Infection with the Fungal Pathogens Aspergillus fumigatus and Fusarium oxysporum. PLoS Pathog. 2013;9:e1003436. doi: 10.1371/journal.ppat.1003436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leal SM, Jr., Vareechon C, Cowden S, Cobb BA, Latge JP, Momany M, Pearlman E. Fungal antioxidant pathways promote survival against neutrophils during infection. J Clin Invest. 2012;122:2482–2498. doi: 10.1172/JCI63239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karthikeyan RS, Leal SM, Jr., Prajna NV, Dharmalingam K, Geiser DM, Pearlman E, Lalitha P. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or fusarium. J Infect Dis. 2011;204:942–950. doi: 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Karmakar M, Roy S, Ramadan RT, Williams SR, Howell S, Shive CL, Han Y, Stopford CM, Rietsch A, Pearlman E. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–4283. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gabrilovich MI, Walrath J, van Lunteren J, Nethery D, Seifu M, Kern JA, Harding CV, Tuscano L, Lee H, Williams SD, Mackay W, Tomashefski JF, Jr., Silver RF. Disordered Toll-like receptor 2 responses in the pathogenesis of pulmonary sarcoidosis. Clin Exp Immunol. 2013;173:512–522. doi: 10.1111/cei.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gendron RL, Liu CY, Paradis H, Adams LC, Kao WW. MK/T-1, an immortalized fibroblast cell line derived using cultures of mouse corneal stroma. Mol Vis. 2001;7:107–113. [PubMed] [Google Scholar]
- 21.Lin M, Carlson E, Diaconu E, Pearlman E. CXCL1/KC and CXCL5/LIX are selectively produced by corneal fibroblasts and mediate neutrophil infiltration to the corneal stroma in LPS keratitis. J Leukoc Biol. 2007;81:786–792. doi: 10.1189/jlb.0806502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sprent J, Surh CD. T cell memory. Annu Rev Immunol. 2002;20:551–579. doi: 10.1146/annurev.immunol.20.100101.151926. [DOI] [PubMed] [Google Scholar]
- 23.Werner JL, Metz AE, Horn D, Schoeb TR, Hewitt MM, Schwiebert LM, Faro-Trindade I, Brown GD, Steele C. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–4946. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaudhary N, Staab JF, Marr KA. Healthy human T-Cell Responses to Aspergillus fumigatus antigens. PLoS One. 2010;5:e9036. doi: 10.1371/journal.pone.0009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oliveira M, Ribeiro H, Delgado JL, Abreu I. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. Int J Biometeorol. 2009;53:61–73. doi: 10.1007/s00484-008-0191-2. [DOI] [PubMed] [Google Scholar]
- 26.Oliveira M, Ribeiro H, Delgado L, Fonseca J, Castel-Branco MG, Abreu I. Outdoor allergenic fungal spores: comparison between an urban and a rural area in northern Portugal. J Investig Allergol Clin Immunol. 2010;20:117–128. [PubMed] [Google Scholar]
- 27.Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol. 2009;37:763–771. doi: 10.1111/j.1442-9071.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 28.Cua DJ, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat Rev Immunol. 2011;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 29.Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 30.Kisand K, Boe Wolff AS, Podkrajsek KT, Tserel L, Link M, Kisand KV, Ersvaer E, Perheentupa J, Erichsen MM, Bratanic N, Meloni A, Cetani F, Perniola R, Ergun-Longmire B, Maclaren N, Krohn KJ, Pura M, Schalke B, Strobel P, Leite MI, Battelino T, Husebye ES, Peterson P, Willcox N, Meager A. Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. J Exp Med. 2010;207:299–308. doi: 10.1084/jem.20091669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puel A, Doffinger R, Natividad A, Chrabieh M, Barcenas-Morales G, Picard C, Cobat A, Ouachee-Chardin M, Toulon A, Bustamante J, Al-Muhsen S, Al-Owain M, Arkwright PD, Costigan C, McConnell V, Cant AJ, Abinun M, Polak M, Bougneres PF, Kumararatne D, Marodi L, Nahum A, Roifman C, Blanche S, Fischer A, Bodemer C, Abel L, Lilic D, Casanova JL. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–297. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Beaucoudrey L, Puel A, Filipe-Santos O, Cobat A, Ghandil P, Chrabieh M, Feinberg J, von Bernuth H, Samarina A, Janniere L, Fieschi C, Stephan JL, Boileau C, Lyonnet S, Jondeau G, Cormier-Daire V, Le Merrer M, Hoarau C, Lebranchu Y, Lortholary O, Chandesris MO, Tron F, Gambineri E, Bianchi L, Rodriguez-Gallego C, Zitnik SE, Vasconcelos J, Guedes M, Vitor AB, Marodi L, Chapel H, Reid B, Roifman C, Nadal D, Reichenbach J, Caragol I, Garty BZ, Dogu F, Camcioglu Y, Gulle S, Sanal O, Fischer A, Abel L, Stockinger B, Picard C, Casanova JL. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–1550. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma CS, Chew GY, Simpson N, Priyadarshi A, Wong M, Grimbacher B, Fulcher DA, Tangye SG, Cook MC. Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. J Exp Med. 2008;205:1551–1557. doi: 10.1084/jem.20080218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez-Santos N, Huppler AR, Peterson AC, Khader SA, McKenna KC, Gaffen SL. Th17 cells confer long-term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol. 2012 doi: 10.1038/mi.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conti HR, Shen F, Nayyar N, Stocum E, Sun JN, Lindemann MJ, Ho AW, Hai JH, Yu JJ, Jung JW, Filler SG, Masso-Welch P, Edgerton M, Gaffen SL. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J Exp Med. 2009;206:299–311. doi: 10.1084/jem.20081463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zelante T, Bozza S, De Luca A, D’Angelo C, Bonifazi P, Moretti S, Giovannini G, Bistoni F, Romani L. Th17 cells in the setting of Aspergillus infection and pathology. Med Mycol. 2009;47(Suppl 1):S162–169. doi: 10.1080/13693780802140766. [DOI] [PubMed] [Google Scholar]
- 38.Werner JL, Gessner MA, Lilly LM, Nelson MP, Metz AE, Horn D, Dunaway CW, Deshane J, Chaplin DD, Weaver CT, Brown GD, Steele C. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect Immun. 2011;79:3966–3977. doi: 10.1128/IAI.05493-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garraud K, Cleret A, Mathieu J, Fiole D, Gauthier Y, Quesnel-Hellmann A, Tournier JN. Differential role of the interleukin-17 axis and neutrophils in resolution of inhalational anthrax. Infect Immun. 2012;80:131–142. doi: 10.1128/IAI.05988-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin JS, Kummer LW, Szaba FM, Smiley ST. IL-17 contributes to cell-mediated defense against pulmonary Yersinia pestis infection. J Immunol. 2014;186:1675–1684. doi: 10.4049/jimmunol.1003303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferretti S, Bonneau O, Dubois GR, Jones CE, Trifilieff A. IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol. 2003;170:2106–2112. doi: 10.4049/jimmunol.170.4.2106. [DOI] [PubMed] [Google Scholar]
- 42.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J Clin Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, Villanueva EC, Shah P, Kaplan MJ, Bruce AT. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Katayama M, Ohmura K, Yukawa N, Terao C, Hashimoto M, Yoshifuji H, Kawabata D, Fujii T, Iwakura Y, Mimori T. Neutrophils are essential as a source of IL-17 in the effector phase of arthritis. PLoS One. 2013;8:e62231. doi: 10.1371/journal.pone.0062231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontao L, Brembilla NC, Masouye I, Kaya G, Prins C, Dupin N, Saurat JH, Chizzolini C, Piguet V. Interleukin-17 expression in neutrophils and Th17 cells in cutaneous T-cell lymphoma associated with neutrophilic infiltrate of the skin. Br J Dermatol. 2011;166:687–689. doi: 10.1111/j.1365-2133.2011.10647.x. [DOI] [PubMed] [Google Scholar]
- 46.Wu SY, Yu JH, Liu FT, Miaw SC, Wu-Hsieh BA. Galectin-3 Negatively Regulates Dendritic Cell Production of IL-23/IL-17-Axis Cytokines in Infection by Histoplasma capsulatum. J Immunol. 2013 doi: 10.4049/jimmunol.1202122. [DOI] [PubMed] [Google Scholar]
- 47.Taylor PR, Roy S, Leal SM, Jr., Sun Y, Howell SJ, Cobb BA, Li X, Pearlman E. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2013 doi: 10.1038/ni.2797. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suryawanshi A, Cao Z, Thitiprasert T, Zaidi TS, Panjwani N. Galectin-1-mediated suppression of Pseudomonas aeruginosa-induced corneal immunopathology. J Immunol. 2013;190:6397–6409. doi: 10.4049/jimmunol.1203501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suryawanshi A, Veiga-Parga T, Rajasagi NK, Reddy PB, Sehrawat S, Sharma S, Rouse BT. Role of IL-17 and Th17 cells in herpes simplex virus-induced corneal immunopathology. J Immunol. 2011;187:1919–1930. doi: 10.4049/jimmunol.1100736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang H, Li H, Li Y, Zou Y, Dong X, Song W, Jia C, Li S, Xi H, Liu D, Wang Y. IL-17 plays a central role in initiating experimental Candida albicans infection in mouse corneas. Eur J Immunol. 2013 doi: 10.1002/eji.201242891. [DOI] [PubMed] [Google Scholar]
- 51.Zou Y, Zhang H, Li H, Chen H, Song W, Wang Y. Strain-dependent production of interleukin-17/interferon-gamma and matrix remodeling-associated genes in experimental Candida albicans keratitis. Mol Vis. 2012;18:1215–1225. [PMC free article] [PubMed] [Google Scholar]
- 52.Ho AW, Gaffen SL. IL-17RC: a partner in IL-17 signaling and beyond. Semin Immunopathol. 2010;32:33–42. doi: 10.1007/s00281-009-0185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, Dana R. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.