Abstract
Aims/Introduction
The aim of the present study was to evaluate the predictive ability of body mass index (BMI), waist circumference (WC), waist‐to‐hip ratio (WHR), waist‐to‐height ratio (WHtR) and body fat percentages (BF%) for the presence of cardiometabolic risk factors, namely type 2 diabetes (DM), hypertension (HTN), dyslipidemia and metabolic syndrome (MS).
Materials and Methods
A total of 2293 subjects aged ≥20 years from rural Bangladesh were randomly selected in a population‐based, cross‐sectional survey. The association of anthropometric indicators with cardiometabolic risk conditions was assessed by using receiver operating characteristic curve analysis and adjusted odds ratios (ORs) for DM, HTN, dyslipidemia and MS.
Results
Area under the curve cut‐off values showed that the association of WHR, BF% and WC was higher than that for other indices for DM, HTN and MS, respectively, for both sexes, and WHtR for men and WHR for women for dyslipidemia. The ORs were highest for WHR for DM and WC for MS for both sexes, and WHtR for men and WC for women for HTN and dyslipidemia, respectively. The optimal cut‐off values for obesity for the present study in men and women showed BMIs of 22 and 22.8 kg/m2, WHRs of 0.93 and 0.87, WHtRs of 0.52 and 0.54, BF% of 21.4 and 32.4%, and WCs of 82 and 81 cm, except for MS, which were 90 for men and 80 for women.
Conclusions
Compared with BMI, measures of central obesity, particularly WHR, WC, WHtR and BF%, showed a better association with obesity‐related cardiometabolic risk factors for both sexes.
Keywords: Anthropometric indicators, Cardiometabolic risk factors, Obesity
Introduction
Once considered a problem only of high‐income countries, obesity continues to be an important clinical and public health problem worldwide. In 2008, the World Health Organisation (WHO) reported that 1.5 billion adults aged 20 and older were overweight, and of these, over 200 million men and nearly 300 million women were obese1. Epidemiological studies have shown overweight and obesity as an independent risk factor of type 2 diabetes (DM), hypertension (HTN), dyslipidemia and cardiovascular disease (CVD)2. Central obesity, which suggests excessive deposition of intra‐abdominal fat, is also found to be an important predictor of cardiometabolic risk. Furthermore, central obesity is assumed to play a pivotal role in the development of the ‘metabolic syndrome’ (MS), a term given to the clustering of CVD risk factors4.
Although there are several instruments to measure total body fat and its distribution5, there is still no ideal method for the measurement of adiposity (diagnostic definitions) or cut‐off points that should satisfy the criteria of being accurate, precise, accessible and acceptable worldwide. The concept of different cut‐offs for different ethnic groups has been proposed by the WHO, because some ethnic groups have higher cardiovascular and metabolic risks at lower body mass index (BMI). This might be because of differences in body shape and fat distribution. Studies have found that for the same age, sex and BMI, south Asians have higher body fat percentage (BF%) than white Caucasians. In Caucasian men, a BMI of 30 kg/m2 corresponds to 25% body fat7, whereas in south Asian men, a BMI of <25 kg/m2 corresponds to 33% body fat8.
Anthropometric measurements still play an important role in clinical practice and epidemiological surveys. BMI is often used to reflect total body fat amounts, whereas the waist circumference (WC), waist‐to‐hip ratio (WHR) and waist‐to‐height ratio (WHtR) are used as surrogates for intra‐abdominal adiposity9. The International Association for the Study of Obesity and the International Obesity Task Force have suggested lower BMI cut‐off values for the definitions of overweight (23.0–24.9 kg/m2) and obesity (25.0 kg/m2 or greater) in Asian populations because of the observed differences between populations12. However, there are few reports and only small studies in the south Asian region based on these cut‐off values. In the present study, we aimed to define and compare the cut‐off values of BMI, WC, WHR, WHtR and BF% for several cardiometabolic risk factors including DM, HTN, dyslipidemia and MS in a rural Bangladeshi population in Chandra, 40 km from Dhaka, the capital city. It should be noted that vast majority (72%) of the Bangladeshi population live in rural areas14.
Materials and Methods
The current cross‐sectional study was carried out during March to December 2009 in an urbanizing rural community ‘Chandra’, 40 km north of the capital city, Dhaka. A total of 10 villages were randomly selected from five areas with a population of approximately 20,000. For the present study, 3,000 individuals were randomly selected, and among them 2,376 (79.2%) participated. The present analysis is based on 2,293 participants (842 men and 1,451 women) for whom all the variables were available. A detailed description of the sample population was described elsewhere15. The inclusion criteria were: aged ≥20 years, willing to participate and being able to communicate. Exclusion criteria included pregnant women, and self‐reported or medical‐recorded history of myocardial infarction, renal disease, liver disease, tuberculosis, malignant diseases and any severe infection at the time of screening. The present study was carried out according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human participants were approved by the National Committee for Medical and Health Research Ethics of Norway and the Ethical Committee of Diabetic Association of Bangladesh for Medical Research. As 52.1% of the adult population in Bangladesh is illiterate14, a witnessed and formally recorded verbal informed consent was obtained from all participants before inclusion in the study to avoid selection bias by literacy. The participants were also verbally informed of their right to withdraw from the study at any stage, or to restrict their data from the analysis. After the verbal information, a printed copy of their rights was given. Once the selection procedure was completed, participants were requested to visit a nearby field center after an overnight fast of 8–14 h. The sociodemographic data was collected by trained staff by interviewing the participants using a predesigned pretested questionnaire.
Blood Pressure and Anthropometrical Measurements
Anthropometric measurements, such as height, weight and waist and hip circumferences, were taken with the participants wearing light clothes and without shoes. The weight was taken to the nearest 0.1 kg by modern electronic digital LCD weighing machines (Best Deluxe Model; Bathroom, Dhaka, Bangladesh) placed on a flat surface. The scales were calibrated everyday against a standard (20 kg). Height was taken while the participants stood in erect posture, touching the occiput, back, hip and heels on a straight measuring wall, while the participants looked straight ahead. BMI was calculated as the weight (kg) divided by square of the height (m2). Waist circumference was measured by placing a tape horizontally midway between the lower border of the ribs and iliac crest on the mid‐axillary line. Hip circumference was measured to the nearest centimeter at the greatest protrusion of the buttocks, just below the iliac crest. WHR and WHtR were then calculated from waist circumference (cm) and height (cm), respectively. In addition, BF% was determined by using Deurenberg's prediction formula for adults16.
Blood pressure was measured in the right arm in both a sitting and standing position. Before the measurement, a 10‐min rest was assured and using standard cuffs for adults fitted with a sphygmomanometer minimized variation in measurement. The systolic BP was determined by the onset of the ‘tapping’ Korotkoff sound (K1). The fifth Korotkoff sound (K5), or the disappearance of Korotkoff sounds, defined the diastolic BP. Two readings were taken 5 min apart, and the mean of the two was taken as the final blood pressure reading of the individual.
Laboratory Tests
On arrival at the field center, an 8‐mL fasting venous blood sample was taken from each participant for measuring fasting plasma glucose (FPG), total cholesterol (TC), low‐density lipoprotein cholesterol (LDL‐C), triglycerides (TG) and high‐density lipoprotein cholesterol (HDL‐C). All participants other than those with known diabetes were then given a 75‐g oral glucose solution (75 g of oral glucose in 250 mL of water) to drink. Another 3 mL of venous blood was collected after 2 h to determine 2‐h post‐oral glucose tolerance test (OGTT). The samples for the plasma glucose test were collected in a tube containing sodium fluoride and potassium oxalate (1:3), and were centrifuged immediately after collection. Separated plasma samples were sent to a laboratory of the Bangladesh Institute of Research and Rehabilitation for Diabetes, Endocrine and Metabolic Disorders (BIRDEM) in ice gel‐packed cooling boxes and stored at −70°C until laboratory assays were carried out. Plasma glucose was measured by the glucose oxidase method using Dimalesion RxL Max (Siemens AG, Erlangen, Germany) on the same day. Quality control of the blood glucose measurement was checked by measuring the 2‐h plasma glucose values using the glucose oxidase methods in every 10th case. The intra‐assay coefficient of variation was 1.24% at a mean of 5.86 mmol/L, and the inter‐assay coefficient of variation was 2.10% at a mean of 5.23 mmol/L. Fasting serum lipid profile was estimated by standard enzymatic procedures (Dimalesion RxL Max). HDL‐C was estimated by the direct assay method and LDL‐C was estimated by Friedewald's formula.
Cut‐off values for general obesity for both sexes were a BMI of ≥25 kg/m2; cut‐off values for central obesity including waist circumference for men and women were ≥90 and ≥80 cm, WHR for men and women were ≥0.90 and ≥0.8012, WHtR for both sexes were ≥0.5017, and BF% for men and women were ≥25% and ≥30%, respectively19.
Diabetes was defined as FPG ≥7.0 mmol/L and/or 2 h after 75‐g oral glucose solution ≥11.1 mmol/L20. In addition, known diabetes was defined by the use insulin or oral antidiabetic medication(s) and self‐reported DM. Individuals were considered to have hypertension if their average systolic blood pressure was ≥140 mmHg or diastolic blood pressure was ≥90 mmHg, or if they were receiving treatment for hypertension21. The presence of MS was defined as the presence of at least three of the following: waist circumference for men and women were >90 and >80 cm, respectively; serum triglycerides ≥1.70 mmol/L; HDL‐C for men <1.04 mmol/L and for women <1.29 mmol/L; measured blood pressure ≥130/85 mmHg; and fasting blood glucose ≥5.6 mmol/L. We used the National Cholesterol Education Program adult treatment panel III criteria modified for Asian subjects as recommended by an American Heart Association‐National Heart Lung Blood Institute statement for defining MS22. Dyslipidemia was defined as serum triglycerides ≥1.70 mmol/L and HDL‐C < 1.04 mmol/L for men and <1.29 mmol/L for women. Physical activity was graded on the ordinal scale of 1–3, corresponding to light, moderate and heavy, according to the activity level of the study population. For the purpose of data analysis, these results were transformed into a binary variable: inactive (grade 1) and active (grade 2 and 3). Smoking habit was classified as either current or non/ex‐smoker.
Statistical Analysis
Means and 95% confidence intervals (CIs) adjusted for age were given for normally distributed continuous variables. Skewed data were logarithm transformed before analysis, and the results were transformed back to the original scale. Percentages and 95% CIs were given for categorical variables. Differences between the groups (men and women) of means and proportions adjusted for age were tested by analysis of covariance (ancova) and logistic regression. Sensitivity and specificity were examined by the receiver operating characteristic (ROC) analysis23, and the areas under the ROC curve (AUC) cut‐off values were calculated for each anthropometrical parameter and risk condition. An AUC of 1 indicates perfect separation between affected and non‐affected participants, and an AUC of 0.5 indicates no discriminative value of the test. Differences between correlated AUCs for each sex were tested by the roccomp command in stata 11 for Windows (StataCorp, College Station, TX, USA) using an algorithm suggested by DeLong et al.25 Summary statistics for the correlated AUCs were also reported by the same command. Additionally, we calculated both crude and adjusted odds ratios (ORs) of respective anthropometric indicators for DM, HTN, dyslipidaemia and MS. Adjusted ORs were obtained by applying logistic regression analysis with adjustments for age, smoking habit, physical inactivity and family history of diabetes. The interaction between a potential confounding variable or an anthropometrical variable and sex in multiple logistic regressions was examined by a likelihood ratio test. Statistical inference is based on 95% CIs and the significance level was set at 0.05. stata 11 for Windows was used to create ROC curves. Otherwise, pasw statistics 18 for Windows (SPSS, Chicago, IL, USA) was used.
Results
Characteristics of the study population are shown in Table 1. There were 842 men and 1,451 women in the study. Men had significantly greater mean of WC, WHR, height, weight and systolic blood pressure than women. WHtR and BF% value were significantly less in men than in women, and BMI was similar in the two groups. Metabolic profiles also differed; men had significantly higher mean FPG and TG values, but lower HDL‐C values than women. Men also had a higher rate of DM, HTN, dyslipidemia, physical activity and smoking habits, but a lower rate of central obesity (defined by WC, WHR, WHtR and BF%) than women. General obesity (defined by BMI), MS and family history of diabetes were similar in the two groups.
Table 1. Characteristics of study population in both sexes.
| Variable | Total | Male | Female | P‐value |
|---|---|---|---|---|
| n | 2293 | 842 | 1451 | |
| Age (years) | 41.8 (41.2, 42.4) | 44.3 (43.3, 45.2) | 40.4 (39.7, 41.1) | <0.001 |
| Smoking habit (%) | 15.9 (14.6, 17.2 | 40.5 (37.2, 43.8) | 1.7 (1.0, 2.3) | <0.001 |
| Physical inactivity (%) | 15.1 (13.8, 16.4) | 35.9 (32.6, 39.1) | 3.1 (2.2, 4.0) | <0.001 |
| Weight (kg) | 54.9 (54.5, 55.3) | 58.5 (57.9, 59,2) | 51.3 (50.8, 51.8) | <0.001 |
| Height (m) | 1.556 (1.543, 1.558) | 1.609 (1.605, 1.613) | 1.503 (1.499, 1.50) | <0.001 |
| BMI (kg/m2) | 22.6 (22.5, 22.8) | 22.6 (22.3, 22.8) | 22.7 (22.5, 22.9) | 0.605 |
| BMI, ≥25 kg/m2 (%) | 26.2 (24.4, 28.0) | 25.2 (22.3, 28.2) | 26.8 (24.5, 29.0) | 0.430 |
| WC (cm) | 80.7 (80.3, 81.1) | 81.7 (81.0, 82.4) | 79.7 (79.2, 80.3) | <0.001 |
| WC, m ≥ 90 cm; f ≥ 80 cm (%) | 39.8 (37.9, 41.8) | 24.4 (21.5, 27.3) | 48.7 (46.2, 51.3) | <0.001 |
| WHR | 0.88 (0.87, 0.89) | 0.90 (0.89, 0.91) | 0.86 (0.85, 0.87) | <0.001 |
| WHR, m ≥ 0.90; f ≥ 0.80 (%) | 71.6 (69.8, 73.4) | 58.6 (55.2, 61.9) | 79.1 (77.0, 81.1) | <0.001 |
| WHtR | 0.52 (0.51, 0.53) | 0.51 (0.50, 0.52) | 0.53 (0.52, 0.54) | <0.001 |
| WHtR, ≥0.50 (%) | 60.1 (58.1, 62.1) | 53.5 (50.1, 56.9) | 64.0 (61.5, 66.5) | <0.001 |
| BF% | 26.3 (26.1, 26.5) | 21.5 (21.2, 21.8) | 31.2 (30.9, 31.4) | <0.001 |
| BF%, m ≥ 25%; f ≥ 30% (%) | 42.9 (41.1, 44.7) | 19.1 (16.7, 21.5) | 57.3 (54.9, 59.6) | <0.001 |
| SBP (mmHg) | 116.2 (115.6, 116.9) | 117.2 (116.2, 118.3) | 115.2 (114.4, 116.1) | 0.002 |
| DBP (mmHg) | 77.1 (76.6, 77.5) | 77.6 (76.9, 78.2) | 76.5 (76.0, 77.1) | 0.499 |
| Hypertension (%) | 15.5 (14.1, 17.0) | 17.5 (15.1, 20.0) | 14.3 (12.5, 16.1) | 0.034 |
| FPG (mmol/L) | 5.2 (5.1, 5.3) | 5.3 (5.2, 5.5) | 5.1 (5.0, 5.2) | 0.002 |
| 2hPG (mmo/L) | 6.3 (6.2, 6.4) | 6.3 (6.1, 6.5) | 6.2 (6.1, 6.4) | 0.499 |
| Diabetes (%) | 7.9 (6.8, 9.0) | 9.1 (7.2, 11.0) | 7.2 (5.8, 8.5) | 0.101 |
| TGa(mmol/L) | 1.38 (1.35, 1.40) | 1.43 (1.39, 1.48) | 1.32 (1.29, 1.36) | <0.001 |
| HDL (mmol/L) | 0.90 (0.89, 0.91) | 0.86 (0.84, 0.97) | 0.93 (0.92, 0.94) | <0.001 |
| Dyslipidemia (%) | 28.7 (26.9, 30.5) | 35.3 (32.1, 38.5) | 24.8 (22.6, 27.0) | <0.001 |
| Metabolic syndrome (%) | 30.7 (28.8, 32.6) | 30.0 (26.9, 33.1) | 31.1 (28.7, 33.5) | 0.569 |
| Family history of DM (%) | 17.6 (15.6, 19.5) | 17.7 (15.1, 20.3) | 17.6 (15.6, 19.5) | 0.687 |
2hPG, 2‐h plasma glucose; BF%, body fat percentage; BMI, body mass index; DM, diabetes mellitus; FPG, fasting plasma glucose; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
Data are mean (95% confidence interval) or percentage (95% confidence interval) adjusted for age as indicated.
Geometric mean (95% confidence interval) for triglyceride (TG). Dyslipidemia: fasting TG ≥150 mg/dL and high‐density lipoproteins (HDL) <40 mg/dL (male), <50 mg/dL (female). Metabolic syndrome: diagnosed according to National Cholesterol Education Program adult treatment panel III criteria guideline22. Diabetes: diagnosed according to World Health Organization 20 criteria20. Hypertension: systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg21.
The AUC cut‐off values for men and women are presented in Table 2. For men, the optimal cut‐offs for BMI related to DM, HTN, dyslipidemia and MS ranged from 21.2 to 23.6 kg/m2; for WC 79.0 to 90.0 cm, for WHtR from 0.51 to 0.53, for BF% from 21.1 to 22.5% and for WHR the optimal cut‐off was 0.93. For women, the optimal cut‐offs for BMI ranged from 21.8 to 22.8 kg/m2, for WC from 80.0 to 82.0 cm, for WHR from 0.86 to 0.89, for WHtR from 0.53 to 0.54 and for BF% from 32.1 to 34.9%.
Table 2. Cut‐off values, sensitivity and specificity for the association of anthropometric parameters with diabetes mellitus, hypertension, dyslipidemia and metabolic syndrome using the National Cholesterol Education Program adult treatment panel III criteria modified for Asian subjects.
| BMI | WC (cm) | WHR | WHtR | BF% | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cut‐offs (kg/m2) | Sn (%) | Spe (%) | Cut‐offs (cm) | Sn (%) | Spe (%) | Cut‐offs | Sn (%) | Spe (%) | Cut‐offs | Sn (%) | Spe (%) | Cut‐offs | Sn (%) | Spe (%) | |
| Male | |||||||||||||||
| DM | 21.2 | 82.5 | 41.2 | 82.0 | 76.2 | 53.7 | 0.93 | 68.8 | 60.9 | 0.53 | 63.8 | 66.4 | 22.5 | 65.0 | 65.2 |
| HTN | 22.0 | 71.7 | 52.0 | 79.0 | 78.6 | 44.9 | 0.93 | 54.1 | 63.4 | 0.52 | 62.9 | 60.5 | 21.4 | 73.4 | 57.9 |
| Dyslipidemia | 22.0 | 74.5 | 59.3 | 82.0 | 76.5 | 56.3 | 0.93 | 56.9 | 70.7 | 0.51 | 72.9 | 60.9 | 21.1 | 65.9 | 61.3 |
| MS | 23.6 | 70.8 | 77.5 | 90.0 | 55.2 | 94.3 | 0.93 | 68.6 | 71.9 | 0.52 | 78.5 | 73.1 | 21.4 | 78.2 | 67.4 |
| Female | |||||||||||||||
| DM | 21.8 | 77.2 | 46.5 | 82.0 | 67.3 | 62.5 | 0.87 | 84.2 | 54.5 | 0.54 | 72.3 | 55.9 | 34.9 | 49.5 | 77.1 |
| HTN | 22.8 | 64.5 | 57.8 | 81.0 | 64.5 | 61.2 | 0.89 | 55.8 | 65.4 | 0.54 | 65.9 | 60.4 | 32.4 | 66.0 | 64.5 |
| Dyslipidemia | 21.9 | 69.1 | 50.1 | 81.0 | 61.8 | 64.1 | 0.86 | 72.5 | 58.0 | 0.53 | 69.1 | 55.2 | 32.1 | 60.4 | 64.8 |
| MS | 22.8 | 74.4 | 68.4 | 80.0 | 86.7 | 71.9 | 0.87 | 80.6 | 66.8 | 0.54 | 81.9 | 71.0 | 32.5 | 70.0 | 75.2 |
BF%, body fat percentage; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; Sn, sensitivity; Spe, specificity; MS, metabolic syndrome; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
The calculated AUCs for predicting DM, HTN, dyslipidemia, and MS by BMI, WC, WHR, WHtR and BF% for men and women are shown in Figure 1, and their associations are shown in Table 3.
Figure 1.
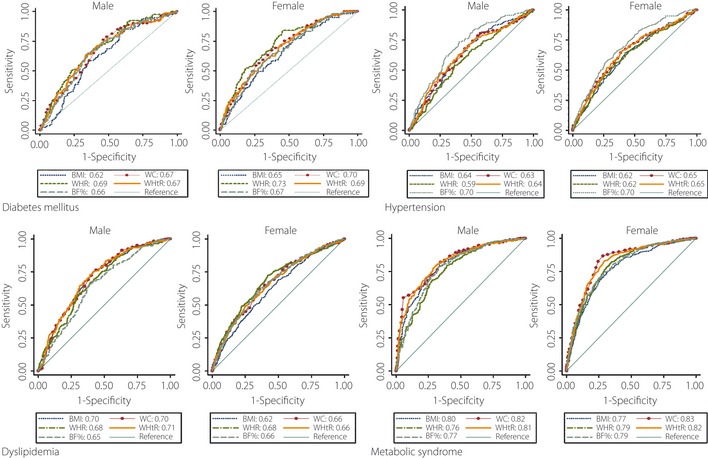
Receiver operating characteristics curve for body mass index (BMI), wait circumference (WC), waist‐to‐hip ratio (WHR), waist‐to‐height ratio (WHtR) and body fat percentages (BF%) values to predict diabetes mellitus, hypertension, dyslipidemia and metabolic syndrome for males and females.
Table 3. Association of anthropometric variables with diabetes mellitus, hypertension, dyslipidemia and metabolic syndrome.
| BMI | WC | WHR | WHtR | BF% | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | AUC | 95% CI | |
| Male | ||||||||||
| DM | 0.62 | 0.56, 0.68 | 0.67* | 0.61, 0.73 | 0.69 * | 0.63, 0.75 | 0.67* | 0.61, 0.73 | 0.66 | 0.60, 0.72 |
| HTN | 0.64* | 0.59, 0.69 | 0.63* | 0.58, 0.66 | 0.59 | 0.54, 0.64 | 0.64* | 0.59, 0.69 | 0.70 * | 0.66, 0.75 |
| Dyslipidemia | 0.70*,† | 0.67, 0.74 | 0.70* | 0.67, 0.74 | 0.68 | 0.64, 0.72 | 0.71* | 0.67, 0.74 | 0.65 | 0.61, 0.69 |
| MS | 0.80* | 0.76, 0.83 | 0.82 * | 0.79, 0.85 | 0.76 | 0.72, 0.89 | 0.81* | 0.78, 0.84 | 0.77 | 0.74, 0.81 |
| Female | ||||||||||
| DM | 0.65 | 0.60, 0.71 | 0.70* | 0.65, 0.75 | 0.73 * | 0.68, 0.78 | 0.69 | 0.64, 0.74 | 0.67 | 0.62, 0.72 |
| HTN | 0.62 | 0.58, 0.67 | 0.65* | 0.61, 0.69 | 0.62 | 0.58, 0.67 | 0.65* | 0.61, 0.69 | 0.70 * | 0.66, 0.74 |
| Dyslipidemia | 0.62 | 0.59, 0.66 | 0.66* | 0.63, 0.70 | 0.68 * | 0.65, 0.71 | 0.66* | 0.63, 0.69 | 0.66* | 0.63, 0.69 |
| MS | 0.77 | 0.74, 0.79 | 0.83 ** | 0.80, 0.85 | 0.79 | 0.77, 0.82 | 0.82** | 0.79, 0.84 | 0.79 | 0.77, 0.82 |
Bold values indicate the highest AUCs. *P < 0.05. * * P < 0.01 for the comparison of area under the receiver operating characteristics curve (AUC) for anthropometric indicators in predicting the cardiometabolic condition. † the comparison of corresponding AUCs for males and females (*P < 0.05). BF%, body fat percentage; BMI, body mass index; DM, diabetes mellitus; HTN, hypertension; MS, metabolic syndrome; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
For men, regarding DM, the AUC value for WHR was significantly higher than for BMI, WC, WHtR and BF%; regarding HTN, the AUC value for BF% was significantly higher than for BMI, WC, WHR and WHtR; regarding dyslipidemia, the AUC value for WHtR was significantly higher than for WHR and BF%; and regarding MS, the AUC value for WC was significantly higher than for BMI, WHR and BF%.
For women, regarding DM, the AUC value for WHR was significantly higher than for BMI and BF%; regarding HTN, the AUC value for BF% was significantly higher than for BMI, WC, WHR and WHtR; regarding dyslipidemia, the AUS value for WHR was only significantly higher than for BMI; and regarding MS, the AUC value for WC was significantly higher than BMI, WHR, WHtR and BF%. However, the AUC values for BMI and WHtR were significantly higher for men than women only for dyslipidaemia.
Significant interactions were found between age and sex (P < 0.001), physical inactivity and sex (P < 0.001), smoking and sex (P < 0.001), and between anthropometrical variables and sex (P < 0.001 for WHR, WC and BF%) in the multivariate analyses of logistic regression. Therefore, the statistical results were reported in the table(4a and 4b) for each sex.
Table 4. Crude and adjusted odds ratios of body mass index, waist circumference, waist‐to‐hip ratio, waist‐to‐height‐ratio and body fat percentage for predicting diabetes mellitus, hypertension, dyslipidemia and metabolic syndrome for (a) males and (b) females.
| DM | HTN | Dyslipidemia | MS | |||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | P‐value | 95% CI | P‐value | 95% CI | P‐value | 95% CI | P‐value | |
| (a) Males | ||||||||
| BMI (≥25 kg/m2) | ||||||||
| Crude | 1.53 (0.93–2.52) | 0.091 | 1.90 (1.31, 2.76) | 0.001 | 3.20 (2.32–4.43) | <0.001 | 7.01 (4.97–9.88) | <0.001 |
| Adjusted | 1.50 (0.90–2.53) | 0.116 | 1.95 (1.32–2.87) | 0.001 | 3.21 (2.31–4.47) | <0.001 | 6.87 (4.82–9.79) | <0.001 |
| WC (≥90 cm) | ||||||||
| Crude | 2.27 (1.40–3.66) | 0.001 | 2.17 (1.50–3.14) | <0.001 | 3.16 (2.28–4.37) | <0.001 | 12.47 (8.63–18.03) | <0.001 |
| Adjusted | 2.09 (1.28–3.43) | 0.003 | 2.16 (1.47–3.18) | <0.001 | 3.14 (2.26–4.38) | <0.001 | 12.02 (8.24–17.52) | <0.001 |
| WHR (≥0.90) | ||||||||
| Crude | 3.60 (1.99–6.52) | <0.001 | 1.83 (1.26–2.66) | 0.001 | 3.08 (2.26–4.21) | <0.001 | 5.84 (4.04–8.48) | <0.001 |
| Adjusted | 3.21 (1.76–5.88) | <0.001 | 1.69 (1.15–2.48) | 0.007 | 3.12 (2.27–4.28) | <0.001 | 5.38 (3.70–7.84) | <0.001 |
| WHtR (≥0.50) | ||||||||
| Crude | 3.26 (1.90–5.63) | <0.001 | 2.67 (1.83–3.89) | <0.001 | 4.21 (3.08–5.75) | <0.001 | 8.62 (5.90–12.59) | <0.001 |
| Adjusted | 2.97 (1.70–5.18) | <0.001 | 2.55 (1.72–3.77) | <0.001 | 4.35 (3.16–6.00) | <0.001 | 7.97 (5.43–11.69) | <0.001 |
| BF% (≥25%) | ||||||||
| Crude | 1.78 (0.94–3.57) | 0.092 | 2.59 (2.12–4.50) | <0.001 | 1.86 (1.33–2.59) | <0.001 | 5.07 (3.57–7.19) | <0.001 |
| Adjusted | 1.60 (0.92–2.78) | 0.094 | 2.35 (1.54–3.57) | <0.001 | 2.14 (1.47–3.12) | <0.001 | 5.24 (3.52–7.78) | <0.001 |
| (b) Females | ||||||||
| BMI (≥25 kg/m2) | ||||||||
| Crude | 2.42 (1.60–3.64) | <0.001 | 2.10 (1.54–2.89) | <0.001 | 2.09 (1.61–2.69) | <0.001 | 5.42 (4.22–6.96) | <0.001 |
| Adjusted | 2.55 (1.69–3.87) | <0.001 | 2.42 (1.75–3.35) | <0.001 | 2.22 (1.71–2.89) | <0.001 | 6.38 (4.89–8.31) | <0.001 |
| WC (≥90 cm) | ||||||||
| Crude | 3.45 (2.17–5.49) | <0.001 | 2.73 (1.97–3.78) | <0.001 | 2.68 (2.08–3.45) | <0.001 | 15.56 (11.37–21.30) | <0.001 |
| Adjusted | 3.47 (2,18–5.53) | <0.001 | 2.88 (2.07–4.02) | <0.001 | 2.74 (2.13–3.54) | <0.001 | 17.95 (12.93–24.94) | <0.001 |
| WHR (≥0.90) | ||||||||
| Crude | 5.53 (2.23–13.72) | <0.001 | 1.72 (1.13–2.63) | 0.011 | 2.55 (1.78–3.64) | <0.001 | 10.83 (6.46–18.17) | <0.001 |
| Adjusted | 5.35 (2.16–13.31) | <0.001 | 1.65 (1.07–2.53) | 0.022 | 2.49 (1.74–3.57) | <0.001 | 10.78 (6.41–18.14) | <0.001 |
| WHtR (≥0.50) | ||||||||
| Crude | 3.48 (1.99–6.01 | <0.001 | 2.47 (1.72–3.56) | <0.001 | 2.61 (1.98–3.46) | <0.001 | 9.96 (6.99–14.20) | <0.001 |
| Adjusted | 3.47 (1.98–6.06) | <0.001 | 2.54 (1.75–3.69) | <0.001 | 2.64 (1.98–3.50) | <0.001 | 10.58 (7.38–15.18) | <0.001 |
| BF% (≥25%) | ||||||||
| Crude | 2.74 (1.71–4.39) | <0.001 | 2.60 (2.23–5.49) | <0.001 | 2.50 (1.93–3.24) | <0.001 | 6.67 (5.04–8.84) | <0.001 |
| Adjusted | 2.46 (1.50–4.05) | <0.001 | 2.83 (1.93–4.17) | <0.001 | 2.13 (1.62–2.81) | <0.001 | 6.18 (4.60–8.29) | <0.001 |
BF%, body fat percentage; BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; HTN, hypertension; MS, metabolic syndrome; WC, waist circumference; WHR, waist‐to‐hip ratio; WHtR, waist‐to‐height ratio.
Adjusted for age, smoking, physical inactivity and family history of diabetes. Age groups used in logistic regression model (20–30 years, 31–40 years, 41–50 years and ≥51 years).
Table 4a and 4b show the ORs of different anthropometric indicators for predicting DM, HTN, dyslipidemia, and MS using both crude and adjusted logistic regression analysis after adjustment for age, smoking habit, physical inactivity and family history of diabetes. The ORs were highest for WHR for DM and WC for MS for both sexes. In contrast, the ORs were highest for WHtR for men and WC for women for HTN and dyslipidemia, respectively. Crude and adjusted ORs were very similar.
Discussion
This is the first population‐based cross‐sectional study that attempted to define and comparatively evaluate cut‐off values of BMI, WC, WHR, WHtR and BF% for a few important cardiometabolic risk factors including DM, HTN, dyslipidemia and MS in a rural population of Bangladesh who can be marked as an Asian Indian population. The optimal cut‐off values for BMI ranged between 21.2 kg/m2 and 23.6 kg/m2 for men, and 21.8 kg/m2 and 22.8 kg/m2 for women to indicate risk for DM. These values were apparently lower than the values for the Western population (BMI ≥25 kg/m2 for overweight and BMI ≥30 kg/m2 for obesity) and for the Asia–Pacific population13 (BMI ≥23 kg/m2 for overweight and BMI ≥25 kg/m2 for obesity) that have been previously recommended. Optimal values of WC fell into a wide range of 79–90 cm for men and 80–82 cm for women. The optimal WC cut‐off values of 90 cm for men and 80 cm for women for MS were similar to the cut‐off levels recommended by the International Diabetes Federation (IDF) for the Asian population26. However, IDF values remained lower than optimal WHR values of 0.93 for men and 0.87–0.89 for women in the present study. Optimal WHtR levels of 0.51–0.53 for men and 0.53–0.54 for women were higher than the present standard for WHtR of ≥0.50 for both sexes17, and the optimum level of BF% 21.1–21.4% for men were lower than the standard level of ≥25% for men, but the optimal cut‐off values of 32.1–34.9 for women were higher than the standard level of ≥30% for women19. In the present analysis, more women had central obesity than men, as defined by the WHO for the Asian population13. This might be a consequence of the division of labor by sex in this community. Manual labour is sustained by male physical labor.
In the present study, the most sensitive indices were WHR for DM, BF% for HTN and WC for MS for both sexes; whereas WHtR for men and WC for women were most sensitive for dyslipidemia. Study findings support that central obesity is an important indicator for predicting cardiometabolic risk compared with general obesity as measured by BMI in the Asian population, which has been verified by a number of studies as well as pathophysiological mechanisms. From a pathophysiological point of view, central obesity has been found to play a vital role in the pathogenesis of insulin resistance27, increased plasma leptin, low plasma adiponectin28 and stimulation of inflammatory cytokines29; all of these factors lead to the development of atherosclerosis, type 2 diabetes, HTN, dyslipidemia and MS. Also, although BMI is an acceptable approximation of total body fat at the population level, and can be used to estimate the relative risk of disease in most people, it is not always an accurate predictor of body fat or fat distribution, particularly in muscular individuals, because of differences in body‐fat proportions and distribution. Previous reports have shown a stronger association of cardiovascular disease risk factors with central obesity than with general obesity in different Asian populations and Bangladeshi subjects7, which is in agreement with the present results for DM, HTN, dyslipidemia and MS.
The AUC values for WC, WHR, WHtR and BF% were higher for women than for men for most risk indicators, and the present findings were similar to recent meta‐analyses that Lee et al.18 and Dong et al.36 carried out.
The present study had some limitations. This was a cross‐sectional study; therefore, our data only showed the associations with present risk factors, but did not directly predict the future risk of cardiovascular events. More prospective or longitudinal studies, however, are required to determine the future risk of development of cardiometabolic risk factors related to obesity in the south Asian population. Subject exclusion based on self‐reported personal medical history was another limitation of the present study, and which might cause selection bias. It is of note that AUCs of ROC analyses were not adjusted by age in the present study. Therefore, the relationship between each anthropometric measure and different cardiometabolic risk factors might be confounded by the influence of aging, underestimating the actual predictive value calculated in ROC analysis.
In conclusion, we projected various anthropometric indices of obesity associated with the risk related to cardiometabolic threats. It should be noted that the risk factors themselves are based on arbitrary cut‐offs, and do not necessarily indicate a clinical condition, especially like DM, hypertension, dyslipidemia and MS. Thus, the recommended cut‐off values show levels of the anthropometric indices above which the population are screened for cardiometabolic risk. The present data suggested that a BMI of 22 kg/m2 for men and 22.8 kg/m2 for women; a WC of 82 cm for men and 81 cm for women, except for MS which were 90 cm for men and 80 cm for women; a WHR of 0.93 for men and 0.87 for women; a WHtR of 0.52 for men and 0.54 for women; and 21.4% for men and 32.4% for women were optimal cut‐offs for defining general and central adiposity in the adult population of Bangladesh. The present study finding proposed that indices of central obesity predicted better cardiometabolic risk factors than general obesity defined by BMI for both men and women. We therefore recommend that the cut‐off values in use for defining obesity as a risk indicator should be readjusted for the population in question.
Acknowledgments
We acknowledge the contribution of our survey team members, the village leaders and volunteers for their continuous effort in the collection of data. We are grateful to all participants in the study for their active co‐operation. We also express our admiration to the authority of the Diabetic Association of Bangladesh for providing us with local logistic support, and the University of Oslo for their financial support. The authors declare no conflict of interest.
(J Diabetes Invest, doi: 10.1111/jdi.12053, 2013)
References
- 1.World Health Organization . Factsheet No. 311 – ‘Obesity and Overweight’. September 2006, Geneva. Available at: http://www.who.int/mediacentre/factsheets/fs311/en/index.html (accessed January 18 2011).
- 2.Balkau B, Deanfield JE, Despres JP, et al International Day for the Evaluation of Abdominal Obesity (IDEA): a study of waist circumference, cardiovascular disease, and diabetes mellitus in 168,000 primary care patients in 63 countries. Circulation 2007; 116: 1942–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casanueva FF, Moreno B, Rodriguez‐Azeredo R, et al Relationship of abdominal obesity with cardiovascular disease, diabetes and hyperlipidaemia in Spain. Clin Endocrinol (Oxf) 2010; 73: 35–40. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM. Multifactorial causation of obesity: implications for prevention. Am J Clin Nutr 1998; 67: 563–572. [DOI] [PubMed] [Google Scholar]
- 5.Mazess RB, Barden HS, Bisek JP, et al Dual‐energy X‐ray absorptiometry for total body and regional bone mineral and soft tissue composition. Am J Clin Nutr 1990; 51: 1106–1112. [DOI] [PubMed] [Google Scholar]
- 6.Friedl KE, Deluca JP, Marchitelli LJ, et al Reliability of body fat estimations from a four‐compartment model by using density, body water, and bone mineral measurements. Am J Clin Nutr 1992; 55: 764–770. [DOI] [PubMed] [Google Scholar]
- 7.Dudeja V, Misra A, Pandey RM, et al BMI does not accurately predict overweight in Asian Indians in northern India. Br J Nutr 2001; 86: 105–112. [DOI] [PubMed] [Google Scholar]
- 8.Banerji MA, Faridi N, Atluri R, et al Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab 1999; 84: 137–144. [DOI] [PubMed] [Google Scholar]
- 9.Gallagher D, Visser M, Sepulveda D, et al How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 1996; 143: 228–239. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg P, Yap M, Van Staveren WA. Body mass index and percent body fat: a meta‐analysis among different ethnic groups. Int J Obes 1988; 22: 1164–1171. [DOI] [PubMed] [Google Scholar]
- 11.Pouliot MC, Despres JP, Lemieux S, et al Waist circumference and abdominal sagittal diameter: the best simple anthropometric indices of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol 1994; 73: 460–468. [DOI] [PubMed] [Google Scholar]
- 12.Choo V. WHO reassesses appropriate body‐mass index for Asian populations. Lancet 2002; 360: 235. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization, Western Pacific Region . The International Association for the Study of Obesity and the International Obesity Task Force. The Asia–Pacific Perspective: Redefining Obesity and its Treatment. Health Communications Australia Pty Limited, Sydney, Australia, 2000. Available at: www.diabetes.com.au/pdf/obesity_report.pdf (accessed August 23 2006). [Google Scholar]
- 14.CIA‐The World Fact book . Available at:https://www.cia.gov/library/publications/the-world factbook/geos/bg.html. (accessed October 4 2012).
- 15.Hussain A, Rahim MA, Azad Khan AK, et al Type 2 diabetes in rural and urban population: diverse prevalence and associated risk factors in Bangladesh. Diabet Med 2005; 22: 931–936. [DOI] [PubMed] [Google Scholar]
- 16.Deurenberg P, Weststrate JA, Seidell JC. Body mass index as a measure of body fatness: Age‐ and sex‐specific prediction formulas. Br J Nutr 2007; 65: 105–114. [DOI] [PubMed] [Google Scholar]
- 17.Margaret A, Sigrid G. Waist to Height Ratio Is a Simple and Effective Obesity Screening Toll for Cardiovascular Risk Factors: Analysis of Data from the British National Diet and Nurtrition Survey of Adult Aged 19‐64 Years. Obesity Facts 2009; 2: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee CM, Huxley RR, Wildman RP, et al Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta‐analysis. J Clin Epidemiol 2008; 61: 646–653. [DOI] [PubMed] [Google Scholar]
- 19.Hortobagyi T, Israel RG, O'Brien KF. Sensitivity and specificity of the Quetelet index to assess obesity in men and women. Eur J Clin Nutr 1994; 48: 769–775. [PubMed] [Google Scholar]
- 20.World Health Organization . Definition, Diagnosis and Classification of Diabetes Mellitus and its Complications. Report of a WHO consultation, World Health Organization, Geneva, 1999. [Google Scholar]
- 21.WHO International Society of Hypertension . Guidelines for the management of hypertension. guidelines subcommittee. J Hypertens 1999; 17: 151–183. [PubMed] [Google Scholar]
- 22.Grundy SM, Cleeman JI, Daniels SR, et al Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005; 112: 2735–2752. [DOI] [PubMed] [Google Scholar]
- 23.Schouw VDYT, Verbeek ALM, Ruijs JHJ. ROC curves for the initial assessment of new diagnostic tests. Fam Pract 1992; 9: 506–511. [DOI] [PubMed] [Google Scholar]
- 24.Metz CE. Basic principles of ROC analysis. Sem Nucl Med 1978; 8: 283–298. [DOI] [PubMed] [Google Scholar]
- 25.DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988; 44: 837–845. [PubMed] [Google Scholar]
- 26.Alberti KG, Zimmet P, Shaw J, IDF Epidemiology Task Force Consensus Group . The metabolic syndrome: a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 27.Abate N, Chandalia M. Ethnicity, type 2 diabetes & migrant Asian Indians. Indian J Med Res 2007; 125: 251–258. [PubMed] [Google Scholar]
- 28.Abate N, Chandalia M, Snell PG, et al Adipose tissue metabolites and insulin resistance in nondiabetic Asian Indian men. J Clin Endocrinol Metab 2004; 89: 2750–2755. [DOI] [PubMed] [Google Scholar]
- 29.Chan JC, Malik V, Jia W, et al Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA 2009; 301: 2129–2140. [DOI] [PubMed] [Google Scholar]
- 30.Asghar S, Khan AKA, Hussain A, et al Incidence of diabetes in Asian‐Indian subjects: A five year follow‐up study from Bangladesh. Primary Care Diabetes 2011; 5: 117–124. [DOI] [PubMed] [Google Scholar]
- 31.Sayeed MA, Mahtab H, Azad Khan AK, et al Waist‐to‐height is a Better Obesity Index than Body Mass Index and Waist‐to‐hip Ratio for Predicting Diabetes, Hypertension and Lipidemia. Bangladesh Med Res Counc Bull 2003; 29: 1–10. [PubMed] [Google Scholar]
- 32.Pua YH, Ong PH. Anthropometric indices as screening tools for cardiovascular risk factors in Singaporean women. Asia Pac J Clin Nutr 2005; 14: 74–79. [PubMed] [Google Scholar]
- 33.Hayashi T, Boyko EJ, McNeely MJ, et al Minimum waist and visceral fat values for identifying Japanese americans at risk for the metabolic syndrome. Diabetes Care 2007; 30: 120–127. [DOI] [PubMed] [Google Scholar]
- 34.Dalton M, Cameron AJ, Zimmet PZ, et al AusDiab Steering Committee Waist circumference, waist‐hip ratio and body mass index and their correlation with cardiovascular disease risk factors in Australian adults. J Intern Med 2003; 254: 555–563. [DOI] [PubMed] [Google Scholar]
- 35.Yusuf S. Obesity and risk of myocardial infraction: the INTERHEART study‐Author's reply. Lancet 2006; 367: 1054. [DOI] [PubMed] [Google Scholar]
- 36.Dong X, Liu Y, Yang J, et al Efficiency of anthropometric indicators of Obesity for identifying cardiovascular risk factors in a Chinese population. Postgrad Med J 2011; 87: 251–256. [DOI] [PubMed] [Google Scholar]


