Abstract
Aims/Introduction
To determine the prevalence and risk factors of retinopathy and validity of the current diagnostic cut‐offs for diabetes by using data of health check‐up examinees.
Materials and Methods
The study comprises 1,864 Japanese who participated in the general health check‐up program and did not have a previous history of cardiovascular disease. Non‐mydriatic 45° digital fundus photographs were taken twice annually. Multivariate logistic regression model was used to identify risk factors for retinopathy in participants without previously diagnosed diabetes.
Results
The overall prevalence of retinopathy in participants with and without previously diagnosed diabetes were 23.3% (28/120) and 4.2% (74/1,744), respectively. Univariate logistic regression analysis identified age, systolic blood pressure (SBP), fasting plasma glucose (FPG) and hemoglobin A1c (HbA1c) as risk factors for retinopathy. Multivariate logistic regression analysis showed that FPG or both HbA1c and SBP were significant, positive and independent risk factors for retinopathy. The prevalence of retinopathy increased with deterioration of glucose categories (P < 0.001 for FPG or HbA1c). However, a statistically significant increased risk of retinopathy remained only in participants with FPG ≥ 7.0 mmol/L or HbA1c ≥ 6.5% compared with those with the lowest quartile of glucose in the participants without previously diagnosed diabetes after adjusting for age and SBP.
Conclusions
The prevalence of retinopathy was 4.2%, and FPG or both HbA1c and SBP were positive and independent risk factors for retinopathy in health check‐up examinees without previously diagnosed diabetes. The FPG 7.0 mmol/L or HbA1c 6.5% seems to be appropriate to diagnose diabetes in view of its association with retinopathy.
Keywords: Fasting plasma glucose, Hemoglobin A1c, Retinopathy
Introduction
The International Expert Committee of the American Diabetes Association (ADA), the European Association for the Study of Diabetes and the World Health Organization (WHO) have recommended that hemoglobin A1c (HbA1c), fasting plasma glucose (FPG) and 2‐h plasma glucose (2‐h PG) on a 75‐g oral glucose tolerance test (OGTT) be included in the diagnosis of diabetes1. The Japan Diabetes Society (JDS) has tackled the issue of HbA1c from its own standpoint and reviewed existing OGTT data from 6,658 Japanese subjects4. In the JDS report, a very high correlation between FPG and HbA1c was seen, with a FPG of 7.0 mmol/L corresponding to a HbA1c of 6.5%. In the same manner, a correlation was identified between OGTT 2‐h PG value and HbA1c, with an OGTT 2‐h PG value of 11.1 mmol/L corresponding to a HbA1c of 6.4%. Furthermore, the prevalence of diabetic retinopathy has substantially increased at a HbA1c level of 6.5%5. Then, since spring 2010, a HbA1c of 6.5% has been included as a component of the diagnosis of diabetes and solely used for epidemiological purposes in Japan4.
Retinal signs, such as isolated microaneurysms, retinal hemorrhages, hard exudates and cotton wool spots, are occasionally observed in subjects without clinically diagnosed diabetes6. The prevalence of retinopathy has been reported to be 4.8–15.5% in subjects without diabetes. However, these reports used different definitions of diabetes, such as FPG alone, a combination of FPG and 2‐h PG, non‐fasting glucose, current use of diabetes medications or a self‐reported history of diabetes. None of the aforementioned studies applied HbA1c to diagnose diabetes. Furthermore, almost all of the reports examined white populations; only a few were from Asia6. Thus, the aim of the present study was to determine the prevalence of retinopathy and its risk factors in the Japanese health check‐up examinees not previously diagnosed with diabetes. Furthermore, the validity of current diagnostic cut‐offs on FPG and HbA1c was evaluated, with respect to the prevalence of retinopathy.
Participants
From February 2006 to January 2007, a total of 3,804 men and 2,208 women participated in a general health check‐up program at Saitama‐ken Saiseikai Kurihashi (SSK) Hospital in Saitama, Japan, and have been followed up in the Kurihashi Lifestyle Cohort Study to clarify the relationship between risk factors and lifestyle‐related diseases and mortality. Of those, 1,387 men and 588 women aged 21–85 years had glucose measurements (FPG and HbA1c) and retinal photographs taken twice a year. Participants were excluded if they had anemia (hemoglobin <13.0 g/dL for men or <12.0 g/dL for women; n = 164) or a previous history of cardiovascular events (n = 34) and if retinal grading did not meet between two independent ophthalmologists (CS and AH; n = 42). The remaining 1,351 men and 513 women were used for data analysis.
FPG ≥7.0 mmol/L or HbA1c ≥6.5% was used to diagnose newly diagnosed diabetes according to the JDS, ADA and WHO criteria2.
Materials and Methods
Participants were classified into six groups according to glucose concentration based on quartiles in the non‐diabetic glucose range, newly diagnosed diabetes for FPG or for HbA1c and previous diagnosed diabetes (FPG categories: <4.9, 4.9–5.1, 5.2–5.4, 5.5–6.9, ≥7.0 mmol/L and previously diagnosed diabetes; HbA1c category: <5.4, 5.4–5.5, 5.6–5.7, 5.8–6.4, ≥6.5% and previously diagnosed diabetes).
Measurement of Variables
The health check‐up procedures at SSK Hospital included biochemical laboratory tests and a self‐administered questionnaire regarding smoking status and medical history. Smoking status was classified into three categories (never, past and current smoker). The height, weight, blood pressure and waist circumference of all participants were measured. Body height was measured to the nearest 0.1 cm with the participant standing without shoes. The participants were requested to wear light indoor clothes before their bodyweight was measured to the nearest 0.1 kg. Blood pressure was measured in a sitting position after 5 min of rest using an automatic sphygmomanometer (Medical Electronics Sphygmomanometer TM‐2655P; A&D Co. Ltd, Tokyo, Japan). Body mass index (BMI) was calculated as the bodyweight (in kg) divided by the body height squared (in meters).
Blood samples were obtained in the morning after a 10‐h fast. FPG (glucose oxidase method), HbA1c (high‐performance liquid chromatographic method), total cholesterol (TC; oxidase method), triglyceride (TG; enzymatic method), high‐density lipoprotein (HDL) cholesterol (direct method), uric acid (UA; uricase peroxidase method) and white blood cell (WBC) count (multi‐angle polarized scatter separation method) were measured in the hospital laboratory. Fasting immunoreactive insulin (F‐IRI), concentrations were measured by immune‐radiometric assay at a commercial laboratory. The mean coefficient of variation of HbA1c was 1.16%. HbA1c values were estimated as National Glycohemoglobin Standardization Program equivalent values calculated from the formula HbA1c (%) = 1.02 × HbA1c (JDS) (%) + 0.25%17. Anthropometric and laboratory data at the first examination were used for data analysis.
Fundus Examination
On two occasions per year, a retinal photograph centered at the optic disc and macula was taken of one eye of each participant, using a 45° 6.3‐megapixel digital non‐mydriatic camera with a 10D single‐lens reflex back. Two retinal photographs per one participant were used for retinal grading by two independent masked ophthalmologists (CS and AH). The presence of retinopathy was defined by the following conditions: microaneurysms, any retinal hemorrhage, and soft and hard exudates.
Statistical Analysis
The χ2‐test was used to compare proportions, and Student's t‐test was used to compare means between two groups.
The univariate logistic regression analysis was carried out to test the association between an independent variable and the presence of retinopathy in participants without previously diagnosed diabetes. The independent variables tested in the model were FPG (1 mmol/L), HbA1c (1%), age (10 years), sex, systolic blood pressure (SBP; 10 mmHg), TC (1 mmol/L), TG (1 mmol/L), HDL2 cholesterol (1 mmol/L), BMI (1 kg/m2), UA (1 μmol/L), WBC count (1000/μL), F‐IRI (1 μU/mL) and smoking status. Subsequently, multivariate logistic regression analysis was carried out to test whether variables identified as significant in the aforementioned univariate model were independently associated with the presence of retinopathy.
The proportions of the participants with retinopathy were calculated in the six glucose categories described earlier, and a logistic regression model was used to test the effect of glucose category on retinopathy. In this model, FPG and HbA1c were treated as categorical variables, and six glucose categories were coded as 1–6 for each of FPG and HbA1c. Thus, the category with the lowest value was coded as one followed by the next lowest value coded as two, and so on. The category of ‘previously diagnosed diabetes’ was coded as six. To examine the appropriate cut‐off for the prediction of retinopathy, each of the categories 2–5 was compared with category one, the lowest quartile of the non‐diabetic glucose range; that is, FPG category ‘<4.9’ or HbA1c category ‘<5.4’.
The Statistical Package for Social Sciences for Windows (version 17.0; SPSS, Chicago, IL, USA) was used for all statistical analyses. All reported P‐values are two‐tailed, and P < 0.05 was considered statistically significant.
The study was approved by the Institutional Review Board of SSK Hospital and Tokyo Women's Medical University, and informed consent was obtained from the study participants.
Results
The overall prevalence of retinopathy in participants with and without previously diagnosed diabetes was 23.3% (28/120) and 4.2% (74/1,744), respectively.
Participant Characteristics According to Retinal Condition in Participants Without Previously Diagnosed Diabetes
In the data analysis of 1,744 participants without previously diagnosed diabetes, participants with retinopathy were more likely to be women than men, older and had higher blood pressure, FPG and HbA1c than those without retinopathy (Table 1). No difference was found in lipid profiles, smoking status, WBC counts or F‐IRI between participants with retinopathy and those without.
Table 1. Clinical characteristics by presence or absence of retinopathy in 1,744 participants without previously diagnosed diabetes.
| Retinopathy absent | Retinopathy present | P‐value | |
|---|---|---|---|
| n | 1,670 | 74 | – |
| Men (%) | 72.2 | 62.2 | 0.044 |
| Age (years) | 51 ± 8 | 53 ± 7 | 0.016 |
| Body mass index (kg/m2) | 23.4 ± 3.0 | 23.8 ± 2.7 | 0.319 |
| Current smokers (%) | 50.7 | 50.0 | 0.499 |
| Systolic blood pressure (mm Hg) | 126 ± 17 | 131 ± 17 | 0.006 |
| Diastolic blood pressure (mm Hg) | 77 ± 12 | 81 ± 13 | 0.011 |
| Fasting plasma glucose (mmol/L) | 5.3 ± 0.7 | 5.7 ± 1.5 | <0.001 |
| HbA1c (%) | 5.2 ± 0.5 | 5.4 ± 0.8 | 0.001 |
| Total cholesterol (mmol/L) | 5.36 ± 0.88 | 5.42 ± 0.89 | 0.579 |
| Triglyceride (mmol/L) | 1.24 ± 0.73 | 1.28 ± 0.71 | 0.680 |
| HDL cholesterol (mmol/L) | 1.46 ± 0.39 | 1.47 ± 0.38 | 0.738 |
| Fasting immunoreactive insulin (IU/mL) | 6.9 ± 4.1 | 7.1 ± 4.1 | 0.785 |
| Uric acid (μmol/L) | 326 ± 83 | 318 ± 76 | 0.414 |
| White blood cell count (/μL) | 5,470 ± 1,560 | 5,410 ± 1,520 | 0.747 |
The χ2‐test was used to compare proportions and Student's t‐test was used to compare means between two groups. Data are means (standard deviations) or proportions. HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein.
Risk Factors for Retinopathy in Participants Without Previously Diagnosed Diabetes
Table 2 shows risk factors identified for retinopathy in the univariate logistic regression analysis. Age, SBP, HbA1c and FPG were revealed as positive risk factors for retinopathy.
Table 2. Univariate logistic regression analysis to predict the presence of retinopathy in 1,744 participants without previously diagnosed diabetes.
| Independent variables | Beta‐coefficients | Standard errors | P‐values |
|---|---|---|---|
| Age (10 years) | 0.358 | 0.148 | 0.016 |
| Sex (women vs men) | 0.456 | 0.246 | 0.064 |
| Systolic blood pressure (10 mmHg) | 0.178 | 0.065 | 0.006 |
| HbA1c (1%) | 0.479 | 0.155 | 0.002 |
| Fasting plasma glucose (1 mmol/L) | 0.337 | 0.960 | <0.001 |
| Body mass index (1 kg/m2) | 0.037 | 0.037 | 0.318 |
| Total cholesterol (1 mmol/L) | 0.074 | 0.133 | 0.579 |
| HDL cholesterol (1 mmol/L) | 0.102 | 0.305 | 0.737 |
| Triglyceride (1 mmol/L) | 0.062 | 0.151 | 0.680 |
| Smoking status (current or past vs never) | −0.029 | 0.238 | 0.904 |
| Uric acid (1 μmol/L) | −0.001 | 0.001 | 0.414 |
| Fasting immunoreactive insulin (1 μU/mL) | 0.013 | 0.047 | 0.785 |
| White blood cell count (1/μL) | −0.003 | 0.008 | 0.747 |
HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein.
In the multivariate model (table 3), either FPG (model 1) or HbA1c (model 2), but not both, was used as a variable as they are tightly associated with each other: Thus, in model 1, FPG was used; while in model 2, HbA1c was used in place of FPG, in addition to age and SBP, which were associated with retinopathy in the univariate analysis. The multivariate logistic regression model showed that FPG (P = 0.003; model 1) or both SBP (P = 0.03; model 2) and HbA1c (P = 0.011; model 2) were significant, positive and independent risk factors for retinopathy (Table 3).
Table 3. Multivariate logistic regression analysis to predict the presence of retinopathy in 1,744 participants without previously diagnosed diabetes.
| Independent variables | Beta‐coefficients | Standard errors | P‐values |
|---|---|---|---|
| Model 1 | |||
|
Age (10 years) Systolic blood pressure (10 mmHg) Fasting plasma glucose (1 mmol/L) |
0.287 | 0.154 | 0.062 |
| 0.130 | 0.068 | 0.055 | |
| 0.295 | 0.099 | 0.003 | |
| Model 2 | |||
|
Age (10 years) Systolic blood pressure (10 mmHg) HbA1c (1%) |
0.271 | 0.154 | 0.078 |
| 0.150 | 0.067 | 0.030 | |
| 0.412 | 0.163 | 0.011 | |
Model 1 includes age, systolic blood pressure and fasting plasma glucose as independent variables. Model 2 includes age, systolic blood pressure and hemoglobin A1c (HbA1c) as independent variables.
Prevalence of Retinopathy by Glucose Categories on FPG or HbA1c
Figure 1 shows the relationship between the crude prevalence of retinopathy and the six glucose categories based on FPG or HbA1c. The prevalence of retinopathy increased with deterioration of glucose categories for each of HbA1c and FPG (P < 0.001 for FPG or HbA1c category) (Figure 1) and FPG or HbA1c category was significantly associated with retinopathy. When no adjustment was made, the statistically significant increase in the risk of retinopathy was observed in participants with the category of FPG ≥5.5 mmol/L or HbA1c ≥6.5% in comparison with participants in the category of the lowest FPG or HbA1c. However, this statistically significant increased risk of retinopathy remained only in participants with FPG ≥7.0 mmol/L or HbA1c ≥6.5% compared with participants with the lowest FPG or HbA1c after adjusting for age and SBP.
Figure 1.
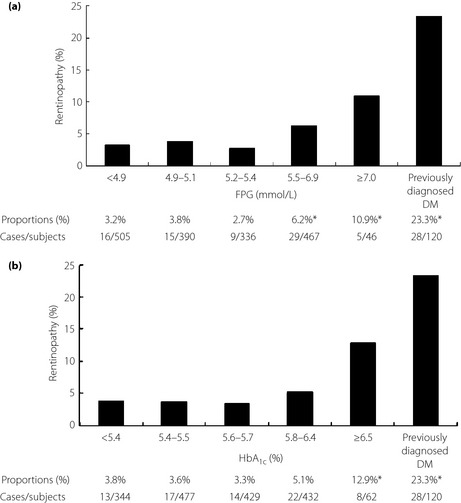
The prevalence of retinopathy based on six glucose categories for (a) fasting plasma glucose (FPG) and (b) hemoglobin A1c (HbA1c). *P < 0.05. DM, diabetes mellitus.
Discussion
The current study has shown that the prevalence of retinopathy in Japanese health check‐up examinees without previously diagnosed diabetes was low at 4.2%, and FPG or both HbA1c and SBP were positive and independent risk factors for retinopathy in this group. The significantly increased adjusted risk for retinopathy in comparison with the lowest quartile for FPG or HbA1c was shown only in the current newly diagnostic glucose categories for FPG or HbA1c.
The prevalence of retinopathy in non‐diabetes varies between different Asian ethnic groups, being 6.0% in the Singapore Malay Eye Study6, 17.2% in Chinese in the Multi‐Ethnic Study of Atherosclerosis7 and 9.0% in the Funagata Study from Japan8. As for diabetic retinopathy in subjects with diabetes and non‐diabetes, it has been reported as 2.3% in the Hisayama Study from Japan18. The reason for the variation in prevalence might be a result of differences in the retinal grading methodology used, the studies’ diagnostic criteria for diabetes, the distribution of clinical characteristics and the frequency of examinations. These factors might in part explain the difference in the prevalence of retinopathy within the same ethnic group. A combination of FPG and 2‐h PG was used in the two Japanese population‐based studies8, whereas either FPG or HbA1c was used to diagnose diabetes in the present study. Participants in the Funagata Study were non‐diabetics8, whereas those in the Hisayama Study included both diabetics and non‐diabetics18, and those in the present study were subjects without previously diagnosed diabetes. Furthermore, participants in the Hisayama Study18 and the present study were approximately 10 years younger than those in the Funagata Study8. Alternatively, the participants analyzed in the present study received health check‐ups twice annually, and the potential risks for complications might be low.
Previous studies have identified various risk factors for retinopathy in subjects without diabetes as a history of heart attack6, the presence of stroke9, SBP6, hypertension6, BMI6, internal carotid arterial intima–media thickness7, carotid arterial plaque9, age8, glucose intolerance8 and dyslipidemia11. These findings suggest that retinopathy and cardiovascular diseases (CVD) share a common pathophysiology. In the current study, age, glucose indicators and SBP had a significant positive and independent relationship to retinopathy in participants without previously diagnosed diabetes or CVD. In contrast, the presence of hypertension based on the combination of systolic and diastolic blood pressure was not an independent risk factor of retinopathy among non‐diabetics of the Funagata Study, where participants with previous CVD were included8. This shows that the magnitude of the same risk factor for retinopathy varies even in the same ethnicity. In the present study, SBP was an independent risk factor for retinopathy in the model including age and HbA1c as covariates, whereas it was a borderline risk factor in the model including age and FPG as covariates. The reason is not known; however, this might be a result of a lower proportion of newly diagnosed diabetes by FPG than HbA1c. Furthermore, SBP was no longer an independent risk factor for retinopathy when excluding participants taking antihypertensive medications in the present study (data not shown).
Some reports have shown the significant sex difference for having retinopathy6. The retinal signs were consistently more prevalent among men than women in the Singapore Malay Eye Study6 and Beaver Dam Eye Study19. This sex difference might be a reflection of the higher CVD risks in men than women20. However, the present results were in contrast to the findings of the Singapore Malay Eye Study and Beaver Dam Eye Study, as sex was not identified as a significant risk factor in logistic regression models. The reason is not known, but this might be because of a selection bias, as our participants had been repeatedly examined in health check‐ups. Further research will be required to clarify the sex difference in the prevalence of retinopathy.
The limitations of the present study should be noted. First, only one single angle retinal photograph per one health check‐up examination was used for the present study, and this might have led to misclassification of retinopathy. However, two independent well‐trained ophthalmologists have confirmed the retinal findings. Furthermore, we selected people who have examined their retina on two occasions per year. This might have increased cumulative time information about the retina and helped to distinguish drusen and hard exudates. Thus, our grading might be justified. Retinal fundus photography is a common, inexpensive and useful tool for assessing early atherosclerotic vascular changes in a general health check‐up program, such as that used in Japan. Second, this was a cross‐sectional study and therefore needs to be replicated using longitudinal data. Third, our participants were general health check‐up examinees, so we had no 2‐h PG data to analyze. The Evaluation of Screening and Early Detection Strategies for Type 2 Diabetes and Impaired Glucose Tolerance (DETECT‐2) Study21 reported a narrow threshold range for diabetic retinopathy was identified for FPG and HbA1c but not for 2‐h PG. While the Hisayama Study from Japan has suggested that measuring FPG or HbA1c was as useful as 2‐h PG for the diagnosis of diabetes18, as the area under the receiver operating characteristic curves for 2‐h PG was slightly, but not significantly, larger than that for FPG or HbA1c with respect to the prevalence of diabetic retinopathy. Thus, even without 2‐h PG data, we believe our data were worthwhile. Fourth, the DETECT‐2 Study has suggested that the current diabetes diagnostic level for FPG could be lowered from 7.0 mmol/L to 6.5 mmol/L, with regard to the prevalence of retinopathy21. The lowered diagnostic cut‐off for FPG of 6.4 mmol/L (and HbA1c of 6.1%) has also been suggested in the Hisayama Study18. However, it was inclusive in the present study because we could not focus on diabetic retinopathy or further stratify our participants because of the small sample size.
In conclusion, the prevalence of retinopathy was 4.2%, and FPG or both HbA1c and SBP were positive and independent risk factors for retinopathy in health check‐up examinees without previously diagnosed diabetes. FPG 7.0 mmol/L or HbA1c 6.5% seem to be appropriate cut‐off values to diagnose diabetes with respect to prevalence of retinopathy.
Acknowledgements
The present study was supported by grants to TN from the Japan Diabetes Society, the Tokyo Women's Medical University Association, the Yayoi Yoshioka Research Fund, and the Yazuya Food and Health Research Foundation. We appreciate the participation of subjects and the staff from the Kurihashi Lifestyle Cohort Study, particularly Junko Oya, Yayoi Yamamoto, Midori Hasegawa and Yasuhiro Endo. We also thank Professor Shigehiko Kitano at the Diabetes Center, Tokyo Women's Medical University Hospital and Naoyuki Kamatani, MD, PhD, a specialist of medical statistics at StaGen Co. Ltd. This paper was presented at the 54th Annual Meeting of the Japan Diabetes Society in Sapporo, Japan and the 12th Symposium of the International Diabetes Epidemiology Group in Sharjah, United Arab Emirates. We have no conflict of interest.
(J Diabetes Invest, doi: 10.1111/jdi.12044, 2013)
References
- 1.International Expert Committee . International expert committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care 2009; 32: 1327–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care 2010; 33(Suppl. 1): S62–S69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization . Use of glycated haemoglobin (HbA1c) in the diagnosis of diabetes mellitus. Abbreviated report of a WHO consultation. WHO/NMH/CHP/CPM/11.1. [PubMed]
- 4.The Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes Mellitus . Report of the committee on the classification and diagnostic criteria of diabetes mellitus. J Diabetes Invest 2010; 1: 212–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito C, Maeda R, Ishida S, et al Importance of OGTT for diagnosing diabetes mellitus based on prevalence and incidence of retinopathy. Diabetes Res Clin Pract 2000; 49: 181–186 [DOI] [PubMed] [Google Scholar]
- 6.Jeganathan VS, Cheung N, Tay WT, et al Prevalence and risk factors of retinopathy in an Asian population without diabetes: the Singapore Malay eye study. Arch Ophthalmol 2010; 128: 40–45 [DOI] [PubMed] [Google Scholar]
- 7.Ojaimi E, Nguyen TT, Klein R, et al Retinopathy signs in people without diabetes: the multi‐ethnic study of atherosclerosis. Ophthalmology 2011; 118: 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawasaki R, Wang JJ, Rochtchina E, et al Cardiovascular risk factors and retinal microvascular signs in an adult Japanese population: the funagata study. Ophthalmology 2006; 113: 1378–1384 [DOI] [PubMed] [Google Scholar]
- 9.Wong TY, Klein R, Sharrett AR, et al The prevalence and risk factors of retinal microvascular abnormalities in older persons: the cardiovascular health study. Ophthalmology 2003; 110: 658–666 [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Barr EL, Tapp RJ, et al Retinopathy in persons with impaired glucose metabolism: the Australian diabetes obesity and lifestyle study. Am J Ophthalmol 2005; 140: 1157–1159 [DOI] [PubMed] [Google Scholar]
- 11.Van Leiden HA, Dekker JM, Moll AC, et al Blood pressure, lipids, and obesity are associated with retinopathy: the hoorn study. Diabetes Care 2002; 25: 1320–1325 [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Klein BE, Moss SE, et al Hypertension and retinopathy, arteriolar narrowing, and arteriovenous nicking in a population. Arch Ophthalmol 1994; 112: 92–98 [DOI] [PubMed] [Google Scholar]
- 13.Yu T, Mitchell P, Berry G, et al Retinopathy in older persons without diabetes and its relationship to hypertension. Arch Ophthalmol 1998; 116: 83–89 [DOI] [PubMed] [Google Scholar]
- 14.Chao JR, Lai MY, Azen SP, et al Retinopathy in persons without diabetes: the Los Angeles latino eye study. Invest Ophthalmol Vis Sci 2007; 48: 4019–4025 [DOI] [PubMed] [Google Scholar]
- 15.Hubbard LD, Brothers RJ, King WN, et al Methods for evaluation of retinal microvascular abnormalities associated with hypertension sclerosis in the atherosclerosis risk in communities study. Ophthalmology 1999; 106: 2269–2280 [DOI] [PubMed] [Google Scholar]
- 16.Cugati S, Cikamatana L, Wang JJ, et al Five‐year incidence and progression of vascular retinopathy in persons without diabetes: the blue mountaions eye study. Eye (Lond) 2006; 20: 1239–1245 [DOI] [PubMed] [Google Scholar]
- 17.Kashiwagi A, Kasuga M, Araki E, et al International clinical harmonization of glycated hemoglobin in Japan: from Japan diabetes society to national glycohemoglobin standardization program values. J Diabetes Invest 2012; 3: 39–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyazaki M, Kubo M, Kiyohara Y, et al Comparison of diagnostic methods for diabetes mellitus based on prevalence of retinopathy in a Japanese population: the hisayama study. Diabetologia 2004; 47: 1411–1415 [DOI] [PubMed] [Google Scholar]
- 19.Klein R, Klein BE, Moss SE, et al Blood pressure, hypertension and retinopathy in a population. Trans Am Opthalmol Soc 1993; 91: 207–222 [PMC free article] [PubMed] [Google Scholar]
- 20.Wilkins JT, Ning H, Berry J, et al Lifetime risk and years lived free of total cardiovascular disease. JAMA 2012; 308: 1795–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colagiuri S, Lee CM, Wong TY, et al DETECT‐2 collaboration writing group glycemic thresholds for diabetes‐specific retinopathy: implications for diagnostic criteria for diabetes. Diabetes Care 2011; 34: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]


