Abstract
Metabolic syndrome is defined as a cluster of glucose intolerance, hypertension, dyslipidemia and central obesity with insulin resistance as the source of pathogenesis. Although several different combinations of criteria have been used to define metabolic syndrome, a recently published consensus recommends the use of ethnic‐specific criteria, including waist circumference as an indicator of central obesity, triglyceride and high‐density lipoprotein (HDL) cholesterol as indicators of dyslipidemia, and blood pressure greater than 130/85 mmHg. The definition of dysglycemia, and whether central obesity and insulin resistance are essential components remain controversial. Regardless of the definition, the prevalence of metabolic syndrome is increasing in Western and Asian countries, particularly in developing areas undergoing rapid socioenvironmental changes. Numerous clinical trials have shown that metabolic syndrome is an important risk factor for cardiovascular disease (CVD), type 2 diabetes mellitus and all‐cause mortality. Therefore, metabolic syndrome might be useful as a practical tool to predict these two major metabolic disorders. Comprehensive management of risk factors is very important to the improvement of personal and public health. However, recent studies have focused on the role metabolic syndrome plays as a risk factor for CVD; its importance in the prediction of incident diabetes is frequently overlooked. In the present review, we summarize the known evidence supporting metabolic syndrome as a predictor for type 2 diabetes mellitus and CVD. Additionally, we suggest how metabolic syndrome might be useful in clinical practice, especially for the prediction of diabetes.
Keywords: Metabolic syndrome, Risk factor, Type 2 diabetes mellitus
Introduction
The clustering of glucose intolerance, hypertension, dyslipidemia and obesity, particularly central obesity, has been termed metabolic syndrome1. Ever since metabolic syndrome was described by Reaven4 in 1988, various definitions have been published and revised, and numerous studies have explored its pathophysiology. When the concept of metabolic syndrome was first proposed, the primary pathological process was believed to be insulin resistance or hyperinsulinemia5. In addition, many etiological factors have been linked to the development and progression of metabolic syndrome, including an altered inflammatory state9, visceral adipose tissue abnormalities11, and the activation of the sympathetic nervous system12.
Although metabolic syndrome contains several unresolved matters, including ambiguous criteria, the inclusion of diabetes, a unifying mechanism and its role as a ‘syndrome’13, its worldwide prevalence has increased rapidly into one of the biggest health problems. Metabolic syndrome is known to increase cardiovascular morbidity and mortality, type 2 diabetes, and all‐cause mortality14. The desired clinical response to metabolic syndrome is improved individual and national public health, and a mitigation of negative outcomes through comprehensive management.
Most studies agree that cardiovascular disease (CVD) is the major outcome of metabolic syndrome15. However, whether type 2 diabetes mellitus is also a major outcome of metabolic syndrome or one of its components is a topic of debate. Many reports have confirmed a strong relationship between metabolic syndrome and incident diabetes.
The present review describes various definitions and changes in the prevalence of the metabolic syndrome, and the significance of metabolic syndrome as a risk factor of type 2 diabetes mellitus and CVD. Finally, we propose the clinical usefulness inherent to metabolic syndrome, especially as a predictor of incident diabetes.
Various Definitions of the Metabolic Syndrome
Although most medical communities agree that obesity, hypertension, dyslipidemia and abnormal glucose tolerance should be factored into the diagnosis, no standard criteria have been set for metabolic syndrome. Several clinical definitions have been proposed and used in clinical practice (Table 1).
Table 1. Various definitions of metabolic syndrome.
| WHO (1998)1 | EGIR (1999)2 | NCEP‐ATP III (2001)3 | AACE (2003)18 | IDF (2005)20 | IDF (2009)21 | |
|---|---|---|---|---|---|---|
| Definition | Type 2 diabetes, IFG, IGT or insulin resistance plus at least two of the criteria below | Fasting hyperinsulinemia (highest 25%), plus at least two criteria below | At least three criteria below | Specific clinical factorsa plus at least two criteria below | Central obesity plus at least two criteria below | At least three criteria below |
| Glucose | IFG, IGT, type 2 diabetes | FPG ≥6.1 mmol/L (excludes diabetes) | FPG ≥110 mg/dL (includes diabetes; FPG ≥100 mg/dL, modified in 2006) | IFG (FPG 110–125 mg/dL) or IGT (excludes diabetes) | FPG ≥100 mg/dL (includes diabetes) | FPG ≥100 mg/dL (includes diabetes) |
| Abdominal obesity | WHR >0.9 in men and >0.85 in women or BMI >30 kg/m2 | WC ≥94 cm in men and ≥80 cm in women | WC >102 cm in men and >88 cm in women | BMI ≥25 kg/m2 | Ethnic‐specific definitionb | Population‐ and country‐specific definitionc |
| BP | BP ≥140/90 mmHg | BP ≥140/90 mmHg or treated for hypertension | BP ≥130/85 mmHg or treatedfor hypertension | BP ≥130/85 mmHg | BP ≥130/85 mmHg or treated for hypertension | BP ≥130/85 mmHg or treated for hypertension |
| TG | TG ≥150 mg/dL and/or HDL‐C <35 mg/dL in men | TG ≥150 mg/dL or HDL‐C <39 mg/dL or treated for dyslipidemia | TG ≥150 mg/dL or treated for dyslipidemia | TG >150 mg/dL | TG ≥150 mg/dL or treated for dyslipidemia | TG ≥150 mg/dL or treated for dyslipidemia |
| HDL‐C | And HDL‐C <39 mg/dL in women | HDL‐C <40 mg/dL in men or HDL‐C <50 mg/dL in women or treated for dyslipidemia | HDL‐C <40 mg/dL in men or HDL‐C <50 mg/dL in women | HDL‐C <40 mg/dL in men or HDL‐C <50 mg/dL in women or treated for dyslipidemia | HDL‐C <40 mg/dL in men or HDL‐C <50 mg/dL in women or treated for dyslipidemia | |
| Other | Microalbuminuria (UAER >20 μg/min) |
AACE, American Association of Clinical Endocrinologists; BMI, body mass index; BP, blood pressure; EGIR, European Group for the Study of Insulin Resistance; FPG, fasting plasma glucose; HDL‐C, high‐density lipoprotein cholesterol; IDF, International Diabetes Federation; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NCEP‐ATP III, National Cholesterol Education Program Adult Treatment Panel III; TG, triglyceride; UAER, urinary albumin excretion rate; WHO, World Health Organization; WHR, waist‐to‐hip ratio.
Diagnosis of cardiovascular diseases (CVD), hypertension, polycystic ovary syndrome, non‐alcoholic fatty liver disease, or acanthosis nigricans; family history of type 2 diabetes, hypertension or CVD; history of gestational diabetes or glucose intolerance; non‐Caucasian ethnicity; sedentary lifestyle; waist circumference (WC) >40 inches in men and WC >35 inches in women; age >40 years.
Europe, ≥94 cm in men and ≥80 cm in women; South Asian and Chinese, ≥90 cm in men and ≥80 cm in women; Japanese, ≥85 cm in men and ≥90 cm in women; South and Central America, South Asian recommendations until more specific data become available; Sub‐Saharan Africa, Eastern Mediterranean and Middle East populations, European data until more specific data becomes available.
WC thresholds are recommended based on organization and risk of metabolic complications.
The first formalized definition of metabolic syndrome was introduced in 1998 by a group who was consulted by the World Health Organization (WHO) for a definition of diabetes1. The diagnostic criteria included markers of abnormal glucose metabolism or insulin resistance, plus at least two out of four risk factors, which included obesity, hypertension, elevated triglycerides and/or reduced high‐density lipoprotein (HDL) cholesterol, and microalbuminuria. Insulin resistance, as measured by the homeostasis model assessment of insulin resistance (HOMA‐IR) or the euglycemic hyperinsulinemic clamp technique, is a key factor of the WHO diagnostic criteria that does not exclude type 2 diabetes mellitus. The diagnostic criteria posed by the European Group for the Study of Insulin Resistance (EGIR) in 19992 and by American Association of Clinical Endocrinologists (AACE) in 200318 also emphasized the presence of insulin resistance. In the three aforementioned definitions listed, both impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) were noted as markers of abnormal glucose metabolism. However, in contrast to the WHO definition, patients with type 2 diabetes were not included in the EGIR and AACE criteria.
The most commonly used criteria emerged from the National Cholesterol Education Program Adult Treatment Panel III (NCEP‐ATP III) in 20013. The presence of three of the five risk factors warrants a metabolic syndrome diagnosis. Under the direction of the American Diabetes Association (ADA), the definition of dysglycemic factor was changed from a fasting plasma glucose (FPG) higher than 110 mg/dL in 2001 to a FPG higher than 100 mg/dL in 200619. The International Diabetes Federation (IDF), American Heart Association (AHA), and National Heart, Lung, and Blood Institute (NHLBI) define metabolic syndrome as central obesity based on waist circumference plus two or more additional metabolic risk factors20. Central obesity criteria were revised in 2005 and 2009; they applied different classification criteria based on ethnicity and risk factor status of CVD. Type 2 diabetes is included; however, the IGT criteria are not in NCEP or IDF. These approaches possess the strength of simplicity and the practicality of their components. In contrast, these approaches are limited, because they underestimate the prevalence of IGT and insulin resistance14.
Prevalence of Metabolic Syndrome
The worldwide prevalence of metabolic syndrome is increasing. In the USA, age‐adjusted prevalence increased from 29.2% in the National Health and Nutrition Examination Survey (NHANES) III to 34.2% in NHANES 1999–200622. Prevalence is significantly higher in women, especially younger women aged 20–39 years. This increasing trend has been observed in Asian countries as well. Age‐adjusted prevalence in the South Korea NHANES (KNHANES) 1998 was 24.9%, and increased to 31.3% in the KNHANES 2007 with the application of revised NCEP criteria23. Distinct and rapid increases in prevalence occur in women aged at least 50 years, after menopause, whereas metabolic syndrome in men aged at least 60 years decreases gradually; and the prevalence in adolescents increased from 6.8% in KNHANES 1998 to 9.2% in KNHANES 200124, to 13.0% in KNHANES 200525. In China, the prevalence of metabolic syndrome increased persistently as well26.
Variance in the prevalence is a result of the use of differing criteria and inclusion of different ethnicities. In a meta‐analysis in 2007, Nestel et al.27 reported prevalence ranges of 10–30% in several Asian countries, including South Korea, China, Singapore, Taiwan, Hong Kong and the Philippines. For Japan, the diagnostic criteria for central obesity differ from other Asian countries, with waist circumference measurements of more than 85 cm for males and 90 cm for females. Based on this definition, prevalence was 22.8% for men and 8.7% for women in the Japanese National Health and Nutrition Survey (NHNS) 200328. However, when other criteria were applied (waist circumference ≥85 cm for males and ≥ 80 cm for females), the prevalence in females was increased from 8.7 to 19.2%. The prevalence of metabolic syndrome was 22.0% based on IDF, 16.9% based on NCEP and 23.3% based on modified NCEP criteria from the Nantong Metabolic Syndrome Study (NMSS) that was carried out in China in 2007–200826.
Metabolic Syndrome as a Predictor of CVD
Numerous studies have confirmed the prognostic significance of metabolic syndrome on cardiovascular outcomes, including some negative results (Table 2).
Table 2. Metabolic syndrome and relative risk of cardiovascular disease.
| References | Year | Definition | Population | n | F/U (years) | Adjusted RR (95% CI) |
|---|---|---|---|---|---|---|
| Wilson et al.62 | 1999 | ≥3 of the 6 metabolically linked risk factors | Framingham Offspring Study (USA population; age 18–74 years) | 2,406 men and 2,569 women | 16.0 |
2.39 (1.56–3.36) in men 5.90 (2.54–13.73) in women |
| Isomaa et al.15 | 2001 | WHO | Botnia Study in Finland and Sweden, including diabetes (age 35–70 years) | 3,928 | 6.9 | 2.96 (2.36–3.72) |
| Lakka et al.29 | 2002 |
WHO NCEP |
Kuopio Ischemic Heart Disease Risk Factor Study (Finnish men without diabetes; age 42–60 years) |
1,209 | 11.4 |
2.83 (1.43–5.59) 2.27 (0.96–5.36) |
| Resnick et al.63 | 2003 | NCEP | Strong Heart Study (non‐diabetic American Indians) | 2,283 | 7.6 | 1.11 (0.79–1.56) |
| Malik et al. 64 | 2004 | Modified NCEP | United States population in NHANES II (age 30–75 years) | 6,255 | 13.3 | 2.02 (1.42–2.89) |
| Hu et al.65 | 2004 | Modified WHO | DECODE study (participants of 11 prospective European cohort studies without diabetes; age 30–89 years) | 6,156 men and 5,356 women | 8.8 |
2.26 (1.61–3.17) in men 2.78 (1.57–4.94) in women |
| Wilson et al.33 | 2005 | NCEP | Framingham Offspring Study (Fourth examination of the cohort excluding diabetes; age 22–81 years) | 3,323 | 8.0 | 2.88 (1.99–4.16) |
| Takeuchi et al.66 | 2005 | Modified NCEP | Tanno and Sobetsu Study (middle‐aged Japanese men excluding diabetes) | 808 | 6.0 | 2.23 (1.14–4.34) |
| Andreadis et al.67 | 2007 | NCEP | Mediterranean hypertensive population including diabetes | 1,007 | 2.1 | 2.26 (1.27–4.02) |
| Meig et al.46 | 2007 |
EGIR NCEP IDF |
Framingham Offspring Study (Fifth examination cohort participants) | 2,803 | 11.6 |
2.0 (1.6–2.7) 1.3 (0.9–1.9) no IR group 2.3 (1.7–3.1) IR group 1.6 (1.1–2.2) no IR group 2.2 (1.6–3.0) IR group |
| Song et al.68 | 2007 | Modified NCEP | Women's Health Study (female adults, age ≥45 years) | 25,626 | 10.0 |
2.40 (1.71–3.37) in BMI <25 3.01 (2.30–3.94) in BMI 25–29.9 2.89 (2.19–3.80) in BMI ≥30 |
| Ingeisson et al.69 | 2007 | NCEP | Framingham Offspring Study (Sixth examination cohort participants) | 1,945 | 7.2 | 1.61 (1.12–2.33) |
| Ninomiya et al.30 | 2007 | NCEP | Hisayama Study (Japanese including diabetes; age ≥40 years) | 2,452 | 14.0 |
1.86 (1.32–2.62) in men 1.70 (1.22–2.36) in women |
| Kokubo et al.70 | 2008 | Modified NCEP Japanese | Urban Japanese (age 30–79 years) | 5,332 | 11.5 |
1.75 (1.27–2.41) in men 1.90 (1.31–277) in women 2.92 (1.54–5.55) in men under 60 years |
| Hwang et al.31 | 2009 | Modified NCEP | Korean (age 20–78 years) | 2,435 | 8.7 |
1.98 (1.3–3.03) in men 4.14 (1.78–9.14) in women |
| Arnlov et al.71 | 2010 | Modified NCEP | Uppsala Longitudinal Study of adult men (ULSAM) without diabetes (age 50 years) | 1,758 | 30.0 |
1.63 (1.11–2.37) in BMI <25 1.74 (1.32–2.30) in BMI 25–29.9 2.55 (1.82–3.58) in BMI ≥ 30 |
CI, confidence interval; BMI, body mass index; EGIR, European Group for the Study of Insulin Resistance; F/U, follow‐up period; IDF, International Diabetes Federation; IR, insulin resistance; NCEP, National Cholesterol Education Program; NHANES, National Health and Nutrition Examination Survey; RR, relative risks; WHO, World Health Organization.
The results of the Botnia study on 4,483 middle‐aged participants in Finland and Sweden showed a marked increase in cardiovascular mortality in participants with metabolic syndrome during a 6.9‐year follow‐up period (12.0 vs 2.2%, P < 0.001)15. In the Kuopio Ischemic Heart Disease Risk Factor Study, metabolic syndrome was associated with a 2.5 to 2.8‐fold greater risk of death from any cardiovascular cause29. However, the relative risks (RR) and statistical significance varied with differing definitions of metabolic syndrome. The RR associated with WHO definitions was significant in all adjustment models; however, when the NCEP criteria (waist circumference over 94 cm) were used, no statistical significance was found in the association between RR and CVD mortality after adjustment for conventional risk factors, such as age, examination year, low‐density lipoprotein (LDL) cholesterol, smoking status and family history of coronary heart disease. A meta‐analysis on the 87 studies that used NCEP or revised NCEP definitions confirmed that metabolic syndrome is associated with a twofold increase in cardiovascular outcomes17. The RR was 2.35 (95% confidence interval [CI] 2.02–2.73) for all CVD, 2.40 (95% CI 1.87–3.08) for CVD mortality, 1.99 (95% CI 1.61–2.46) for myocardial infarction and 2.27 (95% CI 1.80–2.85) for stroke. A few studies on Asian populations produced similar results30. The Hisayama Study, 14‐year prospective study that included 2,452 middle‐aged Japanese individuals, confirmed that the hazard ratio (HR) of CVD events was 1.86 (95% CI 1.32–2.62) in men with metabolic syndrome and 1.70 (95% CI 1.22–2.36) in women, after multivariable adjustment30.
CVD predictability tended to vary by sex and numbers of components31. Wilson et al.32 determined that the RR of coronary heart diseases was significantly higher in women than in men, although they did share the same number of metabolic risk factors. In that study, the presence of three or more metabolic risk factors increased the risk for coronary heart diseases (CHD) 2.5‐fold in men and approximately sixfold in women during the 16‐year follow‐up period of middle‐aged adults. In the presence of two risk factors, the RR was approximately 2.0 in men and 3.0 in women. A study of 2,435 Korean participants (age range 20–78 years) showed that the odds ratios (OR) for CVD were higher in women (OR 4.04; 95% CI 1.78–9.14) than in men (OR 1.98; 95% CI 1.30–3.03)31. In the Beaver Dam Study, the incidence of CVD was 2.5% in a group to have 0 components of the metabolic syndrome by WHO definition and 14.9% in four more risk factors16. The OR was 1.95 (95% CI 0.91–4.16) in the group with one risk factor, 2.05 (95% CI 0.96–4.40) in the group with two risk factors, 2.70 (95% CI 1.22–5.98) in the group with three risk factors and 5.86 (95% CI 2.51–13.66) in the group with four or more risk factors. In the Framingham Heart Study Offspring Study, the age‐adjusted RR for CVD gradually increased as the number of risk factors increased in both men and women33. In men, the RR was 1.48 (95% CI 0.69–3.16) for one or two components and 3.99 (95% CI 1.89–8.41) for three or more components. In women, the RR was 3.39 (95% CI 1.31–8.81) for one or two components and 5.95 (95% CI 2.20–16.11) for three or more components.
Metabolic Syndrome as a Predictor of Type 2 Diabetes
Many large‐scale clinical trials and meta‐analyses reported that the presence of metabolic syndrome, regardless of definition, was highly predictive of new‐onset type 2 diabetes in many different populations (Table 3). Some studies showed that the RR for incident diabetes is higher than it is for CVD34. Based on a meta‐analysis of 42,419 participants from 16 cohorts, the average estimated RR for incident diabetes was 3.5–5.2, and did not differ appreciably with each definition. In contrast, the RR for CVD was 1.5–2.034. The Insulin Resistance Atherosclerosis Study (IRAS) showed that the OR for diabetes development based on the NCEP and IDF definitions was similar to the WHO definition, despite the use of modified risk factors35. The study of Aboriginal Canadians showed a prevalence of diabetes three to fivefold higher than in non‐Aboriginal Canadians, and metabolic syndrome had associated with incident diabetes regardless of the use of the NCEP criteria (OR 2.03; 95% CI 1.1–3.75) or IDF criteria (OR 2.14; 95% CI 1.29–3.55) to define metabolic syndrome36. In contrast, Cameron et al.37 reported a higher OR for the WHO criteria (OR 4.6; 95% CI 3.5–6.0) compared with EGIR criteria (OR 3.2; 95% CI 2.3–4.3), NCEP criteria (OR 3.1; 95% CI 2.3–4.0) and IDF criteria (OR 3.0; 95% CI 2.2–4.2). In addition, the OR for incident diabetes in a study of 4,756 Iranian subjects was highest using the WHO criteria (OR 11.0; 95% CI 7.9–15.3) during the 3.6‐year follow‐up period38. In that study, the OR using the IDF criteria was 4.3 (95% CI 3.0–6.0), the original NCEP criteria (FPG ≥ 110 mg/dL) was 3.7 (95% CI 2.7–5.1), and using modified NCEP criteria (FPG ≥ 100 mg/dL) was 4.9 (95% CI 3.5–6.9). According to a meta‐analysis carried out by Ford et al.34, the random‐effects summary RR was 5.17 (95% CI 3.99–6.69) for the WHO 1999 definition, 4.45 (95% CI 2.41–8.22) for the EGIR 1999 definition, 3.53 (95% CI 2.84–4.39) for the NCEP 2001 definition and 4.42 (95% CI 3.30–5.92) for the IDF 2005 definition.
Table 3. Metabolic syndrome and relative risk of type 2 diabetes mellitus.
| References | Year | Definition | Population | n | F/U (years) | Adjusted RR or HR (95% CI) |
|---|---|---|---|---|---|---|
| Sattar et al.48 | 2003 | Modified NCEP | West of Scotland Coronary Prevention Study (male adults) | 5,974 | 4.9 |
7.26 (2.25–23.4) in 3 components 24.4 (7.53–79.6) in 4 components 7.65 (5.99–9.31) in IFG components |
| Wilson et al.33 | 2005 | NCEP | Framingham Offspring study (middle‐aged adults) | 3,323 | 8.0 |
11.0 (8.1–14.9) in metabolic syndrome including IFG 5.0 (3.7–6.8) in metabolic syndrome excluding IFG |
| Wang et al.72 | 2007 |
WHO EGIR AACE IDF Modified NCEP |
Beijing Project (part of the National Diabetes Survey Population) | 541 | 5.0 |
2.39 (1.51–3.77) 1.88 (1.08–3.27) 2.97 (1.85–4.76) 2.05 (1.27–3.30) 2.33 (1.47–3.70) 2.61 (1.61–4.24) in IFG component |
| Cheung et al.40 | 2007 |
Modified NCEP IDF |
Hong Kong Cardiovascular Risk Factor Prevalence Study cohort | 1,679 | 6.4 |
4.1 (2.8–6.0) 3.5 (2.3–5.2) 5.1 (3.0–8.7) in IFG components |
| Cameron et al.37 | 2007 |
WHO EGIR NCEP IDF |
A longitudinal survey in Mauritius | 3,685 | 5.0 |
4.6 (3.5–6.0) 3.2 (2.3–4.3) 3.1 (2.3–4.0) 3.0 (2.2–4.2) 3.3 (2.6–4.3) in IFG component |
| Cameron et al.42 | 2008 |
WHO EGIR IDF NCEP |
Australian Diabetes, Obesity, and Lifestyle (AusDiab) study (adults, age ≥25 years) | 5,842 | 5.0 |
7.8 (5.5–11.0) 7.4 (5.2–10.4) 5.5 (3.9–7.6) 6.4 (4.6–9.0) 3.05 in IFG component |
| Ley et al.36 | 2009 |
NCEP IDF |
Sandy Lake Health and Diabetes Project | 492 | 10 |
2.03 (1.10–3.75) 2.14 (1.29–3.55) 2.30 (1.40–3.77) per 1 mmol/L increment of FPG |
| Salminen et al.51 | 2012 | IDF | Populations of Lieto in Finland (age ≥64 years) | 1,117 | 9 |
3.15 (1.89–5.25) 5.16 (2.68–9.93) in IFG component |
CI, confidence interval; EGIR, European Group for the Study of Insulin Resistance; F/U, follow‐up period; FPG, fasting plasma glucose; HR, hazard ratio; IDF, International Diabetes Federation; IFG, impaired fasting glucose; IGT, impaired glucose tolerance; NCEP, National Cholesterol Education Program; NHANES, National Health and Nutrition Examination Survey; RR, relative risks; WHO, World Health Organization.
To test which criteria enable improved predictability for the development of diabetes, we reviewed several statistical analyses that varied according to sensitivity, specificity, positive predictive values (PPVs), negative predictive values (NPVs) and the area under the receiver operating characteristics curve (aROC). The sensitivity ranged from 0.224 to 0.722, and the specificity ranged from 0.613 to 0.93937. PPVs ranged from 0.078 to 0.36 and NPVs ranged from 0.90 to 098337. A factor analysis study of 1,918 Pima Indians confirmed that the WHO definition led to superior sensitivity and specificity compared with the NCEP definition, because the former weights the presence of insulin resistance43. Also, in a longitudinal survey of 3,198 Mauritius subjects, the WHO definitions resulted in a higher value of sensitivity (42.1%) and PPV (26.8%) compared with the IDF and NCEP definitions37. However, differences among the aROCs (range 0.68–0.86) were small and insignificant, despite the differing criteria14. The predictability of metabolic syndrome for incident diabetes was superior to the predictability associated with either the Framingham Risk Score (FRS)47 or classical clinical risk factors excluding laboratory parameters, such as FPG, triglyceride, HDL‐cholesterol and blood pressure33.
Several studies examined that the number of metabolic syndrome components associated with the risk of type 2 diabetes16. According to a substudy on 3,323 members of the Framingham Heart Study Offspring Study, the RR for type 2 diabetes had increased with the number of metabolic syndrome components when the NECP criteria were applied33. The adjusted RR for participants with three abnormalities or four more abnormalities was 4.56 (95% CI 2.48–8.78) and 10.88 (95% CI 5.77–20.50), respectively, in the British Regional Heart study47. In the West of Scotland Coronary Prevention study, Sattar et al.48 used the NCEP definition based on body mass index (BMI) instead of waist circumference, with or without the inclusion of C‐reactive protein (CRP). The estimated RR for participants with three abnormalities or four more abnormalities was 7.26 (95% CI 2.25–23.40) and 24.4 (95% CI 7.53–79.6). In the Beaver Dam study, Klein et al.16 used a modified WHO definition to determine that the OR for the incidence of diabetes was 9.37 (95% CI 2.22–39.59) in the group with three abnormalities and 33.67 (95% CI 7.93–142.96) in the group with four or more abnormalities. Another study that was not based on one of the major definitions also reported that the RR relates to three or more risk factors49.
Among the components of metabolic syndrome, IFG has been shown as the strongest predictor for type 2 diabetes development37. Subjects with metabolic syndrome, which included the IFG trait, showed a RR of 11.0 (95% CI 8.1–14.9), whereas the RR for subjects excluding IFG were 5.0 (95% CI 3.7–6.8) in the Framingham Offspring Study33. Lorenzo et al.41 showed that the OR of incidental diabetes was 5.03 (95% CI 3.39–7.48) in participants with metabolic syndrome excluding IFG, 7.07 (95% CI 3.32–15.1) in participants without metabolic syndrome including IFG, and 21.0 (95% CI 13.1–33.8) in participants with metabolic syndrome including IFG when the NCEP criteria were used. The trend is also similar to the IDF definition (4.51 [95% CI 3.05–6.68] vs 10.5 [95% CI 5.50–24.3] vs 21.5 [95% CI 13.3–34.8]). In a recently published study on older populations in Finland, the HR of each metabolic syndrome component for the development of type 2 diabetes was 1.75 (95% CI 1.04–2.95) for the obesity factor, 1.34 (95% CI 0.78–2.31) for the triglyceride factor, 1.60 (95% CI 0.91–2.81) for the HDL‐cholesterol factor, 1.87 (95% CI 0.45–7.76) for the blood pressure factor and 5.16 (95% CI 2.68–9.93) for the IFG factor51. The strong relationship between metabolic syndrome with IFG and incident type 2 diabetes mellitus was not predictive of CVD. Whether other components (except for FPG) are related to incident diabetes remains controversial. Hwang et al.31 reported that a dramatic decrease in the risk of incident diabetes was observed in men after the initial FPG was adjusted, and metabolic syndrome without IFG was not associated with incident diabetes in women. However, the individual components of metabolic syndrome associated independently with risk for incident diabetes. As aforementioned, metabolic syndrome without IFG associated significantly with risk for incident diabetes; however, the RR in this case was less than the RR in metabolic syndrome with IFG.
A few studies have shown that the incorporation of some markers not of traditional metabolic syndrome components can be used as new metabolic syndrome components. In the European Investigation into Cancer and Nutrition (EPIC)‐NL, Monitoring Project on Risk Factors for Chronic Diseases (MORGEN) study, the predictive ability of type 2 diabetes in the extended model with high sensitivity of CRP (hsCRP) was slightly better than the predictive ability of the standard model of metabolic syndrome52. Furthermore, several studies have considered additional features, such as markers of liver function, uric acid and albumin20. However, more research is required to confirm the validity of these new markers.
Clinical Interpretations of Metabolic Syndrome
Some concern has emerged with regard to the lack of certainty inherent to metabolic syndrome, its pathogenesis and its value as a risk marker of CVD13. Nevertheless, the syndrome is used widely and conveniently in clinical practice and research fields; an important aspect of its clinical significance is the ‘visualization’ of the risk for CVD and type 2 diabetes development. By receiving a diagnosis of metabolic syndrome, patients might become motivated to actively carry out lifestyle modifications, and physicians can implement the focused risk management and comprehensive implementation approaches available to them to mitigate major complications.
The debate has continued on the inclusion of type 2 diabetes mellitus in definitions of metabolic syndrome (Figure 1)55. Early detection of individuals at high risk for type 2 diabetes is essential not only for the prevention of diabetes itself, but also to decrease associated cardiovascular complications. As aforementioned, metabolic syndrome is ideal as a predictor of incident diabetes. With the inclusion of diabetes in the defining criteria, metabolic syndrome loses its clinical advantage as a predictor for the development of diabetes. In addition, physicians should not expect effects from concurrent prevention measures for incident type 2 diabetes and its complications to overlap with active intervention of metabolic syndrome. Therefore, heavy consideration should be given to the exclusion of diabetes from the definition, and more focus should fall on the role of metabolic syndrome as an intervention tool for diabetes prevention.
Figure 1.
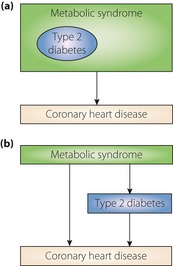
Concept of metabolic syndrome according to its major clinical outcome. (a) Classical concept of metabolic syndrome including type 2 diabetes as one of the main components. (b) Proposed concept that type 2 diabetes mellitus is regarded as a major outcome of metabolic syndrome.
Among the five components of metabolic syndrome, IFG is particularly superior for its ability to predict incident diabetes33; the other components can predict CVD better than or similarly to IFG33. Thus, metabolic syndrome with IFG is complimentary, allowing the prediction of CVD and diabetes; the populations in this group require extra care in management.
The role of metabolic syndrome in patients who have been diagnosed with diabetes is a topic many believe should not be ignored. Alexander et al.55 reported that in the USA, over 80% of participants aged 50 years or older with diabetes also have metabolic syndrome. Most patients with type 2 diabetes possess multiple risk factors for CVD other than hyperglycemia. Because CVD is the leading cause of death in diabetic patients56, careful attention should be exercised with regard to all modifiable risk factors. Many clinical studies have confirmed that adequate control of blood pressure and lipid profiles can reduce cardiovascular risk effectively58. However, diabetes itself is a strong risk factor for CVD, and type 2 diabetes mellitus is well‐known for its similar risks to coronary heart disease61. Consequently, the value of metabolic syndrome in diabetic patients is relatively weak compared with its value in non‐diabetic subjects.
In conclusion, metabolic syndrome is immensely useful as a clinical tool to predict diabetes and CVD, especially in high‐risk groups with metabolic syndrome that includes IFG. Exclusion of diabetes mellitus in metabolic syndrome is important to maximize the prevention effect of CVD with preceding diabetes mellitus. Further studies are required in several areas, including unified classification, ambiguous pathogenesis, the ‘syndrome’ role and the development of a more effective model.
Acknowledgements
The authors state no conflicts of interest.
(J Diabetes Invest, doi: 10.1111/jdi.12075, 2013)
References
- 1.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998; 15: 539–553 [DOI] [PubMed] [Google Scholar]
- 2.Balkau B, Charles MA. Comment on the provisional report from the WHO consultation. European Group for the Study of Insulin Resistance (EGIR). Diabet Med 1999; 16: 442–443 [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285: 2486–2497 [DOI] [PubMed] [Google Scholar]
- 4.Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37: 1595–1607 [DOI] [PubMed] [Google Scholar]
- 5.Fontbonne A, Charles MA, Thibult N, et al Hyperinsulinaemia as a predictor of coronary heart disease mortality in a healthy population: the Paris Prospective Study, 15‐year follow‐up. Diabetologia 1991; 34: 356–361 [DOI] [PubMed] [Google Scholar]
- 6.Després J, Lamarche B, Mauriège P, et al Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996; 334: 952–957 [DOI] [PubMed] [Google Scholar]
- 7.Yip J, Facchini FS, Reaven GM. Resistance to insulin‐mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab 1998; 83: 2773–2776 [DOI] [PubMed] [Google Scholar]
- 8.Hu G, Qiao Q, Tuomilehto J, et al Plasma insulin and cardiovascular mortality in non‐diabetic European men and women: a meta‐analysis of data from eleven prospective studies. Diabetologia 2004; 47: 1245–1256 [DOI] [PubMed] [Google Scholar]
- 9.Chen SJ, Yen CH, Huang YC, et al Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One 2012; 7: e45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devaraj S, Valleggi S, Siegel D, et al Role of C‐reactive protein in contributing to increased cardiovascular risk in metabolic syndrome. Curr Atheroscler Rep 2010; 12: 110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carr DB, Utzschneider KM, Hull RL, et al Intra‐abdominal fat is a major determinant of the National Cholesterol Education Program Adult Treatment Panel III criteria for the metabolic syndrome. Diabetes 2004; 53: 2087–2094 [DOI] [PubMed] [Google Scholar]
- 12.Lambert GW, Straznicky NE, Lambert EA, et al Sympathetic nervous activation in obesity and the metabolic syndrome–causes, consequences and therapeutic implications. Pharmacol Ther 2010; 126: 159–172 [DOI] [PubMed] [Google Scholar]
- 13.Kahn R, Buse J, Ferrannini E, et al The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2005; 28: 2289–2304 [DOI] [PubMed] [Google Scholar]
- 14.Aguilar‐Salinas CA, Rojas R, Gómez‐Pérez FJ, et al The metabolic syndrome: a concept hard to define. Arch Med Res 2005; 36: 223–231 [DOI] [PubMed] [Google Scholar]
- 15.Isomaa B, Almgren P, Tuomi T, et al Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 2001; 24: 683–689 [DOI] [PubMed] [Google Scholar]
- 16.Klein BE, Klein R, Lee KE. Components of the metabolic syndrome and risk of cardiovascular disease and diabetes in Beaver Dam. Diabetes Care 2002; 25: 1790–1794 [DOI] [PubMed] [Google Scholar]
- 17.Mottillo S, Filion KB, Genest J, et al The metabolic syndrome and cardiovascular risk a systematic review and meta‐analysis. J Am Coll Cardiol 2010; 56: 1113–1132 [DOI] [PubMed] [Google Scholar]
- 18.Bloomgarden ZT. American Association of Clinical Endocrinologists (AACE) consensus conference on the insulin resistance syndrome: 25–26 August 2002, Washington, DC. Diabetes Care 2003; 26: 933–939 [DOI] [PubMed] [Google Scholar]
- 19.Genuth S, Alberti KG, Bennett P, et al Follow‐up report on the diagnosis of diabetes mellitus. Diabetes Care 2003; 26: 3160–3167 [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome–a new worldwide definition. Lancet 2005; 366: 1059–1062 [DOI] [PubMed] [Google Scholar]
- 21.Alberti KG, Eckel RH, Grundy SM, et al Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009; 120: 1640–1645 [DOI] [PubMed] [Google Scholar]
- 22.Mozumdar A, Liguori G. Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 2011; 34: 216–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim S, Shin H, Song JH, et al Increasing prevalence of metabolic syndrome in Korea: the Korean National Health and Nutrition Examination Survey for 1998–2007. Diabetes Care 2011; 34: 1323–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HM, Park J, Kim HS, et al Prevalence of the metabolic syndrome in Korean adolescents aged 12–19 years from the Korean National Health and Nutrition Examination Survey 1998 and 2001. Diabetes Res Clin Pract 2007; 75: 111–114 [DOI] [PubMed] [Google Scholar]
- 25.You MA, Son YJ. Prevalence of metabolic syndrome and associated risk factors among Korean adolescents: analysis from the Korean national survey. Asia Pac J Public Health 2012; 24: 464–471 [DOI] [PubMed] [Google Scholar]
- 26.Cai H, Huang J, Xu G, et al Prevalence and determinants of metabolic syndrome among women in Chinese rural areas. PLoS One 2012; 7: e36936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nestel P, Lyu R, Low LP, et al Metabolic syndrome: recent prevalence in East and Southeast Asian populations. Asia Pac J Clin Nutr 2007; 16: 362–367 [PubMed] [Google Scholar]
- 28.Nishimura R, Nakagami T, Tominaga M, et al Prevalence of metabolic syndrome and optimal waist circumference cut‐off values in Japan. Diabetes Res Clin Pract 2007; 78: 77–84 [DOI] [PubMed] [Google Scholar]
- 29.Lakka HM, Laaksonen DE, Lakka TA, et al The metabolic syndrome and total and cardiovascular disease mortality in middle‐aged men. JAMA 2002; 288: 2709–2716 [DOI] [PubMed] [Google Scholar]
- 30.Ninomiya T, Kubo M, Doi Y, et al Impact of metabolic syndrome on the development of cardiovascular disease in a general Japanese population: the Hisayama study. Stroke 2007; 38: 2063–2069 [DOI] [PubMed] [Google Scholar]
- 31.Hwang YC, Jee JH, Oh EY, et al Metabolic syndrome as a predictor of cardiovascular diseases and type 2 diabetes in Koreans. Int J Cardiol 2009; 134: 313–321 [DOI] [PubMed] [Google Scholar]
- 32.Wilson PW. Estimating cardiovascular disease risk and the metabolic syndrome: a Framingham view. Endocrinol Metab Clin North Am 2004; 33: 467–481 [DOI] [PubMed] [Google Scholar]
- 33.Wilson PW, D'Agostino RB, Parise H, et al Metabolic syndrome as a precursor of cardiovascular disease and Type 2 diabetes mellitus. Circulation 2005; 112: 3066–3072 [DOI] [PubMed] [Google Scholar]
- 34.Ford ES, Li C, Sattar N. Metabolic syndrome and incident diabetes: current state of the evidence. Diabetes Care 2008; 31: 1898–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanley AJ, Karter AJ, Williams K, et al Prediction of type 2 diabetes mellitus with alternative definitions of the metabolic syndrome: the Insulin Resistance Atherosclerosis Study. Circulation 2005; 112: 3713–3721 [DOI] [PubMed] [Google Scholar]
- 36.Ley SH, Harris SB, Mamakeesick M, et al Metabolic syndrome and its components as predictors of incident type 2 diabetes mellitus in an Aboriginal community. CMAJ 2009; 180: 617–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cameron AJ, Zimmet PZ, Soderberg S, et al The metabolic syndrome as a predictor of incident diabetes mellitus in Mauritius. Diabet Med 2007; 24: 1460–1469 [DOI] [PubMed] [Google Scholar]
- 38.Hadaegh F, Ghasemi A, Padyab M, et al The metabolic syndrome and incident diabetes: assessment of alternative definitions of the metabolic syndrome in an Iranian urban population. Diabetes Res Clin Pract 2008; 80: 328–334 [DOI] [PubMed] [Google Scholar]
- 39.Schmidt MI, Duncan BB, Bang H, et al Identifying individuals at high risk for diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 2005; 28: 2013–2018 [DOI] [PubMed] [Google Scholar]
- 40.Cheung BM, Wat NM, Man YB, et al Development of diabetes in Chinese with the metabolic syndrome: a 6‐year prospective study. Diabetes Care 2007; 30: 1430–1436 [DOI] [PubMed] [Google Scholar]
- 41.Lorenzo C, Williams K, Hunt KJ, et al The National Cholesterol Education Program ‐ Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007; 30: 8–13 [DOI] [PubMed] [Google Scholar]
- 42.Cameron AJ, Magliano DJ, Zimmet PZ, et al The metabolic syndrome as a tool for predicting future diabetes: the AusDiab study. J Intern Med 2008; 264: 177–186 [DOI] [PubMed] [Google Scholar]
- 43.Hanson RL, Imperatore G, Bennett PH, et al Components of the “metabolic syndrome” and incidence of type 2 diabetes. Diabetes 2002; 51: 3120–3127 [DOI] [PubMed] [Google Scholar]
- 44.Lorenzo C, Okoloise M, Williams K, et al The metabolic syndrome as predictor of type 2 diabetes: the San Antonio heart study. Diabetes Care 2003; 26: 3153–3159 [DOI] [PubMed] [Google Scholar]
- 45.Stern MP, Williams K, González‐Villalpando C, et al Does the metabolic syndrome improve identification of individuals at risk of type 2 diabetes and/or cardiovascular disease? Diabetes Care 2004; 27: 2676–2681 [DOI] [PubMed] [Google Scholar]
- 46.Meigs JB, Rutter MK, Sullivan LM, et al Impact of insulin resistance on risk of type 2 diabetes and cardiovascular disease in people with metabolic syndrome. Diabetes Care 2007; 30: 1219–1225 [DOI] [PubMed] [Google Scholar]
- 47.Wannamethee SG, Shaper AG, Lennon L, et al Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med 2005; 165: 2644–2650 [DOI] [PubMed] [Google Scholar]
- 48.Sattar N, Gaw A, Scherbakova O, et al Metabolic syndrome with and without C‐reactive protein as a predictor of coronary heart disease and diabetes in the West of Scotland Coronary Prevention Study. Circulation 2003; 108: 414–419 [DOI] [PubMed] [Google Scholar]
- 49.Elwood PC, Pickering JE, Fehily AM. Milk and dairy consumption, diabetes and the metabolic syndrome: the Caerphilly prospective study. J Epidemiol Community Health 2007; 61: 695–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Macchia A, Levantesi G, Borrelli G, et al A clinically practicable diagnostic score for metabolic syndrome improves its predictivity of diabetes mellitus: the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI)‐Prevenzione scoring. Am Heart J 2006; 151: e7–e754, e17. [DOI] [PubMed] [Google Scholar]
- 51.Salminen M, Kuoppamäki M, Vahlberg T, et al Metabolic syndrome defined by modified International Diabetes Federation criteria and type 2 diabetes mellitus risk: a 9‐year follow‐up among the aged in Finland. Diab Vasc Dis Res 2013; 10: 11–16. doi: 10.1177/1479164112442077 [DOI] [PubMed] [Google Scholar]
- 52.Povel CM, Beulens JW, van der Schouw YT, et al Metabolic syndrome model definitions predicting type 2 diabetes and cardiovascular disease. Diabetes Care 2013; 36: 362–368. doi: 10.2337/dc11‐2546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hanley AJ, Williams K, Festa A, et al Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes 2005; 54: 3140–3147 [DOI] [PubMed] [Google Scholar]
- 54.Pladevall M, Singal B, Williams LK, et al A single factor underlies the metabolic syndrome: a confirmatory factor analysis. Diabetes Care 2006; 29: 113–122 [DOI] [PubMed] [Google Scholar]
- 55.Alexander CM, Landsman PB, Grundy SM. Metabolic syndrome and hyperglycemia: congruence and divergence. Am J Cardiol 2006; 98: 982–985 [DOI] [PubMed] [Google Scholar]
- 56.Morrish NJ, Wang SL, Stevens LK, et al Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 2001; 44(Suppl 2): S14–S21 [DOI] [PubMed] [Google Scholar]
- 57.Woodward M, Zhang X, Barzi F, et al The effects of diabetes on the risks of major cardiovascular diseases and death in the Asia‐Pacific region. Diabetes Care 2003; 26: 360–366 [DOI] [PubMed] [Google Scholar]
- 58.Grundy SM, Cleeman JI, Merz CN, et al Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004; 110: 227–239 [DOI] [PubMed] [Google Scholar]
- 59.Buse JB, Ginsberg HN, Bakris GL, et al Primary prevention of cardiovascular diseases in people with diabetes mellitus: a scientific statement from the American Heart Association and the American Diabetes Association. Diabetes Care 2007; 30: 162–172 [DOI] [PubMed] [Google Scholar]
- 60.Gaede P, Lund‐Andersen H, Parving HH, et al Effect of a multifactorial intervention on mortality in type 2 diabetes. N Engl J Med 2008; 358: 580–591 [DOI] [PubMed] [Google Scholar]
- 61.Haffner SM, Lehto S, Ronnemaa T, et al Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 1998; 339: 229–234 [DOI] [PubMed] [Google Scholar]
- 62.Wilson PW, Kannel WB, Silbershatz H, et al Clustering of metabolic factors and coronary heart disease. Arch Intern Med 1999; 159: 1104–1109 [DOI] [PubMed] [Google Scholar]
- 63.Resnick HE, Jones K, Ruotolo G, et al Insulin resistance, the metabolic syndrome, and risk of incident cardiovascular disease in nondiabetic american indians: the Strong Heart Study. Diabetes Care 2003; 26: 861–867 [DOI] [PubMed] [Google Scholar]
- 64.Malik S, Wong ND, Franklin SS, et al Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation 2004; 110: 1245–1250 [DOI] [PubMed] [Google Scholar]
- 65.Hu G, Qiao Q, Tuomilehto J, et al Prevalence of the metabolic syndrome and its relation to all‐cause and cardiovascular mortality in nondiabetic European men and women. Arch Intern Med 2004; 164: 1066–1076 [DOI] [PubMed] [Google Scholar]
- 66.Takeuchi H, Saitoh S, Takagi S, et al Metabolic syndrome and cardiac disease in Japanese men: applicability of the concept of metabolic syndrome defined by the National Cholesterol Education Program‐Adult Treatment Panel III to Japanese men–the Tanno and Sobetsu Study. Hypertens Res 2005; 28: 203–208 [DOI] [PubMed] [Google Scholar]
- 67.Andreadis EA, Tsourous GI, Tzavara CK, et al Metabolic syndrome and incident cardiovascular morbidity and mortality in a Mediterranean hypertensive population. Am J Hypertens 2007; 20: 558–564 [DOI] [PubMed] [Google Scholar]
- 68.Song Y, Manson JE, Meigs JB, et al Comparison of usefulness of body mass index versus metabolic risk factors in predicting 10‐year risk of cardiovascular events in women. Am J Cardiol 2007; 100: 1654–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ingelsson E, Sullivan LM, Murabito JM, et al Prevalence and prognostic impact of subclinical cardiovascular disease in individuals with the metabolic syndrome and diabetes. Diabetes 2007; 56: 1718–1726 [DOI] [PubMed] [Google Scholar]
- 70.Kokubo Y, Okamura T, Yoshimasa Y, et al Impact of metabolic syndrome components on the incidence of cardiovascular disease in a general urban Japanese population: the suita study. Hypertens Res 2008; 31: 2027–2035 [DOI] [PubMed] [Google Scholar]
- 71.Arnlöv J, Ingelsson E, Sundström J, et al Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle‐aged men. Circulation 2010; 121: 230–236 [DOI] [PubMed] [Google Scholar]
- 72.Wang JJ, Li HB, Kinnunen L, et al How well does the metabolic syndrome defined by five definitions predict incident diabetes and incident coronary heart disease in a Chinese population? Atherosclerosis 2007; 192: 161–168 [DOI] [PubMed] [Google Scholar]


